Abstract
Beginning with basic stereotactic operative methods in neurosurgery, intraoperative navigation and image guidance systems have since become the norm in that field. Following the introduction of image guidance into spinal surgery, there has been a dramatic increase in its utilization across disciplines and pathologies. Spine tumor surgery encompasses a wide range of complex surgical techniques and treatment strategies. Similarly to deformity correction and trauma surgery, spine navigation holds potential to improve outcomes and optimize surgical technique for spinal tumors. Recent data demonstrate the applicability of neuro-navigation in the field of spinal oncology, particularly for spinal stabilization, maximizing extent of resection and integration of minimally invasive therapies. The rapid introduction of new, less invasive and ablative surgical techniques in spine oncology coupled with the rising incidence of spinal metastatic disease make it imperative for spine surgeons to be familiar with the indications for and limitations of imaging guidance. Herein we provide a practical, current concepts narrative review on the use of spinal navigation in three areas of spinal oncology: a) extent of tumor resection, b) spinal column stabilization, and c) focal ablation techniques.
Keywords: image guidance, navigation, minimally invasive, spinal metastases, spinal tumors
Introduction
Treatment goals of spinal tumor surgery include local tumor control, preservation or restoration of both neurologic function and spinal stability, and quality of life improvement. With an aging population and improved systemic treatment options, the incidence of spine tumor patients requiring surgery is rising [12,17,67]. Recent advancements include integration of technology such as stereotactic radiosurgery, minimally invasive surgical (MIS) techniques, improved spinal stabilization methods, and percutaneous ablation systems [69].
Spinal navigation has been used in the degenerative, deformity, and trauma populations for years with data supporting improved hardware placement accuracy, reduced screw placement time and decreased risk of reoperation [36,49,29]. Furthermore, it facilitates MIS operations, which in the spine tumor population has been shown to decrease the risk of wound complications, expedite recovery, and shorten time to radiation (RT) and systemic therapy [25,24,13,51]. A variety of intraoperative navigational tools are now available, including 2- or 3- dimensional systems which can be portable or permanent installed in a ‘hybrid’ OR. Imaging modalities of these systems range from fluoroscopic based systems like Ziehm Vision RFD 3D™ (Ziehm Imaging GmbH, Nuremberg, Germany) to computed tomography (CT) devices such as the cone-beam O-arm™ (Medtronic, Minneapolis, MN) and Airo® (BrainLab AG, Munich, Germany). As navigation and image-guidance technology continues to mature, its use is beginning to take hold in spine oncology.
The current objective is to provide a practical, current concepts review of how spinal navigation and image-guidance can be used in the treatment of spinal tumors. This narrative review aims to address the following three areas: (a) extent of tumor excision, (b) spinal column stabilization, and (c) focal ablation. Detailed cases are provided to illustrate navigation principles.
Materials and Methods
To describe the use of navigation and image-guidance in spinal tumor surgery, a practical, narrative review was undertaken. The study was exempt from institutional review board approval as it did not involve human subjects research. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Relevant articles were searched using the PubMed, Embase, and Google Scholar databases. The reference lists of collected studies were scanned for other pertinent articles. The MEDLINE search terms included: “minimally invasive spine spinal tumor” OR “navigation”OR “stereotactic” OR “image-guidance” OR “fluoroscopy” OR “separation surgery” OR “spine oncology” OR “spine metastases.” Since the a-priori objective was to conduct a narrative review, a systematic literature search was not done. Rather, a concise summary of key navigation concepts in spine tumor surgery was synthesized. Detailed case presentations with corresponding figures are provided. The manuscript is divided into three main sections: a) extent of tumor excision, b) stabilization, and c) ablation. Within each section, sub-sections of background, intraoperative use, and representative cases are included.
Results
I. EXTENT OF TUMOR RESECTION
Complete tumor removal with appropriate surgical margins represents the main goal of surgery for primary spinal column tumors. For metastatic tumors of the spine, the key surgical goal is spinal cord and nerve root decompression, laying the foundation for optimal adjuvant radiation therapy, which can generally be achieved with subtotal tumor excision [4].
A). Primary Tumors
Background & Justification
The treatment of primary spinal tumors is in evolution and the optimal treatment strategy is debatable. Traditionally primary tumors such as osteogenic sarcomas [53], chondrosarcomas [18], and chordomas [27,59] had been operated to achieve an en bloc tumor excision with marginal or wide margins. The Enneking system classifies an Enneking Appropriate (EA) resection as wide or marginal, compared to an Enneking Inappropriate (EI) resection that is intralesional. Achieving an EA resection requires careful planning of the exposure, meticulous osteotomy trajectories, and visualization of complex three-dimensional anatomy already distorted by tumor. With a cogent understanding of the relevant anatomy and tumor margins, navigation can play a crucial role in defining a plane of dissection and osteotomy location, thus minimizing the extent of surgical resection. The use of frameless stereotactic navigation can be used to navigate drill bits or osteotomes [14]. in order to ensure adequate bony resection while encountering challenging anatomy. Image-guidance assists in maintaining the osteotomy in line with the previously made plan which can be crucial to maintaining the tumor margin integrity. This is often done through intra-operative CT navigation, but the combination of MR and CT images has been reported in a large series of sacral chordomas [66,2].
Intraoperative Use
Real time navigation systems require a reference array placed firmly on a bony surface. A reference clamp is placed at an adjacent spinous process for thoracic lesions, at the posterior superior iliac spine (PSIS) for lumbar or sacral tumors or directly on the Mayfield head holder for cervical tumors. Initial exposure requires meticulous care to avoid violating the tumor capsule, as often navigation cannot be used until adequate bony landmarks are subperiosteally dissected. For some systems, additional anatomical landmarks are then chosen for 3-D registration. Though a learning curve exists, this process can be done quickly, as one study reported 19.2 minutes for full registration [66]. Upon successful registration, instruments can be used to determine the plane of dissection. Though this is most reliable in the bony landmarks of the sacrum or pelvis, it can be useful for soft tissue planes such as the subcutaneous tissue, erector spinae, gluteus muscles, and pelvic viscera. One additional study noted that CT images are most useful to identify proximal level of tumor involvement, sacroiliac joints, and other bony landmarks, while MRI is best used to identify the inferior sacrum and soft tissue involvement [66]. Previous biopsy tracts can also be excised with certainty, as can previous intralesional surgeries if relevant. One of the earliest series of navigation in spine tumor surgery reported successful screw placement and tumor localization and excision in 7 patients with both primary and metastatic spine tumors. At 18 months follow-up, no LR had occurred and one case of revision surgery was needed for a C1 pedicle screw [2].
Sacral and pelvic tumors represent a unique set of challenges given the complex, 3-D anatomy of the region and large tumor size. Large tumors are often in close proximity to critical structures such as the ureters, rectum, and iliac vessels, iestructures not commonly seen in the spine operative corridor. Even the most experienced surgeons may have difficulty in achieving wide or marginal margins [34]. First reported by Dosenbrock and colleagues, several authors have reported the benefit of using navigation during sacral tumor operations [14-16,66,34]. In the only paper to show improved outcomes with navigated sacral tumor resection, Jeys et al. [35] described a 9% intralesional resection rate in 23 primary sacral tumors using navigation compared to their previously published rate of 29% without navigation. For large sacral tumors done in two stages, anterior sacral bone cuts are made in the first, anterior stage. In fact, the distal sacrum falls steeply away from the surgeon anteriorly after S1, and stereotaxy to know the precise location of the drill or osteotome in the anterior S2-S4 region can be very useful when operating deep in the pelvis with vessels nearby [1]. Yang et al. [66] reported the use of navigation in the resection of 26 sacral chordomas, of which 18 were wide, 4 marginal, and 4 intralesional resections. Interestingly, the difference between the preoperative planned bone cuts to the tumor edge and resected specimen to the tumor edge for EA resections was +4.1mm (range 1.9-6.8mm) compared to the EI resections distances, where the four excised specimens were shorter than that of the preoperative plans by −2.0mm (range −1.1-3.0mm). The authors concluded that computer-assisted navigation allowed for execution of preoperative planning with minimal registration deviation.
Representative Case
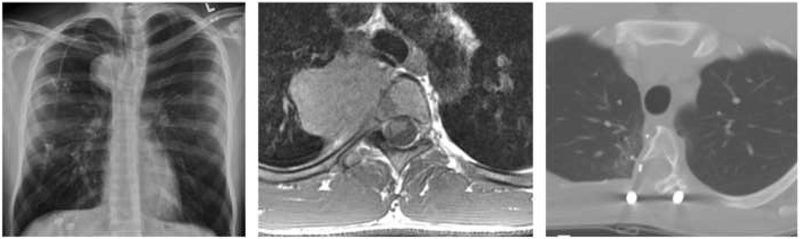
A 22 year-old male presented with back pain after weightlifting and on further workup was found to have the right thoracic mass shown in Figure 1. This tumor—ultimately diagnosed as a chondrosarcoma—was invading only a portion of the T4 and T5 vertebral bodies, but intraoperative navigation allowed for resection with clean margins while sparing the patient a complete two-level corpectomy.
Fig. 1.
Intraoperative navigation used to optimize extent of resection. Representative case of a 22 year-old male with a T4-5 chondrosarcoma. (a) Chest x-ray at diagnosis, (b) axial T1 post-contrast MRI demonstrating invasion into the T4 vertebral body, (c) post-operative axial CT scan depicting partial corpectomy achieved with navigation assistance.
B). Metastatic Tumors
Background & Justification
With the integration of spinal stereotactic radiosurgery (SRS), separation surgery has become the preferred treatment for metastatic epidural spinal cord compression (MESCC) [4,5]. Using this combined hybrid therapy of separation surgery with concomitant radiosurgery, [4] extensive cytoreductive surgery is no longer necessary, as SRS provides reliable tumor control regardless of volume and histology [65]. The goal of separation surgery is to provide separation between the tumor and spinal dura to allow adequate doses of SRS to be delivered postoperatively [45]. The thecal sac is reconstituted with 2-4mm of space between tumor and the spinal cord and/or nerve roots. SRS can then be initiated safely without fear of under-dosing the tumor, leading to inadequate tumor control, or of overdosing the nearby organs at risk (OAR) resulting in undesired toxicity [7,38].
Intraoperative Use
With the transition of surgical treatment of spinal metastases to less invasive procedures, methods to minimize surgical exposure, operative time, and complications become key. Hence, the role for image-guidance is currently under exploration. Separation surgery is a posterolateral approach in which circumferential epidural tumor separation can be achieved via bilateral pedicle resection. Ventral tumor separation is challenging and methods to ensure adequate ventral decompression include resection of the posterior longitudinal ligament (PLL). Primarily, the role of image guidance in surgery for spinal metastases is to aid in instrumented stabilization and minimalizing exposure to radiation [35]. However, when direct visualization is not possible, image-guidance, in the form of neuronavigation or ultrasound, becomes the most reliable means of visualizing the anterior epidural space. Subsequently, navigated probes and curettes in conjunction with intraoperative ultrasound (US) can facilitate achievement and verification of ventral decompression [4,47].
Representative Case
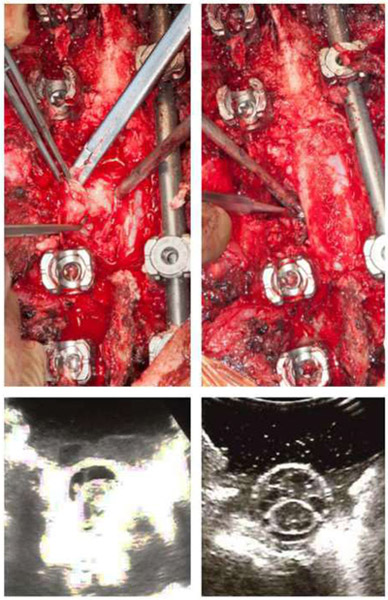
Figure 2 demonstrates an example case of metastatic epidural spinal cord compression in a 76-year-old gentleman with a known history of renal cell carcinoma who presented with isolated back pain. His workup revealed a T4 mass invading the pedicle and posterior elements with high grade spinal cord compression. He underwent a posterior decompression with circumferential separation using intraoperative navigation and ultrasound.
Fig. 2.
Navigational assistance and ultrasound for resection of spinal metastasis. Intraoperative photographs and ultrasound images during separation surgery for a renal cell carcinoma metastasis at T4 with high-grade epidural spinal cord compression. (a) Photograph of decompression of the posterior most portion of the tumor, (b) photograph of ventral decompression portion of the procedure, using intraoperative navigation, (c) intraoperative axial ultrasound view demonstrating echodense material surrounding the spinal cord, (d) post-resection axial ultrasound indicating successful decompression of the thecal sac and sufficient separation.
II. SPINAL COLUMN STABILIZATION
Background & Justification
Spinal instability represents an independent surgical indication for surgery since radiation or systemic therapies do not restore spinal stability. Determination of mechanical integrity is a clinical and radiographic decision facilitated by the Spinal Instability Neoplastic Score (SINS) developed by the Spinal Oncology Study Group (SOSG) [22]. Cancer patients typically have poor bone quality secondary to the osteolytic tumors, radiation, chemotherapy, malnutrition and other age and disease related comorbidities. Consequently, arthrodesis is unlikely and stabilization is principally achieved through spinal instrumentation and/or cement augmentation. Given the previously mentioned high morbidity in metastatic spine tumor patients, a trend towards MIS approaches is gaining favor. With less invasive approaches, the use of intraoperative navigation becomes essential to hardware placement [29].
Intraoperative Use
Percutaneous screws.
Percutaneous instrumentation allows muscle and ligament sparing alongside improved wound healing. Anterior-Posterior (AP) and lateral fluoroscopic guidance using one or two x-ray machines or intraoperative CT navigation can be used for accurate pedicle screw placement. In the case of tumor-infiltrated or osteoporotic bone, pedicle screw cement augmentation can increase pullout strength, as a pedicle fracture or hardware failure in a short construct can be potentially catastrophic [52,9]. In cases of sacral insufficiency fractures requiring lumbopelvic fixation, percutaneous iliac screws can also be placed with an additional “tear-drop” view to visualize the ilium [39]. Although several studies cite minimal complication rates, improved pain, and decreased time to radiation, [39,46] it is important to note the potential for neurologic injury due to a misplaced screw or cement extravasation always leers [60]. Avoiding these complications with intraoperative image guidance, either fluoroscopic or CT-guided, can be essential. The use of fenestrated percutaneous pedicle screws has also gained favor [3].
Open screws.
The importance well placed pedicle screws is critical particularly when instrumentation is placed before decompression. Intraoperative CT can be a useful adjunct for placement of difficult screws or instances where only unilateral fixation is possible [64]. At the opposite end of the spine, total sacrectomies provide an equally formidable challenge, where pelvic and lumbar pedicle screws bear even more importance [23].
Cement injection.
As stated, cement augmentation — with or without screws — is commonly done by surgeons and interventional pain physicians. Kyphoplasty involves balloon inflation within the vertebral body followed by polymethylmethacrylate (PMMA) injection, [8,24] and vertebroplasty is a similar percutaneous procedure without balloon inflation.[33] A study providing Class I evidence of balloon kyphoplasty compared to non-operative management for treatment of painful metastatic fractures was the Cancer Patient Fracture Evaluation (CAFE) study [6]. The randomized, multicenter trial evaluated 65 patients treated with kyphoplasty compared to 52 treated non-operatively and found a statistically significant improvement in pain, activity, analgesic requirement, and quality of life in the kyphoplasty group. It should be noted that CT guidance alone is not recommended in this population due to the requirement of dynamic imaging. After each cement injection, fluoroscopy is used to ensure cement extravasation has not occurred by comparing to the pre-injection image.
Representative Case
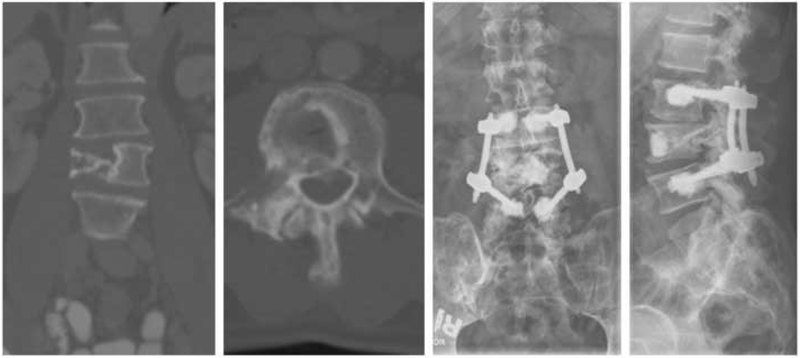
A 61-year-old male with a known history of multiple myeloma presented with mechanical low back pain and was found to have a pathologic L4 compression fracture, shown Figure 3 (A-B). This patient underwent an L4 kyphoplasty and percutaneous L3-5 pedicle screw fixation using fenestrated screws which allowed additional cement augmentation in light of poor bone quality.
Fig. 3.
Stabilization and cement augmentation using navigation. Use of fenestrated percutaneous pedicle screws for cement augmentation. (a) coronal pre-operative CT scan demonstrating a right-sided pathologic compression fracture, (b) axial CT slice at the level of the L4 compression fracture, (c) post-operative AP x-ray depicting pedicle screw construct with evidence of cement administration through fenestrated screws, (d) post-operative lateral x-ray of the same construct which provides a better view of the kyphoplasty cement injected into the L4 vertebral body.
III. ABLATIVE PROCEDURES
Image-guided ablative techniques represent an assortment of treatment modalities for spine tumors and are most often used in the setting of metastatic disease. These techniques may be used for either cytoreduction and/or pain control.
A). Laser Interstitial Thermotherapy
Background & Justification
Image-guided laser interstitial thermal therapy (LITT)—initially developed for ablation of intracranial pathology—has recently been adopted as an alternative to traditional open separation surgery in the management of epidural spine metastases. There have been four reports describing this technique to date, pioneered at M.D. Anderson Cancer Center [57,26,54,56]. The primary indication for the procedure is for cytoreduction of radioresistant tumors in the epidural region, creating space for concomitant radiosurgery. The technique is particularly useful for patients with contraindications to more invasive open separation surgery such as malnutrition or poor performance status. Early results from these studies showed that LITT is effective in reducing epidural tumor burden while improving pain and quality of life measures [57,55].
Intraoperative Use
LITT cases require intraoperative MRI to monitor delivery of the thermal therapy. A pre-operative CT of the spine is used for navigation by way of registration with a spinous process clamp and C-arm fluoroscopy. This navigation system is then used to position the laser probe within the involved epidural space, typically via a transpedicular, vertical, or translaminar approach [56]. Thermal therapy is then administered under real-time intraoperative MRI monitoring; the specific thermal MRI sequence permits assessment of both intensity and spread of heat within the involved tissue [57]. The workflow of these cases has been meticulously described [55].
B). Radiofrequency Ablation
Background & Justification
Metastatic spine patients frequently suffer from biological pain (i.e. night or morning pain, thought to be related to low nighttime cortisol production and different from mechanical pain). For those patients who do not undergo surgical intervention, radiation therapy is only capable of providing partial pain improvement in 48-50% of patients, with complete pain relief in a mere 15-18% [40,58,28]. In these instances, image-guided radiofrequency ablation (RFA) has emerged as a viable option for pain relief.
RFA is a percutaneous procedure involving placement of an electrode into the involved vertebral body to deliver high frequency alternating current into the lesion, producing frictional heating [37,42]. Local tissue temperatures reach 60-100°C, causing protein denaturation and coagulative necrosis of the tumor [42]. Cryoablation is a related technique which rapidly reduces the temperature of surrounding tissues to less then −100°C resulting in ice crystal formation and a resultant osmotic gradient that causes cell injury [50]. Compared to RT alone, RFA can provide rapid relief (including for painful but benign lesions), and can be used synergistically with both cement augmentation or concurrent RT [28,68,42,62,44]. Long-term outcomes are unclear, but this technology may be a useful addition to the minimally invasive methods available for palliative treatment [11].
Intraoperative Use
Several series have endorsed the use of fluoroscopic and CT image guidance with RFA [44,61,42,68]. Tumors in difficult-to-reach areas such as the posterior vertebral body often require a specialized navigation radiofrequency probe [61,30,42], For complex, multi-level lesions, preoperative MRI can be used for navigation to prepare overlapping ablation plans, bipedicular approaches, or secondary trajectories to inject cooling material near neural elements [61].
After preoperative planning, intraoperative imaging is done with either fluoroscopy or CT-guidance. Biplanar fluoroscopy can be used for probe placement and to monitor for cement extravasation, if this is performed. Fluoroscopy provides suboptimal visualization of tumor, however, so for both cryoablation and traditional RFA, probes can be more accurately be positioned using CT guidance.
C). Brachytherapy
Background & Justification
Brachytherapy is the direct application of a radiation source to the region of tumor. Though its use in the spine has been limited, it is one of the oldest radiation techniques, first described in the early 20th century [31]. Modern day indications include salvage treatment for radioresistant tumors in the setting of high grade cord compression in a previously irradiated field or if other treatments cannot be tolerated. Brachytherapy in the spine enables delivery of a therapeutic radiation dose to dural margin using a short-range source and without exceeding spinal cord constraints and may be administered using high energy photon beta-emitting radioisotopes in the form of radioactive seeds, gel foam, or plaque [20,21,41,43]. Positioning of the brachytherapy source is typically performed via an open approach but some sources can be placed in a minimally invasive manner. With improved image-guidance brachytherapy options and applications are likely to grow.
Intraoperative Use
Spinal brachytherapy is administered either directly during open surgery, or percutaneously. In the case of brachytherapy in the setting of open surgery, image-guidance is not required. However, when done percutaneously, either fluoroscopy [48] or CT/MRI-guidance is used [10,32,63]. Once access to the pedicle is gained, catheters are placed into the vertebral body while attempting to minimize radiation dose to adjacent neurological and critical structures [21]. Navigation with CT or MRI allows for pre-operative planning of brachytherapy administration with the assistance of a radiation oncologist [19].
Representative Case
Figure 4 demonstrates the use of a navigational system for insertion of brachytherapy catheters for vertebral body metastasis. The patient was a 62-year-old woman with metastatic thyroid cancer and an associated L3 vertebral body/paravertebral metastasis. She underwent percutaneous single-fraction treatment with iridium-192 delivered percutaneously via flexible afterloading brachytherapy catheters.
Fig. 4.
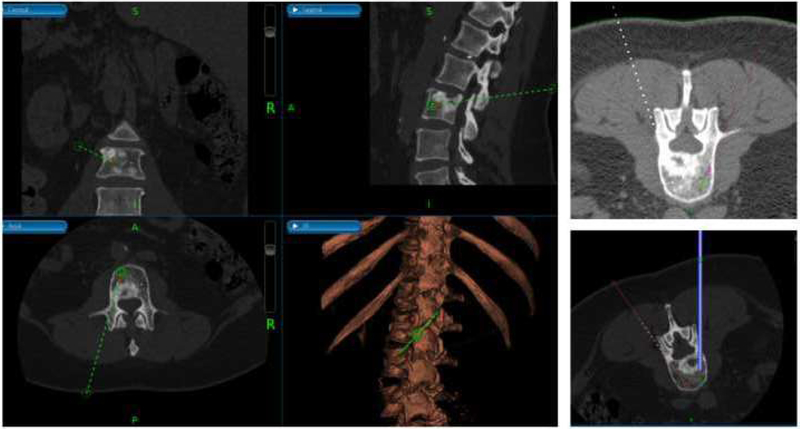
Use of intraoperative navigation for delivery of brachytherapy. (Left) multi-plane view of trajectory planning using intraoperative navigational system, (Right, Top) simulated positioning of brachytherapy source at the tip of the afterloading catheter (Right, Bottom) guided placement of trocar.
Discussion
This current narrative review sought to summarize the utility of neuronavigation in the field of spine oncology. Navigation systems can be helpful in traditional open techniques, as exemplified by its applications in sacral tumor removal yet perhaps the ripest area for navigational techniques is in adjunctive and MIS therapies.
Applications of neuronavigation are rapidly expanding, and large centers continue to add to the considerable advances in the field. One recent article applied the growing field of 3D printing to the problem of spine oncology surgical planning; Jentzsch et al. reported four pelvic sarcomas and the use of navigation for three-dimensional (3D) preoperative planning, printing, and patient specific instruments [34]. In comparing one patient with patient-specific instruments to one with freehand osteotomies, the maximum error in preoperative planning compared to the excised tumor was 0.4mm compared to 2.8mm.
Many of the publications identified this review were case reports, case series, or expert opinions. As the use of navigation in primary spine tumor surgery grows, cohort studies may eventually be available to correlate metrics such as operative time, blood loss, and length of stay between navigated and non-navigated tumors. Furthermore, to our knowledge, no study exists showing the effect of navigation on tumor control, which may be a future area of study [16].
The spread of any new technology is hindered by both ‘late-adopters’ as well as the learning curve necessary to apply the technology. Farfalli and co-authors used navigation in 78 primary sacropelvic tumors and abandoned its use in four (5%) of cases; time spent on navigation significantly improved with experience, however [16]. As this technology becomes more widespread, high volume centers continue to disseminate methods of improving overall workflow and addressing potential pitfalls. Especially in the setting of large, complex operations, experienced surgeons using new technology should not be discouraged.
Several noteworthy arguments against the use of navigation warrant discussion. One major impediment is cost; the fixed cost of the equipment as well as variable costs of additional instruments, maintenance, and staff training are often considered prohibitive. Although there is additional reimbursement for the use of navigation, this amount is minimal [29]. On the other hand, cost savings through decreased operative time and avoidance of reoperation (including possible malpractice claims) are difficult to quantify [29].
Conclusion
While innovative intraoperative navigation equipment and techniques are continually produced, their applications in spine oncology grow concomitantly. Navigation assists with maximal safe resection in debulking and separation surgeries, improved accuracy with instrumentation and targeted local ablative therapies. In light of growing interest in minimally invasive procedures with decreased morbidity alongside direct visualization, intraoperative navigation is poised to become a critical tool for spine tumor surgeons.
Footnotes
Financial/Material Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Al Eissa S, Al-Habib AF, Jahangiri FR (2015) Computer-Assisted Navigation During an Anterior-Posterior En Bloc Resection of a Sacral Tumor. Cureus 7 (11):e373. doi: 10.7759/cureus.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandiera S, Ghermandi R, Gasbarrini A, Barbanti Brodano G, Colangeli S, Boriani S (2013) Navigation-assisted surgery for tumors of the spine. Eur Spine J 22 Suppl 6:S919–924. doi: 10.1007/s00586-013-3032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzilai O, Fisher CG, Bilsky MH (2018) State of the Art Treatment of Spinal Metastatic Disease. Neurosurgery 82 (6):757–769 [DOI] [PubMed] [Google Scholar]
- 4.Barzilai O, Laufer I, Robin A, Xu R, Yamada Y, Bilsky MH (2018) Hybrid Therapy for Metastatic Epidural Spinal Cord Compression: Technique for Separation Surgery and Spine Radiosurgery. Oper Neurosurg (Hagerstown). doi: 10.1093/ons/opy137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzilai O, Laufer I, Yamada Y, Higginson DS, Schmitt AM, Lis E, Bilsky MH (2017) Integrating Evidence-Based Medicine for Treatment of Spinal Metastases Into a Decision Framework: Neurologic, Oncologic, Mechanicals Stability, and Systemic Disease. J Clin Oncol 35 (21):2419–2427. doi: 10.1200/JCO.2017.72.7362 [DOI] [PubMed] [Google Scholar]
- 6.Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schechtman K, Tillman JB, Bastian L, Ashraf T, Vrionis F, Cancer Patient Fracture Evaluation I (2011) Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 12 (3):225–235. doi: 10.1016/S1470-2045(11)70008-0 [DOI] [PubMed] [Google Scholar]
- 7.Bilsky MH, Laufer I, Matros E, Yamada J, Rusch VW (2014) Advanced lung cancer: aggressive surgical therapy vertebral body involvement. Thoracic surgery clinics 24 (4): 423–431. doi: 10.1016/j.thorsurg.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 8.Burton AW, Mendoza T, Gebhardt R, Hamid B, Nouri K, Perez-Toro M, Ting J, Koyyalagunta D (2011) Vertebral compression fracture treatment with vertebroplasty and kyphoplasty: experience in 407 patients with 1,156 fractures in a tertiary cancer center. Pain Med 12 (12): 1750–1757. doi: 10.1111/j.1526-4637.2011.01278.x [DOI] [PubMed] [Google Scholar]
- 9.Burval DJ, McLain RF, Milks R, Inceoglu S (2007) Primary pedicle screw augmentation in osteoporotic lumbar vertebrae: biomechanical analysis of pedicle fixation strength. Spine (Phila Pa 1976) 32 (10): 1077–1083. doi: 10.1097/01.brs.0000261566.38422.40 [DOI] [PubMed] [Google Scholar]
- 10.Cao Q, Wang H, Meng N, Jiang Y, Jiang P, Gao Y, Tian S, Liu C, Yang R, Wang J, Zhang K (2014) CT-guidance interstitial (125)Iodine seed brachytherapy as a salvage therapy for recurrent spinal primary tumors. Radiat Oncol 9:301. doi: 10.1186/s13014-014-0301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi D, Bilsky M, Fehlings M, Fisher C, Gokaslan Z (2017) Spine Oncology-Metastatic Spine Tumors. Neurosurgery 80 (3S):S131–S137. doi: 10.1093/neuros/nyw084 [DOI] [PubMed] [Google Scholar]
- 12.Choi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C, Melcher R, Tomita K, Global Spine Tumor Study G (2010) Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J 19 (2) :215–222. doi: 10.1007/s00586-009-1252-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahele M, Fehlings MG, Sahgal A (2011) Stereotactic radiotherapy: an emerging treatment for spinal metastases. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques 38 (2):247–250 [DOI] [PubMed] [Google Scholar]
- 14.Dasenbrock HH, Clarke MJ, Bydon A, McGirt MJ, Witham TF, Sciubba DM, Gokaslan ZL, Wolinsky JP (2012) En bloc resection of sacral chordomas aided by frameless stereotactic image guidance: a technical note. Neurosurgery 70 (1 Suppl Operative):82–87; discussion 87–88. doi: 10.1227/NEU.0b013e31822dd958 [DOI] [PubMed] [Google Scholar]
- 15.Drazin D, Bhamb N, Al-Khouja LT, Kappel AD, Kim TT, Johnson JP, Brien E (2017) Image-guided resection of aggressive sacral tumors. Neurosurg Focus 42 (1):E15. doi: 10.3171/2016.6.FOCUS16125 [DOI] [PubMed] [Google Scholar]
- 16.Farfalli GL, Albergo JI, Ritacco LE, Ayerza MA, Milano FE, Aponte-Tinao LA (2017) What Is the Expected Learning Curve in Computer-assisted Navigation for Bone Tumor Resection? Clin Orthop Relat Res 475 (3):668–675. doi: 10.1007/s11999-016-4761-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehlings MG, Nater A, Holmer H (2014) Cost-effectiveness of surgery in the management of metastatic epidural spinal cord compression: a systematic review. Spine (PhilaPa 1976) 39 (22 Suppl 1):S99–S105. doi: 10.1097/BRS.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 18.Fisher CG, Versteeg AL, Dea N, Boriani S, Varga PP, Dekutoski MB, Luzzati A, Gokaslan ZL, Williams RP, Reynolds JJ, Fehlings MG, Germscheid NM, Bettegowda C, Rhines LD (2016) Surgical Management of Spinal Chondrosarcomas. Spine (Phila Pa 1976) 41 (8):678–685. doi: 10.1097/BRS.0000000000001485 [DOI] [PubMed] [Google Scholar]
- 19.Folkert MR, Bilsky MH, Cohen GN, Voros L, Oh JH, Zaider M, Laufer I, Yamada Y (2015) Local recurrence outcomes using the (3)(2)P intraoperative brachytherapy plaque in the management of malignant lesions of the spine involving the dura. Brachytherapy 14 (2):202–208. doi: 10.1016/j.brachy.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 20.Folkert MR, Bilsky MH, Cohen GN, Zaider M, Dauer LT, Cox BW, Boland PJ, Laufer I, Yamada Y (2012) Intraoperative 32P high-dose rate brachytherapy of the dura for recurrent primary and metastatic intracranial and spinal tumors. Neurosurgery 71 (5): 1003–1010; discussion 1010–1001. doi: 10.1227/NEU.0b013e31826d5ac1 [DOI] [PubMed] [Google Scholar]
- 21.Folkert MR, Bilsky MH, Cohen GN, Zaider M, Lis E, Krol G, Laufer I, Yamada Y (2013) Intraoperative and percutaneous iridium-192 high-dose-rate brachytherapy for previously irradiated lesions of the spine. Brachytherapy 12 (5):449–456. doi: 10.1016/j.brachy.2013.01.162 [DOI] [PubMed] [Google Scholar]
- 22.Foumey DR, Frangou EM, Ryken TC, Dipaola CP, Shaffrey CI, Berven SH, Bilsky MH, Harrop JS, Fehlings MG, Boriani S, Chou D, Schmidt MH, Polly DW, Biagini R, Burch S, Dekutoski MB, Ganju A, Gerszten PC, Gokaslan ZL, Groff MW, Liebsch NJ, Mendel E, Okuno SH, Patel S, Rhines LD, Rose PS, Sciubba DM, Sundaresan N, Tomita K, Varga PP, Vialle LR, Vrionis FD, Yamada Y, Fisher CG (2011) Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol 29 (22):3072–3077. doi: 10.1200/JCO.2010.34.3897 [DOI] [PubMed] [Google Scholar]
- 23.Garvey PB, Clemens MW, Rhines LD, Sacks JM (2013) Vertical rectus abdominis musculocutaneous flow-through flap to a free fibula flap for total sacrectomy reconstruction. Microsurgery 33 (l):32–38. doi: 10.1002/micr.21990 [DOI] [PubMed] [Google Scholar]
- 24.Gerszten PC (2014) Spine metastases: from radiotherapy, surgery, to radiosurgery. Neurosurgery 61 Suppl 1:16–25. doi: 10.1227/NEU.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 25.Gerszten PC, Mendel E, Yamada Y (2009) Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine 34 (22 Suppl):S78–92. doi: 10.1097/BRS.0b013e3181b8b6f5 [DOI] [PubMed] [Google Scholar]
- 26.Ghia AJ, Rebueno NC, Li J, Brown PD, Rhines LD, Tatsui CE (2016) The use of image guided laser interstitial thermotherapy to supplement spine stereotactic radiosurgery to manage metastatic epidural spinal cord compression: Proof of concept and dosimetric analysis. Pract Radiat Oncol 6 (2):e35–38. doi: 10.1016/j.prro.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 27.Gokaslan ZL, Zadnik PL, Sciubba DM, Germscheid N, Goodwin CR, Wolinsky JP, Bettegowda C, Groves ML, Luzzati A, Rhines LD, Fisher CG, Varga PP, Dekutoski MB, Clarke MJ, Fehlings MG, Quraishi NA, Chou D, Reynolds JJ, Williams RP, Kawahara N, Boriani S (2016) Mobile spine chordoma: results of 166 patients from the AOSpine Knowledge Forum Tumor database. J Neurosurg Spine 24 (4):644–651. doi: 10.3171/2015.7.SPINE15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwood TJ, Wallace A, Friedman MV, Hillen TJ, Robinson CG, Jennings JW (2015) Combined Ablation and Radiation Therapy of Spinal Metastases: A Novel Multimodality Treatment Approach. Pain Physician 18 (6):573–581 [PubMed] [Google Scholar]
- 29.Helm PA, Teichman R, Hartmann SL, Simon D (2015) Spinal Navigation and Imaging: History, Trends, and Future. IEEE Trans Med Imaging 34 (8): 1738–1746. doi: 10.1109/TMI.2015.2391200 [DOI] [PubMed] [Google Scholar]
- 30.Hillen TJ, Anchala P, Friedman MV, Jennings JW (2014) Treatment of metastatic posterior vertebral body osseous tumors by using a targeted bipolar radiofrequency ablation device: technical note. Radiology 273 (l):261–267. doi: 10.1148/radiol.14131664 [DOI] [PubMed] [Google Scholar]
- 31.Holm HH (1997) The history of interstitial brachytherapy of prostatic cancer. Semin Surg Oncol 13 (6): 431–437 [DOI] [PubMed] [Google Scholar]
- 32.Huang H, Xu S, Du Z, Li F, Wang L (2014) [Treatment of metastatic thoracolumbar tumors by percutaneous vertebroplasty combined with interstitial implantation of (1)(2)(5)I seeds], Zhonghua Zhong Liu Za Zhi 36 (3):228–231 [PubMed] [Google Scholar]
- 33.Jensen ME, Kallmes DE (2002) Percutaneous vertebroplasty in the treatment of malignant spine disease. Cancer J 8 (2): 194–206 [DOI] [PubMed] [Google Scholar]
- 34.Jentzsch T, Vlachopoulos L, Fumstahl P, Muller DA, Fuchs B (2016) Tumor resection at the pelvis using three-dimensional planning and patient-specific instruments: a case series. World J Surg Oncol 14 (1):249. doi: 10.1186/s12957-016-1006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeys L, Matharu GS, Nandra RS, Grimer RJ (2013) Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Joint J 95-B (10): 1417–1424. doi: 10.1302/0301-620X.95B10.31734 [DOI] [PubMed] [Google Scholar]
- 36.Kotani T, Akazawa T, Sakuma T, Koyama K, Nemoto T, Nawata K, Yamazaki A, Minami S (2014) Accuracy of Pedicle Screw Placement in Scoliosis Surgery: A Comparison between Conventional Computed Tomography-Based and O-Arm-Based Navigation Techniques. Asian Spine J 8 (3): 331–338. doi: 10.4184/asj.2014.8.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurup AN, Callstrom MR (2010) Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Intervent Radiol 27 (3):276–284. doi: 10.1055/s-0030-1261786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laufer I, Iorgulescu JB, Chapman T, Lis E, Shi W, Zhang Z, Cox BW, Yamada Y, Bilsky MH (2013) Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine 18 (3):207–214. doi: 10.3171/2012.11.SPINE12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu G, Hasan MY, Wong HK (2016) Minimally invasive iliac screw fixation in treating painful metastatic lumbosacral deformity: a technique description and clinical results. Eur Spine J. doi: 10.1007/s00586-016-4387-6 [DOI] [PubMed] [Google Scholar]
- 40.Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, Howell D, Konski A, Kachnic L, Lo S, Sahgal A, Silverman L, von Gunten C, Mendel E, Vassil A, Bruner DW, Elartsell W, American Society for Radiation O (2011) Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 79 (4):965–976. doi: 10.1016/j.ijrobp.2010.11.026 [DOI] [PubMed] [Google Scholar]
- 41.Ma X, Wei S, Yang C, Hua Y, Shen J, Cai Z (2015) Gelfoam embolization or 125I seed implantation may be a more effective treatment than surgical treatment for giant benign sacral neurogenic tumors. World J Surg Oncol 13:247. doi: 10.1186/s12957-015-0662-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Wallace AN, Madaelil TP, Jennings JW (2016) Treatment of osseous metastases using the Spinal Tumor Ablation with Radiofrequency (STAR) system. Expert Rev Med Devices 13 (12): 1137–1145. doi: 10.1080/17434440.2016.1256772 [DOI] [PubMed] [Google Scholar]
- 43.Marchese MJ, Nori D, Anderson LL, Hilaris BS (1984) A versatile permanent planar implant technique utilizing iodine-125 seeds imbedded in gelfoam. Int J Radiat Oncol Biol Phys 10 (5):747–751 [DOI] [PubMed] [Google Scholar]
- 44.Morassi LG, Kokkinis K, Evangelopoulos DS, Karargyris O, Vlachou I, Kalokairinou K, Pneumaticos SG (2014) Percutaneous radiofrequency ablation of spinal osteoid osteoma under CT guidance. Br J Radiol 87 (1038):20140003. doi: 10.1259/bjr.20140003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moussazadeh N, Laufer I, Yamada Y, Bilsky MH (2014) Separation surgery for spinal metastases: effect of spinal radiosurgery on surgical treatment goals. Cancer Control 21 (2): 168–174 [DOI] [PubMed] [Google Scholar]
- 46.Moussazadeh N, Rubin DG, McLaughlin L, Lis E, Bilsky MH, Laufer I (2015) Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J 15 (7): 1609–1617. doi: 10.1016/j.spinee.2015.03.037 [DOI] [PubMed] [Google Scholar]
- 47.Nasser R, Drazin D, Nakhla J, Al-Khouja L, Brien E, Baron EM, Kim TT, Patrick Johnson J, Yassari R (2016) Resection of spinal column tumors utilizing image-guided navigation: a multicenter analysis. Neurosurgical focus 41 (2):E15. [DOI] [PubMed] [Google Scholar]
- 48.Qian J, Bao Z, Zou J, Yang H (2016) Effect of pedicle fixation combined with (125)I seed implantation for metastatic thoracolumbar tumors. J Pain Res 9:271–278. doi: 10.2147/JPR.S105284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajasekaran S, Vidyadhara S, Ramesh P, Shetty AP (2007) Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine (Phila Pa 1976) 32 (2):E56–64. doi: 10.1097/01.brs.0000252094.64857.ab [DOI] [PubMed] [Google Scholar]
- 50.Sabel MS (2009) Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 58 (1): 1–11. doi: 10.1016/j.cryobiol.2008.10.126 [DOI] [PubMed] [Google Scholar]
- 51.Sahgal A, Bilsky M, Chang EL, Ma L, Yamada Y, Rhines LD, Letourneau D, Foote M, Yu E, Larson DA, Fehlings MG (2011) Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine 14 (2): 151–166. doi: 10.3171/2010.9.SPINE091005 [DOI] [PubMed] [Google Scholar]
- 52.Sawakami K, Yamazaki A, Ishikawa S, Ito T, Watanabe K, Endo N (2012) Polymethylmethacrylate augmentation of pedicle screws increases the initial fixation in osteoporotic spine patients. Journal of spinal disorders & techniques 25 (2):E28–35. doi: 10.1097/BSD.0b013e318228bbed [DOI] [PubMed] [Google Scholar]
- 53.Schwab J, Gasbarrini A, Bandiera S, Boriani L, Amendola L, Picci P, Ferrari S, Boriani S (2012) Osteosarcoma of the mobile spine. Spine (Phila Pa 1976) 37 (6):E381–386. doi: 10.1097/BRS.0b013e31822fb1a7 [DOI] [PubMed] [Google Scholar]
- 54.Tatsui CE, Belsuzarri TA, Oro M, Rhines LD, Li J, Ghia AJ, Amini B, Espinoza H, Brown PD, Rao G (2016) Percutaneous surgery for treatment of epidural spinal cord compression and spinal instability: technical note. Neurosurg Focus 41 (4):E2. doi: 10.3171/2016.8.FOCUS16175 [DOI] [PubMed] [Google Scholar]
- 55.Tatsui CE, Lee SH, Amini B, Rao G, Suki D, Oro M, Brown PD, Ghia AJ, Bhavsar S, Popat K, Rhines LD, Stafford RJ, Li J (2016) Spinal Laser Interstitial Thermal Therapy: A Novel Alternative to Surgery for Metastatic Epidural Spinal Cord Compression. Neurosurgery 79 Suppl 1 S73–S82. doi: 10.1227/NEU.0000000000001444 [DOI] [PubMed] [Google Scholar]
- 56.Tatsui CE, Nascimento CN, Suki D, Amini B, Li J, Ghia AJ, Thomas JG, Stafford RJ, Rhines LD, Cata JP, Kumar AJ, Rao G (2017) Image guidance based on MRI for spinal interstitial laser thermotherapy: technical aspects and accuracy. J Neurosurg Spine:l–8. doi: 10.3171/2016.9.SPINE16475 [DOI] [PubMed] [Google Scholar]
- 57.Tatsui CE, Stafford RJ, Li J, Sellin JN, Amini B, Rao G, Suki D, Ghia AJ, Brown P, Lee SH, Cowles CE, Weinberg JS, Rhines LD (2015) Utilization of laser interstitial thermotherapy guided by real-time thermal MRI as an alternative to separation surgery in the management of spinal metastasis. Journal of neurosurgery Spine: 1–12. doi: 10.3171/2015.2.SPINE141185 [DOI] [PubMed] [Google Scholar]
- 58.Valesin Filho ES, de Abreu LC, Lima GH, de Cubero DI, Ueno FH, Figueiredo GS, Valenti VE, Monteiro CB, Wajnsztejn R, Fujiki EN, Neto MR, Rodrigues LM (2013) Pain and quality of life in patients undergoing radiotherapy for spinal metastatic disease treatment. Int Arch Med 6 (1):6. doi: 10.1186/1755-7682-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varga PP, Szoverfi Z, Fisher CG, Boriani S, Gokaslan ZL, Dekutoski MB, Chou D, Quraishi NA, Reynolds JJ, Luzzati A, Williams R, Fehlings MG, Germscheid NM, Lazary A, Rhines LD (2015) Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J 24 (5): 1092–1101. doi: 10.1007/s00586-014-3728-6 [DOI] [PubMed] [Google Scholar]
- 60.Versteeg AL, Verlaan JJ, de Baat P, Jiya TU, Stadhouder A, Diekerhof CH, van Solinge GB, Oner FC (2016) Complications After Percutaneous Pedicle Screw Fixation for the Treatment of Unstable Spinal Metastases. Ann Surg Oncol. doi: 10.1245/s10434-016-5156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallace AN, Greenwood TJ, Jennings JW (2015) Use of Imaging in the Management of Metastatic Spine Disease With Percutaneous Ablation and Vertebral Augmentation. AJR Am J Roentgenol 205 (2):434–441. doi: 10.2214/AJR.14.14199 [DOI] [PubMed] [Google Scholar]
- 62.Wallace AN, Tomasian A, Vaswani D, Vyhmeister R, Chang RO, Jennings JW (2016) Radiographic Local Control of Spinal Metastases with Percutaneous Radiofrequency Ablation and Vertebral Augmentation. AJNR Am JNeuroradiol 37 (4):759–765. doi: 10.3174/ajnr.A4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Q, Hu X, Li Z (2014) [Application study of vertebral column metastasis tumor with embedment of (1)(2)(5)I by CT guide], Zhonghua Yi Xue Za Zhi 94 (33):2573–2575 [PubMed] [Google Scholar]
- 64.Wewel JT, Nunna RS, Tan LA, Kasliwal MK, O’Toole JE (2016) Novel reconstruction of the anterior craniocervical junction using an expandable cage with integrated fixation after total C2 spondylectomy for chordoma. J Clin Neurosci 30:157–160. doi: 10.1016/j.jocn.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 65.Yamada Y, Katsoulakis E, Laufer I, Lovelock M, Barzilai O, McLaughlin LA, Zhang Z, Schmitt AM, Higginson DS, Lis E, Zelefsky MJ, Mechalakos J, Bilsky MH (2017) The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus 42 (1):E6. doi: 10.3171/2016.9.FOCUS16369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang YK, Chan CM, Zhang Q, Xu HR, Niu XH (2016) Computer Navigation-aided Resection of Sacral Chordomas. Chin Med J (Engl) 129 (2):162–168. doi: 10.4103/0366-6999.173465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshihara H, Yoneoka D (2014) Trends in the surgical treatment for spinal metastasis and the in-hospital patient outcomes in the United States from 2000 to 2009. The spine journal : official journal of the North American Spine Society 14 (9): 1844–1849. doi: 10.1016/j.spinee.2013.11.029 [DOI] [PubMed] [Google Scholar]
- 68.Yu F, Niu XH, Zhang Q, Zhao HT, Xu LH, Deng ZP (2015) Radiofrequency ablation under 3D intraoperative Iso-C C-arm navigation for the treatment of osteoid osteomas. Br J Radiol 88 (1056):20140535. doi: 10.1259/bjr.20140535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuckerman SL, Laufer I, Sahgal A, Yamada YJ, Schmidt MH, Chou D, Shin JH, Kumar N, Sciubba DM (2016) When Less Is More: The indications for MIS Techniques and Separation Surgery in Metastatic Spine Disease. Spine (PhilaPa 1976) 41 Suppl 20:S246–S253. doi: 10.1097/BRS.0000000000001824 [DOI] [PMC free article] [PubMed] [Google Scholar]