Abstract
A novel oxime platform, the substituted phenoxyalkyl pyridinium oximes (US patent 9,227,937), was invented at Mississippi State University with an objective of discovering a brain-penetrating antidote to highly potent organophosphate anticholinesterases, such as the nerve agents. The goal was reactivation of inhibited brain acetylcholinesterase to attenuate the organophosphate- induced hypercholinergic activity that results in glutamate- mediated excitotoxicity and neuropathology. The currently approved oxime antidote in the US, 2-PAM, cannot do this. Using highly relevant surrogates of sarin and VX that leave acetylcholinesterase phosphylated with the same chemical moiety as their respective nerve agents, in vitro screens and in vivo tests in rats were conducted to identify the most efficacious members of this platform. The most promising novel oximes provided 24-hour survival of lethal level surrogate exposure better than 2-PAM in almost all cases, and two of the oximes shortened the time to cessation of seizure-like behavior while 2-PAM did not. The most promising novel oximes attenuated neuropathology as indicated by immunohistochemical stains for both glia and neurons, while 2-PAM did not protect either glia or neurons. These results strongly suggest that these novel oximes can function within the brain to protect it, and therefore show great promise as potential future nerve agent antidotes.
Keywords: neuroprotection, oxime, nitrophenyl isopropyl methylphosphonate (NIMP), brain penetration, seizure- like behavior cessation, neuropathology protection
Introduction
Organophosphate anticholinesterases are among the most toxic synthetic chemicals known and are well documented as chemical warfare agents that can quickly cause death (Ecobichon, 1996; Tucker, 2007). Some of the organophosphate insecticides are also acutely toxic. Because of their potency as acute toxicants, these organophosphates are of considerable concern not just as chemical weapons, but also as agents of terrorism and as poisons responsible for life-threatening accidents. These organophosphates persistently inhibit acetylcholinesterase (AChE), resulting in the accumulation of the neurotransmitter acetylcholine in synapses and neuromuscular junctions, leading to hypercholinergic activity within both the central and peripheral nervous systems. The lethality caused by these very potent organophosphates is usually the result of respiratory failure induced by spasm of the respiratory muscles, excessive bronchiolar mucus, bronchoconstriction, and inhibition of the brain’s respiratory control center (Taylor, 1990). The central hypercholinergic activity stimulates the glutamatergic excitotoxicity pathways to result in seizures which, when prolonged, can lead to neuropathology (Shih and McDonough, 2003). Therapy with the muscarinic acetylcholine receptor antagonist atropine and oxime AChE reactivators can preserve life by opposing the hypercholinergic activity and by restoring the catalytic function of AChE, respectively (Eddleston and Chowdhury, 2015).
The most effective AChE reactivators are pyridinium oximes. However, the pyridinium group imparts to these oximes a permanent positive charge, which makes them unlikely to penetrate the blood-brain barrier which effectively excludes charged molecules from the brain. One of the greatest deficiencies of this current therapeutic regimen against organophosphate toxicity is the lack of brain penetrability of the oxime currently approved in the US, pralidoxime (2-PAM), as well as the oxime approved in some European countries, HI-6, so the current therapy cannot restore AChE function in the brain and cannot provide central neuroprotection from the seizure-induced damage. Therefore a benzodiazepine, such as diazepam, is used to suppress seizures to combat the brain damage, although benzodiazepine therapy is not always effective in preventing brain damage (Aroniadou-Anderjaska et al., 2016).
However, a brain-penetrating oxime would have the potential to remediate the hypercholinergic activity in the brain, i.e., the initial event in the toxidrome, and likely dampen or ideally eliminate the glutamatergic excitotoxicity and the subsequent prolonged seizures, thereby reducing or preventing the neuropathology. The goal of our research program is to investigate a platform of novel substituted phenoxyalkyl pyridinium oximes as potential brain-penetrating oxime AChE reactivators. The overall objective is prevention or reduction of brain damage that would occur as a result of high level, seizure-inducing levels of organophosphate anticholinesterase exposure. Therefore the goal of this research effort is very consistent with the goals of the NIH CounterACT program which currently supports the continued development of this oxime platform. The present article provides a review of the major findings thus far on this platform of novel oximes.
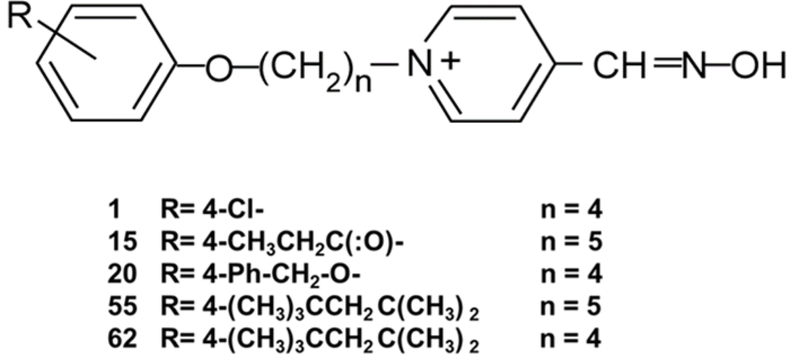
Substituted phenoxyalkyl pyridinium oximes
This platform of oximes was invented by the late Dr. Howard Chambers at Mississippi State University under the support of the Department of Defense’s Defense Threat Reduction Agency. The platform is based upon the pyridinium oxime moiety, because of its efficacy in AChE reactivation. The strategy behind the synthesis of this platform was the addition of an alkyl linker chain of 3–12 carbons between the pyridinium ring and the substituted phenoxy group. The linker alkyl chain is attached to the 4-position of the pyridinium ring. This platform of novel oximes has a wide variety of substitutions on the phenoxy group. The base structure is shown in Figure 1, as are the structures of the more promising oximes that we report on here. The increased lipophilicity of these novel oximes imparted by the alkyl chain was theorized to assist in counterbalancing the positive charge of the quaternary ammonium of the pyridinium ring compared to 2-PAM and other pyridinium oximes, and enhance their ability to cross the blood-brain barrier and/or obtain a therapeutic concentration in the brain. The most effective AChE reactivators in this platform possess alkyl linker chains of 4 or 5 carbons. Over 100 of the oximes in this platform were synthesized, and this review summarizes information on the 5 down-selected novel oximes that have shown the most experimental promise thus far. The oximes that were most efficacious are those with a 3–5 carbon linker group, and these are now patented by Mississippi State University (US patent 9,227,937; patents also issued in Germany, UK, France and Italy) and licensed by Defender Pharmaceuticals.
Figure 1.
Generic structure of the substituted phenoxyalkyl pyridinium oximes (US patent 9,227,937) and the substitutions and linker chain length of the five novel oximes discussed in this paper.
Efficacy of novel oximes as acetylcholinesterase reactivators
The initial studies performed on these oximes were in vitro reactivation screening studies using several organophosphates. Since our laboratories at Mississippi State University are not authorized to use chemical warfare agents, highly relevant surrogates of two of the nerve agents used in terrorist attacks were synthesized: nitrophenyl isopropyl methylphosphonate (NIMP), a surrogate of sarin, and nitrophenyl ethyl methylphosphonate (NEMP), a surrogate of VX. NIMP was first described by Ohta et al. (2006) and NEMP was first described by Fukuto and Metcalf (1959). The NIMP and NEMP used in these experiments were synthesized and characterized in our laboratories (Meek et al., 2012). The advantage of these surrogates for AChE reactivation assays is that they only differ from their respective nerve agents in their leaving groups (i.e., a nitrophenyl group was substituted for the sarin or VX leaving groups, thereby leaving AChE phosphylated by the same chemical moiety as the actual nerve agents. However, while these surrogates are quite potent, they are less toxic than the nerve agents and therefore safer for laboratory personnel. As determined by inhibition kinetics studies in our laboratory (Coban et al., 2016), the potency of these surrogates is greater than for some insecticidal organophosphates, and the surrogates reflect the relative potency of the actual nerve agents as reported in the literature (Worek et al., 2004). Our laboratories also synthesized an additional sarin surrogate, phthalimidyl isopropyl methylphosphonate (PIMP) that is quite labile in aqueous solution, degrading within about 15 min, and has been useful for our in vitro screening studies so that no intact organophosphate remained during the reactivation phase of the assay, thus preventing reinhibition of reactivated AChE.
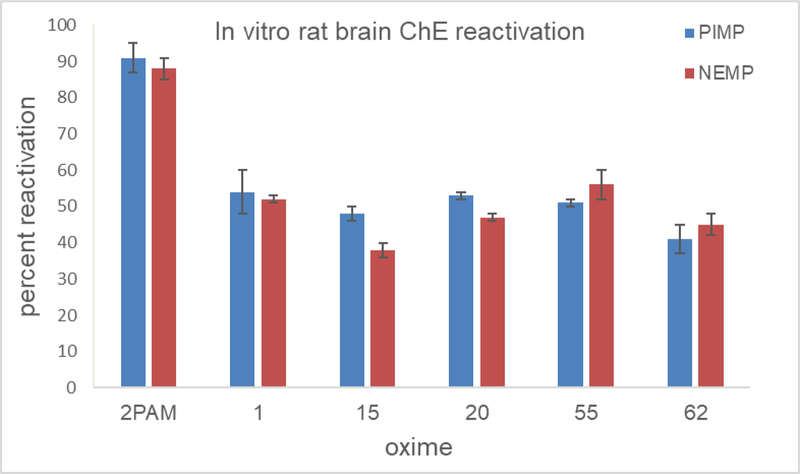
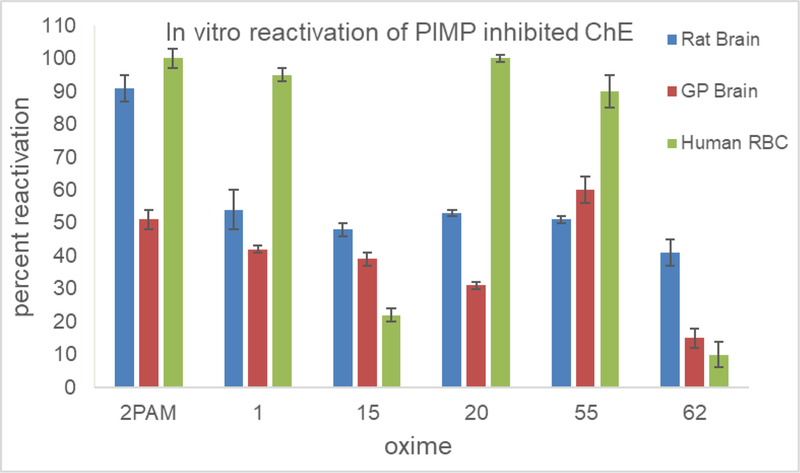
The novel oximes displayed a wide variety of efficacies as reactivators in these screening in vitro tests using homogenates of whole rat brain as a source of AChE inhibited with either PIMP or NEMP, from poor (21–30%) to moderate (30–78%) efficacy compared to 2-PAM and TMB-4 (91–98%); the efficacy screening concentration was set at 0.1 mM and the novel oximes did not exert any inhibition of AChE at this concentration (Chambers et al., 2013). The efficacy for each of the oximes was similar between PIMP and NEMP (Figure 2). Five of the oximes on which we have performed the majority of our tests were also studied for their efficacy as reactivators of PIMP-inhibited AChE from guinea pig brain (since guinea pigs are frequently the test organism in survival studies with actual nerve agents) and with purified human red blood cell AChE (Figure 3). The novel oximes are generally less efficacious than 2-PAM with rat and guinea pig brain preparations, but three of them (Oximes 1, 20, and 55) show efficacy comparable to 2-PAM with the human AChE, which shows promise for their potential as human drugs.
Figure 2.
Percent reactivation in vitro compared to vehicle controls by 0.1 mM 2-PAM or five of the novel oximes of rat brain homogenate acetylcholinesterase inhibited to about 80% by the sarin surrogate phthalimidyl isopropyl methylphosphonate (PIMP) or the VX surrogate nitrophenyl ethyl methylphosphonate (NEMP). Values are means ± SD, N = 3.
Figure 3.
Percent reactivation in vitro compared to vehicle controls by 0.1 mM 2-PAM or five of the novel oximes of acetylcholinesterase inhibited to about 80% by the sarin surrogate phthalimidyl isopropyl methylphosphonate (PIMP). AChE sources were rat brain homogenate, guinea pig (GP) brain homogenate, or commercially available AChE purified from human red blood cells. Values are means ± SD, N = 3.
A few of the more efficacious oximes from the in vitro screens were tested in male rats in vivo which had been challenged with a high sub-lethal dosage of NIMP (0.325 mg/kg) selected to yield about 80% peak brain AChE inhibition and signs of organophosphate acute toxicity. Oximes (either novel or 2-PAM) were administered at the time of peak brain AChE inhibition (1 hour post NIMP challenge). Homogenates of whole brain tissue were assayed using a modified spectrophotometric Ellman technique (Chambers et al., 1988) at 30 min and 2 hr post oxime administration. The oxime dosage tested here was a screening dosage of 0.1 mmoles/kg, and the novel oximes did not exert any lethality at this test dosage. This treatment paradigm and all other in vivo paradigms employed in our laboratories received prior approval from the Mississippi State University Animal Care and Use Committee (Mississippi State University is AAALAC accredited). Because this was a high but sub-lethal dosage of NIMP, no atropine was required to assure survival. This paradigm was developed (i.e., providing oxime therapy at the time of peak brain AChE inhibition) to ensure that most or all of the NIMP was in the process of being cleared at the time of oxime administration and would not have been available to reinhibit reactivated AChE, thereby avoiding possible confounding of the data on reactivation. In these trials several of the novel oximes showed a higher level of brain AChE activity (up to about 25% for the best novel oximes at 30 min and 35% at 2 hr post-NIMP challenge) compared to non-oxime treated controls; 2-PAM and some of the novel oximes provided no brain AChE reactivation. Similar results on novel oxime efficacy were obtained with the VX surrogate NEMP (Chambers et al., 2013 and 2016a).
Effects on survival and seizure-like behavior
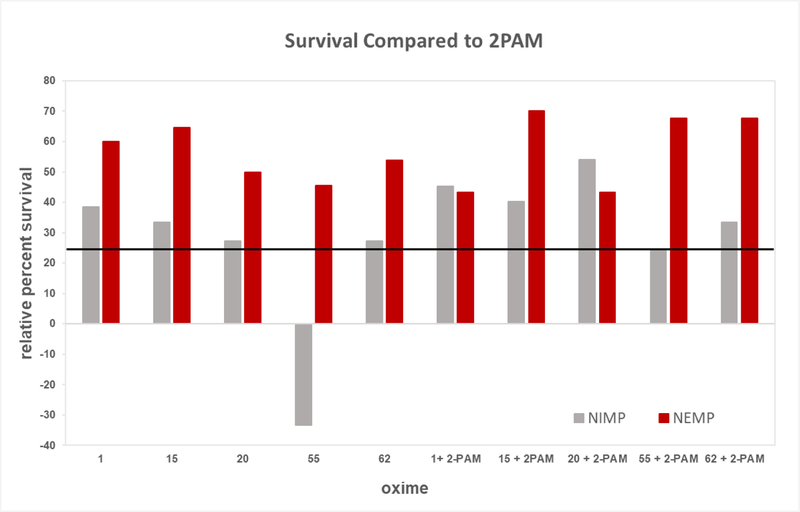
At this point, the NIH CounterACT program initiated support for our oxime development. The most efficacious novel oximes in the above in vivo screening tests with high sub-lethal OP challenge were down-selected for further investigation. A second paradigm was developed using lethal dosages (i.e., LD99) of the nerve agent surrogates NIMP and NEMP in multisol, to compare 24-hour survival and signs of poisoning including seizure-like behavior in male rats with our down-selected novel oximes (Oximes 1, 15, 20, 55, and 62) and with 2-PAM. In this paradigm, oxime in multisol was delivered intramuscularly at 0.146 mmoles/kg, the human equivalent dosage of three autoinjectors administered at the time of initiation of seizure-like behavior, about 30 min, and the novel oximes did not exert any lethality at this dosage. It was also necessary to provide atropine, but the dosages of NIMP and NEMP were such that no survival occurred with atropine alone as therapy, and oxime was also required together with atropine to provide 24-hour survival. A dosage of either NIMP or NEMP was determined that yielded moderate (about 30–40%) survival with 2-PAM therapy so that improved survival efficacy of our novel oximes could be observed if they were superior to 2-PAM. The 24-hour survival of the lethal dosage of NIMP or NEMP was higher for all five of the novel oximes, either alone or in combination with 2-PAM, than 2-PAM alone except for Oxime 55 with NIMP, and they all met or exceeded the criterion of 25% improvement over 2-PAM except for Oxime 55 with NIMP (Figure 4). The survival odds ratios of novel oxime therapy compared to 2-PAM were all statistically significant (P < 0.1), with only the therapy of Oxime 55 alone being worse than 2-PAM alone (Table 1). Some of these data were reported in Chambers et al. (2016b and 2016c).
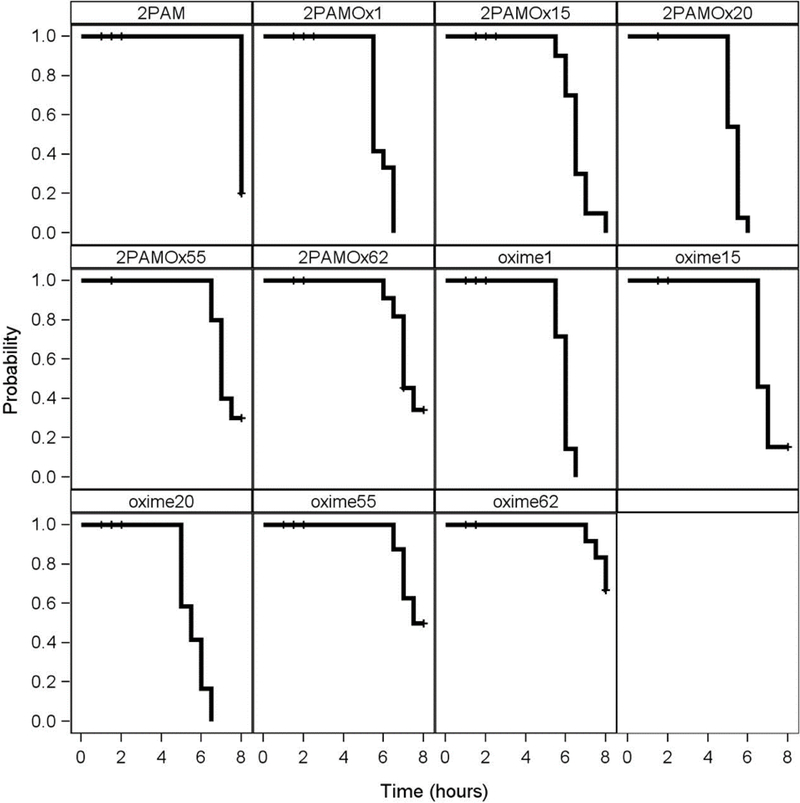
Figure 4.
Relative 24-hour survival imparted by novel oximes alone or in combination with 2-PAM compared to 2-PAM in rats challenged with a lethal subcutaneous dosage of the sarin surrogate nitrophenyl isopropyl methylphosphonate (NIMP; 0.6 mg/kg) or the VX surrogate nitrophenyl ethyl methylphosphonate (NEMP; 0.65 mg/kg). Oximes were delivered intramuscularly at 0.146 mmoles/kg at time of initiation of seizure-like behavior (about 30 min post organophosphate challenge). The percent of 0 would be the same percent survival as 2- PAM. The solid black line was the project’s success criterion of 25% improvement in survival compared to percent survival of 2-PAM alone (which was 40% and 30% for NIMP and NEMP, respectively.) Some of these data were reported in Chambers et al., 2016b. Statistical differences are displayed as Odds Ratios in Table 1.
Table 1:
Twenty four hour survival of male rats challenged with a lethal dosage of a sarin surrogate (nitrophenyl isopropyl methylphosphonate; NIMP; 0.6 mg/kg, subcutaneous) or a VX surrogate (nitrophenyl ethyl methylphosphonate; NEMP; 0.65 mg/kg, subcutaneous), with oxime (0.146 mmol/kg in multisol, intramuscular) and atropine (0.65 mg/kg, in saline, intramuscular) administered at time of initiation of seizure-like behavior, about 30 min post-organophosphate. Odds ratio is in comparison to 2-PAM alone.
| Oxime | NIMP |
NEMP |
||||
|---|---|---|---|---|---|---|
| Surv./ Trted. | %Surv. | Odds Ratio | Surv./ Trted. | %Surv. | Odds Ratio | |
| None | 0/20 | 0 | 0/20 | 0 | ||
| 2-PAM | 8/20 | 40 | 6/20 | 30 | ||
| 1 | 13/20 | 65 | 2.8* | 15/20 | 75 | 6.3* |
| 15 | 12/20 | 60 | 2.3* | 17/20 | 85 | 11.2* |
| 20 | 11/20 | 55 | 1.8* | 12/20 | 60 | 4.0* |
| 55 | 6/20 | 30 | 0.6* | 11/20 | 55 | 2.7* |
| 62 | 11/20 | 55 | 1.8* | 13/20 | 65 | 4.0* |
| 1+ 2-PAM | 11/15 | 73 | 4.1* | 8/15 | 53 | 2.5* |
| 15 + 2PAM | 10/15 | 67 | 3.0* | 15/15 | 100 | 69.1* |
| 20 + 2-PAM | 13/15 | 87 | 9.7* | 8/15 | 53 | 2.5* |
| 55 + 2-PAM | 8/15 | 53 | 1.7* | 14/15 | 93 | 21.6* |
| 62 + 2-PAM | 9/15 | 60 | 2.3* | 14/15 | 93 | 21.6* |
P<0.05. (Data from this experiment also shown in Figure 4.
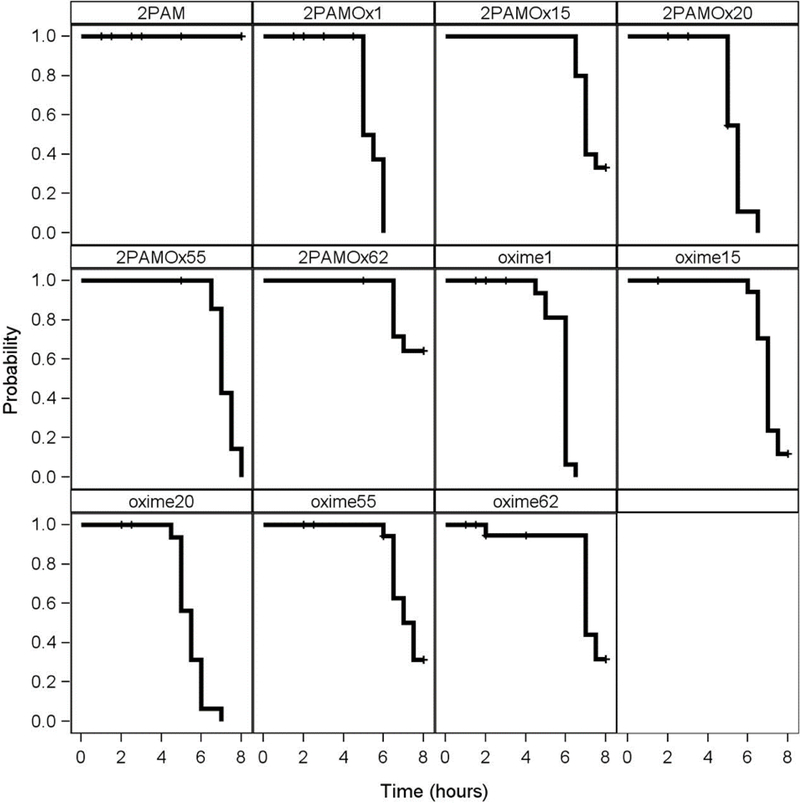
During the above experiments, observations were also made of the signs of poisoning during the 8 hours following OP challenge. With Oximes 1 and 20, either individually or in combination with 2-PAM, there was an earlier cessation of seizure- like behavior after NIMP or NEMP challenge in those rats surviving to that point, than after 2-PAM alone where 8 hour survivors were still displaying seizure- like behavior, as shown with the Kaplan-Meier analysis in Figures 5 and 6. However, Oximes 15, 55, and 62 did not shorten time to cessation of seizure-like behavior compared to 2-PAM.
Figure 5.
Kaplan-Meier time-to-event analysis showing cessation of seizure-like behavior in rats challenged with a subcutaneous dosage of the sarin surrogate nitrophenyl isopropyl methylphosphonate (NIMP; 0.6 mg/kg) followed by 2-PAM or novel oximes alone or in combination with 2-PAM delivered intramuscularly in multisol at 0.146 mmoles/kg at time of initiation of seizure-like behavior (about 30 min post NIMP challenge). Hash marks indicate animal death. Data adapted from data in Chambers et al., 2016b.
Figure 6.
Kaplan-Meier time-to-event analysis showing cessation of seizure-like behavior in rats challenged with a subcutaneous dosage of the VX surrogate nitrophenyl ethyl methylphosphonate (NEMP; 0.65 mg/kg) followed by 2-PAM or novel oximes alone or in combination with 2-PAM delivered intramuscularly in multisol at 0.146 mmoles/kg at time of initiation of seizure-like behavior (about 30 min post NIMP challenge). Hash marks indicate animal death. Data adapted from data in Chambers et al., 2016b.
Experiments are on-going at the time of submission of this manuscript on female rats, and similar results are being obtained. Therefore these behavioral results are highly reflective of brain accumulation by our novel oximes and lack of brain accumulation, as expected, by 2-PAM.
Neuroprotection against brain damage
Following the above behavioral observations suggesting central therapy by our oximes, an experiment was conducted with immunohistochemical staining of glial fibrillary acidic protein (GFAP), a well-known histological marker of damage and scarring within the brain (Chen et al. 2005; Collombet et al., 2007). The high sub-lethal dosages of NIMP or NEMP in DMSO were used for the intraperitoneal challenge in male rats, and Oxime 20 in DMSO as the intramuscular therapy because Oxime 20 had shown the most positive results. Because these OP dosages were sub-lethal, no atropine therapy was provided (Pringle et al., 2018). In these experiments, GFAP was enhanced by NIMP or NEMP alone in both the piriform cortex and the dentate gyrus of the hippocampus and GFAP accumulation was not alleviated by 2-PAM therapy, whereas Oxime 20 reduced GFAP accumulation to the level observed in the vehicle controls. These experiments provided additional evidence that novel Oxime 20 was capable of functioning within the brain and providing neuroprotection against two nerve agent surrogates whereas 2-PAM could not.
Likewise similar protective results, as assessed using the nuclear marker NeuN, were obtained with intramuscular Oximes 20 or 55 (in multisol) of the neurons in the CA1 level of the hippocampus from damage induced by NIMP (in multisol, lethal- level subcutaneous challenge) but not by 2-PAM (Dail et al., 2019).
Potential mechanism for brain accumulation of novel oximes
Because these novel oximes are pyridinium oximes and therefore bear permanent positive charges, they were not expected to readily cross the blood-brain barrier since conventional wisdom declares that charged molecules are excluded from the brain by the blood-brain barrier. We were pleasantly surprised to find the above evidence that some of these pyridinium oximes actually do show protective effects that can only be explained by action within the brain, and not by reactivation of peripheral AChE. The attenuation of the seizure-like behavior and the protection of the architecture of both glial cells and neurons in the brain can only be explained by the presence of the novel oximes in the brain; these results could not be obtained by a reactivator that is acting only on the peripheral nervous system, as evidenced by the results obtained with 2-PAM which is known to not penetrate into the brain at appreciable levels.
However, the blood-brain barrier is not strictly an exclusion barrier through its tight junctions, but it also functions by exporting charged molecules which have diffused into the brain using export transporters such as P-glycoprotein (P-gp). An in vitro experiment was designed to assess the likelihood of some of the oximes to be substrates for P-gp. The experiment compared the in vitro binding efficacy of the oximes to P-gp to the in vivo results on brain AChE recovery following NIMP challenge. The oximes that were the most efficacious in vivo, as indicated by a reduction in AChE inhibition in rats challenged with NIMP (Chambers et al., 2016b), were the poorest substrates for P-gp in an in vitro model system using the rat brain membrane Multi-Drug-Resistance Assay (Dail et al., 2018). These binding results suggest that our most efficacious oximes are less likely to be exported from the brain and therefore may have a longer residence time in the brain facilitating their AChE reactivation potential. While not definitive at this point, these data are suggestive of a mechanism by which our positively charged pyridinium oximes could accumulate in the brain at sufficient levels to be therapeutic.
Summary of data supporting the central neuroprotective ability of novel oximes
As described above, our laboratories have observed several lines of biochemical and functional evidence supporting the conclusion that some members of our platform of novel oximes are capable of accumulating in the brain in sufficient levels to attenuate OP-induced toxicity. Our initial results used an experimental paradigm that produced convincing data that some of our novel oximes could enter the brain and yielded data reflecting a reduction in brain AChE inhibition compared to 2-PAM. The shorter time of cessation of seizure-like behavior by our oximes compared to 2-PAM could only reasonably be caused by action within the brain. Likewise, the preservation of cellular architecture of glial cells and neurons with our oximes following high level organophosphate challenge could only occur following novel oxime action within the brain. In addition, our oximes show appreciable efficacy in promoting survival following lethal levels of two highly relevant nerve agent surrogates. Therefore our platform of substituted phenoxyalkyl pyridinium oximes are showing exceptional promise of therapeutic potential from biochemical, behavioral and histological bases.
Acknowledgements
The work was supported in part by the Department of Defense, Defense Threat Reduction Agency (FA850–05-2–6518/0000169320) through the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. The funding source did not provide input into the study design, conduct, or interpretation. The content of the information does not necessarily reflect the position or the policy of the federal government, and no official endorsement should be inferred.
The work was also supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health [Award Numbers U01NS083430 and U01NS107127] to Mississippi State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The novel oximes described here are patented by Mississippi State University (US patent 9,277,937). They have been recently licensed by Defender Pharmaceuticals, which did not have any input into the studies described.
References
- Aroniadou-Anderjaska V, Figueiredo TH, Apland JP, Prager E, Pidoplichko V, Miller S, and Braga MFM 2016. Long-term neuropathological and behavioral impairments after exposure to nerve agents. Ann. N Y Acad. Sci 1374:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JE, Chambers HW, Meek EC, and Pringle RB 2013. Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates. Chemico-Biol. Interact 203:135–138. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Chambers HW, Funck KE, Meek EC, Pringle RB, and Ross MK 2016a. Efficacy of novel phenoxyalkyl pyridinium oximes as brain-penetrating reactivators of cholinesterase inhibited by surrogates of sarin and VX. Chemico-Biol. Interact 259:154–159. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Meek EC, Bennett JP, Bennett WS, Chambers HW, Leach CA, Pringle RB, and Wills RW 2016b. Novel substituted phenoxyalkyl pyridinium oximes enhance survival and attenuate seizure- like behavior of rats receiving lethal levels of nerve agent surrogates. Toxicology 339:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JE, Meek EC, and Chambers HW 2016c. Novel brain-penetrating oximes for reactivation of acetylcholinesterase inhibited by sarin and VX surrogates. Ann. N.Y. Acad. Sci 1374:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JE, Wiygul SM, Harkness JE, Chambers HW, Effects of acute paraoxon and atropine exposures on retention of shuttle avoidance behavior in rats. Neurosci. Res. Comm, 3:85–92. [Google Scholar]
- Chen Z, Duan RS, Quezada HC, Mix E, Nennesmo I, Adem A, Winblad B,, and Zhu J 2005. Increased microglial activation and astrogliosis after intranasal administration of kainic acid in C57BL/6 mice. J. Neurobiol 62:207–218. [DOI] [PubMed] [Google Scholar]
- Coban A, Carr RL, Chambers HW, Willeford KO, and Chambers JE 2016. Comparison of inhibition kinetics of several organophosphates, including some nerve agent surrogates, using human erythrocyte and rat and mouse brain acetylcholinesterase. Toxicol. Lett 248:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombet JM, Four E, Fauquette W, Burckhart MF, Masqueliez C, Bernabe D, Baubichon D, and Lallement G 2007. Soman poisoning induces delayed astrogliotic scar and angiogenesis in damaged mouse brain areas. Neurotoxicology 28:38–48. [DOI] [PubMed] [Google Scholar]
- Dail MB, Meek EC, Chambers HW, and Chambers JE 2018. In vitro P-glycoprotein activity does not completely explain in vivo efficacy of novel centrally effective oxime acetylcholinesterase reactivators. Drug Chem Toxicol 3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dail MB, Leach CA, Meek EC, Olivier AK, Pringle RB, Green CE, and Chambers JE 2019. Novel brain-penetrating oxime acetylcholinesterase reactivators attenuate organophosphate-induced neuropathology in the rat hippocampus. Toxicol Sci doi: 10.1093/toxsci/kfz060. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Ecobichon DJ (1996). Toxic effects of pesticides. In: Casarett and Doull’s Toxicology: The Basic Science of Poisons (Amdur MO, Doull J, and Klaassen CD, Eds.), McGraw-Hill, New York: pp.643–689. [Google Scholar]
- Eddleston M and Chowdhury F 2015. Pharmacological treatment of organophosphorus insecticide poisoning: the old and the (possible) new. Br J Clin Pharmacol 81:462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto TR and Metcalf RL 1959. The effect of structure on the reactivation of alkylphosphonate esters. J Am Chem Soc 81:372–376. [Google Scholar]
- Meek E, Chambers H, Coban A, Funck K, Pringle R, Ross M, and Chambers J 2012. Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates. Toxicol. Sci 126:525–533. [DOI] [PubMed] [Google Scholar]
- Ohta H, Ohmori T, Suzuki S, Ikegaya H, Sakuraga K and Takatori T 2006. New safe method for preparation of sarin-exposed human erythrocytes acetylcholinesterase using non- toxic and stable sarin analogue isopropyl p-nitrophenyl methylphosphonate and its application to evaluation of nerve agent antidotes. Pharm. Res 23:2827–2833. [DOI] [PubMed] [Google Scholar]
- Pringle RB, Meek EC, Chambers HW, and Chambers JE 2018. Neuroprotection from organophosphate- induced damage by novel phenoxyalkyl pyridinium oximes in rat brain. Toxicol. Sci 166:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T, Duniho S, and McDonough J 2003. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol 188:69–80. [DOI] [PubMed] [Google Scholar]
- Taylor P (1990). Anticholinesterase agents. In: Pharmacological Basis of Therapeutics (Gilman AG, Nies AS, Rall TW, and Taylor P, Eds.). Macmillan, New York: pp. 131–150. [Google Scholar]
- Tucker J 2007. War of Nerves: Chemical Warfare from World War I to Al-Qaeda, Anchor Books. [Google Scholar]
- Worek F, Thiermann H, and Wille T 2016. Oximes in organophosphate poisoning: 60 years of hope and despair. Chem Biol Interact 259:93–98. [DOI] [PubMed] [Google Scholar]