Abstract
Background:
The influx of new oncologic technologies has changed the treatment landscape of renal cell cancer (RCC) in the last decade. This study updated a previously published paper on the economic burden of RCC in the US by using more recent data to examine the impact of various forms of new oncologic technologies on the economic burden of RCC.
Methods:
Using the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database, we employed prevalence and incidence costing approaches to estimate RCC costs from the payer’s perspective. We conducted longitudinal analysis of cost data per patient per month (PPPM) for a prevalence cohort of RCC patients to determine which category of new technology (surgery, radiation, or cancer drugs) was the major cost driver for RCC. We then applied the incidence costing approach to estimate costs related to RCC by care phase (initial, continuing, and terminal) and compared costs between two incidence cohorts to examine how new technology affected the economic burden of RCC over time.
Results:
After controlling for demographic factors, clinical characteristics, neighborhood socioeconomic status, and time trend, we found that rising PPPM costs were driven by new technologies in cancer drugs. Incidence-based analysis showed the annual net cost (2018 US$) for distant stage RCC patients diagnosed between 2002 and 2006 was $51,639, $19,025, $76,603, and $29,045 for the initial, continuing (year 1), terminal (died from RCC), and terminal (died from other causes) care phases, respectively. Costs increased to $70,703, $34,716, $107,989, and $47,538, respectively, for the incidence cohort diagnosed between 2007 and 2011.
Conclusion:
The rising economic burden of RCC was most pronounced among patients with distant stage RCC, and driven primarily by new cancer drugs.
INTRODUCTION
This is the second manuscript of a two-part article to update a previously published two-part article in PharmacoEconomics that provided a comprehensive review of the economic burden of renal cell carcinoma (RCC) in Part I [1] and an estimate of the cost-of-illness (COI) of RCC in the US in Part II [2]. The previous publication analyzed two US databases, 1991–2007 SEER (Surveillance, Epidemiology and End Results)-Medicare [3] and 1996–2007 MarketScan [4], to project the cost impact of the first two targeted therapies approved to treat RCC in the US [2]. It reported that the annual medical cost was $31,000 – $65,000 higher for RCC patients treated with targeted therapies than those who received conventional therapies and concluded that the economic burden was likely to grow with an increasing use of such therapies [2]. Since the original publication, the treatment landscape of RCC has witnessed an explosive growth of new therapies, especially for patients with metastatic RCC (mRCC). As of October 2018, close to 20 treatment alternatives for mRCC have been included in the National Comprehensive Cancer Network Clinical Practice Guidelines for kidney cancer [5]. The influx of new oncologic technologies to treat RCC has motivated us to update our previous review of economic studies for RCC [6] and revisit our previous estimates.
Our previous analysis relied on the 2007 MarketScan databases to glean insight on the potential financial impact of targeted therapies for patients with RCC because of data deficiency of the SEER-Medicare data at the time of the study [2]. That is, although targeted therapy agents such as sorafenib and sunitinib were approved by the Food and Drug Administration (FDA) in late 2005/early 2006, these oral agents were not covered by Medicare until the official launch of the Medicare Prescription Drug Plan, also known as Part D, in 2006. Additionally, the SEER-Medicare data did not release Part D claims (covering year 2007 and forward) until much later. Estimates generated from MarketScan databases were tentative because of the lack of information on tumor characteristics and the date of cancer diagnosis.
The purpose of this article was to update our previous estimate of the economic burden of RCC in the US [2] with more recent releases of the SEER-Medicare data that include Part D claims, thus allowing us to capture costs of targeted oral anticancer medications (TOAMs).
METHODS
Data Source
Data used in our analyses were the 2002–2012 SEER-Medicare database. The SEER-Medicare database links cancer patients in the SEER Program, an epidemiological surveillance system of population-based tumor registries containing data from 18 geographic areas in the US, to Medicare claims and enrollment files [3, 7]. The Medicare program in the US provides health insurance for the elderly and individuals with disability or end-stage-renal disease, with Part A covering inpatient care in hospitals and skilled nursing facilities, hospice, and home health care; Part B for outpatient care, physician services, physical and occupation therapy, and some home health services; and Part D for outpatient prescription drugs. The SEER-Medicare data provide both clinical (e.g., tumor site, stage at diagnosis) and economic information (e.g., Medicare payment) for elderly patients with cancer [3]. The data used in our study include persons with cancer diagnosed in 2011 and before, and Medicare claims for those patients through 2012. This study is exempt from Institutional Review Board at The University of Texas M. D. Anderson Cancer Center because SEER-Medicare data contain de-identified person identifiers.
Approach
Our analysis employed the payer’s perspective because information on out-of-pocket payments is not reliably captured in SEER-Medicare. We applied both prevalence and incidence approaches to estimate the costs of cancer care and assess the impact of new oncologic technologies on costs for patients with RCC. The prevalence approach reports costs of cancer for a specific time period, whereas the incidence approach follows a newly diagnosed cohort to keep track of costs throughout the cancer care continuum [8, 9]. Each costing approach provides different but equally important information to policy makers. The combination of these two costing approaches allowed us to better understand how the diffusion of new oncologic technologies affected the economic burden of RCC. Specifically, we applied the prevalence costing approach to examine the trend of RCC costs over time and to determine which treatment modality (surgery, radiation, or cancer drugs) had new technological advancements that more strongly influenced costs. We then applied the incidence costing approach to assess how the availability of new oncologic technologies, most noticeably TOAMs, changed cancer care costs for newly diagnosed RCC patients.
Ascertainment of Study Cohorts
For the prevalence cohort, we used the 2007–2011 SEER-Medicare database, and identified patients who had RCC and no other cancers from the Patient Entitlement and Diagnosis Summary File (PEDSF, the SEER portion of the SEER-Medicare database) using the site code “kidney” and the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histology codes indicative of RCC (8260, 8310–8312, 8316–8320, 8510, 8959) [10]. Patients were required to have RCC diagnosed before January 1, 2012 and to have been alive at the beginning of 2007. Year 2007 was chosen as the starting year for analyses employing the prevalence approach because claims for outpatient prescription drugs (covered by Medicare Part D) were not available in SEER-Medicare before 2007. In addition, for the completeness of monthly cost information from Medicare claims, patients were required to have Medicare Parts A, B, and D coverage and not be enrolled in a health maintenance organization (HMO) in that month.
For the incidence costing approach, we constructed two incidence cohorts of RCC patients from SEER-Medicare. As in the prevalence cases, patients with RCC were identified from the PEDSF via the above-mentioned ICD-O-3 histology codes. Because Medicare Part D data are only available since 2007, we stratified the incidence cases into two study cohorts: patients who were diagnosed with RCC between 2002 and 2006 vs. those diagnosed between 2007 and 2011. In order to confirm that the patients’ cancer treatment was only for RCC and not any other cancers, we required patients to have a primary diagnosis of RCC and no other cancers. To ensure that patients’ claims data were complete, all patients included in these two cohorts had to be age 65 or older at the time of diagnosis, continuously enrolled in Medicare Parts A & B, and have no HMO enrollment since their RCC diagnosis. For the 2007–2011 incident cohort, we further required patients to be continuously enrolled in Medicare Part D.
Identification of New Oncologic Technologies
We identified surgery (i.e., nephrectomy), radiation, and cancer drugs administered intravenously via the Healthcare Common Procedure Coding System (HCPCS) and ICD-9 procedure codes from Medicare Parts A and B claims. Under Medicare policies, cancer drugs administered intravenously at a physician’s office are covered by Part B; these drugs were identified via the HCPCS codes. Cancer drugs administered orally were identified using National Drug Codes (NDC) codes from Medicare Part D claims. We characterized new oncologic technologies for the treatment modality of surgery as radical or partial nephrectomy performed as laparoscopic procedures, with or without robotic-assistance. New technologies for radiation were exemplified by the use of intensity-modulated radiation therapy, stereotactic body radiation therapy, proton beam radiation therapy, or brachytherapy, whereas new technologies for cancer drugs were captured by the use of targeted therapy (both injectable and oral agents). Targeted therapies captured in our analyses included targeted cancer therapy agents listed in the website of the U.S. National Cancer Institute (NCI) and approved by the U.S. Food and Drug Administration before December 31, 2012, such as bevacizumab, temsirolimus, sorafenib, sunitinib, everolimus, pazopanib, among others.
Analysis
We used the 2007–2011 prevalence cohort of RCC patients to assess the impact of new technologies on the monthly costs of RCC over time. Using patient-month as the unit of analysis, we created three dichotomous variables to indicate whether surgery, radiation, and/or cancer drugs, respectively, were received in a specific month. We added another three dichotomous variables, one for each treatment modality, to capture whether treatment received in that month included new oncologic technologies. Following the convention of cost reporting by the NCI in the US [11, 12], we categorized each patient-month into three care phases: initial, continuing, and terminal. The initial care phase covered the first 12 months following cancer diagnosis, the terminal phase captured the last 12 months of life, and the continuing phase included all the months in between. We applied generalized estimation equation (GEE) with Gamma family and log link to analyze the longitudinal cost data. The dependent variable was monthly medical costs calculated from claims, and covariates included patients’ sociodemographic characteristics (e.g., age, gender, race/ethnicity, neighborhood socioeconomic status [SES]), comorbidity score, geographic region, tumor characteristics (e.g., stage, grade), treatment characteristics (e.g., treatment modality, use of new technology, and phase of care), and time trend. Comorbidity score for each patient was calculated using the Klaubunde algorithm for claims data [13, 14].
We then compared two incidence cohorts of RCC patients, those diagnosed between 2002 and 2006 vs. between 2007 and 2011, to assess how novel therapies introduced after 2006 (targeted therapies) affected the total annual costs of RCC. To attribute costs to care related to RCC, we employed the incremental costing approach which compared the total annual cost of patients with RCC with that of patients in a matched non-cancer control group [15]. We applied 1:1 frequency match to construct a non-cancer control group using age, gender, race, and state of residence as the matching criteria [16]. To estimate the annual costs of RCC for patients in different care phases, we constructed the non-cancer control group for each of the three care phases and further separated patients in terminal care phase by cause of death (RCC vs. other causes). We then calculated the total annual costs by aggregating Medicare payments from all Medicare claims and estimated the annual costs of RCC using the difference in the mean annual costs between patients in the case and control groups for each care phase. We applied non-parametric bootstrapping method, with 1,000 repeated sampling, to derive the 95% confidence intervals of the costs attributable to cancer (i.e., the difference in mean cost between case and control groups) from the 2.5th and 97.5th percentiles [17]. We conducted a subgroup analysis using only patients with distant stage RCC because the financial impact of new cancer drugs could be most pronounced in this subgroup.
All costs were normalized to 2018 US dollars using the medical care services component of the Consumer Price Index. We used SAS 9.4 for data management and STATA 15.1 for the statistical/econometric analyses.
RESULTS
Characteristics of Prevalence and Incidence Cohorts of RCC Patients
A total of 15,227 prevalence cases of RCC were identified from the 2007–2011 SEER-Medicare database, compared to 9,078 and 5,098 patients in the 2002–2006 and 2007–2011 incidence cohorts, respectively (Table 1). Differences in demographic and tumor characteristics between the prevalence vs. incidence cohorts were evident in the distribution of age (P<0.0001), stage (P<0.0001), and year of diagnosis, with a substantially higher proportion of patients in the prevalence cohort in the younger age group (i.e., 65–69 years), localized stage, and being diagnosed before 2007.
Table 1:
Descriptive Statistics of the Study Cohort
| Prevalence Cohort 2007–2011 |
Incidence Cohort diagnosed 2002–2006 |
Incidence Cohort diagnosed 2007–20011 |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | Frequency | Percent | ||||
| Total | 15227 | 100 | 9078 | 100 | 5098 | 100 | |||
| Age group | <0.0001 | ||||||||
| 65–69 | 7736 | 50.80 | 2532 | 27.89 | 1459 | 28.62 | |||
| 70–74 | 3007 | 19.75 | 2284 | 25.16 | 1365 | 26.78 | |||
| 75–79 | 2250 | 14.78 | 2076 | 22.87 | 1008 | 19.77 | |||
| >=80 | 2234 | 14.67 | 2186 | 24.08 | 1266 | 24.83 | |||
| Race group | <0.0001 | ||||||||
| Non-Hispanic white | 11399 | 74.86 | 7251 | 79.87 | 3700 | 72.58 | |||
| Non-Hispanic black | 1248 | 8.20 | 765 | 8.43 | 451 | 8.85 | |||
| Hispanic and non-Hispanic other races | 2580 | 16.94 | 1062 | 11.70 | 947 | 18.58 | |||
| Sex | 0.0002 | ||||||||
| Female | 7334 | 48.16 | 4132 | 45.52 | 2451 | 48.08 | |||
| Male | 7893 | 51.84 | 4946 | 54.48 | 2647 | 51.92 | |||
| Comorbidity score | <0.0001 | ||||||||
| 0 | 6359 | 41.76 | 5313 | 58.53 | 2387 | 46.82 | |||
| 1 | 2759 | 18.12 | 1973 | 21.73 | 1271 | 24.93 | |||
| >=2 | 3105 | 20.39 | 1382 | 15.22 | 1193 | 23.40 | |||
| Missing | 3004 | 19.73 | 410 | 4.52 | 247 | 4.85 | |||
| Region | <0.0001 | ||||||||
| Midwest | 2235 | 14.68 | 1192 | 13.13 | 627 | 12.30 | |||
| Northeast | 2802 | 18.40 | 1810 | 19.94 | 880 | 17.26 | |||
| South | 3753 | 24.65 | 2582 | 28.44 | 1380 | 27.07 | |||
| West | 6437 | 42.27 | 3494 | 38.49 | 2211 | 43.37 | |||
| Neighborhood SES: education*,** | <0.0001 | ||||||||
| First quartile | 4317 | 28.35 | 2270 | 25.01 | 1284 | 25.19 | |||
| Second quartile | 3549 | 23.31 | 2425 | 26.71 | 1286 | 25.23 | |||
| Third quartile | 3472 | 22.80 | 2114 | 23.29 | 1260 | 24.72 | |||
| Fourth quartile | 3888 | 25.54 | 2269 | 24.99 | 1268 | 24.87 | |||
| Neighborhood SES: poverty* | 0.0002 | ||||||||
| First quartile | 4134 | 27.15 | 2460 | 27.10 | 1310 | 25.70 | |||
| Second quartile | 3421 | 22.47 | 2138 | 23.55 | 1276 | 25.03 | |||
| Third quartile | 3650 | 23.97 | 2233 | 24.60 | 1276 | 25.03 | |||
| Fourth quartile | 4021 | 26.41 | 2247 | 24.75 | 1236 | 24.24 | |||
| Stage | <0.0001 | ||||||||
| localized | 10997 | 72.22 | 5354 | 58.98 | 3219 | 63.14 | |||
| regional | 2434 | 15.98 | 1554 | 17.12 | 786 | 15.42 | |||
| distant | 1347 | 8.85 | 1725 | 19 | 892 | 17.50 | |||
| unstaged | 449 | 2.95 | 445 | 4.9 | 201 | 3.94 | |||
| Grade | |||||||||
| well differentiated, NOS |
1834 | 12.04 | 879 | 9.68 | 466 | 9.14 | 0.0001 | ||
| moderately differentiated | 5351 | 35.14 | 2668 | 29.39 | 1755 | 34.43 | |||
| poorly differentiated | 2515 | 16.52 | 1468 | 16.17 | 974 | 19.11 | |||
| undifferentiated | 543 | 3.57 | 408 | 4.49 | 226 | 4.43 | |||
| cell type not determined, not stated or not applicable | 4984 | 32.73 | 3655 | 40.26 | 1677 | 32.90 | |||
| Year of diagnosis | |||||||||
| Before 2002 | 3445 | 22.69 | - | - | |||||
| 2002 | 801 | 5.26 | 1701 | 18.74 | - | ||||
| 2003 | 885 | 5.81 | 1730 | 19.06 | - | ||||
| 2004 | 1016 | 6.67 | 1803 | 19.86 | - | ||||
| 2005 | 1060 | 6.96 | 1897 | 20.90 | - | ||||
| 2006 | 1348 | 8.85 | 1947 | 21.45 | - | ||||
| 2007 | 1443 | 9.48 | - | 946 | 18.56 | ||||
| 2008 | 1461 | 9.59 | - | 989 | 19.40 | ||||
| 2009 | 1349 | 8.86 | - | 998 | 19.58 | ||||
| 2010 | 1237 | 8.12 | - | 1050 | 20.60 | ||||
| 2011 | 1172 | 7.70 | - | 1115 | 21.87 | ||||
| died by 2012 | O.0001 | ||||||||
| No | 10232 | 67.20 | 5534 | 60.96 | 3356 | 65.83 | |||
| Yes | 4995 | 32.80 | 3544 | 39.04 | 1742 | 34.17 | |||
Note:
One patient with a missing value.
High School diploma or more.
SES, socioeconomic status; NOS: not otherwise specified
Pattern of Treatment and Use of New Technologies among Prevalence RCC Cohort
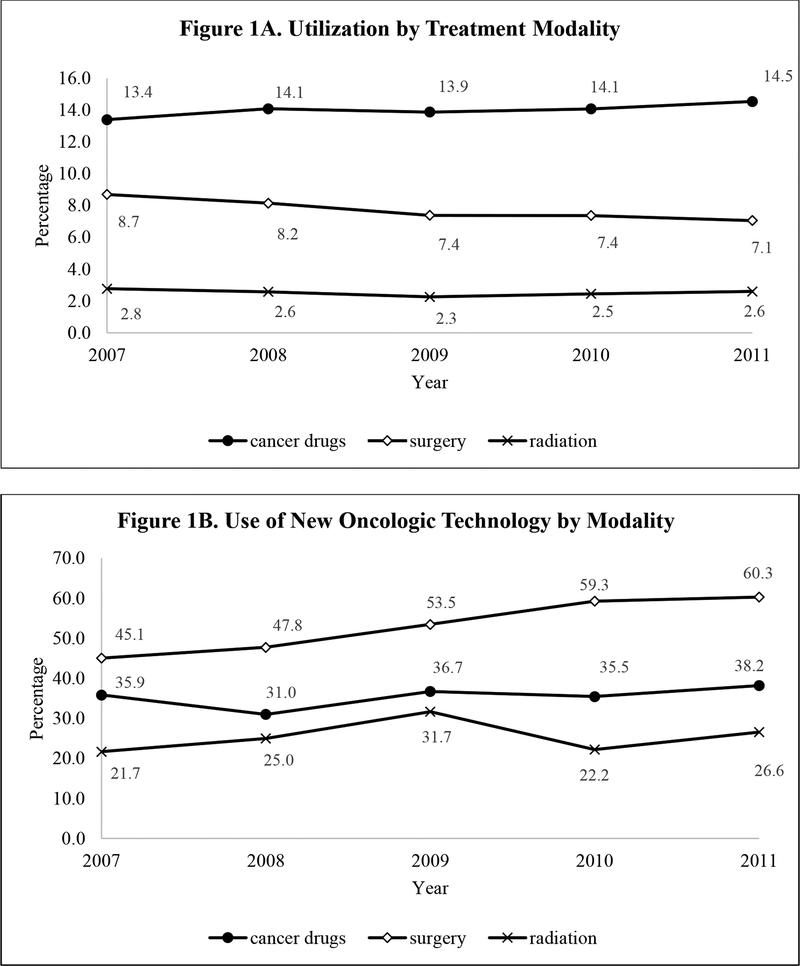
Figure 1A illustrates the proportion of patients who received surgery, radiation, or cancer drugs by calendar year. The decline in the percentage of patients who received surgery over time (from 8.7% in 2007 to 7.1% in 2011) most likely reflects the use of a prevalence cohort because surgical treatment is usually given to newly diagnosed RCC patients. Conversely, the slightly rising trend in the percentage of RCC patients receiving cancer drugs likely reflected the changing mode of drug administration in RCC, from intravenous agents at oncologists’ offices for a defined duration (e.g., 4–6 months) to continuous, sustained treatment with TOAMs. Figure 1A also shows that the use of radiation was uncommon in RCC, averaging < 3% over time, consistent with expected clinical practice. Figure 1B shows the utilization trend of new oncologic technology among RCC patients who received each treatment modality. Growing use of new technologies was most pronounced among surgically treated patients, with the proportion of patients receiving laparoscopic nephrectomies increasing from 45.1% in 2007 to 60.3% in 2011.
Figure 1: Utilization Trend of Treatment and New Oncologic Technologies for Prevalence Cohort of RCC Patients, 2007-2011.
Note: New oncologic technologies for surgery were characterized by laparoscopic procedures, with or without robotic-assistance. New technologies for radiation were exemplified by intensity-modulated radiation therapy, stereotactic body radiation therapy, proton beam radiation, and brachytherapy. New technologies for cancer drugs were captured by targeted therapies.
Impact of New Technology on Cost Trends
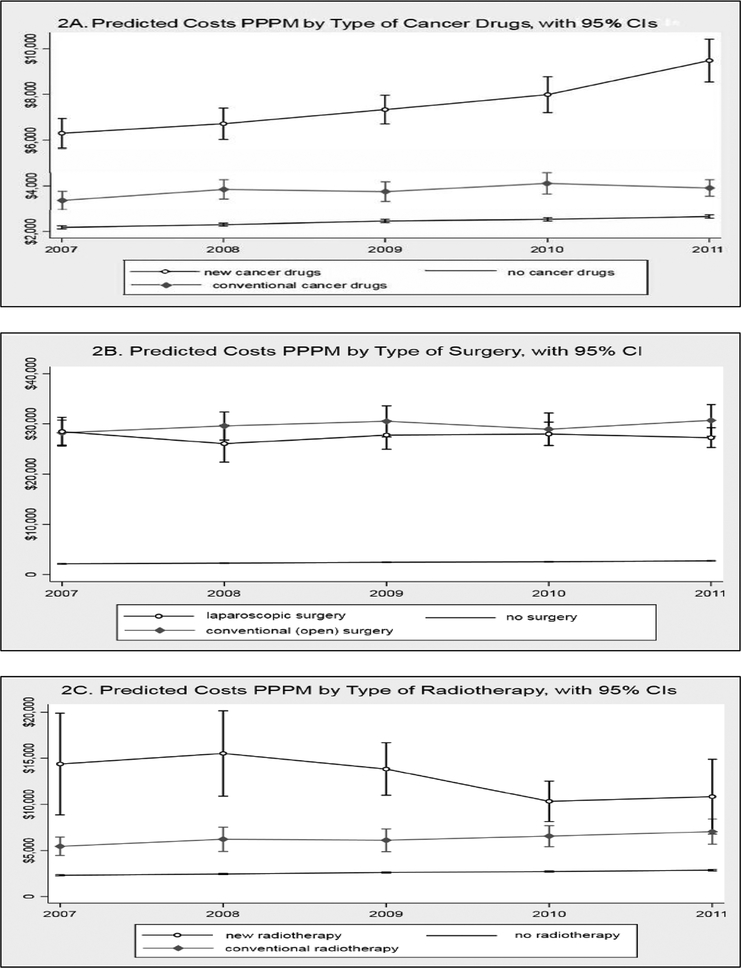
Results from the GEE model are shown in Table 2. The data show a sustained increase in costs per patient per month (PPPM) over time. Compared with costs PPPM in 2007, those in 2008, 2009, 2010, and 2011 were 6.5%, 14.2%, 18.8%, and 25.4% higher, respectively. Significantly higher costs PPPM were found to be associated with the receipt of cancer drugs, radiation, and surgery. Use of new technologies contributed to additional cost increases for cancer drugs and radiation, but not for surgery. Other factors associated with higher costs PPPM included older age, being non-Hispanic black, having comorbidity greater than score 0, residing in Northeast or West region (vs. Midwest), having cancer at distant stage (vs. localized stage), being in the terminal care phase (vs. initial care phase), and died before year 2012. Table 2 also indicates some association between costs PPPM and neighborhood SES, with stronger association found in educational attainment than poverty. To better understand the impact of new technologies on the trend of PPPM costs over time, we used “margins” command in STATA to obtain predicted costs for three scenarios for each treatment modality: without treatment, with conventional treatment, and with new treatment (i.e., of advanced technology). Figure 2 plots the predicted costs PPPM over time for cancer drugs (Figure 2A), surgery (Figure 2B), and radiation (Figure 2C).
Table 2:
Results from the GEE Model, Prevalence Cohort 2007–2011
| Percentage change* | Coefficient | 95% CI | ||
|---|---|---|---|---|
| Age group (reference group: age 65–69) | ||||
| 70–74 | 0.9% | 0.0086 | −0.039 | 0.056 |
| 75–79 | 5.8% | 0.0560 | 0.003 | 0.110 |
| >=80 | 0.8% | 0.0081 | −0.047 | 0.063 |
| Race group (reference group: non-Hispanic white) | ||||
| Non-Hispanic black | 39.8% | 0.3349 | 0.263 | 0.406 |
| Hispanic/non-Hispanic other races | −2.6% | −0.0261 | −0.099 | 0.046 |
| Sex (reference group: female) | ||||
| Male | −5.0% | −0.0509 | −0.088 | −0.014 |
| Comorbidity score (reference group: comorbidity score=0) | ||||
| 1 | 37.7% | 0.3199 | 0.273 | 0.367 |
| >=2 | 120.5% | 0.7907 | 0.743 | 0.838 |
| Missing | 18.1% | 0.1660 | 0.106 | 0.226 |
| Region (reference group: Midwest) | ||||
| Northeast | 13.5% | 0.1265 | 0.063 | 0.190 |
| South | −2.4% | −0.0246 | −0.092 | 0.043 |
| West | 10.8% | 0.1028 | 0.046 | 0.160 |
| Neighborhood SES: education (reference group: first quartile) | ||||
| Second quartile | −9.0% | −0.0945 | −0.149 | −0.040 |
| Third quartile | −10.8% | −0.1142 | −0.184 | −0.044 |
| Fourth quartile | −18.5% | −0.2040 | −0.275 | −0.133 |
| Neighborhood SES: poverty (reference group: first quartile) | ||||
| Second quartile | −6.6% | −0.0679 | −0.119 | −0.017 |
| Third quartile | −3.8% | −0.0387 | −0.104 | 0.027 |
| Fourth quartile | −11.7% | −0.1243 | −0.193 | −0.056 |
| Stage (reference group: localized) | ||||
| Regional | −2.2% | −0.0227 | −0.076 | 0.030 |
| Distant | 29.8% | 0.2609 | 0.192 | 0.330 |
| Unstaged | 6.9% | 0.0669 | −0.039 | 0.173 |
| Grade (reference group: well differentiated) | ||||
| Moderately differentiated | −0.8% | −0.0077 | −0.068 | 0.052 |
| Poorly differentiated | 0.0% | −0.0002 | −0.070 | 0.070 |
| Undifferentiated | 2.6% | 0.0259 | −0.100 | 0.152 |
| Cell type not determined, not stated or not applicable | 4.1% | 0.0404 | −0.024 | 0.104 |
| Phase of care (reference group: initial care phase) | ||||
| Continuing care phase | −31.3% | −0.3758 | −0.412 | −0.339 |
| Terminal care phase | 48.7% | 0.3969 | 0.346 | 0.448 |
| Year of claims (reference group:2007) | ||||
| 2008 | 6.5% | 0.0628 | 0.026 | 0.100 |
| 2009 | 14.2% | 0.1331 | 0.093 | 0.173 |
| 2010 | 18.8% | 0.1724 | 0.129 | 0.216 |
| 2011 | 25.4% | 0.2262 | 0.183 | 0.269 |
| died by 2012 (reference group: alive on December 31, 2012) | ||||
| Yes | 107.2% | 0.7286 | 0.680 | 0.777 |
| Cancer drugs use (reference group: no cancer drugs) | ||||
| Conventional therapy | 57.4% | 0.4538 | 0.391 | 0.516 |
| New cancer drugs | 217.9% | 1.1566 | 1.085 | 1.228 |
| Surgery (reference group: no surgery) | ||||
| Conventional (open) surgery | 1124.7% | 2.5053 | 2.456 | 2.554 |
| New (laparoscopic) surgery | 1024.4% | 2.4199 | 2.370 | 2.470 |
| Radiotherapy (reference group: no radiotherapy) | ||||
| Conventional radiotherapy | 142.9% | 0.8874 | 0.794 | 0.980 |
| New radiotherapy | 389.3% | 1.5878 | 1.430 | 1.746 |
Note:
percentage change (relative to the reference group) was calculated as 100 x [exp(estimated coefficient)-1]
Figure 2:
Predicted Costs Per Patient Per Month (PPPM) by Treatment Modality
The combination of information from Figures 1 and 2 suggested that a major cost driver for RCC was the use of new cancer drugs. We reached this conclusion from the following observations. First, Figure 1A shows that among the three treatment modalities, the impact of radiation is likely to be low because only a small percentage of RCC patients received radiation. This observation suggests that costs of RCC would likely be affected by the utilization pattern and costs of surgery and/or cancer drugs. Next, Figure 2A shows that the PPPM of new cancer drugs not only is higher than that of conventional cancer drugs, the difference grows more pronounced over time, whereas Figure 2B shows that the PPPM is similar between laparoscopic and open surgery and this pattern persists over time. Therefore, even with an increasing proportion of laparoscopic procedures among surgically treated patients (Figure 1B), cost of surgery would likely stay the same; this observation, combined with the pattern in Figure 1A showing that the proportion of patients underwent surgery had not increased over time, suggested that new surgical technology was unlikely to contribute to increase in RCC costs over time. Lastly, the steep increase in PPPM of new cancer drugs observed in Figure 2A will likely be the cost driver of RCC because Figure 1A illustrates an increase in the percentage of RCC patients receiving cancer drugs and Figure 1B shows a trend toward rising percentage of utilizing new cancer drugs among patients who received cancer drugs, which then amplifies the cost impact of new cancer drugs on the overall cost of RCC.
Incidence Costs of RCC
Table 3 presents the mean costs and the associated 95% confidence intervals (CI), by phase of care, for two incidence cohorts of RCC patients: those diagnosed from 2002–2006 vs. 2007–2011, their corresponding matched controls, and the incremental costs. For the 2007–2011 cohort, costs are presented with and without Part D claims. The reason to present costs that excluded Part D claims for this cohort was to form a more compatible basis of comparison with the 2002–2006 cohort because their costs only consist of claims from Medicare Parts A and B. Table 3 presents costs for patients in two categories: all tumor stages combined and subgroup analysis of patients with distant stage RCC since most targeted therapy agents approved by the FDA to treat RCC had been limited to advanced stage. For the initial care phase, incidence costs of RCC at all stages had not increased; the net cost was $34,991 (95% CI: $34,478 – $35,480) for the 2002–2006 cohort and $33,485 (95% CI: $32,862 – $34,197) for the 2007–2011 cohort with Part D claims. However, a large increase in the costs associated with the initial care phase was observed among patients with distant stage RCC, increasing from $51,639 (95% CI: $48,836 – $54,572) in the 2002–2006 cohort to $70,703 (95% CI: $67,412 – $74,167) in the 2007–2011 cohort. It is worth noting that with the exclusion of Part D claims, the initial care costs for the 2007–2011 cohort would be $53,390 (95% CI: $50,412 – $56,816). For the terminal care phase, the net costs for the 2007–2011 cohort were higher than that of the 2002–2006 cohort, regardless of tumor stage and the inclusion or exclusion of Part D claims. For the continuing care phase, the cost pattern was more mixed.
Table 3:
Costs for Patients with RCC in the United States (2018 $US), by Stage, Care Phase, and Time of Diagnosis
| Incidence Cohort: 02–06 | Cohort: 07–11, no Part D claims | Cohort: 07–11, with Part D claims | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All Stages | |||||||||
| RCC cohort | Control cohort | Net Costs | RCC cohort | Control cohort | Net Costs | RCC cohort | Control cohort | Net Costs | |
| Initial Care Phase | |||||||||
| $38,915 (38429, 39391) |
$3,929 (3752,4115) |
$34,991 (34478 – 35480) |
$36,217 (35633, 36843) |
$5,051 (4744, 5372) |
$31,160 (30501,31851) | $40,601 (40032,41231) | $7,108 (6793,7441) | $33,485 (32862,34197) | |
| Continuing Care Phase | |||||||||
| yearl | $11,209 (10930,11478) | $4,542 (4289,4810) |
$6,668 (6278, 7045) |
$11,853 (11477, 12186) |
$5,640 (5287, 6005) |
$6,213 (5692, 6664) |
$16,065 (15674, 16417) |
$7,759 (7373,8123) | $8,315 (7786, 8785) |
| year2 | $10,331 (10011,10651) | $5,269 (4773,5777) |
$5,064 (4468, 5676) |
$11,431 (10913, 11904) |
$6,063 (5276,6919) |
$5,344 (4374, 6269) |
$15,709 (15166, 16239) |
$8,473 (7610,9393) | $7,234 (6194,8186) |
| Terminal Care Phase, Died from RCC | |||||||||
| $70,505 (68847, 72226) | $3,316 (3027, 3628) | $67,188 (65486, 68949) | $84,912 (81982,81878) | $4,822 (4242, 5476) | $80,024 (77073, 82981) | $99,359 (96410, 102196) | $6,814 (6229,7501) | $92,522 (89554, 95460) | |
| Terminal Care Phase, Died from Other Causes | |||||||||
| $80,349 (77861,82671) | $47,319 (45290, 49437) |
$32,990 (29698, 35989 |
$95,120 (91014,99256) | $53,888 (51031,56795) | $41,287 (36078, 46588) |
$102,128 (98014,106298) | $58,660 (55777,61600) | $43,464 (38354, 48745) |
|
| Distant Stage | |||||||||
| RCC cohort | Control cohort | Net Costs | RCC cohort | Control cohort | Net Costs | RCC cohort | Control cohort | Net Costs | |
| Initial Care Phase | |||||||||
| $56,424 (53823, 59197) |
$4,782 (3947, 5725) |
$51,639 (48836, 54572) |
$57,368 (54418,60639) | $3,957 (3279,4681) |
$53,390 (50412,56816) | $77,305 (74113,80727) | $6,580 (5783,7410) | $70,703 (67412,74167) | |
| Continuing Care Phase | |||||||||
| yearl | $22,456 (20551,24348) | $3,427 (2704, 4372) |
$19,025 (16945,21156) | $19,930 (17879,21946) | $3,274 (2693, 3883) |
$16,653 (14335, 18735) |
$40,281 (37386,43134) | $5,643 (4739, 6497) |
$34,716 (31550,37650 |
| year2 | $14,724 (12660, 16612) |
$3,119 (1968, 5476) |
$11,304 (8333, 13945) |
$16,214 (13524, 19111) |
$2,721 (1578,4036) |
$13,514 (10403, 16499) |
$36,163 (32160,40358) | $7,387 (5052,9814) | $28,869 (24159,33526) |
| Terminal Care Phase, Died from RCC | |||||||||
| $79,465 (77147,81919) | $2,857 (2446, 3325) |
$76,603 (74248,79091) | $95,196 (91209,98981) | $3,868 (3292,4414) |
$91,328 (87287,95131) | $114,002 (109,759,117800) | $6,007 (5420, 6577) |
$107,989 (103726, 111767) |
|
| Terminal Care Phase, Died from Other Causes | |||||||||
| $76,551 (70919,81853) | $47,450 (42478,51888) | $29,045 (21590,36351) | $91,018 (82515,99242) | $56,556 (49653, 63659) |
$34,560 (22827, 44855) |
$109,273 (101140,117810) | $62,158 (55211,69308) | $47,538 (35619, 57875) |
|
Note: Numbers in parentheses are 95% confidence intervals (CI) calculated by non-parametric bootstrapping.
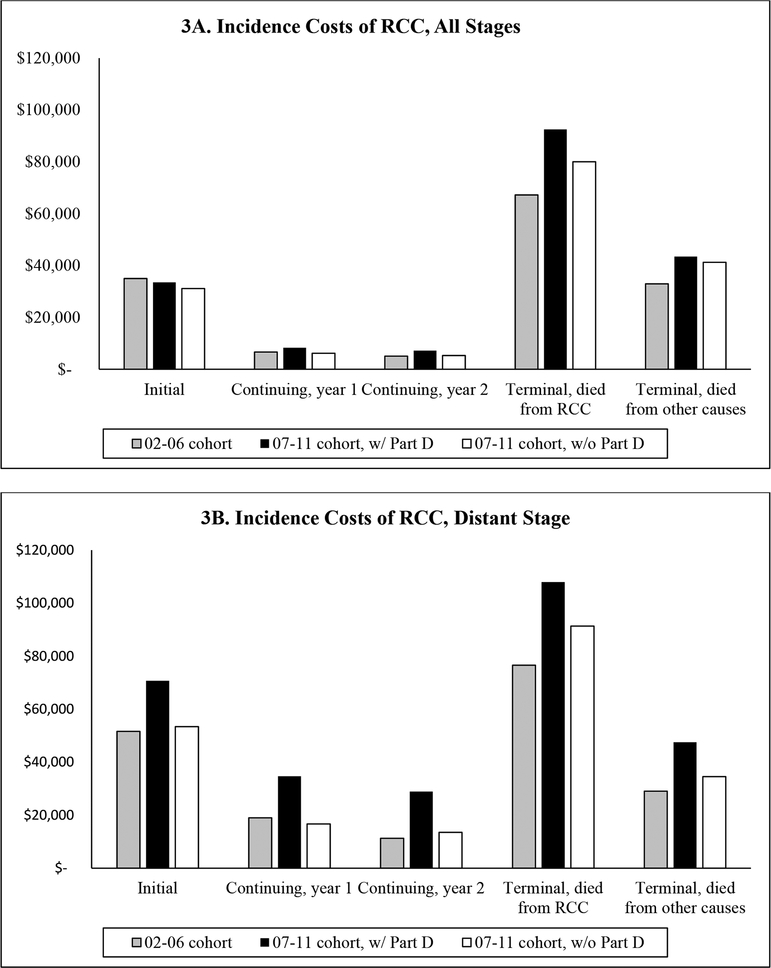
Figure 3 summarizes the net costs for the 2002–2006 cohort, 2007–2011 cohort without Part D, and 2007–2011 cohort with Part D by phase of care for all stages (Figure 3A) and distant stage (Figure 3B). The pattern for RCC patients at all stages showed that compared to the 2002–2006 cohort, the 2007–2011 cohort had similar or even lower Medicare Parts A and B costs for the initial and continuing care phases, but higher costs for the terminal care phase regardless of the cause of death. With the inclusion of Part D claims in the 2007–2011 cohort, higher costs were observed in all care phases compared to the 2002–2006 cohort, except for the initial care phase (Figure 3A). The impact of Part D claims on costs was most pronounced among patients with distant stage RCC. For this subgroup of patients, when excluding Part D claims, the results suggest that the costs associated with the continuing care phases had remained stable or even reduced over time, i.e., if the comparison was limited to costs from Parts A and B claims. However, the inclusion of Part D claims substantially raised the costs in this care phase for the 2007–2011 cohort. For example, Table 3 shows that costs for the first year of the continuing care phase was $16,653 excluding Part D data and $34,716 with Part D for the late cohort.
Figure 3:
Incidence Costs of Renal Cell Cancer, by Cohort and Care Phase
DISCUSSION
In this paper, we used more recent SEER-Medicare data to update previously published estimates of the economic burden of RCC in the US. Analyses employing the prevalence costing approach showed that despite growing use of laparoscopic surgical techniques to treat patients with RCC, new cancer drugs were the major cost driver for growing PPPM costs over time. This finding was then verified by analyses taking the incidence costing approach. The comparison of net costs between the 2002–2006 and 2007–2011 incidence cohorts of RCC patients suggested that the increase in costs for the initial and continuing care phases (from approximately $52,000 to $71,000 and $19,000 to $35,000, respectively) were largely observed among patients with distant stage RCC, for whom the new cancer drugs were indicated.
The comparison between the two incidence cohorts on initial care costs showed a puzzling pattern in that even with the inclusion of Part D claims, costs estimated for the 2007–2011 cohort appeared to be lower than those for the 2002–2006 cohort in the analysis that included all RCC patients (Figure 3A), but were much higher in the subgroup of patients with distant stage RCC (Figure 3B). Our recently published review article [6] (PMID: 30467701, DOI: 10.1007/s40273–018-0746-y) offers insights to understand this pattern. Of the studies we reviewed that documented utilization patterns of new technologies, three noticeable trends were (a) an increasing use of minimally invasive procedures for nephrectomies [18, 19], (b) a trend toward less aggressive care (e.g., ablation or surveillance) for patients with smaller renal masses (< 4cm) [20], and (c) a growing number of RCC patients switching from conventional cancer drugs to targeted therapies [21]. Switching from open to minimally invasive nephrectomies is likely to be cost-neutral or even cost-saving as our review found that minimally invasive procedures had comparable or even lower costs as open surgery [6]. Increasing use of less aggressive strategies to manage patients with smaller renal masses would likely lower the costs for early stage patients because Kowalczy et al. had shown that the costs of ablation or surveillance were substantially less than the costs of nephrectomies [20]. However, switching from conventional cancer drugs to targeted therapies could have substantial financial consequences because many newly approved therapies for mRCC are TOAMs. Research has shown that this class of drugs not only launched at higher prices over time, they also exhibited a pattern of sustained price increase post-launch [22, 23]. The cost trends of surgical treatment (including ablation) largely affect early stage patients whereas the cost impact of new cancer drugs would mostly be limited to late stage patients. Given that over 65% of RCC patients in the incident cohorts had localized stage, reduction in the costs of the initial care phases observed among all RCC patients was most likely driven by the trend of less aggressive treatments for early stage patients, whereas the increase in costs among distant stage patients is likely due to rising prices of oncology drugs.
TOAMs have transformed the delivery of cancer drugs from office-based to home-based settings. For payers, this transformation means that insurance coverage for a proportion of utilization of cancer drugs will be switched from medical to pharmacy benefit. As the number of advanced stage RCC patients receiving TOAMs grew, Medicare spending on oral cancer drugs (covered by Part D) would increase while spending on injectable cancer drugs (covered by Part B) would decrease accordingly. The therapeutic substitution between injectable and oral formulations of cancer drugs and its cost complication were evident from the comparison of the incidence costs of the 2002–2006 cohort with that of the 2007–2011 cohort with vs. without Part D claims (Figure 3B) for the continuing care phase. Neglecting to include Part D claims and limiting the comparison to costs obtained from Parts A and B claims for the sake of data consistency (since 2002–2006 cohort lack Part D data) would have led to the erroneous conclusion that the costs of treating distant stage RCC had reduced over time. However, including medication claims data exposes the reality that increases in the costs of TOAMs outweighed the reduction in costs of Part B-covered injectable cancer drugs and ultimately raised the costs of RCC. Although it is impossible to include Part D claims in the cost comparison between these two incidence cohorts because Medicare Part D program began in 2006, the lack of claims for outpatient prescription drugs should not cause gross underestimation of RCC costs for the 2002–2006 cohort because sorafenib, the first oral cancer drug for RCC, was approved by the FDA in December 2005; thus most patients in this cohort should be treated with injectable cancer drugs. The above discussions highlight the importance of acquiring knowledge on clinical, institutional, and regulatory details when conducting cost studies. Future analyses will likely continue to show cost increases in RCC as a) an increasing number of oral and intravenous drugs are available to advanced RCC, b) expensive new immunotherapy drugs that have entered the market since 2015 (e.g., nivolumab, pembrolizumab) continue to gain regulatory approval and use, and c) the use of both TOAMs and intravenous immunotherapy can extend for years for many advanced RCC patients. A topic ripe for future research is to apply the analytical framework (i.e., the combination of incidence- and prevalence-based costing approach) presented in this study to examine the impact of immunotherapy on the economic burden of RCC using the latest release of SEER-Medicare data or commercial claims data, such as MarketScan or IMS LifeLink database.
Several study limitations warrant discussions. First, the exclusion of beneficiaries enrolled in HMOs made the study cohorts less representative of the Medicare population. This exclusion was necessitated by the completeness of cost information because HMOs are not required to submit detailed, itemized claims of their enrollees to Medicare. Although this is a standard practice for studies using SEER-Medicare data, as the proportion of Medicare beneficiaries enrolled in HMOs continues to grow over time [24], future research needs to explore analytical strategies to integrate HMO enrollees in economic studies using SEER-Medicare or Medicare data. Second, although the inclusion of Part D claims allowed us to assess the cost impact of TOAMs, the requirement of Part D enrollment led to further reduction in sample size. Numbers reported by the NCI showed that during the time period of our study (2007–2011), less than 55% of cancer patients in the SEER-Medicare data enrolled in Medicare Part D [25]. The rate of Part D enrollment eventually rose to 70% in 2015 and continued to rise, making it imperative to replicate our analysis when newer data become available. Lastly, the use of SEER-Medicare limited our study to elderly RCC patients in the US; therefore, findings from our study may not be generalizable to non-elderly patients in the US or patients outside the US. Despite these limitations, our study made a unique contribution to the methodology of medical cost research by demonstrating the use of prevalence and incidence costing approaches as complementary methods to analyze cost data in the context of new oncologic technology diffusions.
CONCLUSION
The influx of new oncologic technologies has changed the treatment landscape of RCC in the last decade. Our analysis identified new cancer drugs as the main cost drivers for elderly patients with RCC. It reported increase in the costs of the continuing and terminal care phases for elderly RCC patients at all stages as well as those with distant stage disease. For the initial care phase, our study found that increase in RCC costs of elderly patients was observed among distant stage patients. This pattern was likely attributable to therapeutic substitution between newly approved novel but pricey TOAMs and injectable cancer drugs.
Data Availability Statement:
Access to the data used in the current study (i.e., SEER-Medicare) is strictly limited to members of the research team who signed the Data Use Agreement (DUA) at the corresponding author’s institution. Per the DUA, authors of this study have no authority to grant data access to SEER-Medicare data nor distribute any subset of the data to individuals outside the research team.
KEY POINTS.
Many new oncologic technologies have become available to patients with renal cell cancer (RCC) in the past two decades. This study examined which technology is likely to be the primary cost driver for the treatment of RCC.
Analyses employing the prevalence costing approach showed a large increase in the use of laparoscopic nephrectomies, although increase in RCC costs were mostly driven by new cancer drugs
Comparisons of costs between two incidence cohorts (2002–2006 vs. 2007–2011) further confirmed the impact of targeted oral anticancer medications on rising costs of RCC
ACKNOWLEDGEMENTS
Dr. Shih contributed to study design, data acquisition, analysis, and interpretation, and drafting the manuscript. She also provided administrative support and acted as the overall guarantor. Dr. Xu contributed to study design, data analysis, and reviewed the manuscript for its scientific content and to ensure the accuracy of the data generation process. Drs. Chien and Geynisman provided clinical insights and conducted critical reviews and revisions of the manuscript. Dr. Kim assisted Dr. Xu in compiling the list of anticancer medications and contributed to critical reviews/comments. Drs. Shen and Li provided statistical expertise and conducted critical reviews and revisions with a special focus on the accuracy of statistical analysis and interpretation. All authors approved the final version that was submitted and agreed to be accountable for all aspects of the work. Drs. Xu, Kim, Shen, and Li have no conflict of interest (either financial or non-financial) to disclose. Dr. Shih received consulting fees for serving on a review panel for Pfizer Inc. Dr. Chien received consulting fees and travel support from AstraZeneca; the consulting activities were not related to this study. Dr. Geynisman received consulting fees from Genentech and AstraZeneca; none of the consulting activities were related to this study.
The authors thank Dr. Gary Deyter, technical writer from the Department of Health Services Research at The University of Texas MD Anderson Cancer Center, for his editorial contribution. We acknowledge funding from the China Medical University Hospital (Chien, CRS-108-054) and the National Cancer Institute (Shih, R01 CA207216, R01 CA225646, R01 CA225647 and CCSG P30 CA016672; Li R01 CA225646; Shen Cancer Center Biostatistics Shared Resource CA016672).
The interpretation and reporting of these data are the responsibilities of the authors and in no way should be viewed as an official policy or interpretation of the NCI. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute (NCI); the Office of Research, Development and Information, Centers for Medicare & Medicaid Services (CMS); Information Management Services (IMS), Inc; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
REFERENCES
- 1.Shih YC, Chien CR, Xu Y, Pan IW, Smith GL, Buchholz TA. Economic burden of renal cell carcinoma: Part I--an updated review. Pharmacoeconomics 2011. April;29(4):315–29. [DOI] [PubMed] [Google Scholar]
- 2.Shih YC, Chien CR, Xu Y, Pan IW, Smith GL, Buchholz TA. Economic burden of renal cell carcinoma in the US: Part II--an updated analysis. Pharmacoeconomics 2011. April;29(4):331–41. [DOI] [PubMed] [Google Scholar]
- 3.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002. August;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 4.Medstat. MarketScan Research Databases User Guide and Database Dictionary: Multi-state Medicaid Database, 1999–2005 Edition. Ann Arbor, Michigan: 2007. [Google Scholar]
- 5.NCCN. NCCN Clinical Practice Guidelines in Oncology: Kidney Cancer NCCN Evidence Blocks Version 2.2019. 2018. [cited 2018 November 22]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney_blocks.pdf
- 6.Chien CR, Geynisman DM, Kim B, Xu Y, Shih YT. Economic Burden of Renal Cell Carcinoma-Part I: An Updated Review. Pharmacoeconomics 2019. March; 37(3): 301–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCI. SEER-Medicare: Brief Description of the SEER-Medicare Database 2018. [cited 2018 December 10]; Available from: https://healthcaredelivery.cancer.gov/seermedicare/overview/
- 8.Lipscomb J, Yabroff KR, Brown ML, Lawrence W, Barnett PG. Health care costing: data, methods, current applications. Med Care 2009. July;47(7 Suppl 1):S1–6. [DOI] [PubMed] [Google Scholar]
- 9.Barlow WE. Overview of methods to estimate the medical costs of cancer. Med Care. 2009. July;47(7 Suppl 1):S33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCI. ICD-O-3 Coding Materials [cited 2018 June 6]; Available from: https://seer.cancer.gov/icd-o-3/
- 11.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011. January 19;103(2):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 2008. May 7;100(9):630–41. [DOI] [PubMed] [Google Scholar]
- 13.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol 2007. August;17(8):584–90. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000. December;53(12):1258–67. [DOI] [PubMed] [Google Scholar]
- 15.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care 2002. August;40(8 Suppl):IV-104–17. [DOI] [PubMed] [Google Scholar]
- 16.Kupper LL, Karon JM, Kleinbaum DG, Morgenstern H, Lewis DK. Matching in epidemiologic studies: validity and efficiency considerations. Biometrics 1981. June;37(2):271–91. [PubMed] [Google Scholar]
- 17.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000. December 15;19(23):3219–36. [DOI] [PubMed] [Google Scholar]
- 18.Golombos DM, Chughtai B, Trinh QD, Thomas D, Mao J, Te A, et al. Minimally invasive vs open nephrectomy in the modern era: does approach matter? World J Urol 2017. October;35(10):1557–68. [DOI] [PubMed] [Google Scholar]
- 19.Bahler CD, Monn MF, Flack CK, Gramm AR, Gardner TA, Sundaram CP. Assessing Cost of Robotic Utilization in Partial Nephrectomy with Increasing Utilization. J Endourol 2018. August;32(8):710–6. [DOI] [PubMed] [Google Scholar]
- 20.Kowalczyk KJ, Choueiri TK, Hevelone ND, Trinh QD, Lipsitz SR, Nguyen PL, et al. Comparative effectiveness, costs and trends in treatment of small renal masses from 2005 to 2007. BJU Int 2013. August;112(4):E273–80. [DOI] [PubMed] [Google Scholar]
- 21.Geynisman DM, Hu JC, Liu L, Tina Shih YC. Treatment patterns and costs for metastatic renal cell carcinoma patients with private insurance in the United States. Clin Genitourin Cancer 2015. April;13(2):e93–100. [DOI] [PubMed] [Google Scholar]
- 22.Shih YC, Smieliauskas F, Geynisman DM, Kelly RJ, Smith TJ. Trends in the Cost and Use of Targeted Cancer Therapies for the Privately Insured Nonelderly: 2001 to 2011. J Clin Oncol 2015. July 1;33(19):2190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih YT, Xu Y, Liu L, Smieliauskas F. Rising Prices of Targeted Oral Anticancer Medications and Associated Financial Burden on Medicare Beneficiaries. J Clin Oncol 2017. August 1;35(22):2482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser Family Foundation. Medicare Advantage 2017 Spotlight: Enrollment Market Update 2017. [cited 2019 January 10]; Available from: https://www.kff.org/medicare/issue-brief/medicare-advantage-2017-spotlight-enrollment-market-update/
- 25.NCI. Number of Part D Enrollees. 2018. 22 October 2018 [cited 2019 January 14]; Available from: https://healthcaredelivery.cancer.gov/seermedicare/aboutdata/enrollees.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to the data used in the current study (i.e., SEER-Medicare) is strictly limited to members of the research team who signed the Data Use Agreement (DUA) at the corresponding author’s institution. Per the DUA, authors of this study have no authority to grant data access to SEER-Medicare data nor distribute any subset of the data to individuals outside the research team.