Abstract
Age-related impairment of angiogenesis likely has a critical role in cerebromicrovascular rarefaction and development of vascular cognitive impairment and dementia (VCID) in the elderly. Recently, we demonstrated that aging is associated with NAD+ depletion in the vasculature and that administration of NAD+ precursors exerts potent anti-aging vascular effects, rescuing endothelium-mediated vasodilation in the cerebral circulation and improving cerebral blood supply. The present study was designed to elucidate how treatment with nicotinamide mononucleotide (NMN), a key NAD+ intermediate, impacts age-related impairment of endothelial angiogenic processes. Using cerebromicrovascular endothelial cells (CMVECs) isolated from young and aged F344xBN rats, we demonstrated that compared with young cells, aged CMVECs exhibit impaired proliferation, cellular migration (measured by a wound-healing assay using electric cell-substrate impedance sensing [ECIS] technology), impaired ability to form capillary-like structures, and increased oxidative stress. NMN treatment in aged CMVECs significantly improved angiogenic processes and attenuated H2O2 production. We also found that pre-treatment with EX-527, a pharmacological inhibitor of SIRT1, prevented NMN-mediated restoration of angiogenic processes in aged CMVECs. Collectively, we find that normal cellular NAD+ levels are essential for normal endothelial angiogenic processes, suggesting that age-related cellular NAD+ depletion and consequential SIRT1 dysregulation may be a potentially reversible mechanism underlying impaired angiogenesis and cerebromicrovascular rarefaction in aging. We recommend that pro-angiogenic effects of NAD+ boosters should be considered in both preclinical and clinical studies.
Keywords: Senescence, Endothelial dysfunction, Vascular contributions to cognitive impairment and dementia, Microcirculation, NAD+ precursor
Introduction
The brain is the most energy-demanding organ, yet, it lacks energy stores. Normal neuronal function is therefore critically dependent on adequate supply of nutrients and oxygen through a dense network of over 600 km of cerebral microvessels. In the brain, the number of endothelial cells is very similar to that of neurons (Garcia-Amado and Prensa 2012) and nearly every neuron is supplied by its own capillary, with an average distance of 8–20 μm between the neuron and the microvessels. Aging-induced functional and structural impairments of the cerebral microcirculatory network have a critical role in the pathogenesis of age-related cognitive decline (Zlokovic 2011; Toth et al. 2013, 2017; Tucsek et al. 2014a, 2014b; Tarantini et al. 2016; Csiszar et al. 2017).
The dynamic balance between angiogenesis (new capillary formation from pre-existing microvessels) and microvascular regression is critical for the maintenance of a healthy cerebral microcirculatory network. Advanced aging is associated with a progressive deterioration of cerebromicrovascular homeostasis, at least in part, due to a significant impairment of endothelial angiogenic processes (Ingraham et al. 2008; Murugesan et al. 2012; Ungvari et al. 2018a). This results in cerebromicrovascular rarefaction/decreased capillary density in the aged brain, which contributes to a decline in cerebral blood flow compromising oxygen and nutrient delivery to the active neurons (Hagstadius and Risberg 1989; Martin et al. 1991; Kawamura et al. 1993; Moeller et al. 1996; Sonntag et al. 1997; Krejza et al. 1999; Lynch et al. 1999; Schultz et al. 1999; Bentourkia et al. 2000; Farkas and Luiten 2001; Khan et al. 2001; Pagani et al. 2002; Riddle et al. 2003; Mitschelen et al. 2009) and the formation of ischemic foci, neuronal dysfunction, demyelination, and, ultimately, to neurodegeneration (Sonntag et al. 1997, 2000; Khan et al. 2001; Ingraham et al. 2008; Warrington et al. 2011, 2012).
Sprouting angiogenesis, which is initiated by VEGF in poorly perfused hypoxic areas of the brain, is critical to satisfy the metabolic requirements of the neuronal tissue. Previous ex vivo studies provide strong evidence that cell-autonomous mechanisms contribute to age-related impairment of sprouting angiogenesis, compromising cellular angiogenic processes induced in response to VEGF in cerebromicrovascular endothelial cells (including endothelial cell proliferation and directed migration, tubulogenesis) (Ungvari et al. 2013; Csiszar et al. 2014). However, the molecular mechanisms, by which aging impairs VEGF-induced endothelial angiogenic processes, remain elusive (Lahteenvuo and Rosenzweig 2012).
NAD+ acts as a coenzyme in electron transfer reactions, as a donor of ADP-ribose moieties in ADP-ribosylation reactions, as a precursor of the second messenger molecule cyclic ADP-ribose, and as a substrate for the longevity assurance factor sirtuin enzymes. Maintenance of NAD+ levels is critical for normal cellular proliferation and function, regulation of mitochondrial metabolism and cellular bioenergetics, adaptive stress responses, and normal activation of pro-survival, anti-aging pathways. With advanced age, there is decreased availability of cellular NAD+ (Massudi et al. 2012; Gomes et al. 2013; Yoshino et al. 2018), which may be a fundamental, evolutionarily conserved contributor to aging processes across tissues. Aging-induced NAD+ depletion was suggested to predispose to a wide range of chronic diseases and pathological conditions associated with old age (Yang et al. 2007; Garten et al. 2009; Bonkowski and Sinclair 2016; de Picciotto et al. 2016; Imai and Guarente 2016; Schultz and Sinclair 2016; Das et al. 2018; Csiszar et al. 2019), including endothelial dysfunction (Csiszar et al. 2019). There is strong preclinical evidence that restoration of cellular NAD+ levels in aged rodents by administration of NAD+ precursors exerts potent anti-aging effects, reversing age-related organ dysfunction (Gomes et al. 2013; Mills et al. 2016; Johnson et al. 2018) and increasing mouse lifespan (Zhang et al. 2016). Recently, we demonstrated that treatment of old mice with nicotinamide mononucleotide (NMN), a key NAD+ intermediate, restores vascular NAD+ levels, rescues endothelium-mediated vasodilation in the cerebral circulation, and improves cerebral blood supply (Tarantini et al. 2019).
The present study was designed to elucidate how NMN treatment impacts age-related impairment of endothelial angiogenic processes. Using cerebromicrovascular endothelial cells (CMVECs) isolated from young and aged F344xBN rats, we tested the hypothesis that chronic treatment of aged endothelial cells with NMN improves angiogenic capacity, including proliferation, migration, and ability to form capillary-like structures.
Materials and methods
Animals and endothelial cell isolation
We used Fischer 344x Brown Norway (F344xBN) rats as a model of aging, since this strain has a lower incidence of age-specific pathology than other rats. In F344xBN rats, the primary effects of aging can be studied without complications caused by age-related pathology. Male, 3- and 24-month-old F344xBN rats were obtained from the National Institute on Aging. All animals were disease-free with no signs of systemic inflammation and/or neoplastic diseases. The rats were housed in an environmentally controlled vivarium under pathogen-free conditions with unlimited access to food and water and a controlled photoperiod (12 h light:12 h dark). All experimental animals were maintained according to National Institutes of Health guidelines, and all animal use protocols were approved by the Institutional Animal Care and Use Committees of the participating institutions. The animals were euthanized with CO2. The brains were rapidly dissected to establish primary cerebromicrovascular endothelial cell (CMVEC) cultures as described.
Establishment and characterization of primary cerebromicrovascular endothelial cell cultures
To assess the effects of NMN on endothelial angiogenic capacity, we measured the effects of NMN on cell proliferation, migration, and tube formation ability in cultured primary CMVECs. The establishment and characterization of the CMVEC strains have been recently reported. In brief, to establish primary cultures of CMVECs, the brains of the 3- and 24-month-old F344xBN rats were removed aseptically, rinsed in ice cold PBS, and minced into ≈ 1 mm2. The tissue was washed twice in ice cold 1X PBS by low-speed centrifugation (50g, 2–3 min). The diced tissue was digested in a solution of collagenase (800 U/g tissue), hyaluronidase (2.5 U/g tissue), and elastase (3 U/g tissue) in 1 mL PBS/100 mg tissue for 45 min at 37 °C in a rotating humid incubator. The digested tissue was passed through a 100-μm cell strainer. The single-cell lysate was centrifuged for 2 min at 70g. After removing the supernatant, the pellet was washed twice in cold PBS supplemented with 2.5% fetal calf serum (FCS), and the suspension was centrifuged at 300g for 5 min at 4 °C. To create an endothelial cell–enriched fraction, the cell suspension was centrifuged using an OptiPrep gradient solution (Axi-Shield, PoC, Norway). Briefly, the cell pellet was resuspended in Hanks’ balanced salt solution (HBSS) and mixed with 40% iodixanol thoroughly (final concentration 17% (v/v) iodixanol solution; ρ = 1.096 g/mL). Two milliliters of HBSS was layered on top and centrifuged at 400g for 15 min at 20 °C. Endothelial cells, which banded at the interface between HBSS and the 17% iodixanol layer, were collected. The endothelial cell–enriched fraction was incubated for 30 min at 4 °C in the dark with anti-CD31/PE (BD Biosciences, San Jose, CA, USA) and anti-MCAM/FITC (BD Biosciences, San Jose, CA, USA). After washing, the cells twice with MACS Buffer (Milltenyi Biotech, Cambridge, MA, USA) anti-FITC and anti-PE magnetic bead labeled secondary antibodies were used for 15 min at room temperature. Endothelial cells were collected by magnetic separation using the MACS LD magnetic separation columns according to the manufacturer’s guidelines (Milltenyi Biotech, Cambridge, MA, USA). The endothelial fraction was cultured on fibronectin coated plates in Endothelial Growth Medium (Cell Application, San Diego, CA, USA) for 10 days. Endothelial cells were phenotypically characterized by flow cytometry (GUAVA 8HT, Merck Millipore, Billerica, MA, USA). Briefly, antibodies against five different endothelial specific markers were used (anti-CD31-PE, anti-erythropoietin receptor-APC, anti-VEGF R2-PerCP, anti-ICAM-fluorescein, anti-CD146-PE), and isotype specific antibody labeled fractions served as negative controls. Flow cytometric analysis showed that after the third cycle of immunomagnetic selection, there were virtually no CD31−, CD146−, EpoR−, and VEGFR2− cells in the resultant cell populations. All antibodies were purchased from R&D Systems (R&D Systems, Minneapolis, MN, USA).
Primary CMVECs were cultured in custom-made Rat Brain Endothelial Cell Growth Medium (Cell Applications, Inc.) with reduced nicotinamide concentration (11.04 μM). Since the results of the assays investigating the endpoints used are affected by the number of viable cells, cell viability of each population was determined as described. To assess the direct effects of NMN on endothelial phenotype, primary CMVECs derived from aged rats were treated with NMN (Santa Cruz, Dallas, TX) in vitro (5 × 10−4 mol/L; for 1 to 5 days).
Cell proliferation assay
Cell proliferation capacity was assessed in CMVECs using the flow cytometry–based Guava CellGrowth assay (Guava Technologies, Inc., Hayward, CA) as previously reported. Briefly, cells were collected, resuspended in PBS containing 0.1% BSA, and stained with 16 μmol/L carboxyfluorescein diacetate succinimidyl ester (CFSE) for 15 min at 37 °C. This dye diffuses into cells and is cleaved by intracellular esterases to form an amine-reactive product that produces a detectable fluorescence and binds covalently to intracellular lysine residues and other amine sources. Upon cell division, CFSE divides equally into the daughter cells halving the CFSE concentration of the mother cell; therefore, there is an inverse correlation between the fluorescence intensity and the proliferation capacity of the cells. After incubation, unbound dye was quenched with serum-containing medium. Then, cells were washed three times and incubated for 24 h with 100 ng/mL VEGF. Finally, cells were collected, washed, stained with propidium iodide (to gate out dead cells), and analyzed with a flow cytometer (Guava EasyCyte 8HT; Millipore, Billerica, MA). The inverse of the fluorescence intensity was used as an index of proliferation.
Assessment of cell migration by ECIS-based wound-healing assay
Electric cell-substrate impedance sensing technology was used to monitor the migration of CMVECs in a wound-healing assay as reported (Applied BioPhysics Inc., Troy, NY). Briefly, CMVECs (2.5 × 105 cells/well) were seeded in 96-well array culture dishes (electric cell-substrate impedance sensing (ECIS), 96W1E) and placed in an incubator (37 °C), and changes in resistance and impedance were continuously monitored. When impedance reached a plateau, cells in each well were subjected to an elevated field pulse (“wounding”) of 5 mA applied for 20 s at 100 kHz, which killed the cells present on the small active electrode due to severe electroporation. The detachment of the dead cells was immediately evident as a sudden drop in resistance (monitored at 4000 Hz) and a parallel increase in conductance. VEGF (100 ng/mL) was immediately added to each well. CMVECs surrounding the active electrode that had not been subjected to the wounding then migrated inward to replace the detached dead cells resulting in resistance recovery (continuously monitored at 4000 Hz for up to 24 h). The time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for cells in each experimental group, and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as μm/h).
Tube formation assay
To investigate the influence of age and NMN on tube formation ability, young, aged, and NMN-treated aged CMVECs were plated on Geltrex Reduced Growth Factor Basement Membrane Matrix (Invitrogen, Carlsbad CA) in Medium 200PRF (Invitrogen, Carlsbad CA). To inhibit sirtuin activity, half of the aged control cells and NMN-treated aged cells were pre-treated with EX-527 (Active Motif Inc., Carlsbad, CA). EX-524 is a potent and selective sirtuin 1 (SIRT1) inhibitor (IC50 38 nM). Briefly, 150 μL/well of Geltrex was distributed in ice-cold 24-well plates. The gel was allowed to solidify while incubating the plates for 30 min at 37 °C. CMVECs were then seeded at a density of 5 × 104 cells/well and placed in the incubator for 24 h. Microscopic images were captured using a Nikon Eclipse Ti microscope equipped with a ×10 phase-contrast objective (Nikon Instruments Inc., Melville, NY). The extent of tube formation was quantified by measuring total tube length in five random fields per well using NIS-Elements microscope imaging software (Nikon Instruments Inc.), as recently reported. The mean of the total tube length per total area imaged (μm tube/mm2) was calculated for each well. Experiments were run in quadruplicates. The experimenter was blinded to the groups throughout the period of analysis.
Measurement of cellular H2O2 production
To assess cellular peroxide production, we used the cell-permeant oxidative fluorescent indicator dye CM-H2DCFDA (5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester, Invitrogen, Carlsbad, CA) as we previously reported. Cells were washed with warm PBS and incubated with CM-H2DCFDA (10 μM, at 37 °C, for 30 min). CM-H2DCFDA fluorescence was assessed by flow cytometry.
Data analysis
Statistical analyses were performed using one-way ANOVA. p < 0.05 was considered statistically significant. Data are expressed as means ± S.E.M.
Results
NMN treatment improves proliferative capacity of aged CMVECs
Proliferation represents a key step in angiogenesis. Proliferative capacity of young and aged CMVECs was compared after incubation with VEGF for 24 h. We found that CFSE fluorescence was significantly increased in aged CMVECs as compared with young CMVECs, indicating that proliferation capacity is impaired by aging (Fig. 1). NMN treatment rescued proliferative capacity of aged CMVECs (Fig. 1).
Fig. 1.
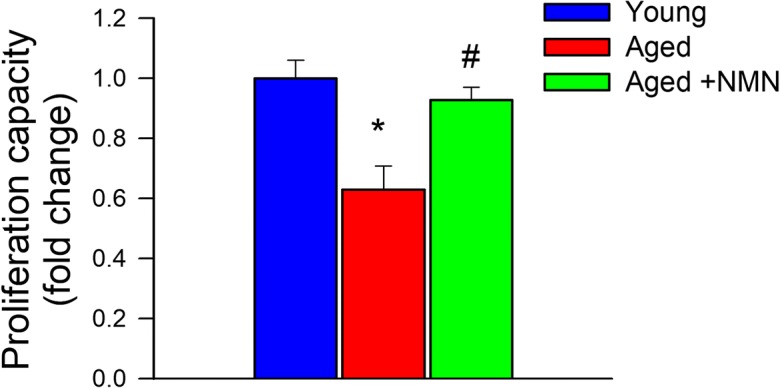
NMN treatment significantly increases proliferation capacity of aged CMVECs. Cell proliferation capacity of CMVECs isolated from aged F344xBN rats is impaired as compared with that of cells isolated from young F344xBN rats, and it is significantly improved by treatment with NMN. Cell proliferation capacity was assessed in primary CMVECs stimulated with VEGF (100 ng/mL) using the flow cytometry–based Guava CellGrowth assay (see “Materials and Methods”). The inverse of the fluorescence intensity of the indicator dye CFSE was used as an index of proliferation capacity of the cells. Data are plotted as means ± S.E.M. (n = 6 in each group); *p < 0.05 vs. control, #p < 0.05 vs. aged
NMN treatment improves migratory capability of aged CMVECs
The migratory capability of vascular endothelial cells has a pivotal role in the maintenance of microvascular integrity and angiogenesis. An ECIS-based wound-healing assay was used to assess the effect of NMN treatment on migratory capability of VEGF-treated CMVECs. We found that aged CMVECs exhibited impaired migratory capability as compared with young CMVECs (Fig. 2). In contrast, migration rate of aged CMVECs with NMN treatment did not differ significantly from that of young CMVECs (Fig. 2).
Fig. 2.
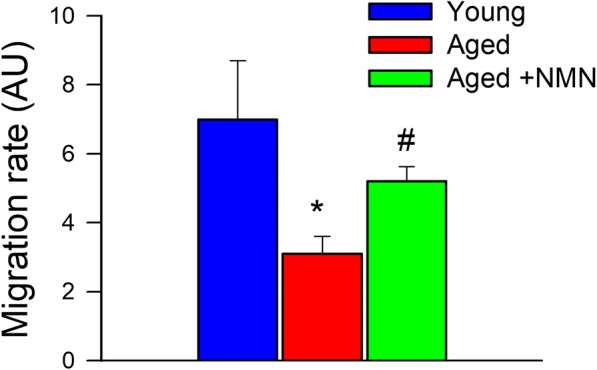
NMN treatment significantly increases migration capacity of aged CMVECs. Migration capacity of CMVECs isolated from aged F344xBN rats is impaired as compared with that of cells isolated from young F344xBN rats, and it is significantly improved by treatment with NMN. VEGF (100 ng/mL)-stimulated cell migration was monitored by electric cell-substrate impedance sensing (ECIS) technology in a wound-healing assay (see “Materials and Methods”). In brief, time course of resistance recovery after wounding (electric pulse of 5 mA for 20 s at 60 kHz) was monitored at 4000 Hz. The time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for each group, and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate. Bar graph depicts the summary data for migration rate in each group. Data are plotted as means ± S.E.M. (n = 5 in each group); *p < 0.05 vs. young control, #p < 0.05 vs. aged
NMN treatment increases formation of capillary-like structures by aged CMVECs
When seeded onto Geltrex matrices, young CMVECs form elaborated capillary networks (Ungvari et al. 2013; Csiszar et al. 2014). Compared with young cells in aged CMVECs, formation of capillary-like structures was significantly impaired (Fig. 3a–e). The finding that treatment with NMN significantly improved formation of capillary-like structures by aged CMVECs (Fig. 3e) suggests that age-related NAD+ deficiency is causally linked to the impaired angiogenic capacity of aged endothelial cells. We found that pharmacological inhibition of SIRT1 significantly inhibited the formation of capillary-like structures by NMN-treated aged CMVECs (Fig. 3e).
Fig. 3.
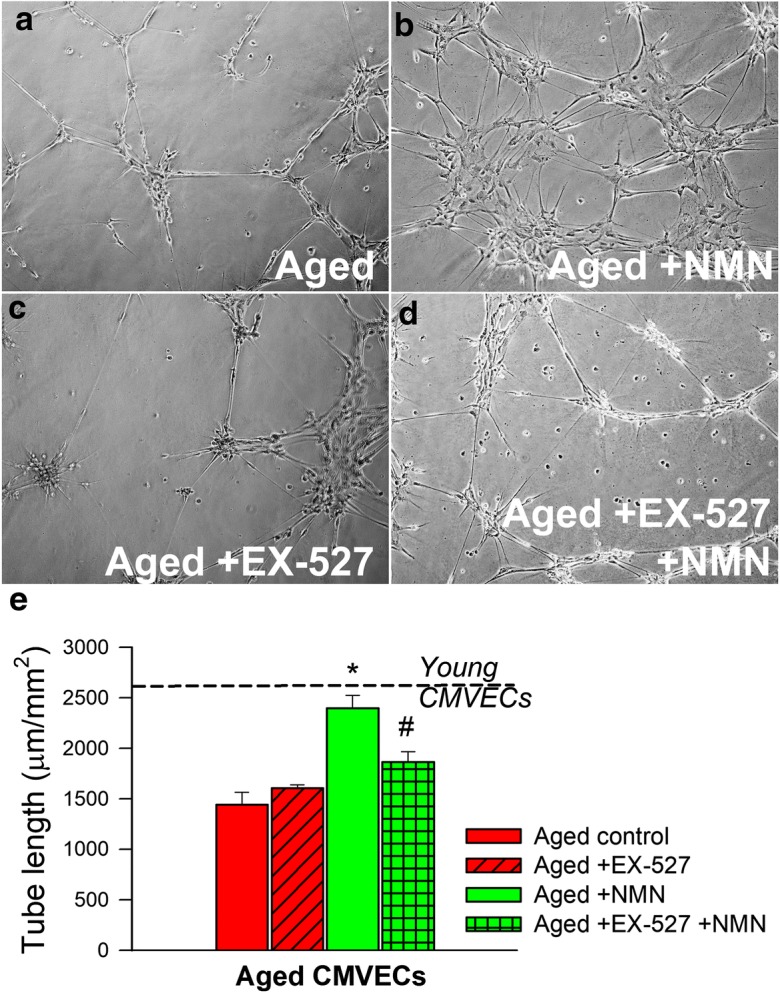
NMN treatment significantly improves the tube formation ability of aged CMVECs. Tube formation ability of CMVECs isolated from aged F344xBN rats is impaired as compared with that of cells isolated from young F344xBN rats (dashed line), and it is significantly improved by NMN treatment. Inhibition of SIRT1 by EX-524 significantly impairs the ability of NMN-treated aged CMVECs to form capillary-like structures, suggesting that the protective effects of NMN are mediated by sirtuin activation. CMVECs were plated on Geltrex matrix–coated wells, and tube formation was induced by treating cells with VEGF (100 ng/mL, for 24 h). Representative examples of capillary-like structures are shown on panels a, b, c, d. Summary data, expressed as total tube length per total area scanned (μm tube/mm2), are shown in panel e. Data are means ± S.E.M. (n = 5 in each group); *p < 0.05 vs. aged control, #p < 0.05 vs. aged + NMN
NMN treatment attenuates oxidative stress in aged CMVECs
Age-related oxidative stress has been implicated in endothelial angiogenic dysfunction (Ungvari et al. 2013). ROS production in young and aged CMVECs was compared by assessing CM-H2DCFDA fluorescence. We found that CM-H2DCFDA fluorescence was significantly increased in aged CMVECs as compared with that in young CMVECs, consistent with the view that endothelial cells in the aged cerebral microcirculation exhibit increased oxidative stress (Fig. 4). NMN treatment resulted in dramatic attenuation of H2O2 production in aged CMVECs (Fig. 4). Recent developments in our understanding of mechanisms of aging (Deepa et al. 2017; Fang et al. 2017; Grant et al. 2017; Konopka et al. 2017; Podlutsky et al. 2017; Cunningham et al. 2018; Habermehl et al. 2018; Kim et al. 2018; Lewis et al. 2018; Masser et al. 2018; Nacarelli et al. 2018; Olecka et al. 2018; Reglodi et al. 2018) and vascular aging processes (Csiszar et al. 2017; Tarantini et al. 2017a, b; Tucsek et al. 2017; Ungvari et al. 2017a, b; Csipo et al. 2018; Fulop et al. 2018; Lee et al. 2018; Reglodi et al. 2018; Sure et al. 2018; Ungvari et al. 2018b, c) highlight the importance of in vitro screening assays that model complex physiological processes for the evaluation of the anti-aging effects of novel pharmacological interventions. The combination of the in vitro assays used in this study, based on rescue of age-related loss-of-function in endothelial cells, could correctly identify the anti-aging effects of caloric restriction (Csiszar et al. 2013, 2014) as well as neuroendocrine factors (Banki et al. 2015).
Fig. 4.
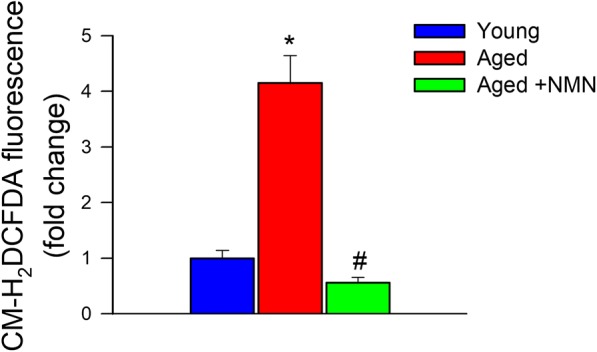
NMN treatment significantly attenuates oxidative stress in aged CMVECs. Cellular peroxide production is significantly increased in cultured primary CMVECs derived from aged F344xBN rats as compared with cells isolated from young F344xBN rats, and it is significantly attenuated by treatment with NMN. Cellular peroxide production was assessed by measuring CM-H2DCFDA fluorescence using a flow cytometry–based approach. Data are plotted as means ± S.E.M. (n = 6 in each group); *p < 0.05 vs. control, #p < 0.05 vs. aged
Discussion
The principal new findings of this study are that (1) age-related decline in cellular NAD+ levels is associated with impaired angiogenic response in aged rat CMVECs, and that (2) restoration of cellular NAD+ levels in aged CMVECs by treatment with NMN confers pro-angiogenic effects, counteracting, at least in part, the adverse effects of aging.
The formation of a new sprout growing out of existing vessels represents the first step in angiogenesis, which is mediated by VEGF-induced stalk cell proliferation and tip cell migration. VEGF also induces in endothelial cell branching and tubulogenesis to create microvascular networks. VEGF-induced proliferation and migration and tube forming capacity of CMVECs decline significantly with age, which are thought to contribute significantly to aging-induced impairment of angiogenesis and, consequentially, microvascular rarefaction (Valcarcel-Ares et al. 2012a, b; Ungvari et al. 2013; Csiszar et al. 2014; Ungvari et al. 2018a, b).
Recently, we demonstrated that age-related decline in NAD+ levels in CMVECs can be reversed by treatment with the NAD+ precursor NMN (Tarantini et al. 2019). This is the first study to demonstrate that treatment with NMN also improves proliferation and rescues migration and tube forming capacity of aged CMVECs. Our studies provide strong evidence that age-related NAD+ depletion compromises endothelial angiogenic responses in the cerebromicrovasculature. Follow-up studies are needed to determine whether in vivo treatment of aged rodents with NMN restores a youthful capillary density in brain regions important for learning and memory and whether NMN positively affects cerebral angiogenesis and/or collateral formation induced by physiological (e.g., exercise, local ischemia) or pharmacological stimuli. As the protective effect of NMN on formation of capillary-like structures by aged CMVECs is prevented by disruption of SIRT1 signaling, it is likely that restoration of NAD+ levels activates sirtuins, which confer pro-angiogenic effects. This concept is supported also by the observation that treatment of aged mice with NMN improves skeletal muscle blood flow by promoting SIRT1-dependent increases in capillary density (Das et al. 2018).
Previous studies established a causal link among age-related oxidative stress, decreased bioavailability of NO, and impaired angiogenic capacity of aged endothelial cells (Koike et al. 2003; Sadoun and Reed 2003; Bach et al. 2005; Reed et al. 2005; Ungvari et al. 2013; Ungvari et al. 2018a, b). Our previous studies demonstrate that increased cellular H2O2 levels promote downregulation of Dicer-dependent angiomirs (pro-angiogenic miRNAs) in aged CMVECs (Ungvari et al. 2013). Further, induction of oxidative stress by downregulation of key antioxidant systems impairs angiogenic potential of endothelial cells (Valcarcel-Ares et al. 2012a, b). Here, we demonstrate that age-related increase in endothelial H2O2 production is effectively attenuated by NMN treatment. This observation extends the findings of our recent studies showing that in vivo treatment with NMN treatment also attenuates age-related mitochondrial oxidative stress in CMVECs restoring NO bioavailability and improving endothelium-mediated vasodilation, suggesting a key role for these mechanisms in NAD+-mediated endothelial protection (Csiszar et al. 2019; Tarantini et al. 2019). Mitochondria-derived O2− is dismutated to H2O2 by manganese superoxide dismutase (MnSOD). H2O2 can readily penetrate the mitochondrial membrane, and its increased cytosolic level is likely responsible for the anti-angiogenic effects associated with mitochondrial oxidative stress. Previous studies provide additional support to this concept by showing that attenuation of mitochondrial oxidative stress using structurally different inhibitors/scavengers of mtROS production (resveratrol, mitoTEMPO) increases cerebral capillary density and/or restores angiogenic potential in aged rodents (Oomen et al. 2009; Miura et al. 2017). Our recent studies also demonstrate that attenuation of mitochondrial oxidative stress (Ungvari et al. 2009; Toth et al. 2014; Tarantini et al. 2018, 2019) also restores endothelium-mediated vasodilation in aged mice. The synergistic functional and structural microvascular protective effects of NMN and mitochondria-targeted antioxidants likely significantly improve cerebral blood flow in aging, contributing to their beneficial effects on cognitive function (Tarantini et al. 2018, 2019). Other age-related mechanisms, which may contribute to the induction of the anti-angiogenic phenotype in CMVECs exacerbating the effects of NAD+ depletion, include age-related IGF-1 deficiency (Sonntag et al. 1997, 2012; Ungvari and Csiszar 2012) and Nrf2 dysfunction (Valcarcel-Ares et al. 2012a, b).
Significant data are available to support the efficacy and translational relevance of NMN and other related NAD+ boosters (e.g., nicotinamide riboside treatment; Yoshino et al. 2018) (Csiszar et al. 2019). Studies are currently underway to determine whether chronic treatment with nicotinamide riboside improves cerebral blood flow (ClinicalTrials.gov Identifier: NCT03482167) in older adults with mild cognitive impairment. If these studies yield positive results, the effects of NMN treatment of organ capillarization in elderly patients should also be investigated.
Funding information
This work was supported by grants from the American Heart Association (ST, MNVA), the Oklahoma Center for the Advancement of Science and Technology (to AC, AY, ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; R01-AG055395), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC, R01-NS100782 to ZU), the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (GM104938, to AY), and the Presbyterian Health Foundation (to ZU, AC, AY).
Compliance with ethical standards
Disclaimer
The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tamas Kiss, Priya Balasubramanian, Marta Noa Valcarcel-Ares and Stefano Tarantini contributed equally to this work.
Contributor Information
Dora Reglodi, Email: dora.reglodi@aok.pte.hu.
Zoltan Ungvari, Email: zoltan-ungvari@ouhsc.edu.
References
- Bach MH, Sadoun E, Reed MJ. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech Ageing Dev. 2005;126(4):467–473. doi: 10.1016/j.mad.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A, Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2015;70(6):665–674. doi: 10.1093/gerona/glu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentourkia M, Bol A, Ivanoiu A, Labar D, Sibomana M, Coppens A, Michel C, Cosnard G, De Volder AG. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. J Neurol Sci. 2000;181(1–2):19–28. doi: 10.1016/s0022-510x(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17(11):679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, Yabluchanskiy A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. Geroscience. 2018;40:337–346. doi: 10.1007/s11357-018-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307(3):H292–H306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68(3):235–249. doi: 10.1093/gerona/gls158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39:359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA, Ungvari ZI. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;316:H1253–H1266. doi: 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham GM, Flores LC, Roman MG, Cheng C, Dube S, Allen C, Valentine JM, Hubbard GB, Bai Y, Saunders TL, Ikeno Y. Thioredoxin overexpression in both the cytosol and mitochondria accelerates age-related disease and shortens lifespan in male C57BL/6 mice. Geroscience. 2018;40:453–468. doi: 10.1007/s11357-018-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173(1):74–89 e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience. 2017;39(2):187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, McFadden S, Darcy J, Hill CM, Huber JA, Verhulst S, Kopchick JJ, Miller RA, Sun LY, Bartke A. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience. 2017;39(3):347–356. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64(6):575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40(5–6):513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Amado M, Prensa L. Stereological analysis of neuron, glial and endothelial cell numbers in the human amygdaloid complex. PLoS One. 2012;7(6):e38692. doi: 10.1371/journal.pone.0038692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20(3):130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CD, Jafari N, Hou L, Li Y, Stewart JD, Zhang G, Lamichhane A, Manson JE, Baccarelli AA, Whitsel EA, Conneely KN. A longitudinal study of DNA methylation as a potential mediator of age-related diabetes risk. Geroscience. 2017;39(5–6):475–489. doi: 10.1007/s11357-017-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermehl Tracy L., Parkinson Kate C., Hubbard Gene B., Ikeno Yuji, Engelmeyer Jennifer I., Schumacher Björn, Mason Jeffrey B. Extension of longevity and reduction of inflammation is ovarian-dependent, but germ cell-independent in post-reproductive female mice. GeroScience. 2018;41(1):25–38. doi: 10.1007/s11357-018-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstadius S, Risberg J. Regional cerebral blood flow characteristics and variations with age in resting normal subjects. Brain Cogn. 1989;10(1):28–43. doi: 10.1016/0278-2626(89)90073-0. [DOI] [PubMed] [Google Scholar]
- Imai SI, Guarente L. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2:16017. doi: 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008;63(1):12–20. doi: 10.1093/gerona/63.1.12. [DOI] [PubMed] [Google Scholar]
- Johnson S, Wozniak DF, Imai S. CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ Aging Mech Dis. 2018;4:10. doi: 10.1038/s41514-018-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura J, Terayama Y, Takashima S, Obara K, Pavol MA, Meyer JS, Mortel KF, Weathers S. Leuko-araiosis and cerebral perfusion in normal aging. Exp Aging Res. 1993;19(3):225–240. doi: 10.1080/03610739308253935. [DOI] [PubMed] [Google Scholar]
- Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE. Growth hormone increases regional coronary blood flow and capillary density in aged rats. J Gerontol A Biol Sci Med Sci. 2001;56(8):B364–B371. doi: 10.1093/gerona/56.8.b364. [DOI] [PubMed] [Google Scholar]
- Kim S, Wyckoff J, Morris AT, Succop A, Avery A, Duncan GE, Jazwinski SM. DNA methylation associated with healthy aging of elderly twins. Geroscience. 2018;40(5–6):469–484. doi: 10.1007/s11357-018-0040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T, Vernon RB, Gooden MD, Sadoun E, Reed MJ. Inhibited angiogenesis in aging: a role for TIMP-2. J Gerontol A Biol Sci Med Sci. 2003;58(9):B798–B805. doi: 10.1093/gerona/58.9.b798. [DOI] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Musci RV, Wolff CA, Reid JJ, Biela LM, Zhang Q, Peelor FF, 3rd, Melby CL, Hamilton KL, Miller BF. Influence of Nrf2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience. 2017;39(2):175–186. doi: 10.1007/s11357-017-9968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol. 1999;172(1):213–218. doi: 10.2214/ajr.172.1.9888770. [DOI] [PubMed] [Google Scholar]
- Lahteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res. 2012;110(9):1252–1264. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Feliers D, Barnes JL, Oh S, Choudhury GG, Diaz V, Galvan V, Strong R, Nelson J, Salmon A, Kevil CG, Kasinath BS. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience. 2018;40(2):163–176. doi: 10.1007/s11357-018-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Rubinstein ND, Buffenstein R. A window into extreme longevity; the circulating metabolomic signature of the naked mole-rat, a mammal that shows negligible senescence. Geroscience. 2018;40(2):105–121. doi: 10.1007/s11357-018-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Cooney PT, Bennett SA, Thornton PL, Khan AS, Ingram RL, Sonntag WE. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging. 1999;20(2):191–200. doi: 10.1016/s0197-4580(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11(4):684–689. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- Masser DR, Hadad N, Porter H, Stout MB, Unnikrishnan A, Stanford DR, Freeman WM. Analysis of DNA modifications in aging research. Geroscience. 2018;40(1):11–29. doi: 10.1007/s11357-018-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7(7):e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitschelen M, Garteiser P, Carnes BA, Farley JA, Doblas S, Demoe JH, Warrington JP, Yan H, Nicolle MM, Towner R, Sonntag WE. Basal and hypercapnia-altered cerebrovascular perfusion predict mild cognitive impairment in aging rodents. Neuroscience. 2009;164(3):918–928. doi: 10.1016/j.neuroscience.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Saitoh SI, Kokubun T, Owada T, Yamauchi H, Machii H, Takeishi Y (2017) Mitochondrial-targeted antioxidant maintains blood flow, mitochondrial function, and redox balance in old mice following prolonged limb ischemia. Int J Mol Sci 18(9). 10.3390/ijms18091897 [DOI] [PMC free article] [PubMed]
- Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996;16(3):385–398. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- Murugesan N, Demarest TG, Madri JA, Pachter JS. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol Aging. 2012;33(5):1004 e1001–1004 e1016. doi: 10.1016/j.neurobiolaging.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. Geroscience. 2018;40:243–256. doi: 10.1007/s11357-018-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olecka M, Huse K, Platzer M. The high degree of cystathionine beta-synthase (CBS) activation by S-adenosylmethionine (SAM) may explain naked mole-rat’s distinct methionine metabolite profile compared to mouse. Geroscience. 2018;40(4):359–360. doi: 10.1007/s11357-018-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Salmaso D, Jonsson C, Hatherly R, Jacobsson H, Larsson SA, Wagner A. Regional cerebral blood flow as assessed by principal component analysis and (99m)Tc-HMPAO SPET in healthy subjects at rest: normal distribution and effect of age and gender. Eur J Nucl Med Mol Imaging. 2002;29(1):67–75. doi: 10.1007/s00259-001-0676-2. [DOI] [PubMed] [Google Scholar]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017;39(2):147–160. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MJ, Bradshaw AD, Shaw M, Sadoun E, Han N, Ferara N, Funk S, Puolakkainen P, Sage EH. Enhanced angiogenesis characteristic of SPARC-null mice disappears with age. J Cell Physiol. 2005;204(3):800–807. doi: 10.1002/jcp.20348. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Atlasz T, Szabo E, Jungling A, Tamas A, Juhasz T, Fulop BD, Bardosi A. PACAP deficiency as a model of aging. Geroscience. 2018;40(5–6):437–452. doi: 10.1007/s11357-018-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2(2):149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51(9):1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- Schultz MB, Sinclair DA. Why NAD(+) declines during aging: it’s destroyed. Cell Metab. 2016;23(6):965–966. doi: 10.1016/j.cmet.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SK, O’Leary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to mid-life adults. Neuroreport. 1999;10(12):2493–2496. doi: 10.1097/00001756-199908200-00011. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Csiszar A, Decabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67A:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138(8):3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Sure VN, Sakamuri S, Sperling JA, Evans WR, Merdzo I, Mostany R, Murfee WL, Busija DW, Katakam PVG. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. Geroscience. 2018;40(4):365–375. doi: 10.1007/s11357-018-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini Stefano, Fulop Gabor A., Kiss Tamas, Farkas Eszter, Zölei-Szénási Dániel, Galvan Veronica, Toth Peter, Csiszar Anna, Ungvari Zoltan, Yabluchanskiy Andriy. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. GeroScience. 2017;39(4):465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38(4):273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17(2):e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39(5–6):601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312(1):H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306(3):H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33(11):1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fulop G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. 2017;39:385–406. doi: 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69(10):1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69(11):1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67A:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297(5):H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123(7):849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39(1):33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15(9):555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE. Aging-induced dysregulation of Dicer1-dependent MicroRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68(8):877–891. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fulop GA, Kiss T, Csiszar A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience. 2017;39(5–6):491–498. doi: 10.1007/s11357-017-9995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Yabluchanskiy A, Tarantini S, Toth P, Kirkpatrick AC, Csiszar A, Prodan CI. Repeated Valsalva maneuvers promote symptomatic manifestations of cerebral microhemorrhages: implications for the pathogenesis of vascular cognitive impairment in older adults. Geroscience. 2018;40(5–6):485–496. doi: 10.1007/s11357-018-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67(8):821–829. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67(8):821–829. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JP, Csiszar A, Johnson DA, Herman TS, Ahmad S, Lee YW, Sonntag WE. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol. 2011;300(3):H736–H744. doi: 10.1152/ajpheart.01024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS One. 2012;7(1):e30444. doi: 10.1371/journal.pone.0030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130(6):1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Baur JA, Imai SI. NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27(3):513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]


