Abstract
Moment-to-moment adjustment of cerebral blood flow (CBF) to neuronal activity via the homeostatic mechanism known as neurovascular coupling (NVC) has an essential role in maintenance of normal brain function. In advanced age cerebromicrovascular endothelial dysfunction impairs NVC responses, which contribute to age-related cognitive decline. Recently, we have shown that pharmacological treatments that attenuate mitochondrial production of reactive oxygen species (ROS) provide significant neurovascular protection, improving NVC responses in aged mice. Transgenic mice that overexpress human catalase localized to the mitochondria (mCAT) are protected from age-related mitochondrial oxidative stress and exhibit a longevity phenotype associated with resistance to several age-related pathologies. The present study was designed to test the hypothesis that mitochondria-targeted overexpression of catalase also confers protection against age-related impairment of NVC responses. To achieve this goal, NVC responses were assessed in aged (24 months old) mCAT mice and compared with those in age-matched wild-type mice and young control mice by measuring CBF responses (laser speckle contrast imaging) evoked by contralateral whisker stimulation. We found that mitochondrial overexpression of catalase resulted in improved NVC in aged mice due to preserved NO-mediated (L-NAME inhibitable) component of the response. Thus, our present and previous findings demonstrate that interventions that boost mitochondrial antioxidative defenses confer significant cerebromicrovascular protective effects, which preserve NVC responses in aged mice. Our findings provide additional proof-of-concept for the potential use of mitochondria-targeted antioxidants as therapy for prevention of vascular cognitive impairment associated with aging.
Keywords: Oxidative stress, Aging, Mitochondria, Cerebral circulation, Vascular cognitive impairment, Endothelial dysfunction
Introduction
Moment-to-moment adjustment of cerebral blood flow (CBF) to neuronal activity via the homeostatic mechanism known as neurovascular coupling (NVC, also known as “functional hyperemia”) has an essential role in maintenance of normal brain function (Tarantini et al. 2017a). NVC is responsible for increased oxygen and nutrient delivery to the activated neurons, for the effective wash-out of toxic metabolites and for maintenance of an optimal humoral microenvironment within the cerebral tissue. There is an increasing appreciation that vascular contributions to cognitive impairment and dementia (termed “VCID”) play a critical role in older individuals (Tarantini et al. 2016; Carlson et al. 2018; Csipo et al. 2018; Gillon et al. 2018; Ungvari et al. 2018a; Csiszar et al. 2017; Tarantini et al. 2017b; Tucsek et al. 2017; Ungvari et al. 2017; Tarantini et al. 2017c, 2018a, b, 2019; Toth et al. 2017). Importantly, NVC responses are impaired both in older adults (Lipecz et al. 2019; Zaletel et al. 2005; Topcuoglu et al. 2009; Stefanova et al. 2013; Fabiani et al. 2013) and aged rodents (Tarantini et al. 2018b, 2019; Toth et al. 2014; Park et al. 2007), which contributes significantly to the progressive age-related decline in cognitive function (Sorond et al. 2011, 2013). Further support for this concept is provided by experimental studies demonstrating that pharmacologically induced NVC dysfunction in young mice mimics important aspects of age-related cognitive impairment (Tarantini et al. 2015, 2017b).
Production of vasodilator NO by the microvascular endothelial cells is stimulated during neuronal activation, which critically contributes to functional hyperemia in the healthy young brain (Toth et al. 2015a; Chen et al. 2014). There is strong evidence that in advanced age, cerebromicrovascular endothelial dysfunction plays a critical role in the genesis of NVC dysfunction (Tarantini et al. 2018b, 2019; Toth et al. 2014). Recent studies demonstrate that therapeutic interventions, which restore endothelium-dependent, NO-mediated vasodilation in aging, have the capacity to rescue NVC responses and thereby improve cognition (Tarantini et al. 2017a, 2018b, 2019; Toth et al. 2014, 2017). A basic tenet in the field of geroscience is that fundamental cellular and molecular mechanisms of aging underlie all the functional changes in each organ system associated with advanced age and contribute to the pathogenesis of age-related diseases (Kennedy et al. 2014; Sierra and Kohanski 2017; Ashpole et al. 2017; Deepa et al. 2017; Fang et al. 2017; Grant et al. 2017; Podlutsky et al. 2017; Unnikrishnan et al. 2017; Lee et al. 2018; Nacarelli et al. 2018; Reglodi et al. 2018). Thus, with respect to age-related cerebrovascular dysfunction, it is predicted that novel treatments that target fundamental cellular and molecular mechanisms of aging could be developed to improve NVC responses for cognitive benefits (Toth et al. 2017).
The mitochondrial theories of aging predict that increased production of mitochondria-derived ROS (mtROS), consequences of mitochondrial stresses (mitochondrial unfolded protein response, alterations of mitochondrial signals, such as dysregulation of mitochondria-derived peptides, mitochondria-derived damage-associated molecular patterns), and related mitochondrial dysfunction (including impaired mitochondrial energy metabolism, age-related NAD+ depletion) are a critical driving force in the aging process. Multiple lines of evidence, ranging from observations in model organisms (Ng et al. 2014; Katsyuba et al. 2018) to studies on rodent lifespan and health span (Tarantini et al. 2018b; Dai et al. 2011, 2014; Siegel et al. 2013; Schriner and Linford 2006; Schriner et al. 2005), support the validity of these interrelated theories. Recently, we have shown that pharmacological treatments that attenuate mtROS production and/or restore mitochondrial function, including treatment with the mitochondrial-targeted antioxidative peptide SS-31 (Tarantini et al. 2018b), the NAD+ precursor nicotinamide mononucleotide (NMN) (which attenuates mitochondrial oxidative stress by improving electron transport chain coupling by activating SIRT1 (Tarantini et al. 2019; Kiss et al. 2019; Csiszar et al. 2019)), and the mitochondria-targeted antioxidant MitoQ (Tarantini et al. 2019), provide significant neurovascular protection, improving NVC responses in aged mice. Importantly, transgenic mice that overexpress human catalase localized to the mitochondria (mCAT) are protected from age-related mitochondrial oxidative stress and mitochondrial dysfunction, which associate with a significant extension of both median and maximum lifespans (by ~ 5 to 5.5 months) (Schriner and Linford 2006; Schriner et al. 2005). mCAT mice also exhibit resistance to several age-related pathologies, including metabolic syndrome and atherosclerosis, cardiac aging, heart failure, skeletal muscle pathology, sensory defect, neurodegenerative diseases, and cancer (Dai et al. 2009, 2017; Ge et al. 2015; Lee et al. 2010; Olsen et al. 2013; Selvaratnam and Robaire 2016; Treuting et al. 2008; Wang et al. 2017). Despite these advances, the effects of mitochondrial catalase overexpression on cerebral vasculature remain unexplored.
The present study was designed to test the hypothesis that mitochondria-targeted overexpression of catalase can improve NVC responses in aged mice. To achieve this goal, NVC responses were assessed in aged (24 month old) mCAT mice and compared with those in young control and aged control mice by measuring whisker-stimulation-induced increases in CBF (assessed by laser speckle contrast imaging; LSCI).
Methods
Experimental animals
Young (3 month old, n = 6) and aged (24 month old, n = 6) male control C57BL/6 mice and transgenic mice that overexpress human catalase localized to the mitochondria (C57BL/6.mCAT; 24 month old, n = 6) were used. Animals were purchased from the Jackson Laboratories (strain name: B6.Cg-Tg(CAG-OTC/CAT)4033Prab/J; Stock number: 016197). The mCAT mice have a CMV enhancer/chicken beta-actin promoter driving the expression of human catalase gene in mitochondria. Animals were housed under specific pathogen-free barrier conditions in the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center under a controlled photoperiod (12-h light; 12-h dark) with unlimited access to water and were fed a standard AIN-93G diet (ad libitum). All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center.
Assessment of neurovascular coupling responses
Neurovascular coupling responses were assessed as described previously (Tarantini et al. 2017c, 2018b). In brief, mice in each group were anesthetized with isoflurane (2% induction and 1% maintenance), endotracheally intubated, and ventilated (MousVent G500; Kent Scientific Co, Torrington, CT). A thermostatic heating pad (Kent Scientific Co, Torrington, CT) was used to maintain rectal temperature at 37 °C (Toth et al. 2014). End-tidal CO2 was controlled between 3.2 and 3.7% to keep blood gas values within the physiological range, as described (Tarantini et al. 2015; Toth et al. 2015b). The right femoral artery was cannulated for arterial blood pressure measurement (Living Systems Instrumentations, Burlington, VT) (Toth et al. 2014). The blood pressure was within the physiological range throughout the experiments (90–110 mmHg). Mice were immobilized and placed on a stereotaxic frame (Leica Microsystems, Buffalo Grove, IL), the scalp and periosteum were pulled aside, and the skull was gently thinned using a dental drill while cooled with dripping buffer. A laser speckle contrast imager (Perimed, Järfälla, Sweden) was placed 10 cm above the thinned skull, and to achieve the highest CBF response, the right whiskers were stimulated for 30 s at 5 Hz from side to side. Differential perfusion maps of the brain surface were captured. Changes in CBF were assessed above the left barrel cortex in six trials in each group, separated by 5-min intervals. To assess the role of NO mediation, CBF responses to whisker stimulation were repeated after administration of the nitric oxide synthase inhibitor Nω-Nitro-l-arginine methyl ester (L-NAME). Changes in CBF were averaged and expressed as percent (%) increase from the baseline value. Experiments lasted 30 min/mouse, which permitted stable physiological parameters to be obtained. In each study, the experimenter was blinded to the genotype of the animals.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. A P value less than 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M.
Results
Mitochondria-targeted overexpression of catalase rescues neurovascular coupling responses in aged mice by restoring NO mediation
CBF responses in the whisker barrel cortex elicited by contralateral whisker stimulation were significantly decreased in aged mice compared with young animals indicating impaired NVC in aging (representative laser speckle contrast images and CBF tracings are shown in Fig. 1 a and b, summary data are shown in Fig. 1c) (Park et al. 2007). We found that mitochondria-targeted overexpression of catalase significantly increased CBF responses induced by contralateral whisker stimulation in aged mice, restoring NVC to levels observed in young mice (Fig. 1). In young animals, administration of the NO synthase inhibitor L-NAME significantly decreased NVC responses, eliminating the differences among the age groups. In untreated aged animals, administration of L-NAME was without effect (Fig. 1a–c). In contrast, in aged mCAT mice, L-NAME significantly decreased CBF responses elicited by whisker stimulation (Fig. 1a–c), suggesting that mitochondria-targeted overexpression of catalase restored the NO mediation of neurovascular coupling in aged animals.
Fig. 1.
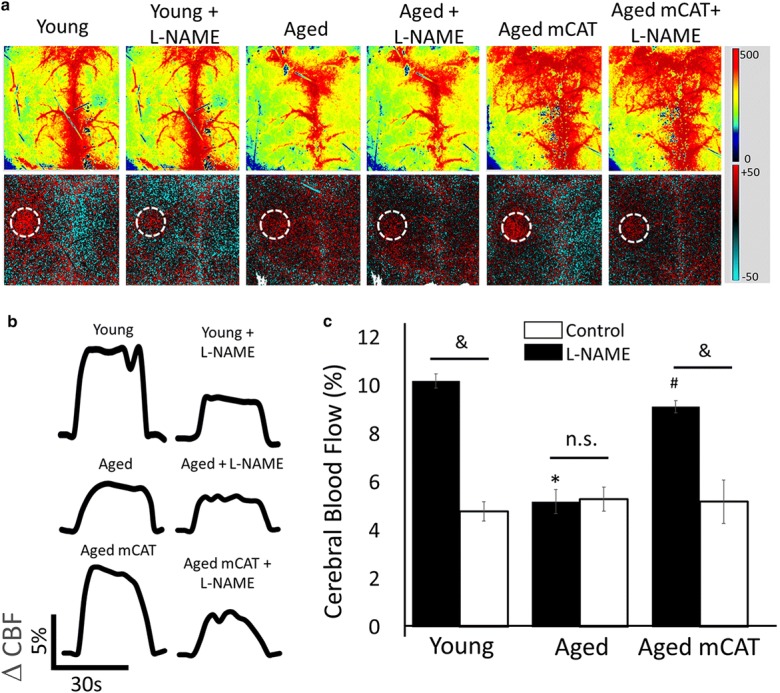
Preserved NO mediated neurovascular coupling responses in aged mCAT mice. Panel a Representative pseudocolor laser speckle flowmetry maps of baseline CBF (upper images) and CBF changes in the whisker barrel field relative to baseline (lower images) during contralateral whisker stimulation (circle, 30 s, 5 Hz) in aged (24 month old) mCAT mice and aged (24 month old) and young (3 month old) control mice. Color bar represents CBF as percent change from baseline. Panel b shows the time-course of CBF changes after the start of contralateral whisker stimulation (horizontal bars). Summary data are shown in panel c. Data are mean ± S.E.M. (n = 4–8 in each group), *P < 0.05 vs. young; #P < 0.05 vs. aged control (one-way ANOVA with post hoc Tukey’s tests)
Discussion
The key finding of this study is that mCAT mice exhibit an antiaging neurovascular phenotype, which results in preserved NVC responses in advanced aging.
Endothelium-derived NO production contributes significantly to normal NVC responses in young mammals. Several lines of evidence support this view, including the observations that genetic deficiency of eNOS, pharmacological inhibition of NO synthesis, endothelial injury, and endothelium-specific depletion of critical vasoprotective factors can similarly impair NVC responses (Tarantini et al. 2018b, 2019; Toth et al. 2014, 2015a; Chen et al. 2014). Strong evidence demonstrates that aging associates with generalized endothelial dysfunction (Csiszar et al. 2019; Csipo et al. 2019; Ungvari et al. 2018b). Extending previous findings in elderly patients and aged laboratory animals, we confirm that aging-induced impairment of cerebromicrovascular endothelial function critically contributes to neurovascular uncoupling in aged mammals (Tarantini et al. 2017a, 2018b, 2019; Park et al. 2007). Here, we demonstrate for the first time that aging-induced decline in NVC is prevented by overexpression of catalase targeted to the mitochondria. These findings extend the results of our earlier investigations, demonstrating that pharmacological treatments, which attenuate mitochondrial oxidative stress, including treatment with SS-31 (Tarantini et al. 2018b), resveratrol (Toth et al. 2014; Ungvari et al. 2009), and MitoQ (Tarantini et al. 2019), also exert significant beneficial effects on NVC responses in aged mice. Importantly, we find that both overexpression of catalase targeted to the mitochondria and treatment with mitochondria-targeted antioxidative peptide SS-31 (Tarantini et al. 2018b) rescue endothelial NO mediation of NVC responses in aged animals. Previous studies show that treatment with mitochondria-targeted antioxidants (MitoQ, SS-31) also rescues agonist-induced, endothelial NO-mediated vasodilation in peripheral arteries in aged mice (Tarantini et al. 2018b; Gioscia-Ryan et al. 2014). MitoQ was also shown to improve endothelial function in peripheral arteries in older adults (Rossman et al. 2018).
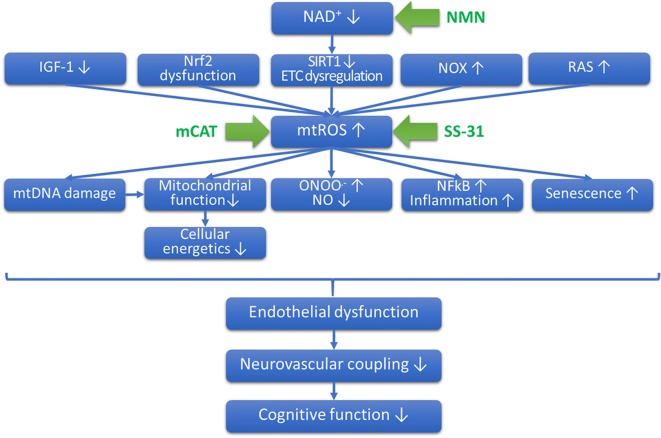
Previous studies suggest that the mechanism(s) by which aging impairs vascular endothelial function involves an increased breakdown of NO by increased levels of superoxide (Toth et al. 2014; Csiszar et al. 2002) (Fig. 2). Although SS-31 and MitoQ are thought to directly reduce superoxide production, mCAT mice express catalase in the mitochondria, which is expected to primarily attenuate the mitochondrial levels of H2O2. As H2O2 readily can penetrate the mitochondrial membranes, it is expected that cytosolic levels of H2O2 will be also significantly attenuated. However, decreased H2O2 levels and increased bioavailability of NO are likely not causally linked. Because direct scavenging of NO is attributed to increased superoxide levels, it is likely that attenuation of H2O2 confers endothelial protection via indirect effects. For example, attenuation of H2O2 levels likely limits oxidative mtDNA damage as well as oxidative modification of lipids and protein damage, which may contribute to the preservation of a youthful mitochondrial function. These factors likely prevent age-related loss of efficiency in mitochondrial electron transport, which should decrease electron leak thereby attenuating mitochondrial superoxide production. Indirect attenuation of mitochondrial superoxide production is expected to increase NO bioavailability. In addition, there is growing evidence that mitochondria-derived ROS regulate the expression/activity of plasma membrane-associated NADPH oxidases in endothelial cells (Dai et al. 2012). Accordingly, in the cerebral vasculature of aged mice, increased mtROS is associated with an upregulation of NADPH oxidases (Toth et al. 2014; Park et al. 2007), which may be reversed by interventions that attenuate mtROS production (Tarantini et al. 2018b; Toth et al. 2014). Thus, it is possible that mitochondria-targeted overexpression of catalase may also alter the interplay between mitochondrial ROS and NADPH oxidases, disrupting a vicious feed-forward cycle that diminishes NO bioavailability, impairing NVC responses. This possibility should be experimentally tested in future studies. An additional method by which mCAT may exert endothelial protective effects involves preservation of mitochondrial/cellular energetics.
Fig. 2.
Proposed role for increased mitochondrial oxidative stress in cerebromicrovascular endothelial impairment and neurovascular dysfunction in aging. The scheme depicts that age-related decline in circulating IGF-1 (Csiszar et al. 2008), impairment of Nrf2-regulated antioxidant mechanisms (Tarantini et al. 2018a; Ungvari et al. 2018b; Dai et al. 2012; Bailey-Downs et al. 2012; Fulop et al. 2018; Ungvari et al. 2011a, b), decline in NAD+ levels and SIRT1 activity and consequential dysregulation of components of the electron transport chain (ETC) (Tarantini et al. 2019), upregulation of NADPH oxidases (NOX) (Park et al. 2007), and the local renin-angiotensin system (RAS) (Dai et al. 2012) act synergistically to promote increased mitochondrial production of reactive oxygen species (mtROS). Mitochondrial oxidative stress promotes mtDNA damage, exacerbating mitochondrial dysfunction, promotes peroxynitrite (ONOO.-) formation reducing bioavailability of NO, promotes NFkB activation (Ungvari et al. 2007), and induces endothelial senescence. All of these mechanisms contribute to endothelial dysfunction, which impair neurovascular coupling responses compromising cognitive function. The model predicts that diverse interventions that attenuate mitochondrial oxidative stress will confer endothelial protection, improving neurovascular coupling responses, thereby preserving cognitive function in old age. Accordingly, overexpression of catalase targeted to the mitochondria (mCAT), treatment with mitochondria-targeted antioxidants (SS-31), and sirtuin-activating treatments (e.g., the NAD+ booster nicotinamide mononucleotide [NMN]) attenuate mtROS production in endothelial cells, improving cerebromicrovascular endothelial function and rescuing neurovascular coupling responses
In conclusion, our present and previous findings demonstrate that interventions that boost mitochondrial antioxidative defenses confer significant cerebromicrovascular protective effects, which rescue NVC responses in aged mice (Fig. 2). Our findings provide additional proof-of-concept for the potential use of mitochondria-targeted antioxidants as therapy for prevention of vascular cognitive impairment associated with aging.
Funding information
This work was financially supported by grants from the American Heart Association (ST), the Oklahoma Center for the Advancement of Science and Technology (to AC, AY, ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; R01-AG055395), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC, R01-NS100782 to ZU), the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (GM104938, to AY), the Presbyterian Health Foundation (to ZU, AC, AY), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (1P20GM125528, sub#5337).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BW, Craft MA, Carlson JR, Razaq W, Deardeuff KK, Benbrook DM. Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. Geroscience. 2018;40:325–336. doi: 10.1007/s11357-018-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM (2014) A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014 Jun 12;3(3):e000787. 10.1161/JAHA.114.000787 [DOI] [PMC free article] [PubMed]
- Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, Yabluchanskiy A (2018) Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. Geroscience [DOI] [PMC free article] [PubMed]
- Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BN, Dzialendzik M, Tarantini S, Balasubramanian P, Kiss T, Yabluchanska V, Silva-Palacios F, Courtney DL, Dasari TW, Sorond F, Sonntag WE, Csiszar A, Ungvari Z, Yabluchanskiy A. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41(2):125–136. doi: 10.1007/s11357-019-00063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A (2017) Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. [DOI] [PMC free article] [PubMed]
- Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA, Ungvari ZI. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;316(6):H1253–H1266. doi: 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chiao YA, Martin GM, Marcinek DJ, Basisty N, Quarles EK, Rabinovitch PS. Mitochondrial-targeted catalase: extended longevity and the roles in various disease ,models. Prog Mol Biol Transl Sci. 2017;146:203–241. doi: 10.1016/bs.pmbts.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience. 2017;39:187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G (2013) Neurovascular coupling in normal aging: a combined optical, ERP and fMRI study. Neuroimage [DOI] [PMC free article] [PubMed]
- Fang Y, McFadden S, Darcy J, Hill CM, Huber JA, Verhulst S, Kopchick JJ, Miller RA, Sun LY, Bartke A. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience. 2017;39:347–356. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Xuang, Pettan-Brewer Christina, Morton John, Carter Katrina, Fatemi Sy, Rabinovitch Peter, Ladiges Warren C. Mitochondrial catalase suppresses naturally occurring lung cancer in old mice. Pathobiology of Aging & Age-related Diseases. 2015;5(1):28776. doi: 10.3402/pba.v5.28776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon A, Nielsen K, Steel C, Cornwall J, Sheard P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. Geroscience. 2018;40:177–192. doi: 10.1007/s11357-018-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CD, Jafari N, Hou L, Li Y, Stewart JD, Zhang G, Lamichhane A, Manson JE, Baccarelli AA, Whitsel EA, Conneely KN. A longitudinal study of DNA methylation as a potential mediator of age-related diabetes risk. Geroscience. 2017;39:475–489. doi: 10.1007/s11357-017-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, Ryu D, Cialabrini L, Matilainen O, Liscio P, Giacche N, Stokar-Regenscheit N, Legouis D, de Seigneux S, Ivanisevic J, Raffaelli N, Schoonjans K, Pellicciari R, Auwerx J. De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature. 2018;563:354–359. doi: 10.1038/s41586-018-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z (2019) Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for prevention of vascular cognitive impairment. GeroScience in press [DOI] [PMC free article] [PubMed]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W, Rabinovitch PS, Santos JH, Petersen KF, Samuel VT, Shulman GI. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Feliers D, Barnes JL, Oh S, Choudhury GG, Diaz V, Galvan V, Strong R, Nelson J, Salmon A, Kevil CG, Kasinath BS. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience. 2018;40:163–176. doi: 10.1007/s11357-018-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipecz Agnes, Csipo Tamas, Tarantini Stefano, Hand Rachel A., Ngo Bich-Thy N., Conley Shannon, Nemeth Gabor, Tsorbatzoglou Alexis, Courtney Donald L., Yabluchanska Valeriya, Csiszar Anna, Ungvari Zoltan I., Yabluchanskiy Andriy. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. GeroScience. 2019;41(3):341–349. doi: 10.1007/s11357-019-00078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C (2018) Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. Geroscience [DOI] [PMC free article] [PubMed]
- Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Olsen RH, Johnson LA, Zuloaga DG, Limoli CL, Raber J. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem. 2013;125:303–313. doi: 10.1111/jnc.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017;39:147–160. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Atlasz T, Szabo E, Jungling A, Tamas A, Juhasz T, Fulop BD, Bardosi A. PACAP deficiency as a model of aging. Geroscience. 2018;40:437–452. doi: 10.1007/s11357-018-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension. 2018;71:1056–1063. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner Samuel E., Linford Nancy J. Extension of mouse lifespan by overexpression of catalase. AGE. 2006;28(2):209–218. doi: 10.1007/s11357-006-9010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Selvaratnam J, Robaire B. Overexpression of catalase in mice reduces age-related oxidative stress and maintains sperm production. Exp Gerontol. 2016;84:12–20. doi: 10.1016/j.exger.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, Kavanagh TJ, Szeto HH, Rabinovitch PS, Marcinek DJ. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell. 2013;12:763–771. doi: 10.1111/acel.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F, Kohanski R. Geroscience and the trans-NIH geroscience interest group, GSIG. Geroscience. 2017;39:1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell’Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81(10):904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Stephan T, Becker-Bense S, Dera T, Brandt T, Dieterich M. Age-related changes of blood-oxygen-level-dependent signal dynamics during optokinetic stimulation. Neurobiol Aging. 2013;34:2277–2286. doi: 10.1016/j.neurobiolaging.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini Stefano, Tran Cam Ha T., Gordon Grant R., Ungvari Zoltan, Csiszar Anna. Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Experimental Gerontology. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zolei-Szenasi D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience. 2017;39(4):465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott ME, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018;73(7):853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini Stefano, Valcarcel-Ares Noa M., Yabluchanskiy Andriy, Fulop Gabor A., Hertelendy Peter, Gautam Tripti, Farkas Eszter, Perz Aleksandra, Rabinovitch Peter S., Sonntag William E., Csiszar Anna, Ungvari Zoltan. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17(2):e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett. 2009;452:17–22. doi: 10.1016/j.neulet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–H1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, Rabinovitch PS, Ladiges WC. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:813–822. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fulop G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z (2017) Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. [DOI] [PMC free article] [PubMed]
- Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Yabluchanskiy A, Tarantini S, Toth P, Kirkpatrick AC, Csiszar A, Prodan CI. Repeated Valsalva maneuvers promote symptomatic manifestations of cerebral microhemorrhages: implications for the pathogenesis of vascular cognitive impairment in older adults. Geroscience. 2018;40:485–496. doi: 10.1007/s11357-018-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnikrishnan A, Jackson J, Matyi SA, Hadad N, Wronowski B, Georgescu C, Garrett KP, Wren JD, Freeman WM, Richardson A. Role of DNA methylation in the dietary restriction mediated cellular memory. Geroscience. 2017;39:331–345. doi: 10.1007/s11357-017-9976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang W, Wang N, Tall AR, Tabas I. Mitochondrial oxidative stress promotes atherosclerosis and neutrophil extracellular traps in aged mice. Arterioscler Thromb Vasc Biol. 2017;37:e99–e107. doi: 10.1161/ATVBAHA.117.309580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol. 2005;20:115–120. [PubMed] [Google Scholar]