Abstract
Preclinical studies provide strong evidence that age-related impairment of neurovascular coupling (NVC) plays a causal role in the pathogenesis of vascular cognitive impairment (VCI). NVC is a critical homeostatic mechanism in the brain, responsible for adjustment of local cerebral blood flow to the energetic needs of the active neuronal tissue. Recent progress in geroscience has led to the identification of critical cellular and molecular mechanisms involved in neurovascular aging, identifying these pathways as targets for intervention. In order to translate the preclinical findings to humans, there is a need to assess NVC in geriatric patients as an endpoint in clinical studies. Functional near-infrared spectroscopy (fNIRS) is a non-invasive neuroimaging technique that enables the investigation of local changes in cerebral blood flow, quantifying task-related changes in oxygenated and deoxygenated hemoglobin concentrations. In the present overview, the basic principles of fNIRS are introduced and the application of this technique to assess NVC in older adults with implications for the design of studies on the mechanistic underpinnings of VCI is discussed.
Keywords: Aging, Neurovascular coupling, Functional near-infrared spectroscopy, fNIRS, Vascular cognitive impairment and dementia, VCI, VCID, Cognitive aging
Introduction
Global population is rapidly aging, and it is now predicted that over 30% of western world will be over the age of 65 by 2050. In these older adults, vascular cognitive impairment (VCI) and dementia are the leading causes of disability and a critical contributing factor to decreased quality of life. Accumulating evidence over the past decade suggests that functional and structural impairment of cerebral microcirculation significantly contributes to age-related cognitive decline (Toth et al. 2017). Among the microvascular mechanisms involved in the pathogenesis of VCI, the importance of age-related impairment of a key cerebral homeostatic mechanism, neurovascular coupling (NVC), has received much attention in the past decade (Faraco et al. 2016; Girouard and Iadecola 2006; Gorelick et al. 2011; Hamel et al. 2016; Iadecola 2004; Nicolakakis and Hamel 2011; Papadopoulos et al. 2016; Park et al. 2007; Park et al. 2014; Tarantini et al. 2017b; Tarantini et al. 2017c; Tarantini et al. 2019; Tarantini et al. 2018a; Tarantini et al. 2018b; Tarantini et al. 2017d; Tong et al. 2012; Toth et al. 2014a). NVC is impaired in animal models of aging and accelerated vascular aging (Tarantini et al. 2017c). Selective experimental disruption of NVC results in cognitive impairment in rodent models, demonstrating a causal role for impaired NVC in cognitive decline (Tarantini et al. 2015b; Tarantini et al. 2017d). Further, preclinical studies provide direct evidence that restoration of NVC by pharmacological interventions is associated with cognitive benefit (Papadopoulos et al. 2016; Tarantini et al. 2019; Tarantini et al. 2018b; Tong et al. 2012). In order to translate these preclinical findings to humans, NVC should be measured as an endpoint in clinical studies. The standard method of NVC assessment in humans is functional magnetic resonance imaging (fMRI). fMRI is widely used in neuropsychological studies, however, it has also major limitations such as the need of immobilization of the patient, the need of highly trained personnel, and high operating costs. Thus, there is an urgent need to adapt easy-to-use, affordable, and convenient methodologies to assess NVC in geriatric patients in an outpatient setting with good sensitivity and repeatability.
Since the development of functional near-infrared spectroscopy (fNIRS) in the mid-80s, the usage of this method to assess changes in cerebral blood flow (CBF) during neuronal activation has been increasing gradually. Because of its safety, affordability, portability, and high temporal resolution, fNIRS has potential for widespread implementation in geroscience research. fNIRS is particularly suited for geriatric patients and combined cognitive/NVC studies involving interactivity. Many excellent studies have been published on improving fNIRS technology, developing and refining data analysis methods, and confirming the validity of the fNIRS-based methods by reproducing the results obtained via other imaging techniques (e.g., fMRI) (Strangman et al. 2002a, 2002b). As fNIRS technology has matured significantly in the past decade, routine measurement of NVC in older humans became feasible.
In this review, a brief overview on the physiology of NVC and the effects of aging on NVC are provided. The role of neurovascular impairment in cognitive decline is considered and the usage of fNIRS-based methods to investigate age-related changes in NVC to identify patients at risk is discussed. The basic principles of fNIRS are introduced and the benefits and the potential limitations of application of this technique to assess NVC in older adults are highlighted. The review is organized into four sections: (1) Neurovascular coupling: age-related changes and role in cognitive decline. (2) Measuring neurovascular coupling in human subjects: from fMRI to fNIRS and (3) perspectives.
Neurovascular coupling: physiological mechanisms, age-related changes, and their role in cognitive decline
Physiology of neurovascular coupling
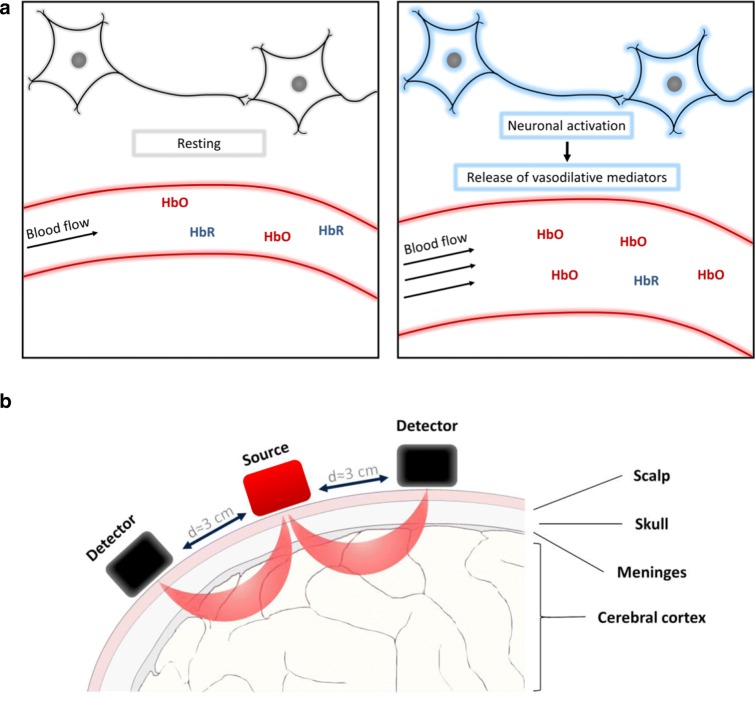
Although the brain accounts for only 2% of the total body mass, it is responsible for 20% of the total oxygen and energy consumption, which makes it the most metabolically active organ in the human body (Tarantini et al. 2017c). During neuronal activation, there is a sudden increase in nutrient and oxygen demand. As the brain does not have significant energy and oxygen reserves, normal brain function depends on an uninterrupted supply of nutrients and oxygen via the cerebral microcirculation. Thus, moment-to-moment adjustment of CBF to neuronal activity via NVC (also known as “functional hyperemia”) has an essential role in maintenance of normal brain function (Tarantini et al. 2017c). NVC is responsible for increased oxygen and nutrient delivery to the activated brain regions, the efficient wash-out of toxic metabolites and maintenance of an optimal humoral microenvironment within the cerebral tissue (Fig. 1a). NVC depends on a coordinated interaction among active neurons, astrocytes, smooth muscle cells, and endothelial cells, which results in prompt dilation of cerebral resistance arterioles with a concomitant significant increase in local cerebral blood flow to the active brain regions (Tarantini et al. 2017a). The rapid influx of oxygenated hemoglobin (HbO) and washout of deoxyhemoglobin (HbR) during NVC processes enables the real-time visualization of functional hyperemia in vivo using fMRI and fNIRS (see below).
Fig. 1.
fNIRS imaging of neurovascular coupling responses in cortical regions in humans. a fNIRS uses near-infrared light to assess neurovascular coupling evoked increases in blood flow by measuring changes in the concentration of oxygenated (HbO) and deoxygenated (HbR) hemoglobin in the brain region immediately below the optodes at baseline and during neuronal activation. HbO and HbR are the main chromophores absorbing near-infrared light and they exhibit distinct absorption spectra. b The surface of the head is irradiated with a combination of near-infrared wavelengths of light generated by the light source (“Source”). Photons returning to the surface of the head after traveling a banana-shaped path (in red) in the tissues are captured by the photodetector (“Detector”) on the scalp. The number and array of the light source and photodetector on the head vary between studies
Age-related changes in neurovascular coupling: role in age-related cognitive decline
There is growing evidence that NVC responses are impaired both in older adults (Fabiani et al. 2013; Lipecz et al. 2019; Stefanova et al. 2013; Topcuoglu et al. 2009; Yang et al. 2017; Zaletel et al. 2005), aged laboratory animals (Park et al. 2007; Tarantini et al. 2019; Tarantini et al. 2018b; Toth et al. 2014b), and animal models of accelerated vascular aging (Toth et al. 2014b), which associate with a significant decline in cognitive function (Sorond et al. 2013a; Sorond et al. 2011). Important in this regard is that pharmacological induction of NVC dysfunction in young mice mimics several aspects of age-related cognitive impairment (Tarantini et al. 2015a; Tarantini et al. 2017d), suggesting that age-related NVC impairment and cognitive decline are causally linked. Indeed, recent studies demonstrate that therapeutic interventions, which improve microvascular function in aging, have the capacity to rescue NVC responses and thereby improve cognition (Tarantini et al. 2017c; Tarantini et al. 2019; Tarantini et al. 2018b; Toth et al. 2017; Toth et al. 2014b).
Measuring neurovascular coupling in human subjects: from fMRI to fNIRS
Among currently used methods to measure NVC in humans, many studies have utilized functional magnetic resonance imaging (fMRI) approach to evaluate functional hyperemia as a proxy measure for neuronal activation. fMRI approach uses the diamagnetic and paramagnetic qualities of HbO and HbR to calculate brain concentration of HbR using a T2* relaxation magnetic resonance signal (Ogawa et al. 1990). This so-called blood-oxygen-level-dependent (BOLD) signal increases with the decrease of HbR concentration, which is commonly interpreted to occur as a result of increased washout of HbR due to local increase in CBF. However, recent studies suggest that fMRI alone may not provide a definitive explanation for the BOLD signal in older adults (Wright and Wise 2018). Arterial spin labeling fMRI (ASL-fMRI) allowing measurement of cerebral blood flow (CBF), showed lower resting CBF in older adults (Restom et al. 2007). Considering inverse correlation between resting CBF and the BOLD signal change (Cohen et al. 2002; Stefanovic et al. 2006) and accounting for the lower resting CBF in older adults, fMRI data suggest a significant vascular component behind the age-related differences in BOLD signal (Zebrowitz et al. 2016). However, due to the complexity of the BOLD signal, there may be several age-related changes that may potentially lead to misinterpretation of the results. In addition, research studies that utilize the fMRI approach for assessment of NVC may often be underpowered due to the high costs associated with the use of equipment. The fMRI approach also imposes additional restrictions due to the prolonged duration of the procedure and limits the choice of methods of neuronal stimulation to those that can be performed in essentially immobilized subject.
In the past two decades, functional near-infrared spectroscopy (fNIRS) has emerged as a promising tool to detect NVC in human subjects, first mainly used as a proxy measure for neuronal activation. In this context, fNIRS has been used in several studies related to autism (Zhang and Roeyers 2019), pediatric studies (Bortfeld 2019), and psychiatry (Ehlis et al. 2014; Grazioli et al. 2019). In the present review, we discuss the potential use of fNIRS-based methods to study NVC responses in translational geroscience research. Similar to the fMRI BOLD signal, the signal measured by fNIRS is dependent on the changes of hemoglobin (Hb) concentration in the cortex. First, we discuss the biophysical principles of fNIRS and describe the methods to assess cerebral blood flow using fNIRS. Some of the technical aspects to separate the cerebral hemodynamic signal from physiological fluctuations in the signal and the potential limitations of the methods are also discussed. Finally, we provide a detailed overview on the benefits of fNIRS-based methods to assess NVC in an outpatient setting and describe future directions of studies using this technique in VCI research.
Biophysical principles of fNIRS
Biological tissues are relatively transparent to light in a part of near-infrared (NIR) window (800–2500 nm), allowing usage of NIR light in physiological measurements. It is indispensable for functional brain imaging studies that NIR light can readily penetrate superficial layers (scalp and skull) towards the brain cortex (Jobsis 1977). In fact, only the wavelength range of 650–950 nm is particularly suitable for studying the in vivo optical properties, given that the majority of photons are absorbed by hemoglobin below 650 nm and by water above 950 nm. HbO and HbR are the chromophores (NIR-absorbers) of main physiological interest since their in vivo dynamics have the greatest impact on the measured signals (compared to cytochromes also absorbing NIR light but in a practically constant manner). The aim of NIRS is to measure the relative or absolute concentration of chromophores (Jobsis 1977) that is explicitly related to detected NIR light attenuation.
Photon paths are usually very complex in a turbid medium which justifies to model it in line with the theory of photon diffusion (Arridge 1999). This is a special case of a model described by radiative transport equation (Chandrasekhar 1960) assuming μs> > μa, where μs is the scattering coefficient and μa is the absorption coefficient. This elastic collision between a NIR-photon and a particle of the illuminated tissue results in an average angle change of 20–30° (Cheong et al. 1990). The consequent anisotropy is incorporated in the definition of the commonly used reduced-scattering coefficient, μs′ (Torricelli et al. 2001), which is inversely proportional to the average distance between two collisions. The aforementioned interaction gave rise to a number of quantification problems related to time-varying scattering loss or heterogeneities in the tissue (partial volume effect) (Obrig and Villringer 2003). Importantly, the considerable scattering of tissues is mainly due to biological membranes and lipid bilayers with an uneven spatial distribution (Cope 1991). Moreover, its degree is influenced by cell volume changes due to slow redistribution of intracranial fluid volumes and accompanying neuronal action potentials (Obrig and Villringer 2003). Nevertheless, scattering phenomena enabled topographic measurement geometries, where the NIR light sources and detectors are arranged in a grid separated by a distance d. Provided that an adult head is sampled with 2.5 cm < d < 6 cm, a predictable amount of detected photons pass through a “banana-shaped” volume (Bunce et al. 2006) restricted to the brain cortex (Fig. 1b). NIR photons are hardly able to penetrate the white matter as they are reflected back from its boundary. Denoting the corresponding optical pathlength by L, its relation to d is simply L = d ∙ DPF, where DPF is differential pathlength factor accounting for the additional path due to scattering (Cope et al. 1988). Of note, the DPF may change with age due to the structural changes of tissue the photons pass through (Scholkmann and Wolf 2013).
The continuous wave NIRS (cwNIRS) method uses multi-wavelength light source with a constant intensity and measures the average decrease in it to quantify attenuation (Jobsis 1977; Scholkmann et al. 2014). In this case, only absorption changes can be measured assuming a constant scattering loss. The relative concentration of HbO and HbR, and their sum, total hemoglobin (HbT), could be assessed with the aid of Beer-Lambert law modified for highly scattering medium (Cope et al. 1988; Kocsis et al. 2006). Its differential form is written as:
| 1 |
from where ∆μa can be expressed:
| 2 |
By rearranging (2), concentration changes are obtained if:
| 3 |
Another important issue is the biological source of the detected signal, the changes of which are assumed to origin from the brain cortex. The contribution of extracerebral tissue to NIRS records is a recognized limitation of non-invasive optical studies of the brain. Various methods have been proposed to address this problem (Hueber et al. 1999; Suzuki et al. 1999). Generally, a better sampling of the brain can be achieved if the separation (d) is increased. However, this reduces the number of detected photons due to increased L, and it is still not able to distinguish the intracerebral component. The principle of spatially resolved spectroscopy is that detectors with small d mainly capture hemodynamics from shallower regions (skin, skull, cerebrospinal fluid) (Suzuki et al. 1999), while the origin of signals measured by detectors further from their corresponding light sources is mainly cerebrocortical (Franceschini et al. 1998). Hence, it is reasonable to assume that if extracerebral change of optical properties influence both records, it can be removed by using the signal coming from a less distant detector.
Cerebral blood flow measurement using fNIRS
Although cwNIRS provides excellent means to monitor changes in hemodynamics in the brain cortex, it is not capable of measuring absolute (baseline) Hb concentration, only the amplitude of the change in Hb concentration. Thus, relative change of cerebral blood flow (rCBF) cannot be measured directly. This drawback is also relevant in fNIRS studies since the locally increased blood flow elicited by neural activity via NVC is represented by and limited to the change of HbO and HbR concentration in the imaged compartment. Since this functional hyperemia is a hallmark of the local hemodynamic response, it may provide an important tool to identify cerebrovascular pathophysiological processes. Therefore, it is important to extend near-infrared optical imaging to enable cerebral perfusion measurements. Several dynamic models have been proposed to address this issue (Huneau et al. 2015) Buxton et al. devised a model specifically applicable to NIRS data, that treats the regional vascular compartment as a lumped representation of the vessels in the probed brain cortex (Buxton et al. 1998). It has been widely used to interpret hemodynamic changes accompanying brain activation captured either by functional magnetic resonance imaging (fMRI) or fNIRS (Cui et al. 2010; Mildner et al. 2001; Mukli et al. 2018). The term “balloon model” was coined to describe the viscoelastic behavior predicted by the underlying equations of the model:
| 4 |
| 5 |
| 6 |
where q, v, and p denote HbR, blood volume, and HbT, respectively. In simulation studies, certain assumptions are necessary about the shape of fin(t), for example, modeling it with a gamma-variate function. This reduces the number of unknown variables rendering the differential equation system (4–6) solvable. It follows that if in vivo data is available about hemoglobin concentration dynamics and its relation to flow changes are also examined in silico, inferences can be made about fin(t). Owing to the lumped nature and possibly violated assumptions of the balloon model (and others), tools enabling a more direct assessment of relative CBF are preferable. In contrast to cwNIRS, frequency-domain multi-distance NIRS (FDMD-NIRS) utilizes light sources that are capable of emitting amplitude-modulated light beams, which also allow measurement of absolute Hb concentration and tissue oxygen saturation (SO2) (Gatto et al. 2006). However, latter method is associated with higher costs and less compact instrumentation.
Separation of the cerebral hemodynamic signal from physiological fluctuations in the signal
Changes in measured HbO and HbR signals due to increases in local CBF evoked by neuronal stimulation can be affected by physiological events related to the cardiac cycle, breathing, and blood pressure fluctuations. There are many methods extant to eliminate such interference, which may arise from the superficial tissue layers (scalp, skull) and also the brain itself. Frequency-based algorithms (e.g., bandpass filtering, low-pass filtering, moving averaging) have been developed to eliminate high-frequency instrument noise and low-frequency drift (Izzetoglu et al. 2005). These approaches can effectively remove interference caused by oscillations related to the cardiac cycle, but usually fail to remove physiological noise signals related to breathing and blood pressure variation. Importantly, physiological noise exhibits broad spatial distribution, while changes in the HbO and HbR signals due to neural activity are localized (e.g., unilaterally in the motor/somatosensory cortex during finger tapping). An increasing number of algorithms have been developed for noise reduction in fNIRS studies that take advantage of these characteristics (Saager and Berger 2008; Saager and Berger 2005; Saager et al. 2011; Zhang et al. 2005). An important emerging approach is to use signals from a channel with a very short distance between the emitter and detector as a reference (Prince et al. 2003). Ideally, such noise reduction techniques should also be applied to real-time processing (Abdelnour and Huppert 2009).
Matching fNIRS signal to neuronal activity during cognitive stimulation
Using fNIRS approach allows to evaluate hemodynamic NVC responses in human brain during neural stimulation. Commonly accepted methods of neural stimulation include performing a cognitive task (e.g., N-back test or similar paradigms) (Sorond et al. 2013b), visual stimulation (Stickland et al. 2019), auditory stimulation (Hendrikx et al. 2019; Schei et al. 2012), or motor tasks such as finger-tapping (Siero et al. 2013), all of which can easily be adapted for studying NVC with fNIRS. In addition, the fNIRS approach allows simultaneous assessment of EEG signal, which provides important information on synchronization of hemodynamic changes with neural activity in corresponding brain regions.
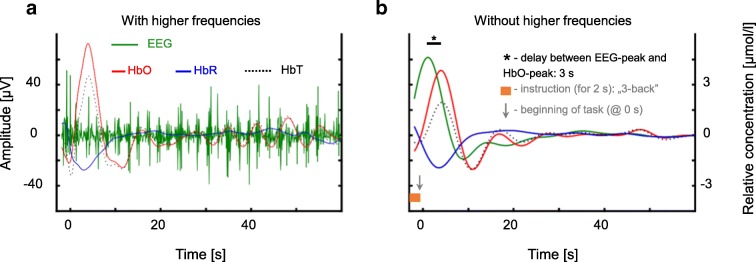
Figure 2 demonstrates the relationship between neural activity related to a cognitive stimulus and the consequential hemodynamic changes. Data obtained from a 26-year-old individual (female, right-handed) performing a 3-back cognitive task were analyzed. The participant was randomly selected from a freely available data repository (Shin et al. 2018) and asked to perform the N-back cognitive task. During the N-back test, participants are required to respond to a sequence of changing letters on the monitor screen by clicking the mouse button upon recognizing the requested pattern (0-back: response was requested when the symbol “X” was presented; 2-back: response was requested when a presented number repeated itself 2 numbers back, e.g., 2-x-2-x; 3-back: response was requested when the number repeated itself 3 numbers back, e.g., 2-x-x-2). Each N-back trial took 40 s with 20-s rest period between trials. The greatest hemodynamic response was observed in the prefrontal cortex during 3-back test when compared to 2-back and 1-back tests. Correlation between EEG and fNIRS signals was assessed in the ranges of 5–14 Hz and 0.01–0.1 Hz (Fig. 2). These data demonstrate rapid hemodynamic responses upon administration of the cognitive task that positively and strongly correlated with the start of neuronal activity measured with EEG.
Fig. 2.
Relationship between bandpass-filtered electroencephalographic (EEG) and near-infrared spectroscopy (NIRS) signals in the prefrontal cortex during N-back task session. The channel position was FP2 for EEG and AFp8 for NIRS. a The EEG signal (amplitude) was filtered between 5 and 14 Hz using a zero-phase 4th order Butterworth filter in order to preserve only higher frequency brain waves related to synchronization and associated with neurovascular coupling (Talukdar et al. 2015). The corresponding oxyhemoglobin (HbO), deoxyhemoglobin (HbR) and total hemoglobin (HbT = HbO + HbR, all are concentrations) time series were obtained by filtering (zero-phase 3rd order Butterworth) NIRS-signals in the 0.02–0.4 Hz frequency band which did not contain high-frequency systemic signals (due to cardiac pulsation, etc.). b Shows the same EEG- and hemodynamic response elicited by cognitive stimulus as in a, but representing the low-frequency oscillations (LFO). Accordingly, all signals were bandpass-filtered with the corresponding lower (0.02 Hz) and higher cutoff frequency (0.1 Hz). Before the task started (indicated by dark-grey arrow) the subject had been instructed to prepare (orange bar) for 3-back task. The accompanying increase in EEG is followed by an increase in HbO with a 3-s delay (black bar, from EEG-peak to HbO-peak). All data shown were obtained from a 26-year-old right-handed female, further details of the measurement can be found in the paper describing the dataset (Shin et al. 2018)
Application of fNIRS to study neurovascular dysfunction in older adults
There is an acute need to establish easy-to-use fNIRS-based methods that could be used in older individuals to assess age-related changes in cerebromicrovascular function by measuring NVC and to use these methods to evaluate treatment effects in clinical investigations. Therefore, a major goal is to develop methods that allow the assessment of the cerebromicrovascular responses independent of changes in neuronal activation. One important challenge is that age-related changes in behavioral performance are associated with changes in neural patterns of activation. Specifically, older participants were shown to exhibit more generalized less specific cerebral activation in response to cognitive tasks and the recruitment of additional frontal regions that are not activated in younger adults (DiGirolamo et al. 2001; Gold et al. 2010; Milham et al. 2002; Sleimen-Malkoun et al. 2014). Thus, identifying the right cognitive challenge is essential for studies attempting to compare NVC in younger and older individuals with the goal to draw conclusions about cerebromicrovascular health.
The sequential finger-opposition/tapping tasks are useful methods to elicit quasi-similar neuronal activation in the primary motor cortex, which is associated with a well-quantifiable fNIRS cortical signal. Several studies demonstrated that NVC elicited by the finger tapping task is consistent over several days (Kashou et al. 2016). Finger tapping task usually produces repeatable fNIRS signals, which are quite reliable for the best optode channel. This is a fine, delicate movement, thus motion artifacts due to head movement are usually not an issue.
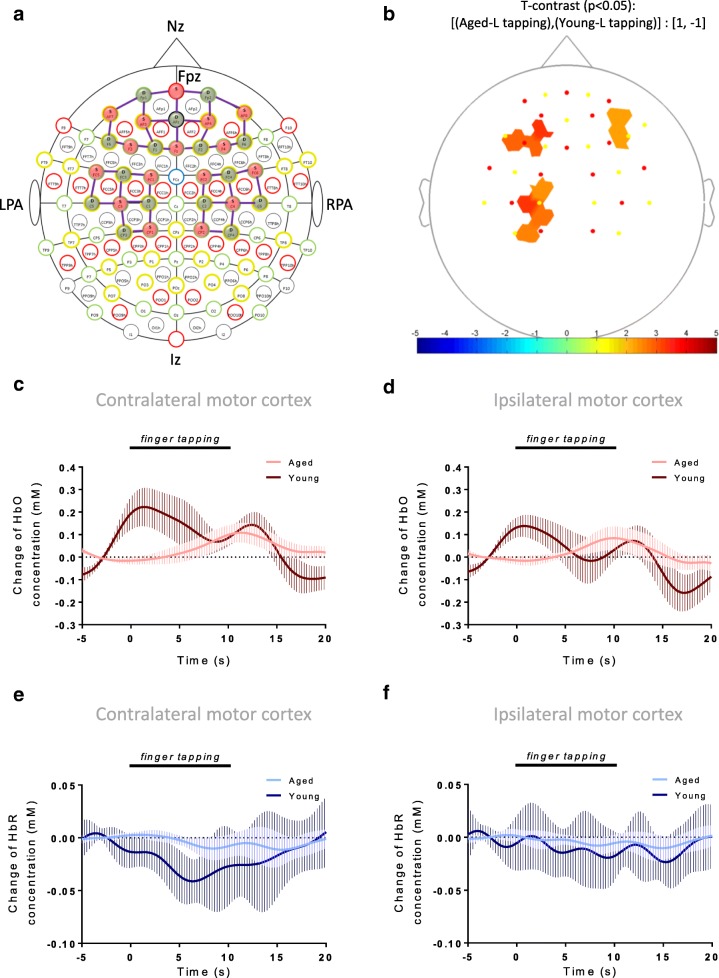
To demonstrate the applicability of the fNIRS approach to assess NVC in older individuals we present our findings obtained in relative healthy older adults using the finger taping task. The data were obtained in a cohort of n = 11 young (32 ± 1.8 years of age, n = 7 males, all participants right-handed) and n = 13 aged (76 ± 2.17 years of age, n = 9 males, all participants right-handed) healthy individuals. Participant selection was taken from an on-going clinical study on healthy aging at the University of Oklahoma Health Sciences Center. All participants provided informed consent prior to participation in the study. To measure fNIRS signal we used the NIRScout platform (NIRx Medical Technologies LLC, NY, USA). We positioned a 128-port Easycap headcap covering the area of the international 10-10 system on the subject’s head. The sagittal line between Fpz and Iz ports on the cap was aligned with the sagittal plane of the head, and the optode in the Fpz port was positioned along this line. The cap was set up with custom spacers that limit the variability of distance between optodes, providing an average source-detector distance of 3 cm. The placement of optodes covered the prefrontal cortex and medial motor cortex extending to the areas of C5 or C6 laterally (Fig. 3a). Measurements were taken in a quiet and darkened room. Each participant was asked to remain silent and as still as possible, aside from the hand movement tasks.
Fig. 3.
Hemodynamic neurovascular coupling responses are altered in older adults during finger-tapping task. To demonstrate the applicability of the fNIRS approach to measure neurovascular coupling in aging, n = 11 young (32 ± 1.8 years of age, n = 7 males, all participants are right-handed) and n = 13 aged (76 ± 2.17 years of age, n = 9 males, all participants are right-handed) healthy individuals were administered finger-tapping task and fNIRS signal was recorded from the motor cortex areas. a Shows optode placement. b Results of group level SPM analysis are shown, where individual general linear modeling (GLM) analysis results are combined into group averages, and averages were compared with a t-contrast of [(aged group, left finger tapping), (young group, left finger tapping)]:[1, -1]. Only channels with p < 0.05 are shown. c-f Show block averages for changes in HbO (panel c, d) and HbR (panel e, f) levels obtained from the motor cortex during left finger tapping task. Data plotted are mean ± SD for channel means per group
All participants were asked to perform a motor task in the form of finger tapping. In brief, upon the auditory command, participants were instructed to tap with the left or right index finger for a duration of 10 s. Left and right finger tapping tasks were alternated with 15-s rest intervals between the tasks. Analysis was performed using NIRSLab software (NIRx Medical Technologies LLC, NY, USA). Saturated channel data and channels with high variable noise (> 7.5% coefficient of variation) were excluded from further analysis. A bandpass filter of 0.05 to 0.2 Hz was applied to filter physiological noise. Measured optical densities were converted to change of hemoglobin concentration using the Beer-Lambert law (Baker et al. 2014). Differential Pathlength Factor (DPF) was adjusted for age with an equation previously suggested (Scholkmann and Wolf 2013). Block averages were then calculated for each channel for each stimulus, and channel means were then averaged for the region of interest for both groups. Concentration changes are relative to the signal recorded 5 to 1 sec prior to start of finger tapping trials. Same, filtered HbO data was used during general linear modeling (GLM) analysis, and canonical hemodynamic response function (hrf) was used as a basis function. During group-level analysis, a t-contrast was used to compare evoked hemodynamic responses of the two groups.
The finger-tapping task is known to be associated with neuronal activation and consequential functional hyperemia predominantly in the primary motor cortex, the supplementary motor area, the pre-motor area, and often the prefrontal cortex. A significant advantage of using this task is that changes in HbO and HbR reflecting NVC responses can be compared in the channels covering the well-defined anatomical areas known to be involved in performing the task.
The summary data presented in Fig. 3 demonstrate that aging is associated with prominent changes in both the amplitude and the time course of NVC related hemodynamic responses. NVC was similar in the right motor cortex during the left finger tapping task in both groups (Fig. 3b). In contrast, the aged group showed a significantly greater activation in the contralateral motor cortex and the prefrontal cortex. When observing NVC as block averages (Fig. 3c–f), we found that the early peak of the HbO signal upon starting the motor task was virtually absent in older adults (Fig. 3c). Interestingly, when observing the HbO signals over the ipsilateral motor cortex (Fig. 3d), we found that the evoked response is indeed similar to the canonical hrf in the aged group, however, the amplitude is lower than in the contralateral motor cortex.
The amplitude of HbR signal reflecting blood washout effectivity was also smaller and the response (if any) was delayed in older adults as compared to younger participants (Fig. 3e). Provided that NVC is spatially heterogenous (Devonshire et al. 2012), amplitudes of evoked NVC may be different depending on the stimulation and the activated corresponding brain region. Recent studies comparing signals from frequency-domain multi-distance NIRS to BOLD fMRI signals provide additional evidence (Fabiani et al. 2014) that in older adults NVC is impaired.
It is common in fNIRS studies (Hirth et al. 1997; Kashou et al. 2016; Obrig and Villringer 1997) to observe two peaks in the HbO response curve (Fig. 3c). Often the first HbO response begins a few seconds prior to the start of the stimulus itself, a phenomenon which has been attributed to mental preparation for the motor task (Kashou et al. 2016). In support of this concept, earlier studies discovered a slow negative electroencephalography activity (termed Bereitschaftspotential or readiness potential) that precede self-initiated movement for up to 2 s and reflects increased neural activity related to readiness, preparation, and execution of movement (Kornhuber and Deecke 1965). Its amplitude correlates positively with movement complexity and its two components demonstrate the hierarchy of the motor system, with the activation of the supplementary motor area preceding the activation of the primary motor cortex (Drenckhahn et al. 2015). These EEG findings accord with the results of whole-scalp magnetoencephalographic studies (Erdler et al. 2000). The early phase of the HbO signal detected in the fNIRS study likely corresponds to these neuronal activities. Interestingly, we have observed in our pilot cohort that this pre-stimulation increase in HbO was more manifest in healthy young individuals, whereas it was virtually absent in the older participants in the present study (Fig. 3a).
As double peaks were not evident in the HbR response upon finger tapping stimulus, HbR signal may be more suitable for studies on cerebromicrovascular aging. Importantly, amplitude features of the HbR signals differ between the different motor areas (Drenckhahn et al. 2015). HbR signal could also be used during GLM analysis, however, the basis function may need to be adjusted to the expected waveform. Most fNIRS studies report statistics based on HbO signal due to the better signal-to-noise ratio, however, HbR signal would essentially be created by the same phenomenon as the BOLD signal captured during an fMRI approach (Strangman et al. 2002a, 2002b). Due to the limited spatial resolution of fNIRS, in our studies we decided to compare amplitude features of the Hb signals in the channels covering the medial motor cortex (Fig. 3c–f) and calculated the average change of concentration within all 10 channels covering each side.
There are several potential caveats that the researchers working with fNIRS studies should be aware of. Interpreting fNIRS signals, the exact anatomical location of the optodes in relation to the supplementary motor area and primary motor cortex, data processing and algorithm of analysis, determination of movement onset, the mode of initiation (self-paced or externally cued) of the finger tapping task (Drenckhahn et al. 2015), and the duration of the stimulus should be considered carefully. Utilization of a digitizer device that records placement of optodes on the head could improve localization of the recorded signal on the brain. However, spatial resolution of fNIRS will not allow localization as accurately as fMRI would. While performing fMRI examinations an anatomical scan is also performed, which can also help identify structural abnormalities contributing to an altered hemodynamic response. On the contrary, fNIRS does not provide anatomical information, so study participants should be carefully screened prior to inclusion in an fNIRS study. Despite the limitations, fNIRS provides a good alternative for NVC examination for assessments that require the participant to be freely moving, or when NVC is measured in a non-hospital setting. Combining fNIRS with other methods, e.g., Transcranial Doppler sonography (TCD) or ASL-fMRI may also provide a good measure of baseline CBF (when not using FDMD-fNIRS), and would also help interpret the extent of the changes in Hb concentration.
There are also potential external confounding factors that should be also considered. For example, there is an evidence that both hair thickness and hair color may affect the fNIRS signals (Kashou et al. 2016), although this may not represent a critical problem in longitudinal study designs.
Perspectives
The advantages of fNIRS for investigating neurovascular function in older adults in an outpatient setting include (i) fewer physical restrictions and limitations, (ii) portability, (iii) repeatability, (iv) large selection of stimulation paradigms to elicit neuronal activity including cognitive tasks, (v) ability to perform neurovascular coupling assessments while moving, (vi) relatively inexpensive instrumentation, (vii) excellent temporal resolution. In addition, fNIRS measurements can be easily combined with simultaneous assessment of other physiological parameters (e.g., gait, cognition, EEG).
We propose that in translational geroscience research fNIRS technology-based measurement of NVC will be particularly useful to assess the effect of various cardiovascular risk factors on the cerebral microcirculation in older adults. Importantly, fNIRS-based NVC measurements can be adapted to longitudinally measure the effect of anti-aging therapeutic approaches and/or lifestyle interventions on the cerebral microcirculation during the course of treatment. The use of fNIRS would potentially allow us to evaluate the effects of medications that were reported to improve the outcomes of age-related diseases, such as metformin in diabetes mellitus (Barzilai et al. 2016) and to repurpose them as drugs that preserve cognitive function in aging. Application of fNIRS-based NVC measurements in self-controlled observational study designs, such as the case-crossover design and the self-controlled case series, is particularly promising. fNIRS may also be a useful tool to test the underlying causes of treatment-associated deteriorations of cognitive function as it is with chemotherapy (Carlson et al. 2018) and whole-brain irradiation (Ungvari et al. 2017; Warrington et al. 2013), which will provide a tool for a development of better and safer medications to tackle these complications in aging.
There are also many exciting and demanding challenges ahead. Although there is a growing number of studies correlating fNIRS- and fMRI-based measurements, for translational geroscience studies it will be highly advantageous to correlate NVC parameters measured by fNIRS with other physiological parameters that reflect microvascular and/or neurovascular health (e.g., CBF and NVC data measured by transcranial Doppler (TCD) sonography (Sorond et al. 2011; Sorond et al. 2008) or dynamic retinal vessel analysis (DVA) (Lipecz et al. 2019). We expect that a comprehensive cerebromicrovascular health index encompassing fNIRS-based NVC, TCD-based NVC and CBF, and DVA-based retinal NVC data can be constructed (Berni et al. 2011). We have recently demonstrated the potential of such an approach, showing that even a peripheral vascular health index used as a surrogate marker of age-related, generalized vascular dysfunction can reliably predict cognitive decline in older adults (Csipo et al. 2019). We expect that a comprehensive cerebromicrovascular health assessment, which encompasses measurements with fNIRS, TCD, and DVA, will be even more reliable and sensitive to predict brain health and cognitive performance, identifying older individuals at risk for vascular cognitive impairment.
Funding information
This work was supported by grants from the American Heart Association (ST), the Oklahoma Center for the Advancement of Science and Technology (to AC, AY, ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; R01-AG055395), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC, R01-NS100782 to ZU, R01-NS085002 to FAS), the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (GM104938, to AY), the Presbyterian Health Foundation (to ZU, AC, AY), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (1P20GM125528, sub#5337).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tamas Csipo, Peter Mukli, Agnes Lipecz and Stefano Tarantini contributed equally to this work.
References
- Abdelnour AF, Huppert T. Real-time imaging of human brain function by near-infrared spectroscopy using an adaptive general linear model. Neuroimage. 2009;46:133–143. doi: 10.1016/j.neuroimage.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arridge SR. Optical tomography in medical imaging. Inverse Problems. 1999;15:R41–R93. doi: 10.1088/0266-5611/15/2/022. [DOI] [Google Scholar]
- Baker WB, Parthasarathy AB, Busch DR, Mesquita RC, Greenberg JH, Yodh AG. Modified Beer-Lambert law for blood flow. Biomed Opt Express. 2014;5:4053–4075. doi: 10.1364/BOE.5.004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni A, Giuliani A, Tartaglia F, Tromba L, Sgueglia M, Blasi S, Russo G. Effect of vascular risk factors on increase in carotid and femoral intima-media thickness. Identification of a risk scale. Atherosclerosis. 2011;216:109–114. doi: 10.1016/j.atherosclerosis.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Bortfeld H. Functional near-infrared spectroscopy as a tool for assessing speech and spoken language processing in pediatric and adult cochlear implant users. Dev Psychobiol. 2019;61:430–443. doi: 10.1002/dev.21818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce SC, Izzetoglu M, Izzetoglu K, Onaral B, Pourrezaei K. Functional near-infrared spectroscopy. IEEE Engineering Med Biol Mag. 2006;25:54–62. doi: 10.1109/MEMB.2006.1657788. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Carlson BW, Craft MA, Carlson JR, Razaq W, Deardeuff KK, Benbrook DM. Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. Geroscience. 2018;40:325–336. doi: 10.1007/s11357-018-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar S (1960) Radiative transfer. Dover Publications, New York
- Cheong W-F, Prahl SA, Welch AJ. A review of the optical properties of biological tissues. IEEE J Quantum Electronics. 1990;26:2166–2185. doi: 10.1109/3.64354. [DOI] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Cope M (1991) The development of a near infrared spectroscopy system and its application for non invasive monitoring of cerebral blood and tissue oxygenation in the newborn infants. Dissertation, University College London
- Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Adv Exp Med Biol. 1988;222:183–189. doi: 10.1007/978-1-4615-9510-6_21. [DOI] [PubMed] [Google Scholar]
- Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BTN, Dzialendzik M, Tarantini S, Balasubramanian P, Kiss T, Yabluchanska V, Silva-Palacios F, Courtney DL, Dasari TW, Sorond F, Sonntag WE, Csiszar A, Ungvari Z, Yabluchanskiy A. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41:125–136. doi: 10.1007/s11357-019-00063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Bray S, Reiss AL. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage. 2010;49:3039–3046. doi: 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire IM, Papadakis NG, Port M, Berwick J, Kennerley AJ, Mayhew JE, Overton PG. Neurovascular coupling is brain region-dependent. Neuroimage. 2012;59:1997–2006. doi: 10.1016/j.neuroimage.2011.09.050. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E. General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Drenckhahn C, Koch SP, Dummler J, Kohl-Bareis M, Steinbrink J, Dreier JP. A validation study of the use of near-infrared spectroscopy imaging in primary and secondary motor areas of the human brain. Epilepsy Behav. 2015;49:118–125. doi: 10.1016/j.yebeh.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Ehlis AC, Schneider S, Dresler T, Fallgatter AJ. Application of functional near-infrared spectroscopy in psychiatry. Neuroimage. 2014;85 Pt 1:478–488. doi: 10.1016/j.neuroimage.2013.03.067. [DOI] [PubMed] [Google Scholar]
- Erdler M, Beisteiner R, Mayer D, Kaindl T, Edward V, Windischberger C, Lindinger G, Deecke L. Supplementary motor area activation preceding voluntary movement is detectable with a whole-scalp magnetoencephalography system. Neuroimage. 2000;11:697–707. doi: 10.1006/nimg.2000.0579. [DOI] [PubMed] [Google Scholar]
- Fabiani Monica, Gordon Brian A., Maclin Edward L., Pearson Melanie A., Brumback-Peltz Carrie R., Low Kathy A., McAuley Edward, Sutton Bradley P., Kramer Arthur F., Gratton Gabriele. Neurovascular coupling in normal aging: A combined optical, ERP and fMRI study. NeuroImage. 2014;85:592–607. doi: 10.1016/j.neuroimage.2013.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G. Neurovascular coupling in normal aging: a combined optical, ERP and fMRI study. Neuroimage. 2014;85 Pt 1:592–607. doi: 10.1016/j.neuroimage.2013.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C. Hypertension enhances Abeta-induced neurovascular dysfunction, promotes beta-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab. 2016;36:241–252. doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini MA, Fantini S, Paunescu LA, Maier JS, Gratton E. Influence of a superficial layer in the quantitative spectroscopic study of strongly scattering media. Appl Opt. 1998;37:7447–7458. doi: 10.1364/AO.37.007447. [DOI] [PubMed] [Google Scholar]
- Gatto R, Hoffman W, Mueller M, Flores A, Valyi-Nagy T, Charbel FT. Frequency domain near-infrared spectroscopy technique in the assessment of brain oxygenation: a validation study in live subjects and cadavers. J Neurosci Methods. 2006;157:274–277. doi: 10.1016/j.jneumeth.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazioli S, Mauri M, Crippa A, Maggioni E, Molteni M, Brambilla P, Nobile M. Light up ADHD: II. Neuropharmacological effects measured by near infrared spectroscopy: is there a biomarker? J Affect Disord. 2019;244:100–106. doi: 10.1016/j.jad.2018.10.100. [DOI] [PubMed] [Google Scholar]
- Hamel E, Royea J, Ongali B, Tong XK. Neurovascular and cognitive failure in Alzheimerʼs disease: benefits of cardiovascular therapy. Cell Mol Neurobiol. 2016;36:219–232. doi: 10.1007/s10571-015-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikx D, Smits A, Lavanga M, de Wel O, Thewissen L, Jansen K, Caicedo A, van Huffel S, Naulaers G. Measurement of neurovascular coupling in neonates. Front Physiol. 2019;10:65. doi: 10.3389/fphys.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth C, Villringer K, Thiel A, Bernarding J, Mühlnickl W, Obrig H, Dirnagl U, Villringer A. Towards brain mapping combining near-infrared spectroscopy and high resolution 3D MRI. Adv Exp Med Biol. 1997;413:139–147. doi: 10.1007/978-1-4899-0056-2_15. [DOI] [PubMed] [Google Scholar]
- Hueber DM, Fantini S, Cerussi AE, Barbieri BB (1999) New optical probe designs for absolute (self-calibrating) NIR tissue hemoglobin measurements vol 3597. BiOS ʼ99 International Biomedical Optics Symposium. SPIE
- Huneau C, Benali H, Chabriat H (2015) Investigating human neurovascular coupling using functional neuroimaging: a critical review of dynamic models. Front Neurosci 9:467. 10.3389/fnins.2015.00467 [DOI] [PMC free article] [PubMed]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimerʼs disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Izzetoglu M, Devaraj A, Bunce S, Onaral B. Motion artifact cancellation in NIR spectroscopy using Wiener filtering. IEEE Trans Biomed Eng. 2005;52:934–938. doi: 10.1109/TBME.2005.845243. [DOI] [PubMed] [Google Scholar]
- Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Kashou NH, Giacherio BM, Nahhas RW, Jadcherla SR. Hand-grasping and finger tapping induced similar functional near-infrared spectroscopy cortical responses. Neurophotonics. 2016;3:025006. doi: 10.1117/1.NPh.3.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis L, Herman P, Eke A. The modified Beer-Lambert law revisited. Phys Med Biol. 2006;51:N91–N98. doi: 10.1088/0031-9155/51/5/N02. [DOI] [PubMed] [Google Scholar]
- Kornhuber HH, Deecke L. Changes in the brain potential in voluntary movements and passive movements in man: readiness potential and reafferent potentials. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965;284:1–17. doi: 10.1007/BF00412364. [DOI] [PubMed] [Google Scholar]
- Lipecz A, Csipo T, Tarantini S, Hand RA, Ngo BTN, Conley S, Nemeth G, Tsorbatzoglou A, Courtney DL, Yabluchanska V, Csiszar A, Ungvari ZI, Yabluchanskiy A. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience. 2019;41:341–349. doi: 10.1007/s11357-019-00078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner T, Norris DG, Schwarzbauer C, Wiggins CJ. A qualitative test of the balloon model for BOLD-based MR signal changes at 3 T. Magn Reson Med. 2001;46:891–899. doi: 10.1002/mrm.1274. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Mukli P, Nagy Z, Racz FS, Herman P, Eke A. Impact of healthy aging on multifractal hemodynamic fluctuations in the human prefrontal cortex. Front Physiol. 2018;9:1072. doi: 10.3389/fphys.2018.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Hamel E. Neurovascular function in Alzheimerʼs disease patients and experimental models. J Cereb Blood Flow Metab. 2011;31:1354–1370. doi: 10.1038/jcbfm.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Near-infrared spectroscopy in functional activation studies. Can NIRS demonstrate cortical activation? Adv Exp Med Biol. 1997;413:113–127. doi: 10.1007/978-1-4899-0056-2_13. [DOI] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Beyond the visible - imaging the human brain with light. J Cereb Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos Panayiota, Tong Xin-Kang, Imboden Hans, Hamel Edith. Losartan improves cerebrovascular function in a mouse model of Alzheimer's disease with combined overproduction of amyloid-β and transforming growth factor-β1. Journal of Cerebral Blood Flow & Metabolism. 2016;37(6):1959–1970. doi: 10.1177/0271678X16658489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Park Laibaik, Koizumi Kenzo, El Jamal Sleiman, Zhou Ping, Previti Mary Lou, Van Nostrand William E., Carlson George, Iadecola Costantino. Age-Dependent Neurovascular Dysfunction and Damage in a Mouse Model of Cerebral Amyloid Angiopathy. Stroke. 2014;45(6):1815–1821. doi: 10.1161/STROKEAHA.114.005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince S, Kolehmainen V, Kaipio JP, Franceschini MA, Boas D, Arridge SR. Time-series estimation of biological factors in optical diffusion tomography. Phys Med Biol. 2003;48:1491–1504. doi: 10.1088/0031-9155/48/11/301. [DOI] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37:430–439. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saager RB, Berger AJ. Direct characterization and removal of interfering absorption trends in two-layer turbid media. J Opt Soc Am A Opt Image Sci Vis. 2005;22:1874–1882. doi: 10.1364/JOSAA.22.001874. [DOI] [PubMed] [Google Scholar]
- Saager R, Berger A. Measurement of layer-like hemodynamic trends in scalp and cortex: implications for physiological baseline suppression in functional near-infrared spectroscopy. J Biomed Opt. 2008;13:034017. doi: 10.1117/1.2940587. [DOI] [PubMed] [Google Scholar]
- Saager RB, Telleri NL, Berger AJ. Two-detector corrected near infrared spectroscopy (C-NIRS) detects hemodynamic activation responses more robustly than single-detector NIRS. Neuroimage. 2011;55:1679–1685. doi: 10.1016/j.neuroimage.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Schei JL, Van Nortwick AS, Meighan PC, Rector DM. Neurovascular saturation thresholds under high intensity auditory stimulation during wake. Neuroscience. 2012;227:191–200. doi: 10.1016/j.neuroscience.2012.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkmann F, Wolf M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J Biomed Opt. 2013;18:105004. doi: 10.1117/1.JBO.18.10.105004. [DOI] [PubMed] [Google Scholar]
- Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, Wolf M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage. 2014;85:6–27. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Shin J, von Luhmann A, Kim DW, Mehnert J, Hwang HJ, Muller KR. Simultaneous acquisition of EEG and NIRS during cognitive tasks for an open access dataset. Sci Data. 2018;5:180003. doi: 10.1038/sdata.2018.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siero JC, Hermes D, Hoogduin H, Luijten PR, Petridou N, Ramsey NF. BOLD consistently matches electrophysiology in human sensorimotor cortex at increasing movement rates: a combined 7 T fMRI and ECoG study on neurovascular coupling. J Cereb Blood Flow Metab. 2013;33:1448–1456. doi: 10.1038/jcbfm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleimen-Malkoun R, Temprado JJ, Hong SL (2014) Aging induced loss of complexity and dedifferentiation: consequences for coordination dynamics within and between brain, muscular and behavioral levels Front Aging Neurosci 6:140. 10.3389/fnagi.2014.00140 [DOI] [PMC free article] [PubMed]
- Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: effects of healthy aging. Cortex. 2008;44:179–184. doi: 10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell'Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond F. A., Hurwitz S., Salat D. H., Greve D. N., Fisher N. D. L. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81(10):904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Stephan T, Becker-Bense S, Dera T, Brandt T, Dieterich M. Age-related changes of blood-oxygen-level-dependent signal dynamics during optokinetic stimulation. Neurobiol Aging. 2013;34:2277–2286. doi: 10.1016/j.neurobiolaging.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Rylander KM, Pike GB. The effect of global cerebral vasodilation on focal activation hemodynamics. Neuroimage. 2006;30:726–734. doi: 10.1016/j.neuroimage.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Stickland R, Allen M, Magazzini L, Singh KD, Wise RG, Tomassini V. Neurovascular coupling during visual stimulation in multiple sclerosis: A MEG-fMRI Study. Neuroscience. 2019;403:54–69. doi: 10.1016/j.neuroscience.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002;17:719–731. doi: 10.1006/nimg.2002.1227. [DOI] [PubMed] [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002;17:719–731. doi: 10.1006/nimg.2002.1227. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Takasaki S, Ozaki T, Kobayashi Y (1999) Tissue oxygenation monitor using NIR spatially resolved spectroscopy. In: Optical tomography and spectroscopy of tissue III. International Society for Optics and Photonics, pp 582-592
- Talukdar MT, Frost HR, Diamond SG. Modeling neurovascular coupling from clustered parameter sets for multimodal EEG-NIRS. Comput Math Methods Med. 2015;2015:830849. doi: 10.1155/2015/830849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, et al. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimerʼs disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini Stefano, Fulop Gabor A., Kiss Tamas, Farkas Eszter, Zölei-Szénási Dániel, Galvan Veronica, Toth Peter, Csiszar Anna, Ungvari Zoltan, Yabluchanskiy Andriy. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. GeroScience. 2017;39(4):465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimerʼs disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fülöp GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O'Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini Stefano, Valcarcel-Ares M Noa, Yabluchanskiy Andriy, Tucsek Zsuzsanna, Hertelendy Peter, Kiss Tamas, Gautam Tripti, Zhang Xin A, Sonntag William E, de Cabo Rafael, Farkas Eszter, Elliott Michael H, Kinter Michael T, Deak Ferenc, Ungvari Zoltan, Csiszar Anna. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood–Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype. The Journals of Gerontology: Series A. 2017;73(7):853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini Stefano, Valcarcel-Ares Noa M., Yabluchanskiy Andriy, Fulop Gabor A., Hertelendy Peter, Gautam Tripti, Farkas Eszter, Perz Aleksandra, Rabinovitch Peter S., Sonntag William E., Csiszar Anna, Ungvari Zoltan. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17(2):e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Süle Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimerʼs disease cerebrovascular and memory deficits. J Neurosci. 2012;32:4705–4715. doi: 10.1523/JNEUROSCI.0169-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett. 2009;452:17–22. doi: 10.1016/j.neulet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Torricelli A, Pifferi A, Taroni P, Giambattistelli E, Cubeddu R. In vivo optical characterization of human tissues from 610 to 1010 nm by time-resolved reflectance spectroscopy. Phys Med Biol. 2001;46:2227. doi: 10.1088/0031-9155/46/8/313. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth Peter, Tarantini Stefano, Tucsek Zsuzsanna, Ashpole Nicole M., Sosnowska Danuta, Gautam Tripti, Ballabh Praveen, Koller Akos, Sonntag William E., Csiszar Anna, Ungvari Zoltan. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. American Journal of Physiology-Heart and Circulatory Physiology. 2014;306(3):H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fülöp GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res. 2013;50:445–457. doi: 10.1159/000354227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ME, Wise RG. Can blood oxygenation level dependent functional magnetic resonance imaging be used accurately to compare older and younger populations? A mini literature review. Front Aging Neurosci. 2018;10:371. doi: 10.3389/fnagi.2018.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Sun Y, Lu Z, Leak RK, Zhang F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev. 2017;34:15–29. doi: 10.1016/j.arr.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol. 2005;20:115–120. [PubMed] [Google Scholar]
- Zebrowitz L, Ward N, Boshyan J, Gutchess A, Hadjikhani N. Dedifferentiated face processing in older adults is linked to lower resting state metabolic activity in fusiform face area. Brain Res. 2016;1644:22–31. doi: 10.1016/j.brainres.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Roeyers H. Exploring brain functions in autism spectrum disorder: a systematic review on functional near-infrared spectroscopy (fNIRS) studies. Int J Psychophysiol. 2019;137:41–53. doi: 10.1016/j.ijpsycho.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brooks DH, Franceschini MA, Boas DA. Eigenvector-based spatial filtering for reduction of physiological interference in diffuse optical imaging. J Biomed Opt. 2005;10:11014. doi: 10.1117/1.1852552. [DOI] [PubMed] [Google Scholar]