Abstract
Docosahexaenoic acid (DHA) is highly concentrated in the brain and its deficiency is associated with several neurological disorders including Alzheimer’s disease. However, the currently used supplements do not appreciably enrich brain DHA, although they enrich most other tissues. We tested the hypothesis that the ability of the dietary carrier to augment brain DHA depends upon the generation of DHA-lysophosphatidylcholine (LPC), the preferred carrier of DHA across the blood brain barrier. We compared the efficacy of DHA-triacylglycerol (TAG), di-DHA phosphatidylcholine (PC) and DHA-LPC to enrich brain DHA following their gavage to normal rats for 30 days, all at a dose of 10 mg DHA/day. The results show that DHA from TAG, which is released as free DHA or monoacylglycerol during digestion, and is absorbed as TAG in chylomicrons, was incorporated preferentially into adipose tissue and heart, but not into brain. In contrast, LPC-DHA increased brain DHA by up to 100%, but had no effect on adipose tissue. Di-DHA PC, which generates both free DHA and LPC-DHA during the digestion, enriched DHA in brain, as well as in heart and liver. Brain-derived neurotrophic factor was increased by di-DHA PC and DHA-LPC, but not by TAG-DHA, showing that enrichment of brain DHA correlated with its functional effect. We conclude that dietary DHA from TAG or from natural PC (sn-2 position) is not suitable for brain enrichment, whereas DHA from LPC (at either sn-1 or sn-2 position) or from sn-1 position of PC efficiently enriches the brain, and is functionally effective.
Keywords: BDNF, Blood brain barrier, Brain omega 3 fatty acids, Intestinal barrier, Neuroinflammatory diseases
1. Introduction
The brain contains very high concentration of DHA, which is essential for the normal development and function of the brain [1]. Deficiency of brain DHA is associated with several neurological diseases including Alzheimer’s, Parkinson’s, schizophrenia, and depression [2,3]. A few studies in animal models of Alzheimer’s disease showed a beneficial effect of dietary DHA on the cognitive behavior [4-7]. However, the amount of DHA needed to show beneficial effect was too high for clinical application. Accordingly, attempts to improve cognition and memory in patients with mild cognitive dysfunction, employing the currently available supplements of DHA have largely failed [8-10]. Several animal studies showed that DHA from these supplements (fish oil, krill oil, algal oil, ethyl esters) was not incorporated appreciably into adult brain, although other tissues were enriched [11-16]. We propose that the inability of these supplements to increase brain DHA is due to the presence of two mutually incompatible natural barriers: 1). The intestinal barrier that releases DHA from most natural carriers as free fatty acid which is consequently absorbed as triacylglycerol (TAG), and 2). The blood brain barrier (BBB), which has a specific transporter (Mfsd2a) that does not transport TAG or free DHA, but instead requires a lysophosphatidylcholine (LPC) form of DHA [17]. We recently showed [18] that both these barriers can be overcome by providing dietary DHA in the form of LPC, which largely escapes intestinal hydrolysis and is absorbed as phospholipid, followed by its transport into the brain through a transporter (Mfsd2a) at the BBB. Although both free DHA and LPC-DHA are taken up by the brain through different mechanisms, the former does not significantly increase the net amount of DHA in the brain, possibly because it is rapidly oxidized [19,20]. It has in fact been estimated that >80% of dietary DHA consumed either as TAG or as natural PC is oxidized by the β-oxidation pathway [20]. On the other hand, our recent studies with normal mice showed feeding LPC DHA nearly doubled the brain DHA levels, whereas feeding equal amount of free DHA had no effect [18]. Furthermore, the mice which were fed LPC-DHA performed much better than the free DHA-fed mice in the Morris water maze test, suggesting that we can not only increase the brain DHA levels by feeding LPC-DHA, but also improve the memory and brain function. The levels of BDNF, the most important neurotrophin for neurogenesis and repair, were increased in most regions of the brain by feeding LPC-DHA, but not by free DHA, indicating one possible mechanism by which oral LPC DHA was effective in improving the memory. In the current study, we compared the efficacy of the commonly used dietary carriers of DHA, namely TAG, and PC with LPC-DHA in enriching brain DHA and improving function in normal male rats,.
The two major natural sources of DHA are TAG and PC. The distribution of DHA among the three positions of TAG can be either random, as in algal oil [21], predominantly in sn-2, as in many fish oils [22] and squid oil [23], or predominantly in sn-1/sn-3 positions as in seal oil [23]. However, the DHA in natural PC is almost always in the sn-2 position, although small percentages of DHA have been reported be present in sn-1 position of roe [24], and krill phospholipids [25]. To determine the effect of the molecular carrier of dietary DHA on its brain accrual, we employed algal oil (DHASCO) in which DHA is distributed equally among the three positions of TAG, synthetic di-DHA PC, in which DHA is present equally at sn-1 and sn-2 positions, and LPC-DHA in which DHA is present only at sn-1 position. The hypothesis tested is that DHA from any position of TAG will not enrich the brain DHA, but will enrich most other tissues because it is absorbed as TAG in the lymph chylomicrons. On the other hand, LPC-DHA would preferentially increase brain DHA because it is absorbed intact (or as sn-1 DHA PC and subsequently converted to LPC-DHA), and is taken up by the transporter Mfsd2a at BBB. Di-DHA PC should increase DHA in the brain as well as the peripheral tissues, since sn-2 DHA would be released as free fatty acid, and sn-1 DHA would be retained as LPC during absorption. It should be therefore half as effective as LPC-DHA in terms of DHA content for the brain enrichment, since only half of DHA (from sn-1 position) would be retained as phospholipid during absorption. Further, we postulate that the increase in brain DHA would result in functional improvement in the brain function. The results presented here confirm that the incorporation profile of TAG-DHA clearly resembles that of free DHA we have reported in mice. While the DHA from dietary TAG-DHA is preferentially incorporated into adipose tissue and heart, the DHA from LPC and from sn-1 position of PC is preferentially incorporated into the brain. As predicted from our model, di-DHA PC was only half as efficient as LPC-DHA for brain enrichment, because the DHA from the sn-2 position behaves similarly to the DHA derived from TAG.
2. Materials and Methods
2.1. Animals:
These studies were approved by the UIC animal care committee (IACUC # 17-115). Male Sprague-Dawley rats (8 week old, 200-250 gm), were purchased from Harlan laboratories (Indianapolis, IN). The animals were housed (2 per cage) in temperature controlled rooms (22±2 °C) with 12 h light/dark cycle, and fed ad lib standard laboratory chow (Teklad #7012, 5.8% fat, contains no DHA, but contains 0.3% 18:3, n-3).). After a one week acclimation, they were randomly divided into 5 groups (10 animals each) and fed for 30 days, by daily gavage, the various DHA compounds dispersed in corn oil as described below. The studies were performed in two batches of 25 animals each (5 rats/treatment).
The amount of DHA administered was 10 mg (30.4 μmol) /day in the form of TAG-DHA, di-DHA PC, or LPC-DHA. An additional dose of 5 mg DHA (15.2 μmol) was included for LPC-DHA to be equivalent to 10 mg di-DHA PC, since only half of the DHA in PC would be expected to be converted to LPC-DHA. The supplements were administered daily to rats by gavage in corn oil for 30 days, while they were fed ad lib normal rodent chow (Teklad LM 485, Envigo, Indianapolis, IN).
2.2. DHA compounds:
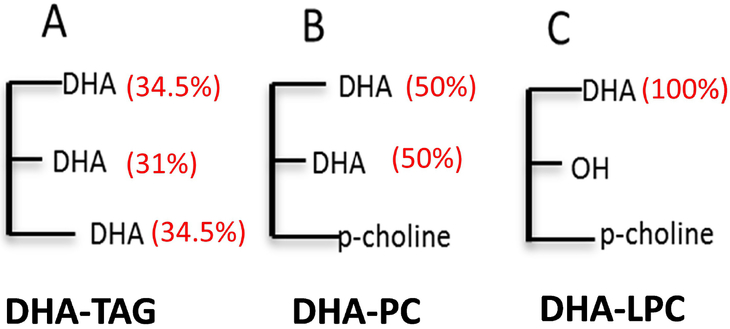
The source of_TAG-DHA was algal oil (DHASCO, DSM Nutritional Products, Columbia, MD). This oil contained 40% of total fatty acid as DHA, which was distributed nearly equally among the three positions of TAG (Fig. 1A). The distribution of DHA among the 3 positions of TAG was 31% in sn-2, and 69% in sn-1 and sn-3. The other major fatty acids in the preparation were 16:0 (14%), 14:0 (4%), 18:1, n-9 (17%), and 22:5, n-6 (11%). Di-DHA PC (Fig. 1B), in which both positions of PC are occupied by equal amounts of DHA, and LPC-DHA (Fig. 1C), in which DHA was present at sn-1 position only, were purchased from Avanti Polar Lipids (Alabaster, AL, USA). All compounds were analyzed for their chemical purity by TLC, and for the fatty acid composition by GC/MS.
Fig.1: Structures of DHA compounds used in the study.
The numbers in parentheses are the percentages of DHA in each position. The DHA in TAG (DHASCO oil) is randomly distributed among the three positions. About 31% of DHA was in sn-2 position, and the rest was equally distributed between sn-1 and sn-3. LPC-DHA and PC-DHA were synthetic preparations, and contained only DHA.
For the preparation of samples for gavage, the DHA compounds in chloroform (equivalent to 684 μmol of DHA) were taken into a glass vial, and the solvent was evaporated under nitrogen. The lipids were dissolved in 200 μl of ethanol and added drop by drop to 5.6 ml of corn oil while stirring for 15 min at room temperature. The ethanol was then evaporated under N2, and the sample was stored at 4 °C under nitrogen . The samples were warmed to room temperature and thoroughly mixed before gavaging to rats (250 μl/day). Fresh dispersions were prepared every 4 days. In the case of the half dose of LPC-DHA (5 mg DHA) the amount of DHA used was decreased by half, but was dispersed in the same volume of corn oil.
2.3. Behavioral studies- Spontaneous Alternation
The Y-maze behavior of the rats was determined as described previously [26], excepting for the dimensions of the apparatus. Rats were placed in one arm of a Y-maze apparatus (56 cm × 56 cm × 56 cm, spaced 120 degrees apart, with a height of 15 cm and width of 10 cm), allowed to explore for 10 min, and the sequence of arm entries were recorded with overhead camera. Spontaneous alternation was calculated (Any-Maze software) as the number of alternations (entries into three different arms consecutively) divided by the total possible alternations (the number of arms entered minus 2) and multiplied by 100.
2.4. Morris Water Maze
Morris water maze (MWM) was conducted as described in [27] with slight modifications. The circular pool was 150 cm in diameter and 59 cm tall, and the circular escape platform was 10 cm in diameter. The pool was filled with water (maintained at 25°C) to 10 cm below the top rim. The pool was divided into equal-sized imaginary quadrants, and the platform placed in the center of one of the quadrants. High contrast visual cues, consisting of different black and white poster board patterns were placed in the four corners for spatial orientation. A single rat was tested in the pool for each testing phase/session. Any-Maze software was used for calculations.
MWM testing was comprised of two phases. (a) Acquisition phase. Rats were trained over the course of 4 days (120 sec trial time, 4 trials each day with a 60 sec inter trial interval) to locate the position of the hidden platform (remains on the hidden platform for >2 sec). Once located, the rat was allowed to remain on the platform for 15 sec before removal from the pool. If the rat did not locate the platform within 120 sec, it was gently guided to the platform and allowed to remain for 15 sec. For each day of the acquisition phase the sequence of quadrant entries varied, but the platform location remained constant. Latency to find the platform (sec) was measured. (b) Probe trial. 24 h following the final acquisition trial, a single 60 sec probe trial was conducted with the platform removed. The latency to the target area (i.e., where platform was located during acquisition phase) and the time spent in the target quadrant were calculated.
2.5. Lipid extraction and analysis:
The rats were fasted overnight, anesthetized with 2% isoflurane, and the blood collected into heparinized tubes by cardiac puncture. The animals were then perfused transcardially with ice-cold phosphate buffer-saline, and the various tissues collected and stored at −80 °C until analysis. Total lipids from the tissues were extracted by the modified Bligh and Dyer procedure, after adding the internal standards of di-17:0 PC and tri-15:0 TAG [18]. Total fatty acids were analyzed by GC/MS following their conversion to methyl esters using methanolic HCl. The analysis was carried out in a Shimadzu QP2010SE GC/MS equipped with a Supelco Omegawax column (30 m × 0.25 mm × 0.25μ). The temperature programming was as follows: 165 °C for 1 min, raised to 210 °C at the rate of 3.5 °C/min, and maintaining at 240 °C for 10 min. Total ion current, in the range of 50-400 m/z was used for quantification of the methyl esters, and the values are expressed as percentage of the total fatty acids. Isomer composition of LPC-DHA in plasma was measured by LC/MS/MS, using a ABSciex QTRAP 6500, as described by Sugasini and Subbaiah [28].
2.6. BDNF analysis
BDNF levels in selected brain regions were determined by ELISA. Rat brain regions (Cortex, hippocampus and striatum) were homogenized in the lysis buffer (Promega Inc, Madison, WI, USA). The homogenates were centrifuged at 10,000 × g, for 20 min, and total protein concentration in supernatant was determined by MicroBCA procedure (Pierce, Rockford, IL, USA) using BSA as standard. Endogenous concentrations of BDNF were quantified using an ELISA kit (BDNF Emax ImmunoAssay System Kit, (Promega Inc, WI, USA) according to manufacturer’s protocol.
2.7. Statistical analyses:
The differences between treatment groups were analyzed one-way ANOVA, with Tukey multiple comparison correction, using Prism software (GraphPad Software, La Jolla California USA).
3. Results
3.1. Body weights
There were no significant differences in the body weights, or the weights of different tissues between the treatment groups (results not shown).
3.2. Behavioral studies
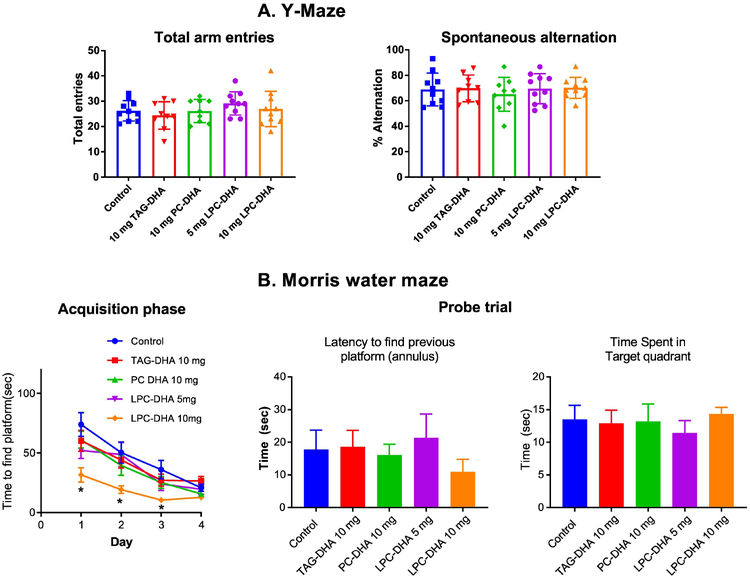
In our previous studies, wild type mice showed a significant improvement in spatial memory following LPC-DHA treatment. In general, wild type rats perform cognitive tasks much better than wild type mice. Therefore, it is difficult to detect changes in cognition using wild type rats after treatment, since their performance is already very high. However, we conducted cognitive analysis to determine whether LPC-DHA treatment results in subtle changes in learning and memory using two behavioral tests: Y-maze test, which measures the exploratory behavior as well as working memory, and Morris water maze test, which is known to be a good indicator of spatial memory. As shown in Fig. 2A, there were no significant differences in between the five groups either in the spontaneous alternation or in number of entries in the Y-Maze apparatus. In the acquisition phase of Morris water maze test, all rats learned the task well by Day 4 (Fig. 2B). Importantly, rats treated with 10 mg LPC-DHA showed the fastest learning time. Indeed, on days 1, 2 and 3 of the acquisition phase, the latency time to find the platform was faster in 10 mg LPC-DHA treated rats compared to vehicle. In the probe test, however, we found no significant differences among the 5 groups either in latency to find the previous platform, or in time spent in the target quadrant (Fig. 2B), consistent with the finding that all groups learned the location of the platform by day 4. These results support that LPC-DHA improves learning in wild type rats and suggest that treatment of neurodegenerative models is required to assess the effect of LPC-DHA on memory.
Fig. 2: Effect of DHA treatments on learning and memory.
A. Y-Maze behavior: Rats were treated with various DHA preparations by gavage for 30 days as described in section 2.2. Y-maze behavior was determined as described in 2.3. Spontaneous alternation was calculated as the number of alternations (consecutive entries into three different arms) divided by the total possible alternations (number of arms entered minus 2) and multiplied by 100. No significant differences were found between treatment groups (n=10 per group).
B. Morris water maze: The test was carried out as described in section 2.4. Rats were trained for 4 consecutive days to locate the hidden platform. On the 5th day, the platform was removed, and the latency to reach the target area, and the time spent in the target quadrant were measured. The data were analyzed by Any-Maze software. The only significant difference observed was between the rats treated with 10 mg LPC-DHA and all the other groups during the acquisition phase (* p<0.05). There were no significant differences between groups in the probe trial. (n=10 per group).
3.3. Effect of the supplements on plasma LPC-DHA:
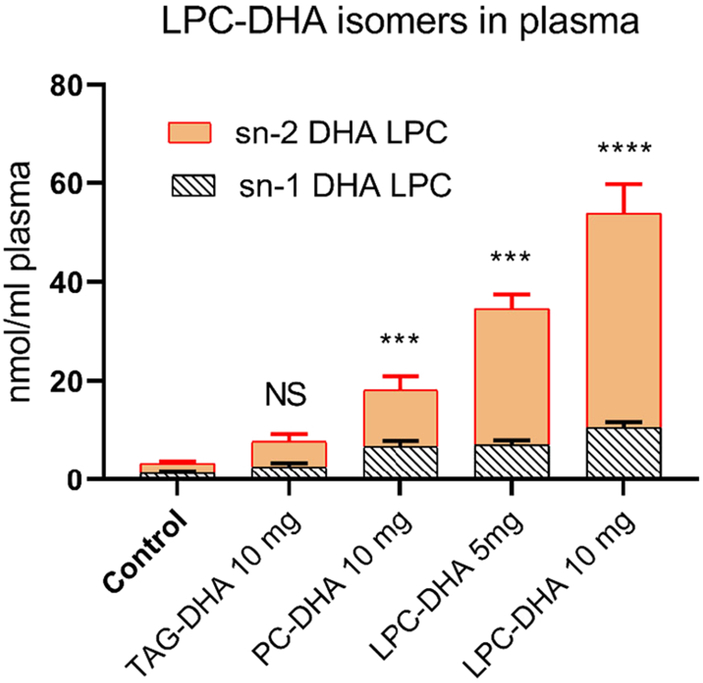
Since LPC-DHA is the major transport form of DHA into the brain through the Mfsd2a pathway [17], we determined the effects of various molecular carriers of dietary DHA on the LPC-DHA level in plasma. We have also analyzed the isomer composition of LPC-DHA in order to distinguish between the absorbed form (sn-1 DHA LPC) and the predominant form secreted by the liver (sn-2 DHA LPC) [29-31]. As shown in Fig. 3, the highest increase in LPC-DHA occurred after feeding 10 mg LPC-DHA (16-fold over control), followed by 5 mg LPC-DHA (10.5-fold), 10 mg PC-DHA (5.5-fold), and 10 mg TAG-DHA (2.5-fold). Interestingly, the isomer composition revealed that the majority of LPC-DHA was present as sn-2 DHA LPC in all groups. Furthermore, the increase in plasma LPC-DHA was mostly due to an increase in the sn-2 DHA isomer. Since the rats were fed sn-1 DHA-LPC, this indicates that the source of plasma LPC-DHA is not the recently absorbed LPC-DHA, but the LPC secreted by the liver, which is known to be sn-2 acyl isomer [29-31]. It may also be noted that the animals were fasted overnight before sacrifice, and therefore it is unlikely that the recently absorbed lipids contributed significantly to the plasma LPC composition. We also found increases in plasma PC and PE species containing DHA, which is similar to the increases in LPC-DHA (Supplementary Figure S1).
Fig. 3: Isomers of LPC-DHA in plasma.
The positional isomers of LPC-DHA were analyzed by LC/MS/MS, as described previously [18]. The results shown are mean ± SD of 5 animals per group. Statistical significance shown is between control (untreated) group and DHA-treated groups for the total LPC-DHA. Similar significance values were obtained when the two isomers were analyzed separately (not shown). *** p< 0.001; **** p< 0.0001.
3.4. Effect of supplements on the DHA and ARA content in systemic tissues
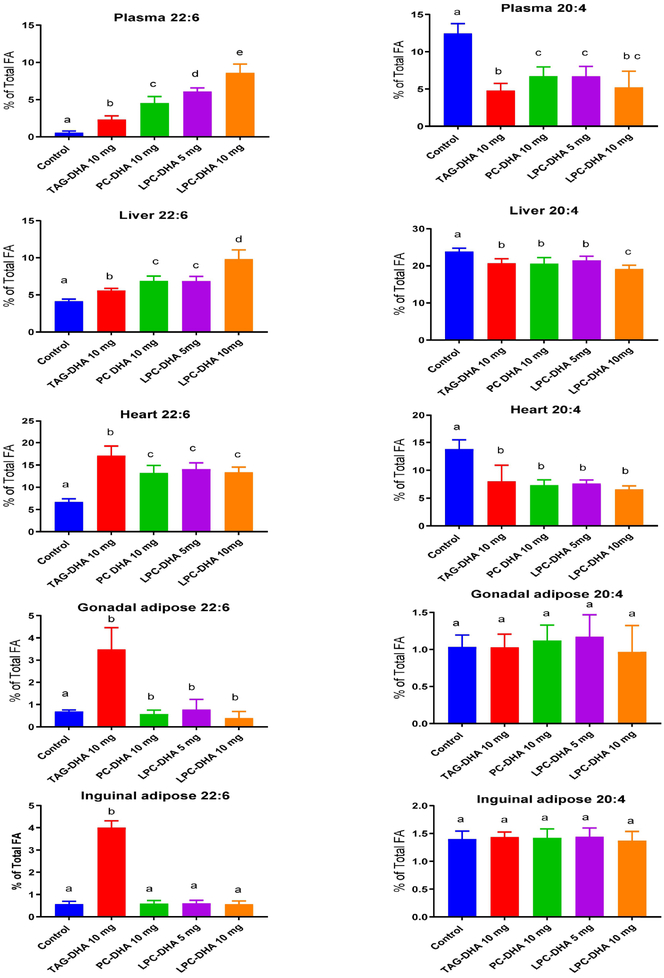
Fig. 4 shows the DHA content of various systemic tissues after 30 day supplementation with the DHA compounds. In addition, the ARA content is shown because of the known reciprocal relationship between these two fatty acids. The differences between the treatments were analyzed by ANOVA and the significance denoted by different letter superscripts on the bars. The magnitude of differences between treatments and their significance levels are shown in Supplementary Figure S2. The total fatty acid composition of all tissues is shown in Supplementary Tables 1-5. In the plasma, the maximum increase in DHA occurred with 10 mg LPC-DHA, followed by 5 mg LPC-DHA and 10 mg PC-DHA. The increase was the lowest with TAG-DHA. However, these levels do not necessarily represent the absorption efficiencies, since the analysis was done in fasted plasma. Instead, these values resemble those of liver DHA, suggesting their hepatic origin. The ARA levels were decreased to similar extent by all preparations.
Fig. 4: Effect of dietary treatments on DHA and ARA levels in systemic tissues.
The total fatty acid composition of plasma and various tissues was analyzed by GC/MS as described in section 2.5. Only the values for DHA and ARA are shown here. The total fatty acid composition is shown in supplementary Tables-1-5. The differences between treatment groups were analyzed by one-way ANOVA with correction by Tukey multiple comparison test (Prism, Graphpad software). Bars with common letter superscripts are not significantly different from each other (n=10 for all groups) The magnitude of differences between individual treatments is shown in Supplemental Figure S2.
In the liver, all preparations increased the DHA content, but 10 mg LPC-DHA was the most efficient. The increase was similar for 10 mg PC and 5 mg LPC, both of which were more efficient than TAG. The decrease in liver ARA was comparable with all preparations except for 10 mg LPC, which decreased more ARA than others.
In contrast to the liver, the DHA content of the heart was elevated most by TAG-DHA. Dietary PC-DHA, and both doses of LPC-DHA elevated heart DHA to similar extent, but less than TAG-DHA. However, the decrease in heart ARA was similar with all preparations.
In both peri-gonadal and inguinal adipose tissues, only TAG-DHA increased the DHA content. PC-DHA, as well as the two doses of LPC-DHA were ineffective in increasing the DHA or decreasing ARA in the adipose tissue.
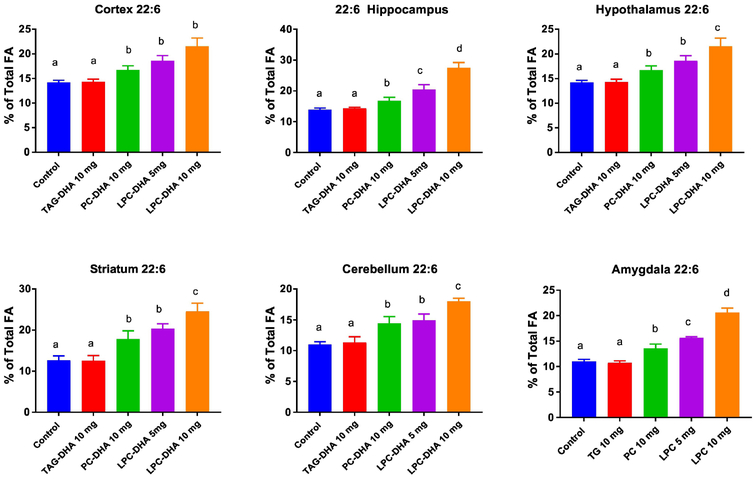
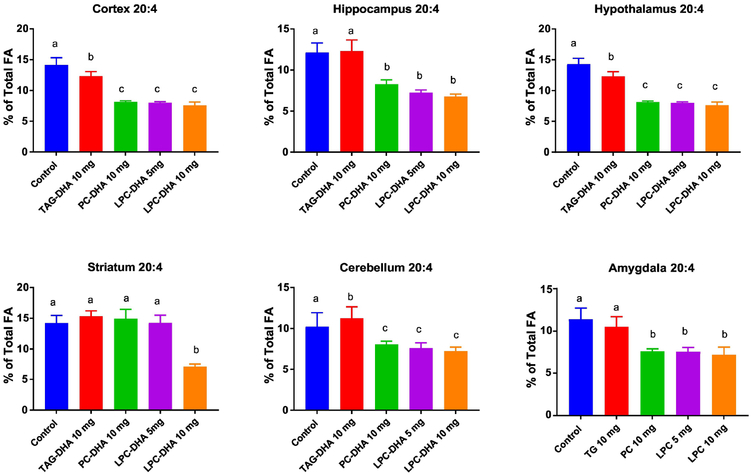
3.5. Effect of the supplements on brain DHA and ARA
Six brain regions were analyzed for the fatty acid composition and the results are shown in Figs. 5 and 6. The differences between the treatments was analyzed by ANOVA, and the magnitude of differences is shown in Supplementary Figures S3 and S4. The total fatty acid compositions of all regions are shown in Supplementary Tables 6-11. As shown in Fig. 5, the DHA content of all brain regions was significantly increased by both di-DHA PC and LPC-DHA, but not by TAG-DHA. As expected, the enrichment was the highest with 10 mg LPC-DHA, whereas the enrichment with 5 mg LPC-DHA was comparable to 10 mg PC-DHA, except in hippocampus and amygdala, where the 5 mg LPC-DHA was slightly more effective. The ARA content was significantly decreased by both PC-DHA and LPC-DHA to a similar extent with no dose dependent effect of LPC-DHA (Fig. 6). The exception was striatum, where only 10 mg LPC-DHA decreased the ARA content. TAG-DHA significantly decreased the ARA content in cortex and hypothalamus, although there was no significant increase in the DHA content of these regions.
Fig. 5. Changes in DHA content of brain regions by DHA supplements.
The DHA content of six brain regions was analyzed by GC/MS, as described in section 2.5. The total fatty acid composition is shown in Supplemental Tables 6-11, in Supporting Information.. Statistical significance between treatments was determined by one-way ANOVA. Bars with common letter superscripts are not significantly different from each other (n=5 for all groups). The magnitudes of differences are shown in Supplemental Figure S3..
Fig. 6. Changes in ARA content of brain regions by DHA supplements.
The percentage composition of ARA in various brain regions was determined by GC/MS (section 2.5). The statistical significance between treatments was determined by one-way ANOVA, applying Tukey multiple comparison correction. Bars with common letter superscripts are not significantly different from each other (n=5 for all groups). The magnitudes of differences are shown in Supplemental Figure S4..
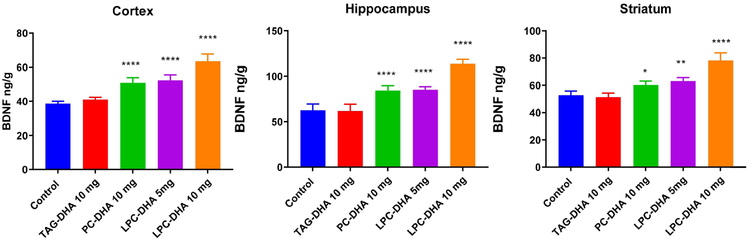
3.6. Brain BDNF levels
Since DHA is known to increase the expression and synthesis of BDNF [32], we determined the BDNF levels by ELISA in selected regions of the brain. As shown in Fig. 7, in all three regions of brain tested (cortex, hippocampus, and striatum) PC-DHA, as well as both doses of LPC-DHA significantly increased BDNF levels, whereas TAG-DHA had no appreciable effect. Furthermore, the increase in BDNF appears to be correlated with the increase in DHA, since the highest increase occurred with 10 mg LPC-DHA, followed by 5 mg LPC-DHA, and 10 mg PC-DHA, a pattern similar to the changes in DHA levels in the same brain regions. These results show that the enrichment of brain DHA through dietary LPC-DHA resulted in functional changes in the brain.
Fig. 7. Effect of dietary DHA on BDNF levels.
BDNF concentrations were determined in cortex, hippocampus, and striatum by ELISA, as described in 2.6. The values shown (ng/g) are mean ± SD of 5 samples per group. Statistical significance shown is between the control and treated groups (one-way ANOVA, with Tukey multiple comparison correction). * p<0.05; ** p<0.01; **** p< 0.0001.
4. Discussion
Omega 3 fatty acid supplements, in the form of fish oil, krill oil, algal oil, and ethyl esters have been beneficially employed for the prevention and treatment of several metabolic disorders, including cardiovascular diseases [33], fatty liver disease [34], and rheumatoid arthritis [35]. Although DHA is highly concentrated in the brain, and its deficiency has been linked to various neurological disorders [2,3,36], the currently available supplements have not been successfully employed for the prevention or treatment of these disorders. The main reason for this is the impermeability of BBB for most major molecular forms of DHA present in the plasma. Majority of plasma DHA occurs in the form of diacyl phospholipids, triacylglycerol, and cholesteryl ester, while smaller amounts occur as LPC, and as free (unesterified) DHA. Of these molecular forms, only the free DHA and LPC-DHA have been shown to cross the BBB, although there is controversy as to which of these two molecular forms is the major supplier of DHA to the brain. The short term kinetic studies of Chen et al in rats [37] suggested that free DHA is the major source of brain DHA, whereas previous studies by Lagarde group suggested that LPC-DHA is the primary source of brain DHA [38]. The recent demonstration of a specific transporter (Mfsd2a) at the BBB that transports LPC-DHA, but not free DHA, and the decrease in brain DHA content in the absence of this transporter [17] appear to support the role of plasma LPC-DHA as the primary molecular form of transport into the brain. However, several previous studies failed to show a significant net increase in brain DHA of normal adult mammals by dietary supplementation. Thus feeding DHA-rich TAG or phospholipid preparations [15], free DHA [14], algal oil [13], krill oil [16], ethyl esters [12], or canned sardines [11] failed to increase the DHA content of the brain in adult animals, although they all increased DHA in other tissues. A few other studies did report an increase in brain DHA and improved brain function after long term feeding of large amounts DHA. Thus, Green et al [39] reported a 20% increase in brain DHA after feeding about 1560 mg DHA/ kg body weight/day (calculated, assuming a 25 g mouse consuming 3 g of diet per day). Chouinard-Watkins et al [40] reported a 9% increase in brain DHA, following feeding of 840 mg/DHA/kg for 3 months, whereas Perez et al [5] reported a 25% increase in brain DHA after feeding 720 mg DHA/kg for 3 months. In contrast, we recently demonstrated that dietary LPC-DHA, either as sn-1 acyl or sn-2 acyl isomer could increase the net amount of brain DHA by almost 100% in normal mice at a very low daily dose (40 mg/kg) in a period of one month [18]. We have also recently demonstrated that the brain EPA levels can be increased by 100-fold, and brain DHA increased by 2-fold, by feeding LPC-EPA, but not free EPA [41].
Whereas our previous study [18] compared LPC-DHA with free DHA, the present study was undertaken to compare LPC-DHA with the most widely used dietary carriers of DHA, namely TAG-DHA and PC-DHA at equal DHA concentrations for their bioavailability to the brain. We hypothesized that TAG-DHA would enrich most tissues except the brain, while LPC-DHA would preferentially enrich the brain. Theoretically, di-DHA PC should provide the combined effects of free DHA and LPC-DHA, and therefore should enrich both brain and peripheral tissues. The results presented here confirm these hypotheses. In addition, we show that the half dose of LPC-DHA (5 mg DHA/day) is equivalent to a full dose (10 mg DHA/day) of di-DHA PC for brain DHA enrichment, since the DHA at the sn-2 position of di-DHA PC would be released as free acid during digestion, and would be absorbed as TAG.
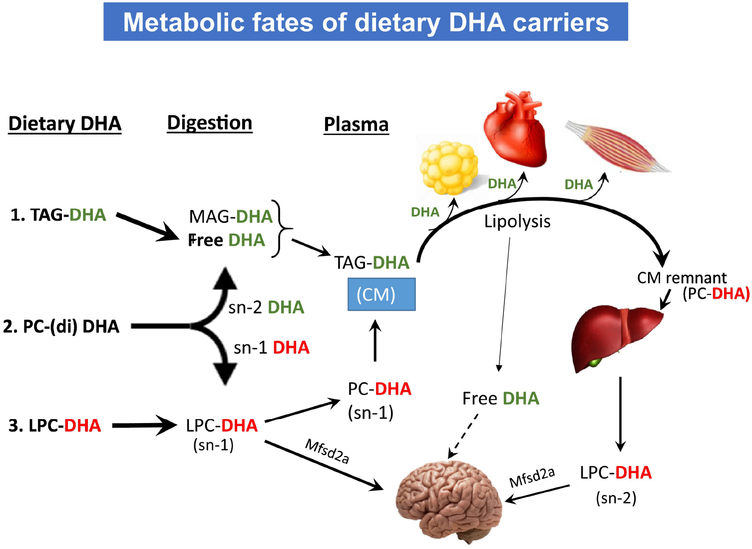
In our previous studies with normal mice [18], we found that only the LPC-DHA could increase the brain DHA at the doses given (40 mg DHA/kg/day for 30 days). The current results in rats, with equivalent dose of DHA confirm the effectiveness of LPC-DHA to enrich brain DHA, whereas TAG-DHA, was ineffectual. Furthermore, they show that the incorporation pattern of TAG-DHA in various tissues closely resembles that of free DHA in our previous study, showing that dietary free DHA can be used to trace the fate of DHA from fish oil and other TAG sources of DHA. We propose the following scheme to explain the differential distribution of DHA among the tissues, following its ingestion in different molecular forms (Fig. 8). It is known that chylomicrons pass through adipose tissue, heart and peripheral tissues before being cleared by the liver in the form of chylomicron remnants [42]. Therefore, it appears that the DHA from TAG-DHA, as well as the DHA from sn-2 position of PC, both of which are absorbed in the form of chylomicron TAG, is partly taken up by the heart, muscle, and adipose tissue first, before being taken up by the liver. This accounts for the higher enrichment of adipose tissue and heart by TAG-DHA, compared to the liver. Dietary LPC-DHA, on the other hand, may be either taken up directly by the brain or first transported to the liver as PC, and subsequently secreted as LPC-DHA into plasma [29,43,44]. The uptake of LPC-DHA by the Mfsd2a pathway leads to net accumulation of DHA in the brain. Free DHA, released by lipolysis is also taken up by the brain [31], but apparently does not increase the brain DHA level. The lack of incorporation of either PC-DHA or LPC-DHA into adipose tissue shows the divergent metabolic pathways of free DHA derived from dietary TAG-DHA and from the phospholipids.
Fig. 8. Divergent metabolic fates of the molecular carriers of dietary DHA.
Proposed mechanisms for the differential effects of the three dietary DHA carriers. 1. DHA from TAG is hydrolyzed by the pancreatic lipase to free DHA and monacyl glycerol (MAG) in the intestine, which are then absorbed as TAG-DHA in chylomicrons (CM). Free DHA is released by lipolysis of the TAG-DHA of CM by lipoprotein lipase at the endothelial surface of various tissues, especially adipose tissue, heart, and skeletal muscle, and is taken up by the tissues. The TAG-depleted CM remnants are taken up by the liver, which assimilates the DHA into cell membrane lipids or incorporates it into VLDL for transport to other tissues. 2. Di-DHA PC is hydrolyzed to free DHA (green) and LPC-DHA (red) by pancreatic phospholipase A2 in the intestine. The free DHA released from PC follows the same metabolic path as the free DHA released from dietary TAG-DHA. The released sn-1 DHA LPC, on the other hand is either incorporated into PC of chylomicrons, or possibly enters the blood directly as LPC.. Part of the DHA taken up by the liver in the form of PC is remodeled and secreted into the blood as sn-2 DHA LPC. Small amounts of sn-2 DHA LPC may also be formed from the TAG-DHA taken up by the liver. Both sn-1 and sn-2 isomers of LPC-DHA are transported by the Mfsd2a pathway into the brain and increase the net content of brain DHA. Free DHA, which is generated by lipolysis and is albumin bound in the plasma, has also been shown to enter the brain apparently by passive diffusion, but unlike LPC-DHA, does not increase the net amount of brain DHA. 3. Dietary LPC-DHA follows the same metabolic pathway as the LPC-DHA released from the hydrolysis of di-DHA PC, and increases brain DHA content.
An unexpected finding in our studies is that the majority of LPC-DHA found in the plasma was sn-2 DHA isomer in all groups of animals, including those fed sn-1 DHA LPC (Fig. 5). In fact, 80% of the LPC-DHA was present as the sn-2 acyl isomer in the animals fed sn-1 LPC-DHA. The exact mechanism by which feeding sn-1 DHA LPC selectively increases the sn-2 DHA LPC in plasma is not clear. Since the analysis was performed in the fasting plasma, the increase is not due to an isomerization of the recently absorbed sn-1 DHA LPC in plasma. Furthermore, the acyl migration from sn-1 to sn-2 position of LPC is not thermodynamically favored [45,46]. Our previous studies, however, showed that sn-2 DHA LPC is more stable than other sn-2 acyl LPC’s in terms of acyl migration [28]. It therefore appears that the increase in sn-2 DHA LPC is due to the secretion of LPC-DHA from the liver, following the absorption of sn-1 DHA LPC, although the exact pathway by which this occurs needs to be elucidated. Another possible mechanism for the formation of sn-2 DHA LPC in the plasma is the cholesterol esterification reaction by lecithin-cholesterol acyltransferase, whose positional specificity is altered in presence of sn-2 DHA PC, producing a saturated cholesterol ester and sn-2 DHA LPC [47,48].
The increase of brain DHA after feeding 10 mg PC-DHA was equal to that of feeding 5 mg LPC-DHA in most cases, as predicted from our hypothesis that only the sn-1 DHA would be available for brain uptake. The exception was hippocampus where the 5 mg dose of LPC-DHA was significantly better than 10 mg PC-DHA. We expected the incorporation of DHA in the liver and heart by 10 mg PC-DHA dose to be higher than 5 mg LPC-DHA dose, since both fatty acids of PC should be incorporated in these tissues. However, we found the incorporation rates to be similar between these two groups, whereas 10 mg LPC-DHA showed the highest incorporation. One possible explanation for this is that the absorption of di-DHA PC is less efficient than that of LPC-DHA, as suggested from the higher plasma DHA content in the case of 5 mg LPC-DHA, compared to 10 mg PC-DHA. There is indeed some indirect evidence that the hydrolysis of DHA from the phospholipids by the pancreatic PLA2 is less efficient than for other fatty acids [24], and therefore the absorption di-DHA PC may be impaired.
During the preparation of this manuscript, Chouinard-Watkins et al [49] published a study in which rats were gavaged with labeled tri-DHA TAG, or di-DHA phospholipids, and the incorporation of the label into brain lipids was determined after a 6 h period. They reported that the incorporation of DHA from the phospholipid was 6-fold higher than DHA from TAG, although the changes in DHA mass were not determined. While these results confirm the preferential uptake of phospholipid DHA by the brain, it is important to show an increase in the mass of DHA, as shown here, because the increase in the label can also occur by a simple exchange with endogenous DHA. Furthermore, since both DHA groups of phospholipids were labeled in the above study, it is likely that the label in the brain was derived primarily from the sn-1 position, according the scheme proposed here (Fig. 8).
Based on the results presented here, we conclude that the most commonly used carriers of DHA, namely TAG-DHA (as in fish oil), sn-2 DHA PC (as in krill oil) or ethyl esters (as in Lovaza®, Omacor®) do not enrich brain DHA, because they are absorbed as TAG, which is not efficiently converted to LPC-DHA in the liver. We show that di-DHA PC is only half as efficient as LPC-DHA on the basis of DHA content, supporting the hypothesis that only DHA from the sn-1 position of PC is transported efficiently into the brain. It should be pointed out that in contrast to PC-DHA, there is no difference between sn-1 and sn-2 isomers of LPC-DHA in their ability to enrich brain DHA. Contrary to previous studies, which showed that even high doses of TAG-DHA only modestly enriched brain DHA, our studies with clinically relevant doses of LPC-DHA show a near doubling of brain DHA. Using the allometric scaling [50], we calculate that the human equivalent daily dose of LPC-DHA for efficient brain enrichment is about 452 mg DHA for a 70 kg person, whereas the dosage using TAG-DHA, based on the previous studies, is about 3.4 g to 8.9 g of DHA per day. It may also be noted that an additional advantage of LPC-DHA is that for each molecule of DHA transported through the Mfsd2a pathway, a molecule of choline is also transported into the brain. Since choline is the essential component of acetylcholine, and is also an epigenetic modifier in the brain [51], this could further contribute to improved brain function. Although saturated LPC has been shown to be pro-inflammatory in many studies [52,53]. LPC-DHA has been shown to be anti-inflammatory in both in vitro and in vivo studies [54,55]. It has in fact been shown to counteract the inflammatory effects of saturated LPC in vivo [56]. The nutraceutical potential of LPC-DHA is therefore worthy of further investigation.
Supplementary Material
Acknowledgements
This research was supported by a VA Merit Review award I01 BX001090, an R21 grant AT00847 from National Center for Complementary and Integrative Medicine, NIH, and by the Office of the Director , NIH, under award number S10 OD010660 (LC/MS equipment) (PVS).
List of Abbreviations
- ARA
Arachidonic acid
- BBB
Blood brain barrier
- BDNF
Brain-derived neurotrophic factor
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- LPC
Lysophosphatidylcholine
- Mfsd2a
Major facilitator superfamily domain-containing protein 2a
- MWM
Morris water maze
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- sn
stereospecifically numbered
- TAG
Triacylglycerol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
All authors declare no conflict of interest
References
- [1].Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, Gu Z, Greenlief CM, Yao JK, Lee JC, Beversdorf DQ. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids 2018; 136: 3–13. doi: 10.1016/j.plefa.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cunnane SC, Chouinard-Watkins R, Castellano CA, Barberger-Gateau P. Docosahexaenoic acid homeostasis, brain aging and Alzheimer's disease: Can we reconcile the evidence? Prostaglandins Leukot Essent Fatty Acids 2013; 88: 61–70. doi: 10.1016/j.plefa.2012.04.006 [doi] [DOI] [PubMed] [Google Scholar]
- [3].Janssen CIF, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Progress in Lipid Research 2014; 53: 1–17. doi: 10.1016/j.plipres.2013.10.002 [DOI] [PubMed] [Google Scholar]
- [4].Arsenault D, Julien C, Tremblay C, Calon F. DHA Improves Cognition and Prevents Dysfunction of Entorhinal Cortex Neurons in 3×Tg-AD Mice. PLoS ONE 2011; 6: e17397. doi: 10.1371/journal.pone.0017397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perez SE, Berg BM, Moore KA, He B, Counts SE, Fritz JJ, Hu YS, Lazarov O, Lah JJ, Mufson EJ. DHA diet reduces AD pathology in young APPswe/PS1delta E9 transgenic mice: Possible Gender Effects. J Neurosci Res 2010; 88: 1026–1040. doi: 10.1002/jnr.22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lim SY, Suzuki H. Changes in Maze Behavior of Mice Occur after Sufficient Accumulation of Docosahexaenoic Acid in Brain. J Nutr 2001; 131: 319–324. doi: 10.1093/jn/131.2.319 [DOI] [PubMed] [Google Scholar]
- [7].Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV. Effect of Dietary n-3 Polyunsaturated Fatty Acids on Brain Lipid Fatty Acid Composition, Learning Ability, and Memory of Senescence-Accelerated Mouse. J Gerontol A Biol Sci Med Sci 2008; 63: 1153–1160. doi: 10.1093/gerona/63.11.1153 [DOI] [PubMed] [Google Scholar]
- [8].Quinn JF, Raman R, Thomas RG. Docosahexaenoic acid supplementation and cognitive decline in alzheimer disease: A randomized trial. JAMA 2010; 304: 1903–1911. doi: 10.1001/jama.2010.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 1538–1544. doi: 10.1016/j.pnpbp.2008.05.015 [DOI] [PubMed] [Google Scholar]
- [10].Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T. Omega-3 fatty acid treatment in 174 patients with mild to moderate alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 2006; 63: 1402–1408. doi: 10.1001/archneur.63.10.1402 [DOI] [PubMed] [Google Scholar]
- [11].Rodrigues PO, Martins SV, Lopes PA, Miguueis S, Alfaia CM, Pinto RMA, Rolo EA, Bispo P, Batista I, Bandarra NM, Prates JAM. Influence of feeding graded levels of canned sardines on the inflammatory markers and tissue fatty acid composition of Wistar rats. Br J Nutr 2014; 112: 309–319. 10.1017/S0007114514000853 [DOI] [PubMed] [Google Scholar]
- [12].Saito M, Ueno M, Kubo K, Yamaguchi M. Dose-Response Effect of Dietary Docosahexaenoic Acid on Fatty Acid Profiles of Serum and Tissue Lipids in Rats. J Agr Food Chem 1998; 46: 184–193. doi: 10.1021/jf970385d [DOI] [PubMed] [Google Scholar]
- [13].Lin YH, Shah S, Salem N Jr. Altered essential fatty acid metabolism and composition in rat liver, plasma, heart and brain after microalgal DHA addition to the diet. J Nutr Biochem 2011; 22: 758–765. doi: 10.1016/j.jnutbio.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaur G, Begg DP, Barr D, Garg M, Cameron-Smith D, Sinclair AJ. Short-term docosapentaenoic acid (22:5 n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br J Nutr 2010; 103: 32–37. doi: 10.1017/S0007114509991334 [doi] [DOI] [PubMed] [Google Scholar]
- [15].Hiratsuka S, Ishihara K, Kitagawa T, Wada S, Yokogoshi H. Effect of Dietary Docosahexaenoic Acid Connecting Phospholipids on the Lipid Peroxidation of the Brain in Mice. J Nutr Sci Vitaminol (Tokyo) 2008; 54: 501–506. doi: 10.3177/jnsv.54.501 [DOI] [PubMed] [Google Scholar]
- [16].Tou JC, Altman SN, Gigliotti JC, Benedito VA, Cordonier EL. Different sources of omega-3 polyunsaturated fatty acids affects apparent digestibility, tissue deposition, and tissue oxidative stability in growing female rats. Lipids in Health and Disease 2011; 10: 179. doi: 10.1186/1476-511X-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014; 509: 503–506. doi: 10.1038/nature13241 [DOI] [PubMed] [Google Scholar]
- [18].Sugasini D, Thomas R, Yalagala PCR, Tai LM, Subbaiah PV. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Scientific Reports 2017; 7: 11263. doi: 10.1038/s41598-017-11766-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Poumes-Ballihaut C, Langelier B, Houlier F, Alessandri JM, Durand G, Latge C, Guesnet P. Comparative bioavailability of dietary alpha-linolenic and docosahexaenoic acids in the growing rat. Lipids 2001; 36: 793–800. doi: 10.1007/s11745-001-0786-5 [DOI] [PubMed] [Google Scholar]
- [20].Kitson AP, Metherel AH, Chen CT, Domenichiello AF, Trepanier MO, Berger A, Bazinet RP. Effect of Dietary Docosahexaenoic Acid (DHA) in Phospholipids or Triglycerides on Brain DHA Uptake and Accretion. J Nutr Biochem 2016; 33: 91–102. doi: 10.1016/j.jnutbio.2016.02.009 [DOI] [PubMed] [Google Scholar]
- [21].Guil-Guerrero JL, Ramos-Bueno RP, Gomez-Mercado F, Rincon-Cervera MA. Positional distribution assessment of essential fatty acids in several fats and oils including plant, fish, and microbial sources and subcutaneous fat of Galician horse. Eur J Lipid Sci Technol 2014; 117: 701–709. doi: 10.1002/ejlt.201400315 [DOI] [Google Scholar]
- [22].Zhang H, Zhao H, Zhang Y, Shen Y, Su H, Jin J, Jin Q, Wang X. Characterization of Positional Distribution of Fatty Acids and Triacylglycerol Molecular Compositions of Marine Fish Oils Rich in Omega-3 Polyunsaturated Fatty Acids. Biomed Res Int 2018; 2018: 3529682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ikeda I, Yoshida H, Tomooka M, Yosef A, Imaizumi K, Tsuji H, Seto A. Effects of long-term feeding of marine oils with different positional distribution of eicosapentaenoic and docosahexaenoic acids on lipid metabolism, eicosanoid production, and platelet aggregation in hypercholesterolemic rats. Lipids 1998; 33: 897–904. doi: 10.1007/s11745-998-0286-7 [DOI] [PubMed] [Google Scholar]
- [24].Murota K, Takagi M, Watanabe Y, Tokumura A, Ohkubo T. Roe-derived phospholipid administration enhances lymphatic docosahexaenoic acid-containing phospholipid absorption in unanesthetized rats. Prostaglandins Leukot Essent Fatty Acids 2018; 139: 40–48. doi: 10.1016/j.plefa.2017.06.011 [DOI] [PubMed] [Google Scholar]
- [25].Winyard PG, Hoem N, Berge K, Reubsaet L. Elucidation of Phosphatidylcholine Composition in Krill Oil Extracted from Euphausia superba. Lipids 2011; 46: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thomas R, Zuchowska P, Morris AW, Marottoli FM, Sunny S, Deaton R, Gann PH, Tai LM. Epidermal growth factor prevents APOE4 and amyloid-beta-induced cognitive and cerebrovascular deficits in female mice. Acta Neuropathol Commun 2016; 4: 111. doi: 10.1186/s40478-016-0387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1: 848–858. doi: 10.1038/nprot.2006.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sugasini D, Subbaiah PV. Rate of acyl migration in lysophosphatidylcholine (LPC) is dependent upon the nature of the acyl group. Greater stability of sn-2 docosahexaenoyl LPC compared to the more saturated LPC species. PLoS ONE 2017; 12: e0187826. doi: 10.1371/journal.pone.0187826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Croset M, Brossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J 2000; 345: 61–67. doi: 10.1042/bj3450061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Graham A, Zammit VA, Christie WW, Brindley DN. Sexual dimorphism in the preferential secretion of unsaturated lysophosphatidylcholine by rat hepatocytes but no secretion by sheep hepatocytes. Biochim Biophys Acta 1991; 1081: 151–158. doi: 10.1016/0005-2760(91)90020-I [DOI] [PubMed] [Google Scholar]
- [31].Scagnelli GP, Cooper PS, VandenBroek JM, Berman WF, Schwartz CC. Plasma 1-palmitoyl-2-linoleoyl phosphatidylcholine. Evidence for extensive phospholipase A1 hydrolysis and hepatic metabolism of the products. J Biol Chem 1991; 266: 18002–18011. [PubMed] [Google Scholar]
- [32].Wu A, Ying Z, Gomez-Pinilla F. The Salutary Effects of DHA Dietary Supplementation on Cognition, Neuroplasticity, and Membrane Homeostasis after Brain Trauma. J Neurotrauma 2011; 28: 2113–2122. doi: 10.1089/neu.2011.1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jump DB, Depner CM, Tripathy S. Omega-3 fatty acid supplementation and cardiovascular disease: Thematic Review Series: New Lipid and Lipoprotein Targets for the Treatment of Cardiometabolic Diseases. J Lipid Res 2012; 53: 2525–2545. doi: 10.1194/jlr.R027904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Di Minno MN, Russolillo AF, Lupoli RF, Ambrosino PF, Di Minno AF, Tarantino G. Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease. World J Gastroenterol 2012; 18: 5839–5847. doi: 10.3748/wjg.v18.i41.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gioxari A, Kaliora AC, Marantidou F, Panagiotakos DP. Intake of -ë-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018; 45: 114–124. doi: 10.1016/j.nut.2017.06.023 [DOI] [PubMed] [Google Scholar]
- [36].Morris M, Evans DA, Bienias JL. Consumption of fish and n-3 fatty acids and risk of incident alzheimer disease. Arch Neurol 2003; 60: 940–946. [DOI] [PubMed] [Google Scholar]
- [37].Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trepanier MO, Lin LE, Ermini L, Post M, Thies F, Bazinet RP. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep 2015; 5: 15791. doi: 10.1038/srep15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thies F, Pillon C, Moliere P, Lagarde M, Lecerf J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Amer J Physiol-Regul Integr C 1994; 36: R1273–R1279. [DOI] [PubMed] [Google Scholar]
- [39].Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-[beta] pathology: a link to Alzheimer's disease? Nat Rev Neurosci 2010; 11: 361–370. 10.1038/nrn2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chouinard-Watkins R, Vandal M, Leveille P, Pincon A, Calon F, Plourde M. Docosahexaenoic acid prevents cognitive deficits in human apolipoprotein E epsilon 4-targeted replacement mice. Neurobiology of Aging 2017; 57: 28–35. [DOI] [PubMed] [Google Scholar]
- [41].Yalagala PCR, Sugasini D, Dasarathi S, Pahan K, Subbaiah PV. Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: potential treatment for depression. J Lipid Res 2019; 60: 566–578. doi: 10.1194/jlr.M090464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Feingold KR GC. Introduction to Lipids and Lipoproteins. In: De Groot LJ, Chrousos G, Dungan K (eds.), Endotext. South Dartmouth (MA): MDText.com, Inc.; 2018. [Google Scholar]
- [43].Brindley DN. Hepatic secretion of lysophosphatidylcholine: A novel transport system for polyunsaturated fatty acids and choline. J Nutr Biochem 1993; 4: 442–449. doi: 10.1016/0955-2863(93)90061-Z [DOI] [Google Scholar]
- [44].Sekas G, Patton GM, Lincoln EC, Robins SJ. Origin of plasma lysophosphatidylcholine: evidence for direct hepatic secretion in the rat. J Lab Clin Med 1985; 105: 190–194. [PubMed] [Google Scholar]
- [45].Pluckthun A, Dennis EA. Acyl and phosphoryl migration in lysophospholipids: importance in phospholipid synthesis and phospholipase specificity. Biochemistry 1982; 21: 1743–1750. doi: 10.1021/bi00537a007 [DOI] [PubMed] [Google Scholar]
- [46].Kielbowicz G, Smuga D, Gladkowski W, Chojnacka A, Wawrzenczyk C. An LC method for the analysis of phosphatidylcholine hydrolysis products and its application to the monitoring of the acyl migration process. Talanta 2012; 94: 22–29. doi: 10.1016/j.talanta.2012.01.018 [DOI] [PubMed] [Google Scholar]
- [47].Subbaiah PV, Liu M, Paltauf F. Role of sn-2 acyl group of phosphatidyl choline in determining the positional specificity of lecithin-cholesterol acyltransfersae. Biochemistry 1994; 33: 13259–13266. [DOI] [PubMed] [Google Scholar]
- [48].Liu M, Bagdade JD, Subbaiah PV. Specificity of lecithin: cholesterol acyltransferase and atherogenic risk. Comparative studies on the plasma composition and in vitro synthesis of cholesteryl esters in 14 vertebrate species. J Lipid Res 1995; 36: 1813–1824. [PubMed] [Google Scholar]
- [49].Rl Chouinard-Watkins, Lacombe RJS Metherel AH, Masoodi M Bazinet RP. DHA Esterified to Phosphatidylserine or Phosphatidylcholine is More Efficient at Targeting the Brain than DHA Esterified to Triacylglycerol. Molecular Nutrition & Food Research 2019; 63: 1801224. doi: 10.1002/mnfr.201801224 [DOI] [PubMed] [Google Scholar]
- [50].Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bekdash RA. Choline and the Brain: An Epigenetic Perspective In: Essa MM, Akbar M, Guillemin G (eds.), The Benefits of Natural Products for Neurodegenerative Diseases. Cham: Springer International Publishing; 2016: 381–399. [Google Scholar]
- [52].Aiyar N, Disa J, Ao Z, Ju H, Nerurkar S, Willette RN, Macphee CH, Johns DG, Douglas SA. Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Molecular and Cellular Biochemistry 2007; 295: 113–120. [DOI] [PubMed] [Google Scholar]
- [53].Sevastou I, Kaffe E, Mouratis MA, Aidinis V. Lysoglycerophospholipids in chronic inflammatory disorders: The PLA2/LPC and ATX/LPA axes. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2013; 1831: 42–60. [DOI] [PubMed] [Google Scholar]
- [54].Huang L, Hung N, Sok DE, Kim M. Lysophosphatidylcholine Containing Docosahexaenoic Acid at the sn-1 Position is Anti-inflammatory. Lipids 2010; 45: 225–236. [DOI] [PubMed] [Google Scholar]
- [55].Hung ND, Kim MR, Sok DE. Oral administration of 2-docosahexaenoyl lysophosphatidylcholine displayed anti-inflammatory effects on zymosan A-induced peritonitis. Inflammation 2011; 34: 147–160. 10.1007/s10753-010-9218-z [doi] [DOI] [PubMed] [Google Scholar]
- [56].Hung ND, Sok DE, Kim MR. Prevention of 1-palmitoyl lysophosphatidylcholine-induced inflammation by polyunsaturated acyl lysophosphatidylcholine. Inflamm Res 2012; 61: 473–483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.