Abstract
Human offspring encounter high amounts of phytoestrogens, such as genistein (GEN), through maternal diet and soy-based formulas. Such chemicals can exert estrogenic activity and thereby disrupt neurobehavioral programming. Besides inducing direct host effects, GEN might cause gut dysbiosis and alter gut metabolites. To determine whether exposure to GEN affects these parameters, California mice (Peromyscus californicus) dams were placed two weeks prior to breeding and throughout gestation and lactation on a diet supplemented with GEN (250 mg/kg feed weight) or AIN93G phytoestrogen-free control diet (AIN). At weaning, offspring socio-communicative behaviors, gut microbiota and metabolite profiles were assayed. Exposure of offspring to GEN induced sex-dependent effects on gut microbiota and metabolites. GEN exposed females were less likely to investigate a novel female mouse when tested in a three-chamber social test. When isolated, GEN males and females exhibited increased latency to elicit their first call, suggestive of reduced motivation to communicate with other individuals. Correlation analyses revealed interactions between GEN-induced microbiome, metabolome, and socio-communicative behaviors. For instance, comparison of GEN males with AIN males revealed the fraction of calls above 20 kHz was associated with daidzein, α-tocopherol, Flexispira spp., and Odoribacter spp. Results suggest early GEN exposure disrupts normal socio-communicative behaviors in California mice, which are otherwise evident in these social rodents. Such effects may be due to GEN disruptions on neural programming but might be attributed to GEN-induced microbiota shifts and resultant changes in gut metabolites. Findings indicate cause for concern that perinatal exposure to GEN might detrimentally affect offspring microbiome-gut-brain axis.
Keywords: Phytoestrogens, Brain, Intestinal bacteria, Diet, Xenoestrogens, Rodent models, Bioinformatics, Autism, ASD
Introduction
Many infants that are fed soy-based formulas are also exposed to plant phytoestrogens/ isoflavones, including genistein (GEN). Additionally, fetuses can be exposed to GEN and other phytoestrogens through the maternal diet. Such chemicals can act similarly to estradiol (E2) and presumably disrupt normal developmental processes, including gonad and brain sexual differentiation. Thus, animal model and human epidemiological studies have been initiated to examine how this chemical affects neonatal development, especially for the reproductive and neurobehavioral systems (Dinsdale and Ward 2010). Early exposure to isoflavones in soy formula is associated with adverse female reproductive consequences later in life (D’Aloisio, et al. 2013; Jefferson and Williams 2011; Upson, et al. 2016). Girls exposed during infancy to soy formula show reduced female-typical play behavior, but comparable disruptions are absent in boys (Adgent, et al. 2011). A potential linkage between feeding infant soy formula and subsequent risk for autistic behaviors has been reported (Westmark 2013). Similarly, animal model studies have reported evidence that early exposure to GEN can lead to reproductive (Cimafranca, et al. 2010; Jefferson, et al. 2007) and behavioral abnormalities such as increased defensive behaviors and demasculinization in male mice (Wisniewski, et al. 2005). Developmental exposure of rodents to GEN is associated with increased anxiety and aggressive behaviors, along with altered brain development (Rodriguez-Gomez, et al. 2014; Santti, et al. 1998).
In pigs, a soy formula diet affects the intestinal epithelial lining, microbial populations, and intestinal epithelial barrier as well as anti-inflammatory markers (Yeruva, et al. 2016). Rodent and human studies suggest that GEN or soy based foods can alter the gut microbiome (Bai, et al. 2016; Cross, et al. 2017; Fernandez-Raudales, et al. 2012; Nakatsu, et al. 2014; Paul, et al. 2017b; Piacentini, et al. 2010; Smith-Brown, et al. 2016; Wu, et al. 2016). Soy-fed neonatal White Dutch Landrace pigs show linkages between diet-responsive intestinal metabolites and gut microbes (Piccolo, et al. 2017). In a human study, the plasma metabolome of vegans living in a Western society and consuming a soy-rich diet differed from that of omnivores, whereas the gut microbial profile between the two groups of individuals was relatively similar (Wu et al. 2016). Other studies in mice have shown that direct consumption of GEN and exposure during pre- or post-natal life is associated with gut microbiota changes, and these alterations are associated with metabolic and cognitive changes (Huang, et al. 2018; Lopez, et al. 2018; Zhou, et al. 2018). Metabolites produced by gut microbes may influence host function (Dodd, et al. 2017; Rosenfeld 2015; Schugar, et al. 2017; van de Wouw, et al. 2017; Zheng, et al. 2017; Zhou and Fang 2018). However, these above studies examining effects of GEN on the gut microbiome did not examine changes in host or microbial metabolites.
It is increasingly being recognized that the gut microbiome through production of metabolites and other potential mechanisms can affect the host’s neurobehavioral state, which has been coined the microbiome-gut-brain axis (Borre, et al. 2014; Clarke, et al. 2013; Rosenfeld 2015; Stilling, et al. 2014). The first evidence associating gut microbiota disturbances and neurobehavioral disorders originated from germ-free (GF) mice that lack a resident gut microbiome. Such mice demonstrate disruptions in the hypothalamic-pituitary-adrenal gland (HPA) axis that is attenuated with bacterial reconstitution or fecal transplantation (Sudo, et al. 2004). GF mice are more anxious, less exploratory, and show cognitive and social deficits (Clarke et al. 2013; Desbonnet, et al. 2014; Diaz Heijtz, et al. 2011; Gareau, et al. 2011; Neufeld, et al. 2011).
Based on past studies, we hypothesized that developmental exposure to GEN might affect the gut microbiome, metabolome and socio-communicative behaviors either directly or indirectly via the microbiome-gut-brain axis. To test this hypothesis, California mice (Peromyscus californicus), who are generally quite social and monogamous, were developmentally exposed to GEN or a phytoestrogen free control diet and the gut microbiota, metabolome, and socio-communicative behaviors were examined at weaning. We chose this species over conventional inbred mouse strains to replicate the genetic diversity present in most human populations.
Materials and methods
Animals and treatments
Adult (60–90 days of age) California mouse females and males, free of common rodent pathogens, were purchased from the Peromyscus Genetic Stock Center (PGSC) at the University of South Carolina (Columbia, SC). After shipment to the University of Missouri, they were placed in quarantine, along with sentinel mice, for a minimum of 8 weeks to ensure that they did not carry any transmittable and zoonotic diseases. No diseases have been identified to date in any sentinel or colony animals. Once the animals were deemed disease-free, they were then moved to the Animal Sciences Research Center (ASRC). At this facility, we currently have our own breeding colony established. Additional animals have been purchased from the PGSC to maintain the outbred status of the line and similar procedures were followed as listed above. All experiments were approved by the University of Missouri Animal Care and Use Committee (Protocol #8693) and performed in accordance with the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
To reduce any background bisphenol A (BPA) exposure, weaned animals were housed either in polystyrene (for breeder pairs) or polypropylene cages (F1 offspring, Allentown, NJ), provided glass water bottles, and BPA-free water. Two weeks prior to breeding, virgin females, 8 to 12 wks of age were randomly assigned to receive one of two diets: 1) a low phytoestrogen AIN 93G diet supplemented with 7% by weight corn oil (as a main fatty acid source) rather than soy oil to minimize potential phytoestrogenic contamination (control) or 2) the same AIN93G diet supplemented with 250 mg/kg GEN, which has previously been shown to affect various parameters in mice (Dolinoy 2006; Dolinoy, et al. 2007; Rosenfeld, et al. 2013). This amount of GEN results in similar circulating concentrations of genistein as in humans consuming soy-enriched diets (Fritz, et al. 2002). Upon weaning (30 days of age) all animals were placed on the AIN diet. This approach was used to replicate the maternal diet exposure of fetuses and neonates to GEN, which can then be transmitted across the placenta and in the milk, respectively. Maternal GEN supplementation was continued until pups were weaned as brain development extends throughout the post-natal period in rodents (Howdeshell 2002; Rice and Barone 2000).
One male and one female offspring from each litter were selected for subsequent analyses. For all analyses, samples from the same individuals were examined to allow for correlation analyses to be performed. Number of replicates included: 5 AIN females, 5 GEN females, 3 AIN males, and 3 GEN males. To determine how many replicates were needed to detect significant differences, especially for the metabolome and microbiome portions of the study, we consulted previous studies in this area beforehand (Javurek, et al. 2016; Okamoto, et al. 2018; Paul, et al. 2017a; Rock, et al. 2018). Moreover, we also did our own Power Analysis with SAS Power and Sample Size application (PSS, SAS analysis, Cary, NC) that verified this number of replicates should be sufficient to detect differences. Based on our previous microbiome and metabolome studies (Javurek et al. 2016; Rosenfeld, et al. 2018; Vieira-Potter, et al. 2018), we also sought to test all samples at the same time to avoid any potential confounding batch effects.
Social behavior testing
Upon weaning (PND30), the pups underwent a sociability and preference for social novelty three-chambered test, designed to identify social deficits (Moy, et al. 2004). We chose this time period to elucidate the potential immediate effects perinatal exposure to GEN might exert on socio-communicative behaviors with deficits in these traits considered one type of autistic-like behaviors in rodent models of autism (Crawley 2007; Silverman, et al. 2010). With the interest as to whether xenoestrogens and endocrine disrupting chemicals as a whole are associated with increased risk of autism in children (Braun, et al. 2014; Schug, et al. 2015), we also sought to test the same life period as to when such disorders are commonly diagnosed in children. The three-chambered test is routinely used to examine for social behavioral deficits in weanling and older mice (Brielmaier, et al. 2014; Ey, et al. 2012; Ryan, et al. 2010; Yang, et al. 2007).
One of the goals of the current project was to also to determine if associations existed between GEN-induced gut microbiota changes (experimental detailed below) and behavioral alterations. Past studies have shown that gut microbiota changes induced by other maternal environmental factors are strongly associated with social behavioral deficits (Bharwani, et al. 2017; Buffington, et al. 2016; Sgritta, et al. 2019). Buffington et al (2016) also established causation of maternal high fat diet (MHFD)-induced gut microbiota changes and social deficits in offspring by supplementing this group of offspring with a single bacterium, Lactobacillus reuteri, that abolished the social deficits and neuropathological changes induced by MHFD. Further, this group confirmed that MHFD-induced gut microbiota changes led offspring social impairments by performing fecal microbial transfer from MHFD mice to germ-free mice not exposed to this in utero change, which resulted in social deficiencies in the recipient mice that recapitulated those seen in donor individuals exposed to the MHFD. With an interest as to whether developmental exposure to GEN-induced gut microbiota may be linked with neurobehavioral changes, we chose to examine socio-communication behaviors that have already proven vulnerable to shifts in gut microbiota resulting from other in utero manipulations.
The test uses a three-chambered apparatus in which openings between the chambers allow the animal to move throughout the apparatus for the duration of the test. The outer two chambers contain wire mesh cups to hold the “stranger” mice, either stranger 1 or stranger 2. The stranger mice originated from different litters than that of the test animal and were of the same sex, approximate age, and treatment group as the test individual. For Trial 1 (habituation trial), the left and right chambers were empty. The pup was placed in the center chamber and video-recorded for a 5-minute acclimation period using a Logitech Carl Zeiss Tessar HD 1080P (Newark, CA) camera mounted onto a Joby- Gorilla Pod Original Tripod (Daymen US Inc., Petaluma, CA). The pup was then removed, and the two stranger mice were placed under their respective cups for a 5-minute acclimation period. For Trial 2, a stranger mouse (stranger 1) was placed on the right side, and the left side contained an empty cup. The test mouse was then placed in the center chamber and recorded for 10 minutes. For Trial 3, the previous mouse (stranger 1) was again placed on the right side, and a novel mouse (stranger 2) was placed on the left side. The test mouse was again placed in the center chamber and recorded for 10 minutes.
For these tests, we opted to use conspecific stranger mice, i.e., those developmentally exposed to the same maternal treatment, to avoid potential confounding effects that could originate due to stranger individuals from different treatment groups being more or less dominant than the experimental animal. In other words, by mixing experimental and stranger mice, any potential social deficits might also be attributed to aversion of the experimental mouse to interact with strangers of different perinatal exposure background, which is of particular concern in California mice where social dominance can be influenced by extrinsic factors (Rosenfeld and Trainor 2014). Between each trial, all animals were returned to their cages and the apparatus was cleaned with a 70% ethanol solution. Data collected from this test were analyzed using Observer software version 11.5 (Noldus, Leesburg, VA), and the animal’s location in the three chambered test was coded along with behaviors such as rearing, grooming, and nose-to-nose contact with stranger 1 mouse (trials 2 and 3) and stranger 2 mouse (trial 3).
Ultrasonic vocalization measurements
Immediately following the sociability test, the animal was placed in a clean, empty cage and transferred to a polypropylene box containing polypropylene panels lined with 2 inches of convoluted acoustic foam paneling (Soundproof Cow, Chambersburg, PA). Audio was recorded for 5 minutes using an Avisoft Bioacoustics CM16/CMPA40–5V microphone (Glienicke, Germany) plugged into a National Instruments USB 6351 data collection board which was further connected to a Dell OptiPlex 7010 (Dell Incorporated, Roundrock, TX). Data were collected using a custom code written by the laboratory of Dr. Katrin Schenk at Randolph College using MATLAB software (MathWorks, Natick, MA) as detailed previously (Johnson, et al. 2018; Johnson, et al. 2017). These recordings capture audible calls as well as ultrasonic vocalizations (USVs) that are above 20 kHz and out of the hearing range for humans. Vocalizations were segmented (separated from the background noise) and the number of syllables, syllable durations, syllable’s median frequencies, average syllable power, and power percent below and above 20 kHz were determined using the analysis program (MATLAB software) designed by Dr. Katrin Schenk.
Collection of fecal samples and isolation of fecal microbial DNA
After the behavioral tests were completed, each animal was placed in a cage alone without any bedding. Four to five fecal boli were collected from each animal and placed in a 7 ml polypropylene vial (Fisher Scientific, St. Louis, MO). As these animals are part of a larger ongoing study to examine for potential longstanding social behavioral deficits, cognitive impairments, and anxiogenic behaviors throughout the lifespan, they were not euthanized at the completion of these experiments. Thus, brain, intestine, and terminal blood samples were not collected at this time.
The fecal microbial DNA was isolated using the PowerFecal DNA Isolation kit (Mo Bio Laboratories, Inc., Carlsbad, CA) according to the manufacturer’s protocol. The quantity of DNA isolated was measured using Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY). The number of replicates (n = 3 to 5 per group) tested is comparable to that used in other maternal diet and offspring gut microbiome studies that showed that such sample sizes can result in statistical differences between offspring groups (Ma, et al. 2014; Thorburn, et al. 2015).
16S rRNA sequencing
The University of Missouri DNA Core Facility prepared bacterial 16S ribosomal DNA amplicons from extracted fecal DNA by amplification of the V4 hypervariable region of the 16S rDNA with universal primers (U515F/806R) flanked by Illumina standard adapter sequences (Caporaso, et al. 2011; Walters, et al. 2011). Universal primer sequences are available at proBase (http://www.microbial-ecology.net/probebase/) (Loy, et al. 2007). A forward primer and reverse primer employing unique, dual indexes were used in each PCR reaction. PCR reactions (50 μl) contained 100 ng of genomic DNA, forward and reverse primers (0.2 μM each), dNTPs (200 μM each), and Phusion High-Fidelity DNA Polymerase (1U). PCR amplification was performed as follows: 98 °C (3:00) + [98 °C (0:15) + 50 °C (0:30) + 72 °C (0:30)] × 25 cycles +72 °C (7:00). Amplified product (5 F06Dl) from each PCR reaction was combined and thoroughly mixed to prepare a single pool. Pooled amplicons were then purified by addition of Axygen AxyPrep MagPCR Clean-up beads (50μl) to an equal volume of 50 μl of the amplicon library pool and incubated at room temperature for 15 minutes. Products were placed on a magnetic stand for 5 minutes and supernatant (95 μl) removed and discarded. Each well was washed by addition of 200 μl of freshly prepared 80% EtOH, incubated at room temperature for 30 seconds, and supernatant removed. Wash steps were repeated once and the plate was allowed to dry on the magnetic stand for 15 minutes. The dried pellet was resuspended in Qiagen EB Buffer (32.5 μl), incubated at room temperature for 2 minutes, and then placed on the magnetic stand for 5 minutes. The supernatant (30 μl) was transferred to a low binding microcentrifuge tube for storage. The final amplicon pool was evaluated using the Advanced Analytical Fragment Analyzer automated electrophoresis system, quantified with a Qubit flourometer using a quant-iT HS dsDNA reagent kit (Invitrogen), and diluted according to Illumina’s standard protocol for sequencing on the MiSeq.
Bioinformatics and amplicon analyses
Paired-end Illumina MiSeq DNA reads were joined using join_paired_ends.py and combined using add_qiime_labels.py from QIIME 1.9.1 (Magoc and Salzberg 2011). Uclust (Edgar 2010) was used to clean contigs and remove those with E > 0.5, (http://drive5.com/usearch/manual/exp_errs.html). Contigs were clustered to 97% identity against DNA sequences in the Greengenes database (DeSantis, et al. 2006), version 13_8, using the QIIME (Caporaso, et al. 2010), version 1.9.1, script pick_open_reference_otus.py, which obviates chimera and PCR error detection with all reads clustered. After OTU picking, we filtered out OTUs with less than two observation counts using the script filter_otus_from_otu_table.py. For alpha-diversity in F1 samples, Chao1 (species richness), Shannon (species diversity) values and rarefaction matrices were calculated and plotted using the alpha_rarefaction.py script in the Qiime package (Caporaso et al. 2010). Measurements of beta-diversity were facilitated by the QIIME script beta_diversity.py and jackknifed_beta_diversity.py with PCoA plots generated. Visualization of taxonomy bar-charts were generated using summarize_taxa_through_plots.py from QIIME packages. Permutational multivariate analysis of variance (PERMANOVA) was used to examine for significant different between groups.
For the subsequent differential abundant OTU and taxonomy analysis, we used the DESeq2 (Love, et al. 2014) and negative binomial Wald test using scripts differential_abundance.py as implemented in QIIME. We selected significant OTUs based on an adjusted p-value at the 0.05 level mapping to the Greengenes database. Regulation was determined by assigned log2 fold change calculated from differential analysis. With these methods, relevant pairwise comparisons were performed, such as F-GEN vs. F-AIN and M-GEN vs. M-AIN.
Gut metabolome analyses:
Metabolomics analyses were performed with the fecal samples from all individuals, as detailed previously (Deda, et al. 2017) and to perform an assessment of how developmental exposure to GEN affects gut bacterial metabolite profiles. An alkane mix was used for quality control (QC) samples and to validate the instrument performance. 10 μl of H2O containing 1μg/μl ribitol (internal standard that was previously validated) and 500μl of 80% methanol were added to 10 ± 0.06 mg of each fecal sample. Samples were vortexed for 5 seconds, sonicated for 15 min shaken for 2 hrs on an orbital shaker at 140 rpms, and finally centrifuged at 13000 g for 15 min. 400 μl of sample volume was collected into a glass vial, dried under a gaseous nitrogen stream, methoximated in pyridine with 40 μl of 15 mg/ml methoxyamine hydrochloride, and then trimethylsilylated with 40 μl MSTFA (N-methyl-N-(trimethyl-silyl)trifluoroacetamide) + 1% TMCS (chlorotrimethylsilane) reagent. The derivatized extracts were analyzed, as described previously (Deda et al. 2017). Metabolic profiling was performed using an Agilent 6890 GC coupled to a 5973N MSD mass spectrometer with a scan range from m/z 50 to 650 (Agilent Technologies, Inc., Santa Clara, CA). Separation was achieved with a temperature program of 80 °C for 2 min, then ramped at 5 °C/min to 315 °C and held at 315 °C for 12 min, a 60 m DB-5MS column (J&W Scientific, 0.25 mm ID, 0.25 um film thickness) and a constant flow of 1.0 ml/min of helium gas. Data were deconvoluted with AMDIS and annotated through mass spectral and retention index matching to our in-house constructed spectra library. The unidentified components were then searched and characterized using spectral matching to a commercial NIST17 mass spectral library. The combined identifications were saved as an. ELU file, and the abundance of the ions were extracted using custom MET-IDEA software (Lei 2012). Abundances were then normalized to the ribitol internal standardand used for statistical comparisons.
Statistical analyses
Behavioral results
Behaviors in the three-chamber social testing and vocalization parameters were analyzed as detailed previously (Johnson et al. 2018; Johnson et al. 2017). Data were analyzed using SAS version 9.4 Software (SAS Institute, Cary, NC). Mean differences were determined using Fisher’s Protected Least Significant Difference (LSD), as described by Steel (Steel 1997). The LSD was only calculated if the overall F test was significant (Chew and United 1977; Saville 1990). All data are presented as actual means (. The error bars for all figures and reported data represent the standard error of the mean (SEM).
Metabolome
Multivariate statistical analyses such as 2D PCA, ANOVA, box plots, and volcano plots were performed with the MetaboAnalyst 3.0 program after data pre-treatments, i.e., normalization to the sum, log transformation and Pareto scaling (http://www.metaboanalyst.ca/). Changes in metabolite abundances were considered statistically significant when their P values were ≤ 0.05. This program was also used to determine the overall metabolite changes in the GEN vs. CON (AIN) groups and pathways predicted to be affected in the GEN male and female offspring. A Venn diagram was generated using http://www.interactivenn.net/ (Heberle, et al. 2015).
Integrative correlation analyses
We used the mixOmics R package (Rohart, et al. 2017) to correlate the bacterial genera changes simultaneously with fecal metabolome and behavioral results, which enabled the integration of the microbiome, metabolome, and mouse behavioral, social and vocalization data. We conducted sparse discriminant analysis with partial least square regression with function ‘block.splsda’. The circos plot was generated using the ‘circosPlot’ function with correlations calculated using the method from González, et al. (González, et al. 2012) and 0.9 correlation was used as the cutoff. It shows together the correlation between each individual features pairs from all four datasets.
Results
Socio-communicative behaviors
As detailed above, F1 male and female California mice were tested in three trials in the three-chamber social test with T1 trial being considered the habituation period. In T2 trial, one stranger mouse placed on one side of the chamber and an empty cup on the other, no treatment differences in location within the apparatus (left, middle, or right) or nose-to-nose contact with Stranger 1 mouse were detected. However, in T3 trial, when a second Stranger mouse was introduced, differences in nose-to-nose contact for females emerged with AIN females predictably spending more time investigating the novel Stranger 2 mouse than Stranger 1 mouse (p = 0.001, Fig. 1). In contrast, GEN-exposed females (F-GEN) spent equal time in this trial with Stranger 1 and Stranger 2 mice, suggesting reduced social behavior or interest in seeking out novel individuals.
Figure 1.
Number of times F1 females engaged in nose-to-nose contact in T3 trial with Stranger 1 and 2 mice. AIN females spent more time investigating a novel, Stranger 2, mouse than previously acquainted mouse, Stranger 1. However, such differences were not observed in GEN-exposed females who spent equal time in this trial in contact with Stranger 1 and Stranger 2 mice, suggestive of reduced social behaviors. *p =0.001 based on time spent with Stranger 2 mouse for AIN females vs. GEN females.
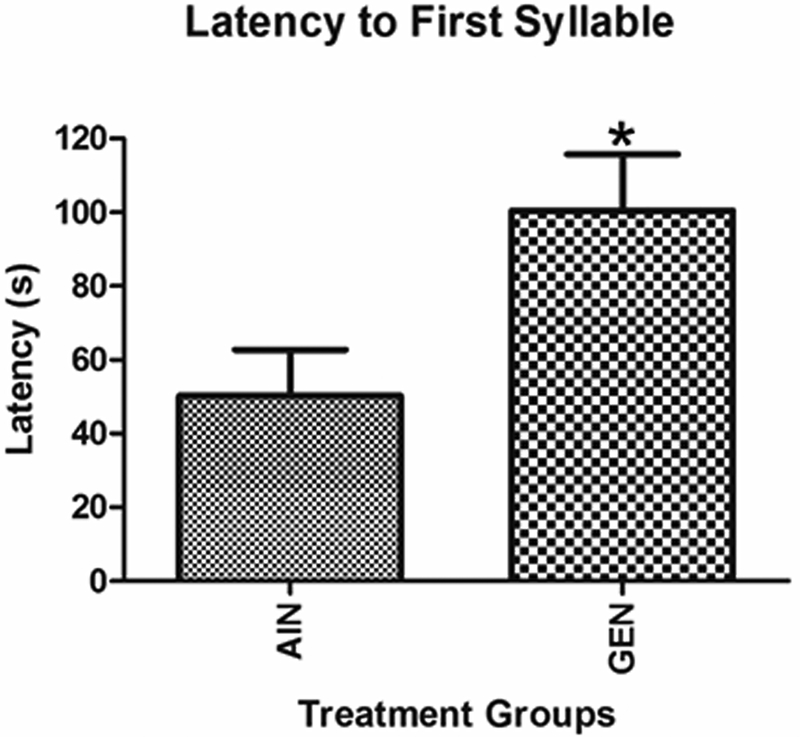
For vocalization responses, a significant main effect based on maternal treatment was detected for latency to exhibit first call (p ≤ 0.05), but no significant interactive effects for maternal treatment X sex were observed. Thus, for this category, male and female data within each treatment group were combined together. When vocalization responses were measured after the three-chambered social test, F1 GEN exposed individuals took twice as long to elicit their first syllable (call) as AIN control mice (p = 0.03, Fig. 2), supporting the idea that developmental exposure to GEN reduces socio-communicative behaviors. None of the other vocalization parameters differed between the two groups or based on offspring sex.
Figure 2.
Latency to first syllable (call). GEN exposed males and females took twice as long to elicit their first syllable (call) as AIN individuals. *p = 0.03.
Gut microbiota
Based on t-test analyses, no overall differences in α-diversity (chao-1 and Shannon analyses) were observed based on developmental exposure of males or females to GEN (Fig. 3). Bar plots of operational taxonomic units (OTU) in F-AIN, F-GEN, M-AIN, and M-GEN revealed some potential but not overt bacterial differences (Fig. 3, as several bacteria are represented by Fig. 3C, a legend for the various bacteria are provided in Supplementary Figs. 1–4 to allow for each to be legible). Weighted PERMANOVA analyses of the PCA plot revealed significant differences based on GEN exposure and offspring sex (p = 0.003) (Fig. 3D). All four groups are shown in this figure to compare the α- and β-diversity across all groups.
Figure 3.
Measures of α- and β-diversity in AIN and GEN exposed F1 males and females. A and B) Developmental exposure of males and females to GEN did not affect overall differences in species richness (as shown by Chao1 results) and diversity (as shown by Shannon results). The data were plotted using phyloSeq R package plot_richness function (McMurdie and Holmes 2013). C) Bar plot analysis of the most abundant bacterial species in all three treatment groups for F1 males and females. D) 3D PCoA analysis F1 male and female offspring results. Weighted PERMANOVA (p = 0.003). All four treatment groups are included in these figures to allow for comparison of α- and β-diversity across all groups.
Further pairwise examination with DESeq2 (Love et al. 2014) and negative binomial Wald test, revealed that select bacteria were altered based on GEN exposure and offspring sex. Relative amounts of Lactobacillus spp., Ruminococcus flavefaciens, Bacteroidales, and Clostridiales were reduced in GEN-exposed females, whereas, Rikenaceae, Ruminococcaceae, Lachnospiraceae, and Lactococcus spp.were elevated in this group (Supplementary File 1). In males, GEN decreased the relative abundance of Flexispira spp., Clostridiales, Bacteroidales, Odoribacter spp., and Desulfovibrionaceae but increased the relative abundance of Lachnospiraceae and Allobaculum spp (Supplementary File 2).
Gut metabolome
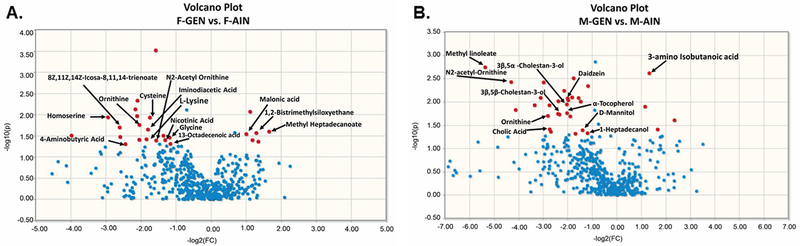
GS-MS data were processed using AMDIS software. Metabolite identifications reported are all level 1 or 2 according to the Metabolomics Standards Initiative and specifically those related to metabolite identification (Sumner, et al. 2007). A total of 528 components were detected and 278 were annotated by searching our in-house and NIST17 MS spectral libraries (Supplementary Files 3 and 4). Two-dimensional (2D) principal component analyses (PCA) revealed male mice developmentally exposed to genistein were separated from the rest of the groups and some separation based on offspring sex (F vs M) and maternal treatment (GEN vs. CON) was evident (Fig. 4). Differential clustering was confirmed by analyzing for significant differences with a Volcano plot for GEN-exposed females vs. AIN control females and GEN-exposed males vs. AIN control males (Fig. 5). The Volcano plot is a combination of fold change and t-test p values. Components with a fold change greater than 2 and a t-test p value less than 0.05 are considered significantly different. We report here p values rather than adjusted p values because while adjusted p value reduces the number of false positives, it reduces the number of true discoveries. Since this is an exploratory experiment, we believe it is appropriate and necessary to use p values in order to avoid missing some significant metabolite differences.
Figure 4.
2D PCA analysis of F1 gut metabolome results. The 2D PCA analysis shows some clustering based on developmental exposure to GEN and offspring sex.
Figure 5.
Volcano plots of metabolites that differ between GEN and AIN females and males. A) This analysis revealed several metabolites that differed between GEN-exposed females and AIN females. B) This analysis revealed several metabolites that differed between GEN-exposed males and AIN males. In each figure, the blue dots represent metabolites that were not significantly different between the two groups, whereas, the red dots represent those that are significantly different between the two groups (p ≤ 0.05). Characterized metabolites are accordingly labeled.
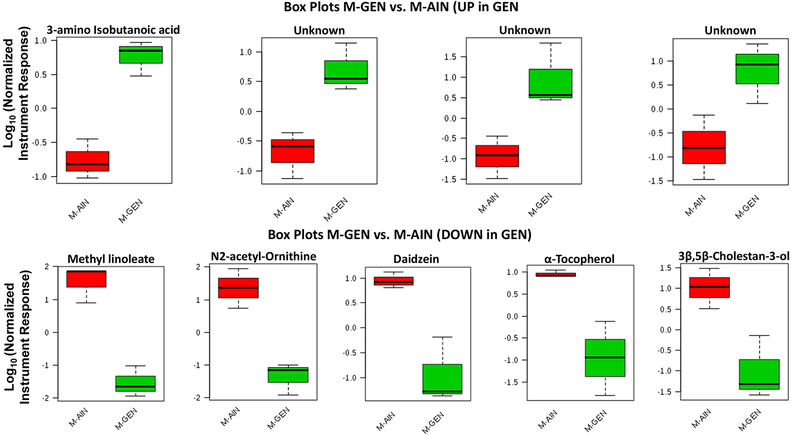
In females, the relative accumulation of several metabolites was altered by developmental exposure to GEN (Supplementary File 5). Malonic acid and methyl heptadecanoate were increased in F-GEN individuals, whereas 4-aminobutyric acid, cysteine, homoserine, and ornithine were decreased in this group (Fig. 6). 3-amino isobutanoic acid was elevated in GEN exposed males (M-GEN), but methyl linoleate, N2-acetyl-ornithine, daidzein, α-tocopherol, and 2β,4β-choestan-3-ol were reduced in this group (Fig. 7). The full list of metabolites altered in M-GEN is detailed in Supplementary File 5.
Figure 6.
Traditional box plots for metabolites elevated in F-GEN vs. F-AIN groups (P < 0.05). The y-axes are log 10 values of the normalized instrument response for the labeled metabolites in F-GEN and F-AIN groups (x-axes). The program arbitrarily assigns color codes for the various groups. The box plot upper and lower brackets represents +/− (1.58*interquartile range-IQR/Squared root of sample size). A complete list of metabolites that differed between these two groups, as well as directionality, is included in Supplementary File 5.
Figure 7.
Traditional box plots for metabolites elevated in M-GEN vs. M-AIN groups (P < 0.05). The y-axes are log 10 values of the normalized instrument response for the labeled metabolites M-GEN vs. M-AIN groups (x-axes). The program arbitrarily assigns color codes for the various groups. The box plot upper and lower brackets represents +/− (1.58*interquartile range-IQR/Squared root of sample size). A complete list of metabolites that differed between these two groups, as well as directionality, is included in Supplementary File 5.
The overview of the significantly changed metabolites can be visualized in Supplementary Fig. 5 showing a number of overlap and unique metabolites that were significantly regulated in each comparison. The data show that very few common metabolites were found to be significantly change based on treatment X sex interactions.
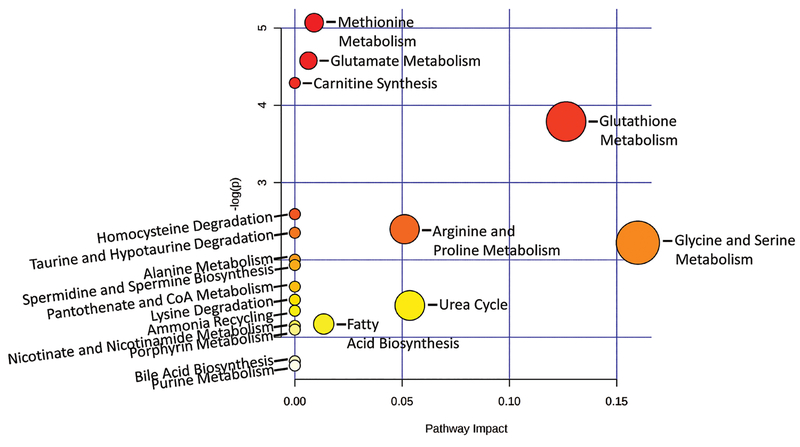
Two pathways significantly affected in F-GEN individuals relative to F-AIN group include methionine and glutamate metabolism and carnitine synthesis (Fig. 8, FDR ≤ 0.05). Supplementary Fig. 6 shows these two pathways and denotes those metabolites within them that were affected by GEN-exposure. After FDR correction, no overall pathways were significantly altered in M-GEN vs. M-AIN.
Figure 8.
Metabolome view of pathways predicted to differ in F-GEN vs. F-AIN groups as determined using Metaboanalyst webserver. For comparison among different pathways, the program calculates the node importance values from a centrality measure, which are further normalized by the sum of the importance pathways. The total or maximum importance of each pathway is 1. The x-axis shows the impact of the set of significantly different metabolites on a pathway and y-axis shows the −log(p) values of this correlation between the set of significantly different metabolites and the pathways.
Integrative correlation analyses between gut microbiota, gut metabolome, and socio-communicative behaviors
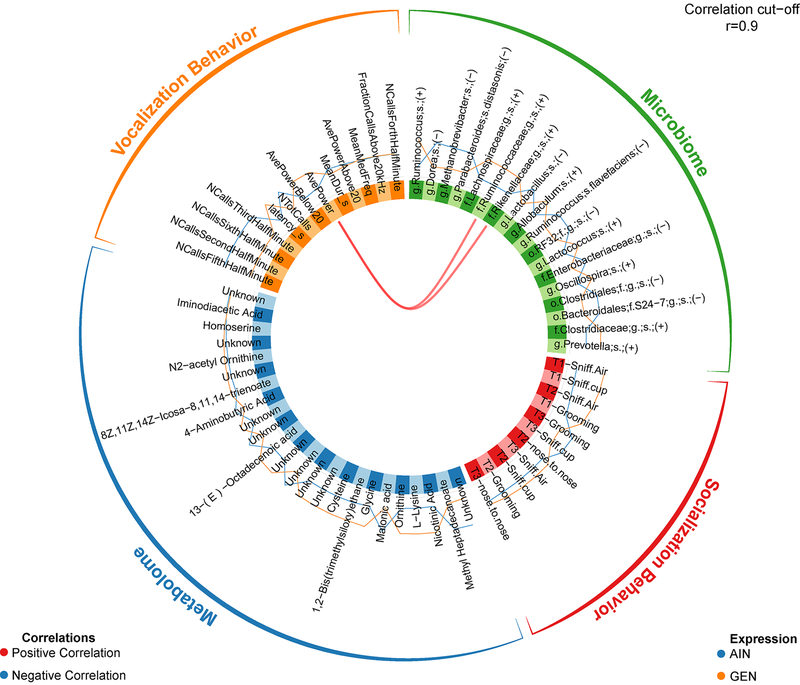
To correlate the gut microbiota changes with gut metabolome and behavioral results, we used the mixOmics R package (Rohart et al. 2017). Four multi-analyses comparisons were tested: F-GEN vs. F-AIN, M-GEN vs. M-AIN, F-GEN vs. M-GEN, and F-AIN vs. M-AIN. Comparison of F-GEN to F-AIN revealed that the only correlations that were significant at 0.9 were average vocalization power positively correlated with Rikenaceae and Ruminococcaceae, both of these bacteria were elevated in F-GEN (Fig. 9). Comparison of M-GEN with M-AIN revealed several significant correlations amongst the gut metabolome, gut microbiome, and behaviors (social and vocalization) at 0.9 (Fig. 10). Some example correlations in GEN exposed males vs. control males include ornithine, 1-heptadecanol, 3β,5β, cholestan-3-ol was associated with Odoribacter spp. and Bacteroidales. 1-heptadecanol was also associated with Flexispira spp. Odoribacter spp., and Flexispira spp. were associated with 3β,5α, cholestan-3-ol and daidzein. All of these bacteria and metabolites were positively associated with fraction of calls above 20 kHz, along with α-tocopherol.
Figure 9.
Circos correlations among taxa altered in fecal microbial community of F-GEN vs. F-AIN individuals and fecal metabolomic changes due to GEN exposure.
Figure 10.
Circos correlations among taxa altered in fecal microbial community of M-GEN vs. M-AIN individuals and fecal metabolomic changes due to GEN exposure.
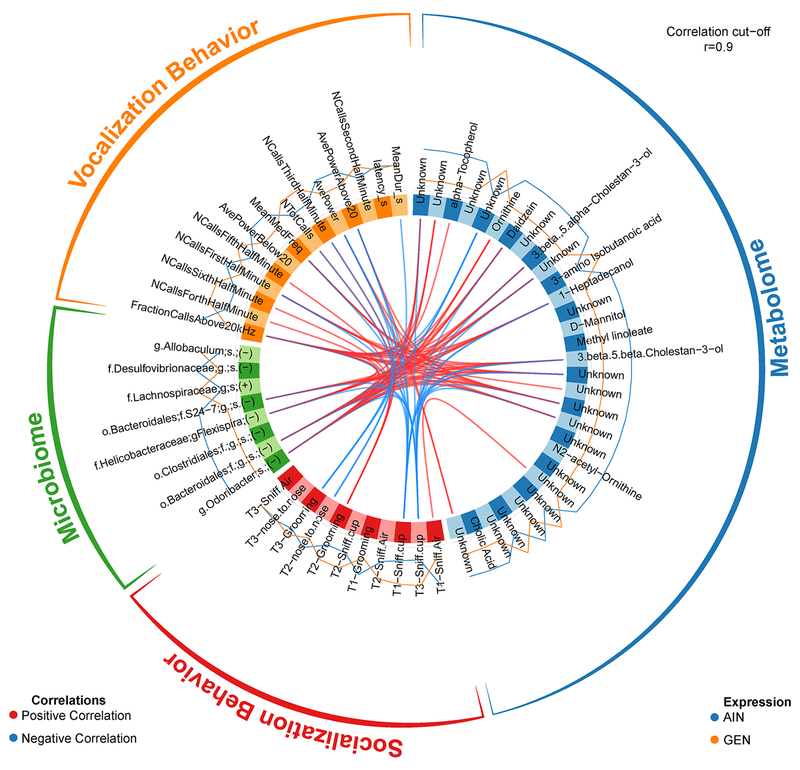
When comparing within GEN and AIN groups, sex differences were evident. Comparison of M-GEN to F-GEN revealed such correlations as valine was associated with Clostridiales and Ruminococcus gnavus (Fig. 11). Cadaverine was associated with Odoribacter spp., Oscillospira spp., and Ruminococcus gnavus. Cholic acid was associated with Rikenellaceae. Total number of calls was associated with cadaverine, Odoribacter spp., Oscillospira spp., and Rumnicoccus gnavus. Latency to produce the first call was associated with cholic acid and Rikenellaceae. Comparison of M-AIN vs. F-AIN revealed fewer significant correlations (Fig. 12). One example was average power above and below 20 kHz was positively associated with alanine. The individual pairwise comparisons for these four analyses are included in Supplementary Figs. 7–10. Supplementary File 6 provides all of the comparisons, and those with correlations ≥ 0.9 are highlighted.
Figure 11.
Circos correlations among taxa altered in fecal microbial community of M-GEN vs. F-GEN individuals and fecal metabolomic changes.
Figure 12.
Circos correlations among taxa altered in fecal microbial community of M-AIN vs. F-AIN individuals and fecal metabolomic changes.
Discussion
The first goal of the current study was to determine whether developmental exposure to GEN induces direct effects on subsequent socio-communicative responses. Secondly, we sought to determine whether early contact with this xenoestrogen might affect gut microbiota, fecal metabolites, and thereby result in indirect CNS effects via the microbiome-gut-brain axis. The current findings support previous human and rodent studies suggesting that phytoestrogens in soy formula and GEN can result in subsequent neurobehavioral disruptions, as we have seen with ethinyl estradiol (estrogen present in birth control pills) and BPA (Jasarevic, et al. 2011; Jasarevic 2013; Johnson, et al. 2015; Williams, et al. 2013). Reduced social behaviors, as evidenced by willingness to interact with a novel mouse, were observed in female California mice developmentally exposed to GEN. GEN-exposed males and females took longer to vocalize when placed in isolation. Current rodent data are similar to human epidemiological reports suggesting that soy infant formula consumption is associated with reduced female-typical play behavior in girls and increases the risk of autistic behaviors (Adgent et al. 2011; Westmark 2013). In male rodents, developmental exposure to GEN increases anxiogenic and aggressive behaviors, in spite of the fact that some reports suggest that this xenoestrogen might disrupt brain masculinization (Rodriguez-Gomez et al. 2014; Santti et al. 1998; Wisniewski et al. 2005). Male rats provided a soy supplemented diet enriched with GEN and daidzein showed similar anxiogenic responses and engaged in less social interactions (Hartley, et al. 2003). Japanese quail (Coturnix japonica) exposed in ovo to GEN showed compromised mating behaviors in adulthood (Viglietti-Panzica, et al. 2007). Our current studies are unique in that we sought to determine whether GEN affects the microbiome-gut-brain axis.
Such behavioral changes might be due to direct effects on the brain, especially the hypothalamus. Early exposure to GEN has been reported to cause vacuolization of oxytocin neurons in the neonatal mouse hypothalamus (Yoshimura, et al. 2011). GEN may alter brain nitrergic and vasopressinergic circuitry systems in rodents and Japanese quail (Ponti, et al. 2017; Scallet, et al. 2003; Viglietti-Panzica et al. 2007). The gonadotropin releasing hormone network and hypothalamic-pituitary-adrenal axis might also be affected by GEN exposure (Arispe, et al. 2013; Levy, et al. 1995; Medigovic, et al. 2012; Mueller and Heger 2014; Trifunovic, et al. 2012; Wojcik-Gladysz, et al. 2005). While socio-communicative deficits observed in GEN-exposed California mice might be attributed to changes in these hypothalamic pathways, such effects could be due to gut dysbiosis and shifts in gut metabolites.
Developmental exposure to GEN led to sex-dependent gut microbiota changes even though xenoestrogen did not affect the overall species richness and diversity. Our expectation was that GEN exposure would lead to similar gut microbiota changes as we previously identified in ethinyl estradiol (EE) exposed female California mice (Javurek et al. 2016). However, the bacterial changes identified in GEN-exposed individuals did not resemble the profile identified in EE-exposed individuals. Instead, select bacteria showed the opposite pattern with the relative amounts of Rikenellaceae and Prevotella spp. increased in GEN-exposed females but reduced in EE-exposed females. Similarly, we previously found that exposure to bisphenol A led to a unique signature pattern of bacteria in male and female California mice (Javurek et al. 2016). A previous study in mice developmentally exposed to GEN showed that members of the Rikenellaceae family were increased by this phytoestrogen (Zhou et al. 2018), although this study did not separate offspring based on sex. Another study reported that perinatal exposure to GEN increased Enterobacteriales in PND 90 female offspring (Huang et al. 2018). This bacterial order was not affected in the current studies with PND 30 offspring.
Previous studies in animals and humans consuming soy products or exposed to GEN indicate that the gut bacterial changes might depend upon type of phytoestrogen exposure, host species examined, and time and duration of exposure to phytoestrogens (Bai et al. 2016; Cross et al. 2017; Fernandez-Raudales et al. 2012; Nakatsu et al. 2014; Paul et al. 2017b; Piacentini et al. 2010; Piccolo et al. 2017; Smith-Brown et al. 2016; Wu et al. 2016; Yeruva et al. 2016). GEN-induced changes in gut microbes might alter host responses, as shown previously (Cross et al. 2017). Rats provisioned with a phytoestrogen-rich diet demonstrated changes in fecal microbiota that were positively associated with improved cardiometabolic outcomes (Cross et al. 2017). In the current studies, GEN-induced gut microbe changes showed sex-dependent associations with socio-communicative behaviors. However, such correlations only indicate that these changes track together; they do not indicate causation. To establish causation that bacterial changes due to GEN exposure result in sociocommunicative alterations, fecal microbial transfer from GEN-exposed individuals into germ-free mice that lack resident gut microbiota and who have not been exposed to GEN would be needed, as was done for fecal samples from offspring exposed to MHFD to germ-free mice with germ-free recipients then developing similar social deficits as the donor mice (Buffington et al. 2016). Unfortunately, our current animal facility does not have the ability to maintain germ-free mice. Thus, we are currently exploring such studies through potential collaborations with those at other Institutes who have a germ-free mouse facility.
By altering the gut microbiota, GEN likely affects gut metabolome profiles. Soy-fed neonatal White Dutch Landrace pigs show linkages between diet-responsive intestinal metabolites and gut microbes (Piccolo et al. 2017). However, the plasma metabolome of vegans living in a Western society and consuming a soy-rich diet differed from that of omnivores, whereas the gut microbial profile between the two groups of individuals was surprisingly similar (Wu et al. 2016). In female California mice, GEN exposure predominantly decreased several metabolites, including 4 (gamma)-aminobutyric acid (GABA), cysteine, homoserine, ornithine, and glycine. Deficiencies in GABAergic synaptic pathways are linked with mouse models of autism and other human neurobehavioral disorders (Chau, et al. 2017; Han, et al. 2012; Politte, et al. 2014; Tso, et al. 2015). Methionine and glutamate metabolism pathways might be disrupted in GEN-exposed females. Disturbances in glutamate metabolism are associated with autism and autistic like behaviors in animal models of this disorder (Bristot Silvestrin, et al. 2013; Fung and Hardan 2015; Wei, et al. 2015). Several discrete metabolites were up- (such as 3-amino-isobutanoic acid) and down-regulated (e.g. methyl linoleate, N2-acetyl-ornithine, daidzein, α-tocopherol, and 3β,5β-choesetan-3-ol) in males.
Importantly, the current studies are the first to show clear sex-dependent linkages between gut metabolites altered in response to developmental exposure to GEN and subsequent socio-communicative disorders. In our recent work, we similarly determined that SOY-induced gut metabolite shifts correlated with host cardiometabolic outcomes (Vieira-Potter et al. 2018). As with the correlations between gut microbes and neurobehavioral responses, the conclusion that changes in select metabolites cause subsequent neurobehavioral alterations cannot be definitively drawn. To establish causation, metabolites identified to be enriched with GEN exposure would need to be administered to control dams and offspring neurobehavioral responses examined throughout the lifespan. Such a post-metabolite approach was done with 4-ethylphenylsulfate (4-EPS), which triggered autistic-like behaviors in control mice (Hsiao, et al. 2013).
Using a multi-Omics approach, we were able to integrate the gut microbiota, metabolome, and behavior results and show sex-dependent inter-relationships between these three categories. For instance, GEN males had reductions in Bacteroidales, and this bacterium was positively associated with 1-heptadecanol and 3μ,5μ, cholestan-3-ol. All of these factors, along with other bacteria and metabolites, were positively associated with aspects of socio-communication behaviors.
Potential limitations of this study include the fact only correlation analysis can be between gut microbiota/metabolite changes and behavioral responses. With the systemic approach used, we cannot tease apart the direct effects GEN might have on the brain vs. those that are due to alterations in gut microbiota/metabolites. This approach was chosen to replicate how offspring are likely exposed to GEN during the pre- and post-natal periods. To disentangle neurobehavioral effects directly due to GEN exposure vs. those mediated by the gut microbiome-metabolome, intracerebroventricular (ICV) injection with radioactive labeled GEN might be performed in neonatal animals, as has been done for other estrogenic compounds (Dominguez-Ordonez, et al. 2016; Dominguez-Ordonez, et al. 2015; Frankiensztajn, et al. 2018). This method is likely, however, to be quite challenging to do for individual fetuses. Radioactive labeled GEN might also be administered via the maternal diet to better trace the systemic distribution of this compound in the offspring.
As the animals were not collected at the end of these current studies, sufficient blood for plasma metabolome studies could not be collected. However, bacterial metabolites might be transmitted to the brain both through the circulatory system and vagal nerve conduction (Bercik, et al. 2011; Bonaz, et al. 2018; Jaglin, et al. 2018; Liu 2017; Petra, et al. 2015; Rosenfeld 2015). Gut microbiota changes are often associated with intestinal system pathologies, including increased gut permeability and influx of inflammatory cells that could also impact the brain (Rosenfeld 2015). Because animals were not euthanized at the completion of these current studies, no assessments could also be made on how developmental exposure to GEN affects the host gastrointestinal system. Future work will explore for such GEN-induced alterations.
Conclusions
Our study is the first to show that developmental exposure to GEN leads to socio-communicative deficits in California mice, especially in females. While such effects might be due to direct disruptions on the neural circuitry, especially in the hypothalamus, the observed changes might also be due to GEN stimulated alterations in the microbiome-gut-brain axis. Multi-integrative correlation analyses revealed several interactions between GEN-induced microbiome, metabolome, and resulting changes in socio-communicative behaviors. Further work is needed to establish how much of the observed neurobehavioral changes are due to direct effects of GEN compared to those attributed to gut dysbiosis/shifts in gut metabolite profiles. Usage of germ-free mice and post-biotic supplementation of GEN-induced metabolites will be useful in teasing apart these diverging mechanisms. Probiotic and post-biotic supplementation approaches might be used to prevent or remediate behavioral disturbances due to early GEN exposure.
Supplementary Material
Supplementary Figures 1. Key for the bacterial species listed in Figure 1.
Supplementary Figure 10. Correlation across microbiome, metabolites, social and vocal behavior at component of M-AIN vs. F-AIN individuals, cutoff = 0.9.
Supplementary File 1. List of bacteria that differ between GEN females and AIN females.
Supplementary File 2. List of bacteria that differ between GEN males and AIN males.
Supplementary File 3. List of detected components. The MS data were deconvoluted by using AMDIS to yield a total of 528 components.
Supplementary File 4. List of identified components. The MS data were deconvoluted by using AMDIS and annotated by searching against our custom in-house and NIST17 MS spectral libraries.
Supplementary File 5. List of significantly changed metabolites. The significant metabolites were determined using a volcano plot that combines fold change (FC>2) and t-test (p < 0.05).
Supplementary File 6. Correlations of Circos plots associated with M-GEN vs. F-GEN groups, M-AIN vs. F-AIN groups, M-GEN vs. M-AIN, and F-GEN vs. F-AIN.
Supplementary Figures 2. Key for the bacterial species listed in Figure 1.
Supplementary Figure 3. Key for the bacterial species listed in Figure 1.
Supplementary Figure 4. Key for the bacterial species listed in Figure 1.
Supplementary Figure 5. Venn diagram showing number of overlap and unique metabolites that were significantly regulated in each comparison based on treatment X sex interactions.
Supplementary Figure 6. Pathway maps of methionine and glutamate metabolism. Pathway maps of A) methionine and B) glutamate metabolism, which are the most significant pathways associated F-GEN vs. F-AIN individuals, as identified in the pathway analysis in Figure 8. Compounds in light blue color are not included in the list of significant metabolites, and the ones with a red circle are altered by GEN-exposure.
Supplementary Figure 7. Correlation across microbiome, metabolites, social and vocal behavior at component of F-GEN vs. F-AIN individuals, cutoff = 0.9.
Supplementary Figure 8. Correlation across microbiome, metabolites, social and vocal behavior at component of M-GEN vs. M-AIN individuals, cutoff = 0.9.
Supplementary Figure 9. Correlation across microbiome, metabolites, social and vocal behavior at component of M-GEN vs. F-GEN individuals, cutoff = 0.9.
Acknowledgements
We thank all of the undergraduate research students who helped take care of the mice.
Funding
The studies were supported by NIEHS 1R01ES025547 (CSR). LWS is supported in part by NSF awards NSF awards 1743594, 1340058, 1639618 and 1139489. The University of Missouri, Office of Research provided initial instrumental and continuing personnel funding for the MU Metabolomics Center.
Footnotes
Declaration of interest
The authors declare they have no actual or potential competing financial interests.
Data Availability
Raw and processed metabolomic data are available at https://sumnerlab.missouri.edu/download/ and within the NIH Metabolomics Workbench database:http://www.metabolomicsworkbench.org/.
References
- Adgent MA, Daniels JL, Edwards LJ, Siega-Riz AM & Rogan WJ 2011. Early-life soy exposure and gender-role play behavior in children. Environmental Health Perspectives 119 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe SA, Adams B & Adams TE 2013. Effect of phytoestrogens on basal and GnRH-induced gonadotropin secretion. Journal of Endocrinology 219 243–250. [DOI] [PubMed] [Google Scholar]
- Bai G, Ni K, Tsuruta T & Nishino N 2016. Dietary Casein and Soy Protein Isolate Modulate the Effects of Raffinose and Fructooligosaccharides on the Composition and Fermentation of Gut Microbiota in Rats. Journal of Food Science 81 H2093–2098. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al. 2011. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology and Motility 23 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Bazin T & Pellissier S 2018. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Frontiers in Neuroscience 12 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, Moloney RD, Clarke G, Dinan TG & Cryan JF 2014. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Advances in Experimental Medicine and Biology 817 373–403. [DOI] [PubMed] [Google Scholar]
- Bristot Silvestrin R, Bambini-Junior V, Galland F, Daniele Bobermim L, Quincozes-Santos A, Torres Abib R, Zanotto C, Batassini C, Brolese G, Goncalves CA, et al. 2013. Animal model of autism induced by prenatal exposure to valproate: altered glutamate metabolism in the hippocampus. Brain Research 1495 52–60. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N & Knight R 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl 1 4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau DK, Choi AY, Yang W, Leung WN & Chan CW 2017. Downregulation of glutamatergic and GABAergic proteins in valproric acid associated social impairment during adolescence in mice. Behavioural Brain Research 316 255–260. [DOI] [PubMed] [Google Scholar]
- Chew V & United S 1977. Comparisons among treatment means in an analysis of variance. [Washington: ]: Dept. of Agriculture, Agricultural Research Service. [Google Scholar]
- Cimafranca MA, Davila J, Ekman GC, Andrews RN, Neese SL, Peretz J, Woodling KA, Helferich WG, Sarkar J, Flaws JA, et al. 2010. Acute and chronic effects of oral genistein administration in neonatal mice. Biology of Reproduction 83 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG & Cryan JF 2013. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry 18 666–673. [DOI] [PubMed] [Google Scholar]
- Cross TL, Zidon TM, Welly RJ, Park YM, Britton SL, Koch LG, Rottinghaus GE, de Godoy MRC, Padilla J, Swanson KS, et al. 2017. Soy Improves Cardiometabolic Health and Cecal Microbiota in Female Low-Fit Rats. Scientific Reports 7 9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aloisio AA, DeRoo LA, Baird DD, Weinberg CR & Sandler DP 2013. Prenatal and infant exposures and age at menarche. Epidemiology 24 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deda O, Chatziioannou AC, Fasoula S, Palachanis D, Raikos N, Theodoridis GA & Gika HG 2017. Sample preparation optimization in fecal metabolic profiling. Journal of Chromatography. B: Analytical Technologies in the Biomedical and Life Sciences 1047 115–123. [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P & Andersen GL 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology 72 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG & Cryan JF 2014. Microbiota is essential for social development in the mouse. Molecular Psychiatry 19 146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H & Pettersson S 2011. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America 108 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale EC & Ward WE 2010. Early exposure to soy isoflavones and effects on reproductive health: a review of human and animal studies. Nutrients 2 1156–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, et al. 2017. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551 648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC 2006. Maternal Genistein alters coat color and protects Avy mouse offsping from obesity by modifying the fetal epigenome. Environmental Health Perspectives 114 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D & Jirtle RL 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proceedings of the National Academy of Sciences of the United States of America 104 13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Ordonez R, Garcia-Juarez M, Lima-Hernandez FJ, Gomora-Arrati P, Blaustein JD, Etgen AM & Gonzalez-Flores O 2016. Estrogen receptor alpha and beta are involved in the activation of lordosis behavior in estradiol-primed rats. Hormones and Behavior 86 1–7. [DOI] [PubMed] [Google Scholar]
- Dominguez-Ordonez R, Garcia-Juarez M, Lima-Hernandez FJ, Gomora-Arrati P, Blaustein JD & Gonzalez-Flores O 2015. Sexual receptivity facilitated by unesterified estradiol: Dependence on estrogen and progestin receptors and priming dose of estradiol benzoate. Behavioral Neuroscience 129 777–788. [DOI] [PubMed] [Google Scholar]
- Edgar RC 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 2460–2461. [DOI] [PubMed] [Google Scholar]
- Fernandez-Raudales D, Hoeflinger JL, Bringe NA, Cox SB, Dowd SE, Miller MJ & Gonzalez de Mejia E 2012. Consumption of different soymilk formulations differentially affects the gut microbiomes of overweight and obese men. Gut Microbes 3 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankiensztajn LM, Gur-Pollack R & Wagner S 2018. A combinatorial modulation of synaptic plasticity in the rat medial amygdala by oxytocin, urocortin3 and estrogen. Psychoneuroendocrinology 92 95–102. [DOI] [PubMed] [Google Scholar]
- Fritz WA, Wang J, Eltoum IE & Lamartiniere CA 2002. Dietary genistein down-regulates androgen and estrogen receptor expression in the rat prostate. Molecular and Cellular Endocrinology 186 89–99. [DOI] [PubMed] [Google Scholar]
- Fung LK & Hardan AY 2015. Developing Medications Targeting Glutamatergic Dysfunction in Autism: Progress to Date. CNS Drugs 29 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G & Sherman PM 2011. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60 307–317. [DOI] [PubMed] [Google Scholar]
- González I, Lê Cao K-A, Davis MJ & Déjean S 2012. Visualising associations between paired ‘omics’ data sets. BioData Mining 5 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO & Catterall WA 2012. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DE, Edwards JE, Spiller CE, Alom N, Tucci S, Seth P, Forsling ML & File SE 2003. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 167 46–53. [DOI] [PubMed] [Google Scholar]
- Heberle H, Meirelles GV, da Silva FR, Telles GP & Minghim R 2015. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 16 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. 2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL 2002. A model of the development of the brain as a construct of the thyroid system. Environmental Health Perspectives 110 Suppl 3 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Xu J, Cai D, Chen SY, Nagy T & Guo TL 2018. Exacerbation of Type 1 Diabetes in Perinatally Genistein Exposed Female Non-Obese Diabetic (NOD) Mouse Is Associated With Alterations of Gut Microbiota and Immune Homeostasis. Toxicological Sciences 165 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglin M, Rhimi M, Philippe C, Pons N, Bruneau A, Goustard B, Dauge V, Maguin E, Naudon L & Rabot S 2018. Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Frontiers in Neuroscience 12 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Sieli PT, Twellman EE, Welsh TH, Schachtman TR, Roberts RM, Geary DC & Rosenfeld CS 2011. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proceedings of the National Academy of Sciences of the United States of America 108 11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Williams SA, Vandas GM, Ellersieck MR, Liao C, Kannan K, Roberts RM, Geary DC, Rosenfeld CS 2013. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Hormones and Behavior 63 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA & Rosenfeld CS 2016. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 7 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E & Newbold RR 2007. Disruption of the developing female reproductive system by phytoestrogens: genistein as an example. Molecular Nutrition & Food Research 51 832–844. [DOI] [PubMed] [Google Scholar]
- Jefferson WN & Williams CJ 2011. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reproductive Toxicology 31 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Farrington MJ, Murphy CR, Caldo PD, McAllister LA, Kaur S, Chun C, Ortega MT, Marshall BL, Hoffmann F, et al. 2018. Multigenerational effects of bisphenol A or ethinyl estradiol exposure on F2 California mice (Peromyscus californicus) pup vocalizations. PloS One 13 e0199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Javurek AB, Painter MS, Peritore MP, Ellersieck MR, Roberts RM & Rosenfeld CS 2015. Disruption of parenting behaviors in California Mice, a monogamous rodent species, by endocrine disrupting chemicals. PloS One 10 e0126284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Painter MS, Javurek AB, Murphy CR, Howald EC, Khan ZZ, Conard CM, Gant KL, Ellersieck MR, Hoffmann F, et al. 2017. Characterization of vocalizations emitted in isolation by California mouse (Peromyscus californicus) pups throughout the postnatal period. Journal of Comparative Psychology 131 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Li H, Chang J, Zhao PX, Sumner LW 2012. MET-IDEA version 2.06; improved efficiency and additional functions for mass spectrometry-based metabolomics data processing. Metabolomics 8 105–110. [Google Scholar]
- Levy JR, Faber KA, Ayyash L & Hughes CL Jr, 1995. The effect of prenatal exposure to the phytoestrogen genistein on sexual differentiation in rats. Proceedings of the Society for Experimental Biology and Medicine 208 60–66. [DOI] [PubMed] [Google Scholar]
- Liu B, Qin L, Liu A, Uchiyama S, Ueno T, Li X & Wang P 2010. Prevalence of the equol-producer phenotype and its relationship with dietary isoflavone and serum lipids in healthy Chinese adults. Journal of Epidemiology 20 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT 2017. The microbiome as a novel paradigm in studying stress and mental health. American Psychologist 72 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P, Sanchez M, Perez-Cruz C, Velazquez-Villegas LA, Syeda T, Aguilar-Lopez M, Rocha-Viggiano AK, Del Carmen Silva-Lucero M, Torre-Villalvazo I, Noriega LG, et al. 2018. Long-Term Genistein Consumption Modifies Gut Microbiota, Improving Glucose Metabolism, Metabolic Endotoxemia, and Cognitive Function in Mice Fed a High-Fat Diet. Molecular Nutrition & Food Research 62 e1800313. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W & Anders S 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy A, Maixner F, Wagner M & Horn M 2007 probeBase--an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Research 35 D800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, Blundell P, Alan Harris R, Frias AE, Grove KL, et al. 2014. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nature Communications 5 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR, Hauck L, Jeffrey BM, Elias V, Humphrey A, Nath R, Perrone A & Bermudez LE 2015. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 300 128–140. [DOI] [PubMed] [Google Scholar]
- Magoc T & Salzberg SL 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27 2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie PJ & Holmes S 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One 8 e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medigovic I, Manojlovic-Stojanoski M, Trifunovic S, Ristic N, Milosevic V, Zikic D & Nestorovic N 2012. Effects of genistein on gonadotropic cells in immature female rats. Acta Histochemica 114 270–275. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J & Crawley JN 2004. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain,and Behavior 3 287–302. [DOI] [PubMed] [Google Scholar]
- Mueller JK & Heger S 2014. Endocrine disrupting chemicals affect the gonadotropin releasing hormone neuronal network. Reproductive Toxicology 44 73–84. [DOI] [PubMed] [Google Scholar]
- Nakatsu CH, Armstrong A, Clavijo AP, Martin BR, Barnes S & Weaver CM 2014. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. Journal of Nutrition 9 e108924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J & Foster JA 2011. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and Motility 23 255–264, e119. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Aoki A, Ueda K & Jinno H 2018. Metabolomic analysis uncovered an association of serum phospholipid levels with estrogen-induced mammary tumors in female ACI/Seg rats. Toxicology Letters 288 65–70. [DOI] [PubMed] [Google Scholar]
- Paul B, Royston KJ, Li Y, Stoll ML, Skibola CF, Wilson LS, Barnes S, Morrow CD & Tollefsbol TO 2017a. Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition. PloS One 12 e018ci9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B, Royston KJ, Li Y, Stoll ML, Skibola CF, Wilson LS, Barnes S, Morrow CD & Tollefsbol TO 2017b. Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition 12 e0189756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P & Theoharides TC 2015. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clinical Therapeutics 37 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini G, Peroni D, Bessi E & Morelli L 2010. Molecular characterization of intestinal microbiota in infants fed with soymilk. Journal of Pediatric Gastroenterology and Nutrition 51 71–76. [DOI] [PubMed] [Google Scholar]
- Piccolo BD, Mercer KE, Bhattacharyya S, Bowlin AK, Saraf MK, Pack L, Chintapalli SV, Shankar K, Adams SH, Badger TM, et al. 2017. Early Postnatal Diets Affect the Bioregional Small Intestine Microbiome and Ileal Metabolome in Neonatal Pigs. Journal of Nutrition 147 1499–1509. [DOI] [PubMed] [Google Scholar]
- Pilsakova L, Riecansky I & Jagla F 2010. The physiological actions of isoflavone phytoestrogens. Physiological Research 59 651–664. [DOI] [PubMed] [Google Scholar]
- Politte LC, Henry CA & McDougle CJ 2014. Psychopharmacological interventions in autism spectrum disorder. Harvard Review of Psychiatry 22 76–92. [DOI] [PubMed] [Google Scholar]
- Ponti G, Rodriguez-Gomez A, Farinetti A, Marraudino M, Filice F, Foglio B, Sciacca G, Panzica GC & Gotti S 2017. Early postnatal genistein administration permanently affects nitrergic and vasopressinergic systems in a sex-specific way. Neuroscience 346 203–215. [DOI] [PubMed] [Google Scholar]
- Rice D & Barone S Jr, 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives 108 Suppl 3 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KD, Horman B, Phillips AL, McRitchie SL, Watson S, Deese-Spruill J, Jima D, Sumner S, Stapleton HM & Patisaul HB 2018. EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocrine Connections 7 305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gomez A, Filice F, Gotti S & Panzica G 2014. Perinatal exposure to genistein affects the normal development of anxiety and aggressive behaviors and nitric oxide system in CD1 male mice. Physiology and Behavior 133 107–114. [DOI] [PubMed] [Google Scholar]
- Rohart F, Gautier B, Singh A & Le Cao K-A 2017. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Computational Biology 13 e1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS 2015. Microbiome disturbances and autism spectrum disorders. Drug Metabolism and Disposition: The Biological Fate of Chemicals 43 1557–1571. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Javurek AB, Johnson SA, Lei Z, Sumner LW & Hess RA 2018. Seminal fluid metabolome and epididymal changes after antibiotic treatment in mice. Reproduction 156 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Sieli PT, Warzak DA, Ellersieck MR, Pennington KA & Roberts RM 2013. Maternal exposure to bisphenol A and genistein has minimal effect on A(vy)/a offspring coat color but favors birth of agouti over nonagouti mice. Proceedings of the National Academy of Sciences of the United States of America 110 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santti R, Makela S, Strauss L, Korkman J & Kostian ML 1998. Phytoestrogens: potential endocrine disruptors in males. Toxicology and Industrial Health 14 223–237. [DOI] [PubMed] [Google Scholar]
- Saville DJ 1990. Multiple Comparison Procedures: The Practical Solution. The American Statistician 44 174–180. [Google Scholar]
- Scallet AC, Wofford M, Meredith JC, Allaben WT & Ferguson SA 2003. Dietary exposure to genistein increases vasopressin but does not alter beta-endorphin in the rat hypothalamus. Toxicological Sciences 72 296–300. [DOI] [PubMed] [Google Scholar]
- Schugar RC, Willard B, Wang Z & Brown JM 2017. Postprandial gut microbiota-driven choline metabolism links dietary cues to adipose tissue dysfunction. Adipocyte 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Brown P, Morrison M, Krause L & Davies PS 2016. Dairy and plant based food intakes are associated with altered faecal microbiota in 2 to 3 year old Australian children. Scientific Reports 6 32385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel RG 1997. Principles and Procedures of Statistics: A Biometrical Approach: McGraw-Hill Higher Education. [Google Scholar]
- Stilling RM, Dinan TG & Cryan JF 2014. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes, Brain, and Behavior 13 69–86. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C & Koga Y 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. Journal of Physiology 558 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW, Fiehn O, Goodacre R, Griffin JL, et al. 2007. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn AN, McKenzie CI & Shen S 2015. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nature Communications 6 7320. [DOI] [PubMed] [Google Scholar]
- Trifunovic S, Manojlovic-Stojanoski M, Ajdzanovic V, Nestorovic N, Ristic N, Medigovic I & Milosevic V 2012. Genistein stimulates the hypothalamo-pituitary-adrenal axis in adult rats: morphological and hormonal study. Histology and Histopathology 27 627–640. [DOI] [PubMed] [Google Scholar]
- Tso IF, Fang Y, Phan KL, Welsh RC & Taylor SF 2015. Abnormal GABAergic function and face processing in schizophrenia: A pharmacologic-fMRI study. Schizophrenia Research 168 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upson K, Harmon QE, Laughlin-Tommaso SK, Umbach DM & Baird DD 2016. Soy-based Infant Formula Feeding and Heavy Menstrual Bleeding Among Young African American Women. Epidemiology 27 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wouw M, Schellekens H, Dinan TG & Cryan JF 2017. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. Journal of Nutrition 147 727–745. [DOI] [PubMed] [Google Scholar]
- Vieira-Potter VJ, Cross TL, Swanson KS, Sarma SJ, Lei Z, Sumner LW & Rosenfeld CS 2018. Soy-Induced Fecal Metabolome Changes in Ovariectomized and Intact Female Rats: Relationship with Cardiometabolic Health. Scientific Reports 8 16896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Mura E & Panzica G 2007. Effects of early embryonic exposure to genistein on male copulatory behavior and vasotocin system of Japanese quail. Hormones and Behavior 51 355–363. [DOI] [PubMed] [Google Scholar]
- Walters WA, Caporaso JG, Lauber CL, Berg-Lyons D, Fierer N & Knight R 2011. PrimerProspector: de novo design and taxonomic analysis of barcoded polymerase chain reaction primers. Bioinformatics 27 1159–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Ding C, Jin G, Yin H, Liu J & Hu F 2015. Abnormal glutamate release in aged BTBR mouse model of autism. International Journal of Clinical and Experimental Pathology 8 10689–10697. [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ 2013. Soy Infant Formula may be Associated with Autistic Behaviors. Autism Open Access 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Jasarevic E, Vandas GM, Warzak DA, Geary DC, Ellersieck MR, Roberts RM & Rosenfeld CS 2013. Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): A monogamous animal model. PloS One 8 e55698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski AB, Cernetich A, Gearhart JP & Klein SL 2005. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiology and Behavior 84 327–334. [DOI] [PubMed] [Google Scholar]
- Wojcik-Gladysz A, Romanowicz K, Misztal T, Polkowska J & Barcikowski B 2005. Effects of intracerebroventricular infusion of genistein on the secretory activity of the GnRH/LH axis in ovariectomized ewes. Animal Reproduction Science 86 221–235. [DOI] [PubMed] [Google Scholar]
- Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, et al. 2016. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 65 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V & Crawley JN 2007. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. International Journal Development Neuroscience 25 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeruva L, Spencer NE, Saraf MK, Hennings L, Bowlin AK, Cleves MA, Mercer K, Chintapalli SV, Shankar K, Rank RG, et al. 2016. Formula diet alters small intestine morphology, microbial abundance and reduces VE-cadherin and IL-10 expression in neonatal porcine model. BMC Gastroenterology 16 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, Yamamoto E & Endo Y 2011. Morphological effects of isoflavones (daidzein and genistein) on hypothalamic oxytocin neurons in the neonatal mouse brain slice cultures. Neuroscience Letters 505 87–92. [DOI] [PubMed] [Google Scholar]
- Zheng H, Powell JE, Steele MI, Dietrich C & Moran NA 2017. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proceedings of the National Academy of Sciences of the United States of America 114 4775–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CB & Fang JY 2018. The regulation of host cellular and gut microbial metabolism in the development and prevention of colorectal cancer. Critical Reviews in Microbiology 1–19. [DOI] [PubMed] [Google Scholar]
- Zhou L, Xiao X, Zhang Q, Zheng J, Li M, Yu M, Wang X, Deng M, Zhai X & Li R 2018. Improved glucose and lipid metabolism in the early life of female offspring by maternal dietary genistein is associated with alterations in the gut microbiota. Frontiers in Endocrinology 9 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1. Key for the bacterial species listed in Figure 1.
Supplementary Figure 10. Correlation across microbiome, metabolites, social and vocal behavior at component of M-AIN vs. F-AIN individuals, cutoff = 0.9.
Supplementary File 1. List of bacteria that differ between GEN females and AIN females.
Supplementary File 2. List of bacteria that differ between GEN males and AIN males.
Supplementary File 3. List of detected components. The MS data were deconvoluted by using AMDIS to yield a total of 528 components.
Supplementary File 4. List of identified components. The MS data were deconvoluted by using AMDIS and annotated by searching against our custom in-house and NIST17 MS spectral libraries.
Supplementary File 5. List of significantly changed metabolites. The significant metabolites were determined using a volcano plot that combines fold change (FC>2) and t-test (p < 0.05).
Supplementary File 6. Correlations of Circos plots associated with M-GEN vs. F-GEN groups, M-AIN vs. F-AIN groups, M-GEN vs. M-AIN, and F-GEN vs. F-AIN.
Supplementary Figures 2. Key for the bacterial species listed in Figure 1.
Supplementary Figure 3. Key for the bacterial species listed in Figure 1.
Supplementary Figure 4. Key for the bacterial species listed in Figure 1.
Supplementary Figure 5. Venn diagram showing number of overlap and unique metabolites that were significantly regulated in each comparison based on treatment X sex interactions.
Supplementary Figure 6. Pathway maps of methionine and glutamate metabolism. Pathway maps of A) methionine and B) glutamate metabolism, which are the most significant pathways associated F-GEN vs. F-AIN individuals, as identified in the pathway analysis in Figure 8. Compounds in light blue color are not included in the list of significant metabolites, and the ones with a red circle are altered by GEN-exposure.
Supplementary Figure 7. Correlation across microbiome, metabolites, social and vocal behavior at component of F-GEN vs. F-AIN individuals, cutoff = 0.9.
Supplementary Figure 8. Correlation across microbiome, metabolites, social and vocal behavior at component of M-GEN vs. M-AIN individuals, cutoff = 0.9.
Supplementary Figure 9. Correlation across microbiome, metabolites, social and vocal behavior at component of M-GEN vs. F-GEN individuals, cutoff = 0.9.