Abstract
OBJECTIVE
Maximal safe tumor resection in language areas of the brain relies on a patient’s ability to perform intraoperative language tasks. Assessing the performance of these tasks during awake craniotomies allows the neurosurgeon to identify and preserve brain regions that are critical for language processing. However, receiving sedation and analgesia just prior to experiencing an awake craniotomy may reduce a patient’s wakefulness, leading to transient language and/or cognitive impairments that do not completely subside before language testing begins. At present, the degree to which wakefulness influences intraoperative language task performance is unclear. Therefore, the authors sought to determine whether any of 5 brief measures of wakefulness predicts such performance during awake craniotomies for glioma resection.
METHODS
The authors recruited 21 patients with dominant hemisphere low- and high-grade gliomas. Each patient performed baseline wakefulness measures in addition to picture-naming and text-reading language tasks 24 hours before undergoing an awake craniotomy. The patients performed these same tasks again in the operating room following the cessation of anesthesia medications. The authors then conducted statistical analyses to investigate potential relationships between wakefulness measures and language task performance.
RESULTS
Relative to baseline, performance on 3 of the 4 objective wakefulness measures (rapid counting, button pressing, and vigilance) declined in the operating room. Moreover, these declines appeared in the complete absence of self-reported changes in arousal. Performance on language tasks similarly declined in the intraoperative setting, with patients experiencing greater declines in picture naming than in text reading. Finally, performance declines on rapid counting and vigilance wakefulness tasks predicted performance declines on the picture-naming task.
CONCLUSIONS
Current subjective methods for assessing wakefulness during awake craniotomies may be insufficient. The administration of objective measures of wakefulness just prior to language task administration may help to ensure that patients are ready for testing. It may also allow neurosurgeons to identify patients who are at risk for poor intraoperative performance.
Keywords: arousal, awake craniotomy, anesthesia, language, brain mapping, surgical technique
The ultimate goal of the surgical management of low- and high-grade gliomas is to achieve maximal safe resection.11,15,17 While there is ample support in the literature for improved overall and progression-free survival with greater extent of resection, the survival benefits of tumor cytoreduction decline in patients with lasting neurological deficits.13 For this reason, neurosurgeons employ awake craniotomies before glioma resection to map out and subsequently preserve, in real time, regions of the brain that are responsible for language, cognitive, and sensorimotor processing.3,4,8,19 An estimated 46%−61% of gliomas are located within presumed eloquent regions of brain. Therefore, awake craniotomy has become an increasingly common and necessary neurosurgical procedure.5,22
Although there are several methods for performing an awake craniotomy, the most common approach invokes the asleep-awake-asleep technique.21 Here, the patient is awakened during surgery as sedating medications used for the initial induction of anesthesia are reduced or stopped completely based on drug metabolism. Performance on language, motor, and cognitive tasks during direct electrical stimulation (DES) mapping informs the removal of noneloquent areas of the brain, with lasting effects on seizure control, cognition, and survival.14
Adequate patient wakefulness and cooperation during the awake stage of the procedure is critical. However, the use of sedatives, opiates, and anxiolytics to improve patient comfort during the procedure may make the delivery and interpretation of intraoperative language tasks a difficult undertaking.2,10
Previous findings suggest that once a patient is taken off anesthesia, the cognitive effects of the drug are eliminated (i.e., an “on-off” phenomenon).18 However, deficits in short-term working memory, language, and attention processing have been reported for up to 3 hours after propofol administration, and drug elimination from the body does not necessarily correlate with cognitive performance.20 Accounts of awake craniotomy performed with dexmedetomidine have further shown discrepancies in patient performance on cognitive tasks (i.e., poor performance on counting and sentence completion tests).2 DES mapping relies on the patient being fully cooperative and alert with a low baseline error rate. The risk of commencing intraoperative mapping for a patient who remains at least partially under the influence of sedation is that functional areas will not be properly identified. This could result in poor functional outcomes following surgery.
These considerations suggest it could be useful to incorporate an objective measure of intraoperative wakefulness to ensure that patients are ready for language testing. For example, since the performance of language tasks varies with arousal, the surgical team may want to assess a patient’s arousal with objective measures before functional mapping begins.6 Commercial measures of arousal such as the bispectral index (BIS) are of limited utility given their poor sensitivity (44%) and relatively modest specificity (74%) for predicting arousal during intraoperative brain mapping procedures.1,7 Further, to the best of our knowledge, no prior studies have sought to quantify intraoperative wakefulness during asleep-awake-asleep craniotomy, especially as it pertains to language processing. We therefore investigated whether performance on any brief wakefulness tasks following the cessation of anesthesia predicts subsequent performance on intraoperative language tasks that guide glioma resection.
Methods
Study Population
Adult patients aged 18–85 years with frontal, temporal, and parietal gliomas were recruited with informed consent for this study in accordance with the University of California, San Francisco (UCSF) institutional review board for human research (UCSF CHR 17–23215). We excluded patients from the final analysis if, during the intraoperative testing period, the surgery team was unable to completely withhold all sedating, anxiolytic, and systemic analgesic medications according to established clinical protocol.13
Tumors were volumetrically analyzed by measuring hyperintense regions on axial T2-weighted FLAIR images for low-grade gliomas and T1-weighted contrast-enhanced MR images for high-grade gliomas. In each case, the tumor was segmented manually across all slices to compute the volume in cubic centimeters using Brainlab software.22 Determination of tumor volume was made without consideration of the patient’s clinical outcome.
The preoperative and intraoperative workflow is summarized in Fig. 1.
FIG. 1.
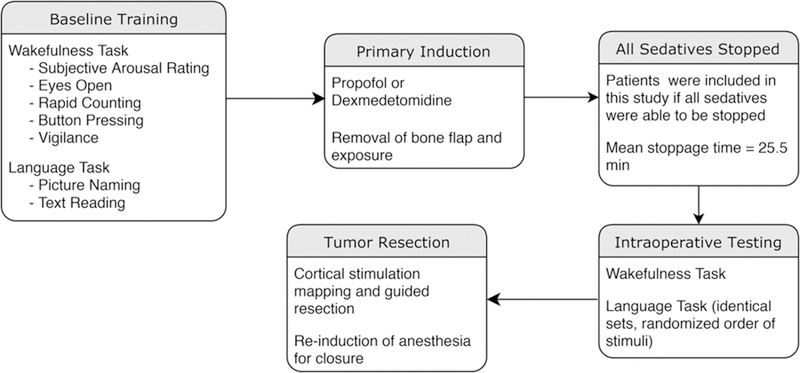
Study workflow including the components of the wakefulness and language tasks, the anesthetic agents applied, and the timing of intraoperative events.
Wakefulness and Language Assessments
Baseline wakefulness and language assessments were collected preoperatively within 24 hours before surgery to ensure patient comfort and familiarity during the procedure.4 The wakefulness measures (“wakefulness tasks”) consisted of 5 components as follows: 1) self-reported level of arousal on a 7-point Likert scale (Fig. 2); 2) visual assessment of the patient’s ability to keep their eyes open for 5 seconds without being prompted (“eyes open”) (Fig. 3A); 3) rapid button pushing on a handheld device over a 5-second period (“button pressing”) (Fig. 3C); 4) counting aloud from 1 to 20 (by ones) as quickly as possible without skipping numbers (“rapid counting”) (Fig. 3B); and 5) designation over 10 trials whether a rapidly flashed box was located on the top or the bottom half of the screen, selecting button 1 for the top half and button 2 for the bottom half of the screen (“vigilance”) during each trial (Fig. 3D). We used Matlab 9.2.0.556344 to record response times, task completion times, and accuracy in each task.
FIG. 2.
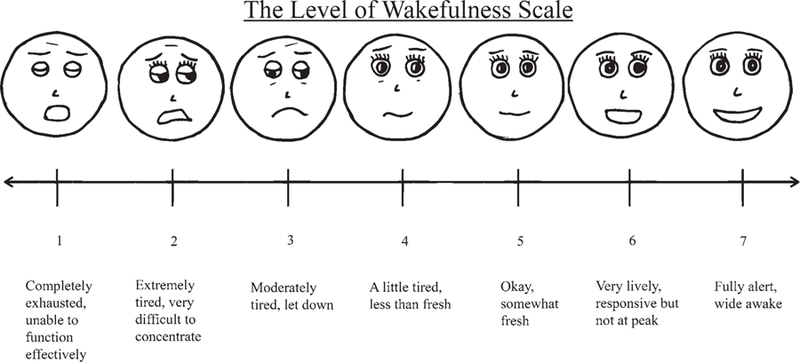
Subjective level of wakefulness scale.
FIG. 3.
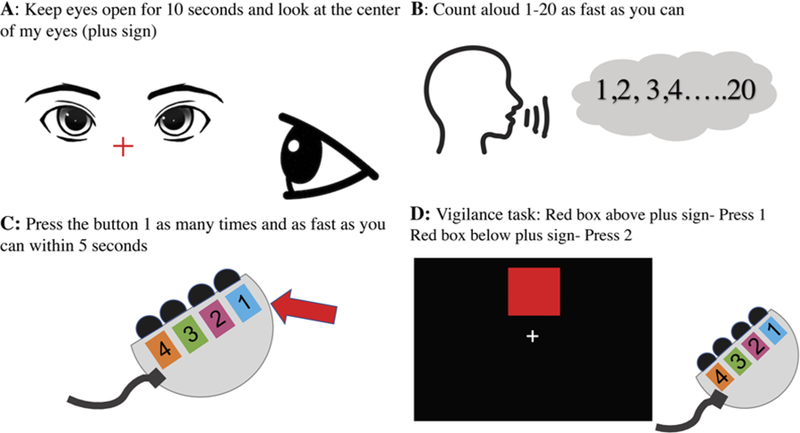
Intraoperative wakefulness tasks assessing a patient’s ability to keep eyes open for 10 seconds (A), count aloud 1–20 by ones as fast as possible (B), press button 1 as many times as possible over 5 seconds (C), and press button 1 when a red indicator box is in the upper half of the screen and button 2 if the red indicator box is in the bottom half of the screen (D).
Next, patients were given standardized picture-naming and text-reading language tasks from the Quick Aphasia Battery (QAB).23 In each trial of the text-reading task, the patient sees and tries to read aloud one of 27 words. In each trial of the picture-naming task, the patient sees and tries to name one of 48 common objects. The tasks were coded such that the order of the stimuli (pictures and text) was always randomized.
Scoring was performed by a speech-language pathologist using the QAB rubric for both picture-naming and text-reading tasks.25 It was not possible for the speech-language pathologist to perform a blinded review of the language tasks given the obvious differences in preoperative and intraoperative settings within the recordings. During the baseline preoperative testing period, patients were permitted to repeat each task multiple times to increase familiarity and to ensure that scores reflected best performance.
Intraoperative Awake Craniotomy Workflow
In the operating room, patients were given low-dose propofol to allow for Mayfield head holder pin placement and insertion of the Foley catheter. According to protocol, the propofol dose was 50–100 µg/kg/min, the dexmedetomidine dose was 0.7–2.0 µg/kg/min, and the remifentanil dose was 0.05–0.1 µg/kg/min.13 We titrated each dose based on patient comfort. In light of established recommendations in the literature, for the propofol-based anesthetic regimen, all medications were withheld for a minimum of 10 minutes prior to intraoperative wakefulness and language testing, while for the dexmedetomidine-based anesthetic regimen, all medications were withheld for a minimum of 15 minutes. Accordingly, even if a patient was found to be clinically ready for testing based on the current standard of care (i.e., able to spontaneously open eyes, respond to surgery team, and subjectively rate their level of wakefulness) before the 10- or 15-minute desired time delay, we still deferred testing until the time threshold was crossed.
Upon awakening, patients were administered the same computerized wakefulness and language tasks that were used during preoperative evaluation. Finally, the neurosurgeon proceeded with stimulation mapping, tumor resection, and closure using a previously published protocol.14
Statistical Analysis
Descriptive statistics are reported as mean value (± standard deviation) for continuous variables. We used the Wilcoxon signed-rank test, the Spearman rank correlation test, and multivariate ANOVA for tests of significance wherever appropriate. All statistical analyses were performed with R Studio 1.1.419.
Results
We recruited 25 patients with low- and high-grade gliomas within the frontal, parietal, and/or temporal lobes of the dominant hemisphere. Four patients were excluded from final analysis because we were unable to stop all anesthesia prior to intraoperative wakefulness and language testing due to increasing anxiety (low-dose anesthesia continued during task administration). Each patient completed all 5 wakefulness task measures during both baseline presurgery and intraoperative testing periods. Wakefulness measures were administered over a mean of 4.88 minutes (SD 1.21 minutes). The preoperative and intraoperative workflow is summarized in Fig. 1.
Glioma diagnoses included 2 patients (10%) with diffuse astrocytoma, 1 (5%) with oligodendroglioma, 1 (5%) with anaplastic oligodendroglioma, 4 (19%) with anaplastic astrocytoma, and 13 (62%) with glioblastoma multiforme. Nine patients (43%) had tumors in the frontal lobe, 3 (14%) in the insula, 3 (14%) in the parietal lobe, and 6 (28%) in the temporal lobe. The mean tumor volume was 22.4 cm3 (SD 18.9 cm3). The intraoperative anesthetic regimen included 13 patients (62%) who received dexmedetomidine anesthesia and 8 (38%) who received propofol (Table 1). For the entire study cohort, the time between stoppage of all anesthetics and commencement of intraoperative wakefulness and language testing ranged from 11 minutes to 72 minutes with a mean of 25.5 minutes (SD 15.5). The mean durations between stopping dexmedetomidine or propofol anesthesia followed by commencement of wakefulness assessments were 30.8 minutes and 17.6 minutes, respectively. Neither the anesthetic agent used (propofol vs dexmedetomidine) nor the time between medication stoppage and intraoperative testing was predictive of task performance in the intraoperative setting (p = 0.0972 and p = 0.3477, respectively). Demographic details are further summarized in Table 1.
TABLE 1.
Patient characteristics, tumor location, diagnosis, tumor volume, anesthetic agent, and time between anesthesia stoppage and intraoperative testing
| Patient No. | Sex | Age (yrs) | Handed | Tumor Location | Hemisphere | WHO Grade | Diagnosis | Tumor Vol (cm3) | Anesthetic | Time Off (mins) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 56 | Lt | Frontal | Rt | IV | GBM | 11.6 | DEX | 49 |
| 2 | M | 60 | Lt | Temporal | Lt | IV | GBM | 31 | DEX | 43 |
| 3 | F | 41 | Rt | Temporal | Lt | II | DA | 26.1 | PROP | 18 |
| 4 | F | 68 | Rt | Insular | Rt | III | AA | 7.1 | DEX | 15 |
| 5 | M | 76 | Rt | Frontal | Lt | IV | GBM | 5.6 | DEX | 15 |
| 6 | F | 64 | Rt | Insular | Lt | III | AA | 6.9 | PROP | 17 |
| 7 | M | 49 | Lt | Parietal | Lt | III | AA | 16.8 | DEX | 72 |
| 8 | M | 78 | Rt | Frontal | Lt | IV | GBM | 58.3 | PROP | 11 |
| 9 | F | 62 | Rt | Temporal | Lt | IV | GBM | 11.9 | DEX | 18 |
| 10 | F | 66 | Rt | Frontal | Lt | IV | GBM | 23.1 | DEX | 15 |
| 11 | F | 26 | Rt | Parietal | Lt | II | OLIG | 37.9 | DEX | 31 |
| 12 | F | 41 | Rt | Frontal | Lt | IV | GBM | 6.9 | PROP | 16 |
| 13 | F | 64 | Rt | Temporal | Lt | IV | GBM | 4.9 | PROP | 23 |
| 14 | F | 63 | Rt | Frontal | Lt | IV | GBM | 14 | PROP | 14 |
| 15 | M | 41 | Rt | Frontal | Rt | IV | GBM | 64.7 | DEX | 15 |
| 16 | M | 57 | Rt | Temporal | Lt | IV | GBM | 10.5 | DEX | 40 |
| 17 | M | 57 | Rt | Temporal | Lt | IV | GBM | 26.1 | DEX | 34 |
| 18 | F | 71 | Rt | Frontal | Rt | IV | GBM | 29.6 | PROP | 20 |
| 19 | M | 55 | Rt | Parietal | Lt | III | AO | 15.5 | DEX | 15 |
| 20 | M | 28 | Rt | Insular | Rt | II | DA | 60.3 | DEX | 38 |
| 21 | M | 47 | Rt | Frontal | Lt | III | AA | 1.89 | PROP | 22 |
AA = anaplastic astrocytoma; AO = anaplastic oligodendroglioma; DA = diffuse astrocytoma; DEX = dexmedetomidine; GBM = glioblastoma; OLIG = oligodendroglioma; PROP = propofol.
Baseline and Intraoperative Wakefulness Task Performance
Subjective Wakefulness
Upon awakening from sedation, patients rated themselves, on average, as “awake, somewhat fresh” with level of alertness rating 5.0. There was no significant difference in subjective (i.e., self-reported) wakefulness between the preoperative baseline and intraoperative testing environments, although there was a trend toward greater subjective wakefulness during intraoperative testing (4.71 preoperative baseline vs 5.38 intraoperative testing, p = 0.0572; Fig. 4A).
FIG. 4.
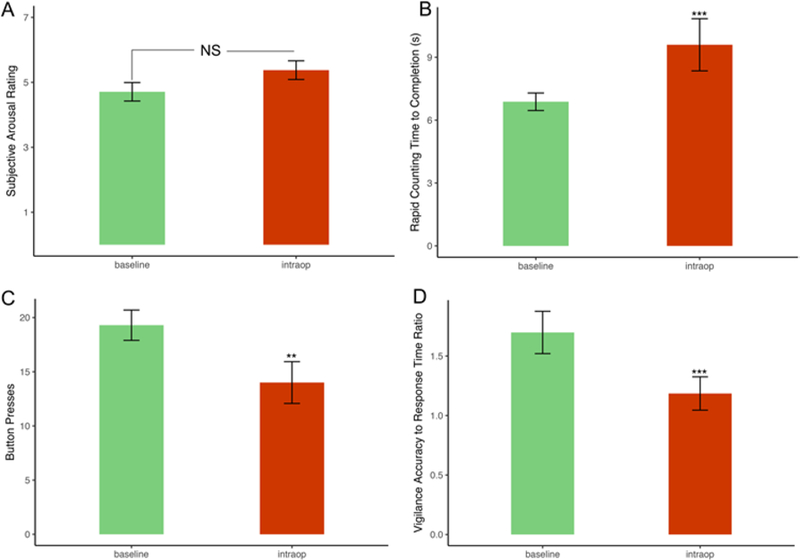
Bar plots showing mean preoperative performance compared to intraoperative performance on 4 of the 5 wakefulness tasks with error bars indicating ± standard error. The eyes-open task was excluded from this figure because all patients were able to successfully complete it in both testing environments. A: Self-reported arousal ratings were not significantly different in the intraoperative setting. B: Patients required on average 2.1 more seconds (s) to count to 20. C: Patients pressed the indicated button on average 5.3 fewer times. D: Patients experienced on average a 30.1% reduction in performance on the vigilance task. *0.01 ≤ p < 0.05, **0.001 ≤ p < 0.01, ***p < 0.001.
Objective Wakefulness
Results for the 4 objective measures of wakefulness were as follows. All of the patients could keep their eyes open for 5 seconds during both testing periods. However, there were statistically significant declines between baseline and intraoperative performance for the remainder of the wakefulness tasks, including rapid counting (8.0 vs 10.1 seconds to count to 20, p < 0.001), button pressing (19.3 vs 14.0 presses within the 5-second time period, p < 0.01), and vigilance (recording worse accuracy to response time ratio, p < 0.001) (Fig. 4B–D).
Language Task Performance
QAB scores for the picture-naming and text-reading tasks were higher in the baseline testing environment than in the intraoperative testing environment (3.70 vs 3.30 with 10.8% decrease, p = 0.032 for picture naming; 3.98 vs 3.87 with 2.8% decrease, p = 0.014 for text reading; Fig. 5A). The bottom quintile of performers on picture naming in the preoperative setting experienced more severe declines in performance on the same task intraoperatively (Fig. 5B). This phenomenon was not observed for text reading as all study subjects were readily able to read text during baseline and intraoperative settings after stopping sedation (Fig. 5C).
FIG. 5.
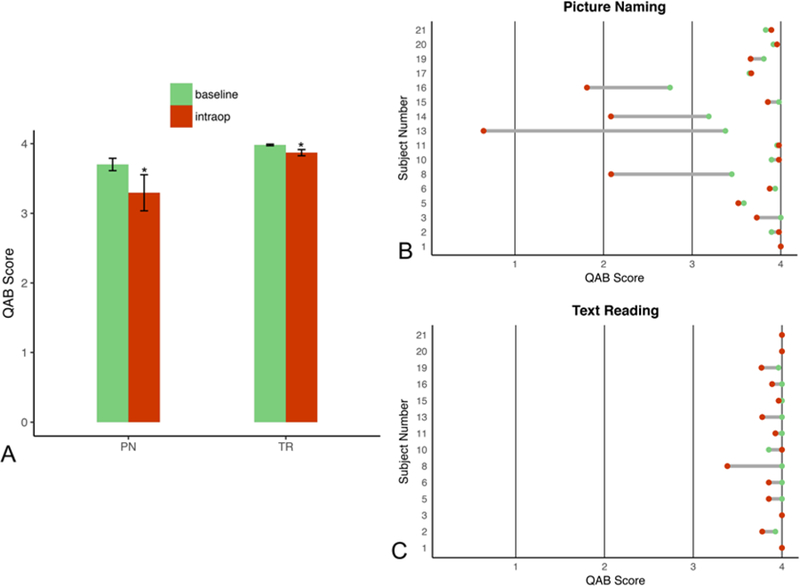
Language task performance across baseline preoperative and intraoperative testing environments. A: Bar plots showing a mean 10.8% decrease in performance on picture naming (PN) and a mean 2.8% decrease on text reading (TR) intraoperatively, with error bars indicating ± standard error. B: Dumbbell plot indicating individual performances on picture naming; the bottom quintile of performers on preoperative examination suffered from the largest declines in intraoperative performance. C: Dumbbell plot of text reading with a relatively small variation in performance. *p < 0.05.
Associations Between Wakefulness and Intraoperative Language Task Performance
We next determined whether any measures of wakefulness correlated with intraoperative language task performance. First, patients’ subjective assessments of their own wakefulness in the intraoperative setting were not predictive of language task performance (p = 0.720). Second, both rapid counting (Spearman’s rank correlation coefficient −0.56, p = 0.01) and vigilance (Spearman’s rank correlation coefficient 0.45, p = 0.04) predicted intraoperative declines in picture-naming performance (Table 2). Finally, we did not conduct similar correlations for the text-reading task. This is because there were no statistically significant declines in intraoperative text reading between baseline presurgery and intraoperative testing periods.
TABLE 2.
Factors predictive of decline in picture-naming performance from baseline
| Predictor | Correlation Coefficient (ρ) | p Value |
|---|---|---|
| Subjective arousal rating | −0.101 | 0.72 |
| Rapid counting | −0.56 | 0.01 |
| Button pressing | −0.35 | 0.10 |
| Vigilance performance | 0.45 | 0.04 |
Boldface type indicates statistical significance.
Discussion
The surgical management of gliomas requires the surgeon to balance the benefits of cytoreduction against the consequences of violating functional cortical and subcortical areas. To the best of our knowledge, no prior study has sought to assess patient performance during intraoperative language tasks by appraising mixed (i.e., linguistic and nonlinguistic) cognitive measures of wakefulness. In our study, we found that performance on 3 of the 4 objective wakefulness measures—button pressing, rapid counting, and vigilance—declined in the intraoperative setting. Further, performance declines on 2 of these measures (rapid counting and vigilance) correlated with performance declines during subsequent language testing. In contrast, neither of the clinical measures that are commonly used—eyes open or subjective wakefulness—correlated with language task performance in the intraoperative setting. The correlations we observed are notable as all of the patients were allowed to repeat the tasks multiple times during the initial training period, which tends to improve performance and would therefore bias one to observe better (rather than worse) performance in the intraoperative setting.
These findings are consistent with prior data indicating that anesthetics do not exert a simple “on-off” effect on cognition. Certain cognitive domains (not limited to language) remain impaired after patients are deemed clinically “awake” based on subjective measures and drug half-life.20 While this may complicate to some extent the postoperative management of any patient receiving anesthesia, we believe this effect to be exceedingly consequential for patients who must participate in cognitive tasks that ultimately guide resection of brain parenchyma.
It is important to mention that differences between baseline and intraoperative performance did not vary with the two anesthetic agents we employed. Our interpretation of this finding is, of course, limited by our inability to randomize the anesthetic regimen we administered. Nevertheless, this finding is consistent with prior reports suggesting similar safety and efficacy profiles of dexmedetomidine and propofol during awake craniotomies.16,21,23
It is also important to note that the time between anesthesia stoppage and the start of intraoperative testing, which was determined by when the patient could be roused by auditory/physical stimulation and consented to engaging in the tasks, did not predict performance. These observations, along with the fairly wide range of time delays across our sample, imply that a prespecified delay following the cessation of anesthesia is likely inadequate for ensuring optimal language task performance during awake craniotomy.
Within the language task itself, there was some divergence in scores. First, both overall performance variability and the magnitude of the performance decline in the intraoperative (vs preoperative) setting were greater for picture naming than for text reading. Second, several of the patients experienced sharp declines for picture naming but not for text reading. These findings may be explained, in part, by well-established reports in the literature documenting greater time requirements for object naming than for text reading despite rigorous training efforts aimed at achieving the contrary.9 While performance of these two tasks ultimately relies on some common motor and nonmotor neural pathways, picture naming seemingly utilizes additional cognitive operations involving visual recognition and lexical linkage via memory as opposed to graphemic processing.24 We speculate that these additional cognitive operations may be more easily disrupted by changes in wakefulness.
We observed the greatest declines in picture-naming performance during the intraoperative setting in the lowest quintile of performers (identified during the baseline preoperative session). While our interpretation is limited by cohort size, this finding may indicate that patients with lower baseline scores are more vulnerable to language declines during intraoperative testing. If this effect persists in a more robust future study, it may provide an opportunity to intervene and manage these patients differently, either by offering patients more time to recover from sedation, or by avoiding the use of early sedation (i.e., by using the awake-awake-awake approach as opposed to asleep-awake-asleep).12
Presently, common methods implemented by the neurosurgical team to assess a given patient’s fitness to engage in intraoperative testing subsequent to anesthesia stoppage include observing and instructing the patient to keep their eyes open for a predefined length of time, asking them to count to a certain number by ones, and requesting them to rate their own level of arousal. Our study demonstrates that some of these tests may be inadequate on their own. Performance on eyes open was perfect across both testing environments for all patients despite several experiencing stark declines in language task performance during intraoperative testing (i.e., patients 8 and 13). Although the rapid counting task was predictive of performance on intraoperative picture naming, without recording the time required for task completion and comparing it to an established baseline or standard, it may be difficult to appreciate whether or not a given performance is acceptable. Finally, patients subjectively reported that they were actually more “awake” in the intraoperative setting (although this finding was not statistically significant). This implies that self-reports of wakefulness are not reliable.
The primary objective of this study was to determine whether objective measures of wakefulness are able to predict intraoperative language declines during asleep-awake-asleep craniotomy for glioma resection. Among the components of the wakefulness task, the vigilance component was likely the most demanding. This task required the patient to conduct rapid visuospatial processing of the stimulus, store it in short-term memory, ignore irrelevant distractors, and make a decision regarding the correct motor response. It is likely that other wakefulness measures (i.e., repetitive button pressing) were either too simple to be sensitive to the lingering effects of the anesthesia and/or relied on neural pathways that differ greatly from those involved in language function. We foresee an opportunity moving forward to assess patient wakefulness prior to brain mapping using assessments of rapid counting and vigilance. Interpretation of these measures may offer better clarity of intraoperative language-mapping results and enhance patient outcomes.
Limitations
We note that our conclusions are limited by our sample size and our inability to standardize the anesthetic agents used, perform blinded scorings of the tasks, and truncate the 24-hour time delay between testing environments. We are therefore unable to offer a head-to-head comparison of intraoperative picture-naming and text-reading task performance with the use of propofol versus dexmedetomidine. Interpretations of these data are also limited by the fact that baseline testing was not performed in the precise manner mimicking the operative room given the inability to place a patient in the Mayfield head holder without first offering intravenous analgesic medications. Furthermore, 4 patients were excluded from final analysis due to clinical inability to stop all anesthesia for the requisite time prior to intraoperative testing, which may have biased our analyses toward the null given the prevalence of the ceiling effect among patients who were able to complete testing. Mitigating the ceiling effect would potentially require the administration of more complex language and cognitive tasks in future investigations (as opposed to those used in this study intended to mirror current clinical practice). However, we acknowledge the possibility that increasing task complexity may lead to higher rates of task failure and further limitations in generalizability.
Conclusions
In the present study, we found that brief measures of wakefulness outperform standard clinical assessments with regard to predicting a patient’s fitness for intraoperative language testing during awake language-mapping craniotomy. The administration of objective measures of wakefulness just prior to language task administration may help to ensure that patients are ready for testing.
Acknowledgments
This study was funded in part by NINDS NIH grant no. 1K08NS110919–01 (for S.L.H.J.).
ABBREVIATIONS
- DES
direct electrical stimulation
- QAB
Quick Aphasia Battery
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, Selvidge JA, et al. : Anesthesia awareness and the bispec tral index. N Engl J Med 358:1097–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bekker AY, Kaufman B, Samir H, Doyle W: The use of dexmedetomidine infusion for awake craniotomy. Anesth Analg 92:1251–1253, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Brown T, Shah AH, Bregy A, Shah NH, Thambuswamy M, Barbarite E, et al. : Awake craniotomy for brain tumor resection: the rule rather than the exception? J Neurosurg Anesthesiol 25:240–247, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Burnand C, Sebastian J: Anaesthesia for awake craniotomy. Contin Educ Anaesth Crit Care Pain 14:6–11, 2014 [Google Scholar]
- 5.Chang EF, Smith JS, Chang SM, Lamborn KR, Prados MD, Butowski N, et al. : Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg 109:817–824, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chang WH, Pei YC, Wei KC, Chao YP, Chen MH, Yeh HA, et al. : Intraoperative linguistic performance during awake brain surgery predicts postoperative linguistic deficits. J Neurooncol 139:215–223, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte V, L’Acqua C, Rotelli S, Stocchetti N: Bispectral index during asleep-awake craniotomies. J Neurosurg Anesthesiol 25:279–284, 2013 [DOI] [PubMed] [Google Scholar]
- 8.De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS: Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol 30:2559–2565, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Ferrand L: Why naming takes longer than reading? The special case of Arabic numbers. Acta Psychol (Amst) 100:253–266, 1999 [Google Scholar]
- 10.Ghazanwy M, Chakrabarti R, Tewari A, Sinha A: Awake craniotomy: a qualitative review and future challenges. Saudi J Anaesth 8:529–539, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groshev A, Padalia D, Patel S, Garcia-Getting R, Sahebjam S, Forsyth PA, et al. : Clinical outcomes from maximum-safe resection of primary and metastatic brain tumors using awake craniotomy. Clin Neurol Neurosurg 157:25–30, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Hansen E, Seemann M, Zech N, Doenitz C, Luerding R, Brawanski A: Awake craniotomies without any sedation: the awake-awake-awake technique. Acta Neurochir (Wien) 155:1417–1424, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Hervey-Jumper SL, Berger MS: Maximizing safe resection of low- and high-grade glioma. J Neurooncol 130:269–282, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Hervey-Jumper SL, Li J, Lau D, Molinaro AM, Perry DW, Meng L, et al. : Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg 123:325–339, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Li YM, Suki D, Hess K, Sawaya R: The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 124:977–988, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Mack PF, Perrine K, Kobylarz E, Schwartz TH, Lien CA: Dexmedetomidine and neurocognitive testing in awake craniotomy. J Neurosurg Anesthesiol 16:20–25, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Martino J, Gomez E, Bilbao JL, Dueñas JC, Vázquez-Barquero A: Cost-utility of maximal safe resection of WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien) 155:41–50, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Meng L, Berger MS, Gelb AW: The potential benefits of awake craniotomy for brain tumor resection: an anesthesiologist’s perspective. J Neurosurg Anesthesiol 27:310–317, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Sacko O, Lauwers-Cances V, Brauge D, Sesay M, Brenner A, Roux FE: Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery 68:1192–1199, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Sanou J, Goodall G, Capuron L, Bourdalle-Badie C, Maurette P: Cognitive sequelae of propofol anaesthesia. Neuroreport 7:1130–1132, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Shen SL, Zheng JY, Zhang J, Wang WY, Jin T, Zhu J, et al. : Comparison of dexmedetomidine and propofol for conscious sedation in awake craniotomy: a prospective, double-blind, randomized, and controlled clinical trial. Ann Pharmacother 47:1391–1399, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. : Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Stevanovic A, Rossaint R, Veldeman M, Bilotta F, Coburn M: Anaesthesia management for awake craniotomy: systematic review and meta-analysis. PLoS One 11:e0156448, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valente A, Pinet S, Alario FX, Laganaro M: “When” does picture naming take longer than word reading? Front Psychol 7:31, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson SM, Eriksson DK, Schneck SM, Lucanie JM: A quick aphasia battery for efficient, reliable, and multidimensional assessment of language function. PLoS One 13:e0192773, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]


