Abstract
We examined whether individual differences in weight gain during exposure to a “junk-food” diet were related to differences in later relapse-like behavior in a rat model. Following free access to a junk-food diet for 7 weeks, rats were trained to press a lever for palatable food pellets. Following extinction training, rats were tested for cue- and pellet priming-induced reinstatement. Results showed that rats prone to obesity while on the junk-food diet displayed greater pellet priming-, but not cue-, induced reinstatement relative to obesity-resistant rats, suggesting that obesity vulnerability is a factor determining one’s chances for some types of relapse.
Keywords: DIET-INDUCED OBESITY, FOOD SEEKING, REINSTATEMENT, RELAPSE
1. Introduction
Obesity is a pandemic and approaching tobacco use as the leading cause of preventable death in industrialized societies. A leading cause of obesity is the increased availability of highly processed, highly palatable foods [1, 2], which are typically high in calories, sugar, and saturated fat [3]. Although many individuals attempt to limit their intake of unhealthy foods through dieting, most relapse to their old, unhealthy eating habits relatively quickly [4, 5]. In fact, the World Obesity Federation recently referred to obesity as a “chronic relapsing progressive disease process.” [6]. Thus, as with the treatment of drug addiction, relapse prevention is a difficult challenge in the treatment of obesity. The development of improved treatment strategies requires a better understanding of the environmental, neural, and phenotypic determinants of relapse vulnerability.
To this end, we examined whether individual differences in weight gain during exposure to a “junk-food” diet were related to differences in later relapse-like behavior in rats. First, rats were separated into obesity phenotypes using a diet-induced obesity model that exposes outbred rats to a junk-food diet that results in excessive weight gain in a subgroup of rats [7, 8]. Next, rats were tested for relapse-like behavior using the reinstatement model of relapse [9, 10]. This classic model has been used extensively to study the environmental and neuropharmacological determinants of relapse and has face and predictive validity [11–16]. Because vulnerability to diet-induced obesity is associated with addiction-like neurobehavioral changes [17, 18], we hypothesized that obesity-prone (OP) rats would display greater relapse to palatable food seeking relative to their obesity-resistant (OR) counterparts.
2. Methods
2.1. Subjects and apparatus
Data were collected from experimentally naïve adult male, Sprague-Dawley rats (n = 36) weighing 280–310 g at the commencement of procedures and supplied by Envigo (Indianapolis, IN). Four rats were excluded from the study due to unreliable food-reinforced responding during training. Rats were housed under standard laboratory conditions (12-hr light cycle from 7:00 AM to 7:00 PM) with ad libitum access to standard chow (Lab Diet 5P07) and water in their home cages for the duration of the study. During the 7-week junk-food diet (see below) rats also had ad libitum access to a junk food mash. Rats were pair-housed upon arrival and until the end of the junk-food period, and then house individually for the remainder of the study. All procedures were in compliance with NIH guidelines and were approved by the Bloomsburg University Institutional Animal Care and Use Committee.
All testing was conducted between 8:00 AM and 6:00 PM and occurred in standard modular operant conditioning chambers (Coulbourn Instruments, Whitehall, PA) that were housed in sound-attenuating, ventilated cubicles and connected to a PC with the Graphic State software interface system (Coulbourn Instruments). Each chamber is equipped with an active and an inactive response lever. Responses on the inactive lever are recorded, but have no programmed consequences. Chambers also include a house light, a row of multicolored LED cue lamps (above active lever), a tone generator, and a food tray (between the two response levers).
2.2. Junk-food diet and obesity classification
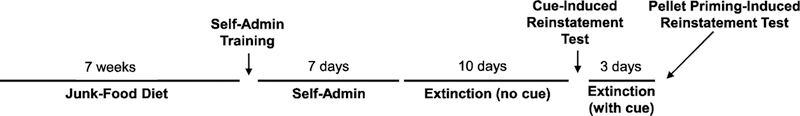
The study began by giving rats 7 weeks of ad libitum access to a mash made of Ruffles original potato chips (40 g), Chips Ahoy original chocolate chip cookies (130 g), Jif smooth peanut butter (130 g), Nesquik powdered chocolate flavoring (130 g), powdered Lab Diet 5P07 (200 g; % of calories: 18.56% protein, 15.79% fat, 65.65% carbohydrates), and water (180 ml). This diet was chosen based on previous studies [7, 18]. Rats did not have access to regular chow during this period. K-means clustering based on weight gain after 7 weeks on the junk-food diet was used as an unbiased method to identify OR and OP rats [19]. See Fig. 1 for schematic of experimental design.
Fig. 1.
Schematic representation of the experimental design for assessing the relationship between obesity phenotype and reinstatement of palatable food-seeking behavior.
2.3. Palatable food self-administration
Following the 7-week junk-food diet, rats were returned to a standard chow (Lab Diet 5P07) diet and trained to press the lever for food reinforcers contingent upon a fixed-ratio (FR)-1 schedule of reinforcement over the course of 1 to 2 days (this training period ended when 80 reinforcers were earned). Following initial training, rats responded on an FR-1 schedule during daily 2-hr sessions for 7 days. Each lever press resulted in delivery of a 45-mg food pellet containing 12.7% fat, 66.7% carbohydrate, and 20.6% protein (Catalogue # 1811155, TestDiet). This pellet type was chosen based on pellet preference tests conducted by Pickens et al. [20], in which it was determined to be the most preferred pellet. Delivery of the pellet was accompanied by a tone + flashing cue light conditioned stimulus (CS) presented for 5 s, which was followed by a 20-s time-out period signaled by illumination of the house light.
2.4. Extinction training and reinstatement testing
On the day following the last self-administration session, daily 2-hr extinction sessions began and continued for 10 days. During the extinction sessions, responses were recorded but had no programmed consequences (i.e., no CS or pellets). Following extinction, rats underwent 2-hr CS-induced reinstatement sessions. Conditions were identical to those of the self-administration sessions, except that lever presses did not lead to pellet delivery. To extinguish lever pressing before pellet priming-induced reinstatement sessions, rats underwent three daily sessions of extinction training (2 hr, with cue) that were identical to CS-induced reinstatement sessions. Next, animals underwent within-session pellet priming-induced reinstatement testing. The testing consisted of four consecutive 1-h sessions that were each identical to the CS-induced reinstatement sessions except that rats received two and four non-contingent pellets within the first minute of sessions 3 and 4, respectively. Session 2 served as the 0-pellet baseline. Data from session 1 were not used for the pellet priming analysis. This within-session procedure is based on previous studies with cocaine and pellet priming [21–24].
2.5. Statistical analyses
Data were analyzed using mixed factorial ANOVAs. The main dependent variable was lever pressing. Body weight while on the junk-food diet also was used as a dependent variable. Within-subjects factors included week of junk-food diet (0 through 7), session (CS-induced reinstatement session and preceding extinction session), number of pellets (0, 2, and 4; for pellet priming-induced reinstatement), extinction day (1 through 10), and lever (active and inactive). The between-subjects factor was obesity phenotype (OR or OP) as determined by K-means clustering. Because the factorial ANOVAs resulted in multiple main and interaction effects, we report only significant effects that are important for interpretation. All ANOVAs were followed by Bonferroni post-tests for multiple comparisons.
3. Results
3.1. Weight gain
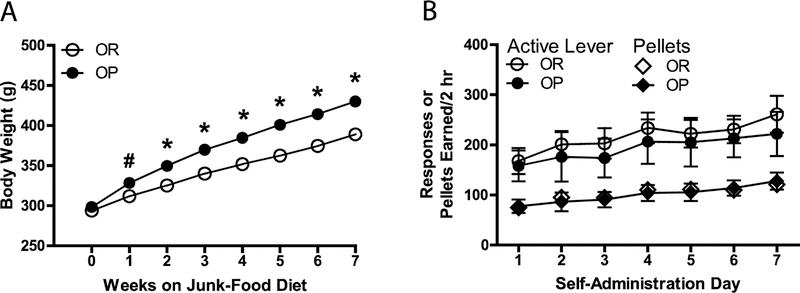
Consistent with previous studies in rodents (e.g., [7, 25]), OR (n = 26) and OP (n = 6) rats did not differ in weight when given ad libitum access to standard chow (week 0; see Fig. 2A); however, after only one week of ad libitum access to the junk food diet, there was a significant difference in weight between groups, and this difference increased with each successive week [week X obesity phenotype interaction, F(7, 210) = 38.43, p = .000; main effect of obesity phenotype, F(1, 30) = 33.13, p = .000]. Because rats in the present study were pair-housed during the junk-food period, we could not measure food intake, but previous studies in rodents indicate that OP animals have greater caloric intake while on an obesogenic diet compared with OR rats (e.g., [17, 25–27]).
Fig. 2.
(a) Body weight across 7 weeks of a continuous junk-food diet in OR and OP rats. Rats were identified as OR or OP by means of K-means clustering based on weight gain after 7 weeks on the junk-food diet. (b) Responses and pellets earned across seven days of 2-hr palatable food self-administration sessions in OR and OP groups. Rats responded on an FR-1 schedule of reinforcement, and delivery of the pellet was accompanied by a tone + flashing cue light CS presented for 5 s, which was followed by a 20-s time-out period signaled by illumination of the house light. #p < .05 and *p < .001 compared to OR group, Bonferroni post-test. All data in figure are represented as mean ± SEM. Points without error bars indicate the SEM is too small to illustrate.
3.2. Self-administration and extinction
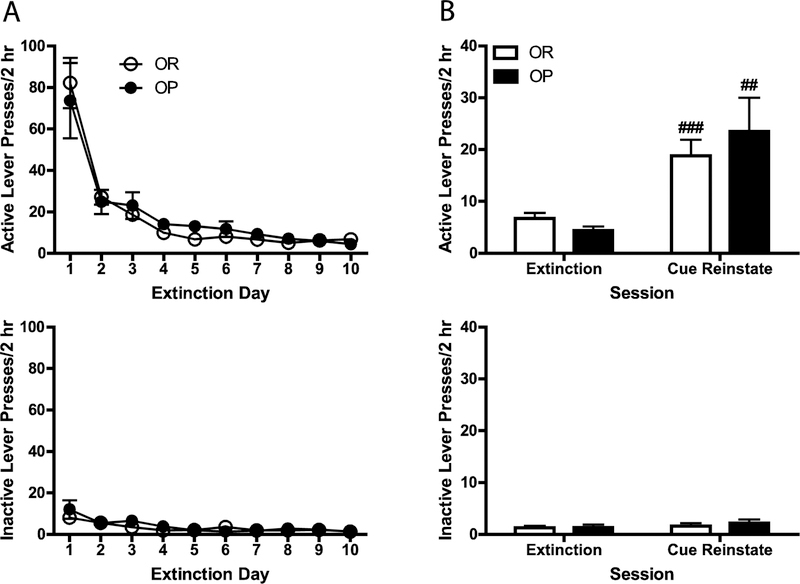
As shown in Fig. 2B, mean response rates and pellets earned were not significantly different across subsequent self-administration sessions for OR and OP groups. Mean response rates also were not significantly different across subsequent extinction sessions for OR and OP groups (see Fig.3A).
Fig. 3.
(a) Mean (± SEM) active and inactive lever presses across 10 days of 2-hr extinction training in OR and OP groups. Responses had no programmed consequences (i.e., no CS or pellets). (b) Mean (+ SEM) active and inactive lever presses during the last extinction session and CS-induced reinstatement testing in OR and OP rats. During reinstatement testing, conditions were identical to those of the self-administration sessions, except that lever presses did not lead to pellet delivery. ##p < .01 and ###p < .001 compared to extinction, Bonferroni post-test.
3.3. Reinstatement
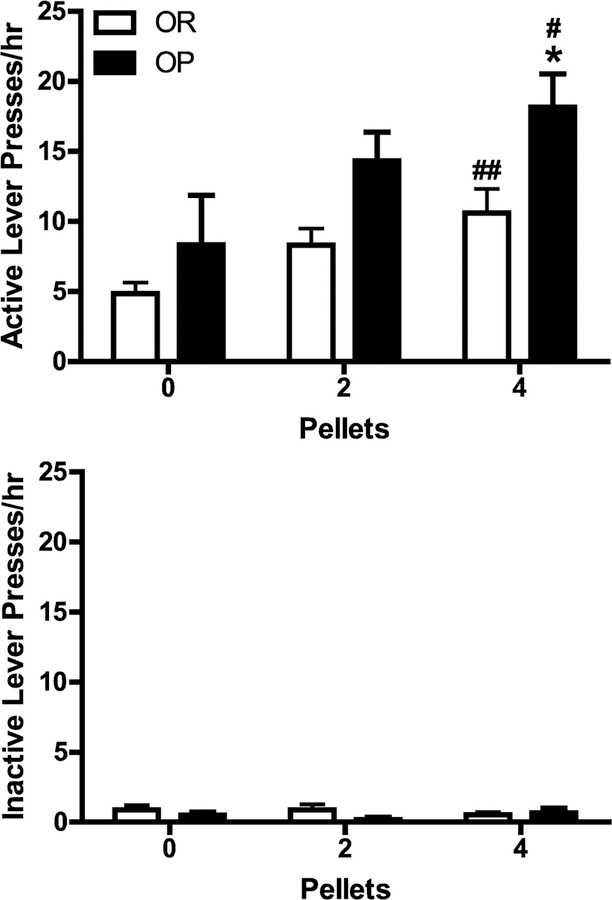
As shown in Fig. 3B, active, but not inactive, lever responding increased significantly during CS-induced reinstatement tests compared to the last extinction session for both OR and OP groups [main effect of session, F(1, 30) = 22.66, p = .000; lever X session interaction, F(1, 30) = 23.05, p = .000], and there was no significant difference in the magnitude of reinstatement between groups. Active, but not inactive, lever responding also increased as a function of pellet priming for both OR and OP groups [main effect of priming, F(2, 60) = 7.09, p = .002; priming x lever interaction, F(2, 60) = 8.24, p = .001; see Fig. 4]. However, active, but not inactive, lever responding was higher for all pellet conditions for the OP group relative to the OR group [main effect of obesity phenotype, F(1, 30) = 7.55, p = .010; obesity phenotype x lever interaction, F(1, 30) = 9.34, p = .005]. The difference in responding between obesity phenotypes reached statistical significance only in the four-pellet condition according to the results of the Bonferroni post-tests. Thus, although active lever pressing was higher in all pellet conditions for obesity-prone relative to obesity-resistant rats (i.e., main effect of obesity phenotype), the greatest difference was observed in the four-pellet condition.
Fig. 4.
Mean (+ SEM) active and inactive lever presses during pellet priming-induced reinstatement testing in OR and OP rats. The testing consisted of four consecutive 1-h sessions that were each identical to the CS-induced reinstatement sessions except that rats received two and four non-contingent pellets within the first minute of sessions 3 and 4, respectively. Session 2 served as the 0-pellet baseline. *p < .05 compared to OR group in 4-pellet condition; #p < .05 and ##p < .01 compared to 0-pellet condition, Bonferroni post-test.
4. Discussion
The present results are the first to show that rats susceptible to diet-induced obesity show increased relapse-like behavior as assessed using the extinction/reinstatement model. To our knowledge, only two studies to date have assessed reinstatement of food seeking in animals with a history of obesogenic diet exposure, but neither study separated animals into obesity phenotypes. Chen et al. [23] reported that female rats with a history of cafeteria diet displayed decreased cue- and pellet priming-induced reinstatement compared with chow-fed rats. Similarly, Burokas et al. [28] found that male mice with a history of obesogenic diet exposure also showed decreased cue-induced reinstatement compared to chow-fed mice (pellet priming-induced reinstatement was not tested). Because a chow-only group was not included in the present study, it is not possible to determine whether rats exposed to a junk-food diet that are OP have increased vulnerability or, alternatively, whether OR rats have reduced relapse vulnerability, a possibility that is suggested by the aforementioned studies. It will be important to test this possibility directly in future studies.
There appear to be overlapping neural mechanisms driving drug and food craving and relapse, especially with regard to the dopamine motive system [29–31]. Systemic and intra-nucleus accumbens injections of the dopamine D1-like receptor antagonist SCH-23390 block cue-induced reinstatement of food seeking [32, 33], and intra-dorsal medial prefrontal cortex injections of the drug attenuate pellet priming-induced reinstatement of food seeking [34]. Using a method that allows identification of two distinct reward-associated ensembles within the same animal, it was recently shown that cue-induced seeking of either alcohol or saccharin activated ensembles consisting of largely overlapping neuronal populations within prefrontal cortex [35]. Moreover, relative to OR rats, OP rats show enhanced responsivity of the mesolimbic dopamine system, as suggested by greater cocaine-induced locomotor activation [18, 26]. Behaviorally, OP rats display increased breakpoints on a progressive ratio schedule of palatable food reinforcement after the development of obesity [17, 27], as well as heightened conditioned approach to a sucrose cue before development of obesity [7]. The present results extend these findings by showing that OP rats also display increased pellet priming-induced food-seeking behavior during extinction, adding to the growing literature suggesting that excessive eating has much in common with drug addiction, including behavioral phenotypes and underlying physiological and neuroanatomical mechanisms [29, 36–38].
It is noteworthy that the increase in food seeking that we observed in OP rats was specific to pellet priming-induced reinstatement, in that no significant differences in self-administration, extinction responding, or cue-induced reinstatement were observed between OP and OR groups. These results support evidence that distinct neural mechanisms underlie food-reinforced operant responding (i.e., food self-administration) vs. reinstatement of food seeking [33, 39], extinction vs. reinstatement [40–42], and cue-vs. pellet priming- and drug priming-induced reinstatement [34, 43]. With regard to this latter distinction, although our results may appear incompatible with evidence that OP rats attribute more motivational value to food cues [7], it is noteworthy that food-associated discrete cues (CSs) were present during food priming in the present study. Thus, our results are compatible with the argument that priming by exposure to the primary reinforcer increases seeking by enhancing the incentive motivational properties of reward-associated cues [44, 45]. This hypothesis should be tested directly in future studies.
In summary, we used a diet-induced obesity model to show that OP rats display greater pellet priming-induced reinstatement of palatable food seeking compared with OR rats. From a translational perspective, our results suggest that those individuals with the greatest need for dietary treatment may also be those at greatest risk for diet recidivism. Future studies should investigate the environmental and neuropharmacological mechanisms underlying increased relapse vulnerability in OP individuals. Such investigations may lead to more targeted treatments for relapse to unhealthy eating habits in humans.
Highlights.
The relationship between obesity vulnerability and relapse was investigated.
Following junk-food diet exposure, relapse tests were conducted using a rat model.
Food-primed reinstatement was greater in obesity-prone vs. obesity-resistant rats.
Cue-induced reinstatement did not differ between obesity phenotypes.
Thus, obesity vulnerability may predict some types of relapse vulnerability.
Acknowledgements:
This work was supported by the National Institutes of Health (NIDA R15 DA035432 to KTB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Poti JM, Braga B, and Qin B, Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Curr Obes Rep, 2017. 6(4): p. 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall KD, et al. , Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab, 2019. 30(1): p. 67–77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poti JM, et al. , Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr, 2015. 101(6): p. 1251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skender ML, et al. , Comparison of 2-year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. Journal of the American Dietetic Association, 1996. 96(4): p. 342–6. [DOI] [PubMed] [Google Scholar]
- 5.Kramer FM, et al. , Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. International Journal of Obesity, 1989. 13(2): p. 123–36. [PubMed] [Google Scholar]
- 6.Bray GA, Kim KK, and Wilding JPH, Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev, 2017. 18(7): p. 715–723. [DOI] [PubMed] [Google Scholar]
- 7.Robinson MJ, et al. , Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacology, 2015. 40(9): p. 2113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin BE, et al. , Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol, 1997. 273(2 Pt 2): p. R725–30. [DOI] [PubMed] [Google Scholar]
- 9.Calu DJ, et al. , The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology, 2014. 76(Pt B): p. 395–406. doi: 10.1016/j.neuropharm.2013.04.030 Epub 2013 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaham Y, et al. , The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology, 2003. 168: p. 3–20. [DOI] [PubMed] [Google Scholar]
- 11.Bachteler D, et al. , The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology, 2005. 30(6): p. 1104–10. [DOI] [PubMed] [Google Scholar]
- 12.Le AD, et al. , Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology, 1999. 21(3): p. 435–44. [DOI] [PubMed] [Google Scholar]
- 13.Leri F, et al. , Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology, 2004. 29(7): p. 1312–20. [DOI] [PubMed] [Google Scholar]
- 14.Liu X and Weiss F, Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. Journal of Neuroscience, 2002. 22(18): p. 7856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor EC, et al. , The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology (Berl), 2010. 208(3): p. 365–76. doi: 10.1007/s00213-009-1739-5 Epub 2009 Dec 5. [DOI] [PubMed] [Google Scholar]
- 16.Sorge RE, Rajabi H, and Stewart J, Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology, 2005. 30(9): p. 1681–92. [DOI] [PubMed] [Google Scholar]
- 17.Brown RM, et al. , Addiction-like Synaptic Impairments in Diet-Induced Obesity. Biol Psychiatry, 2017. 81(9): p. 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oginsky MF, et al. , Eating ‘Junk-Food’ Produces Rapid and Long-Lasting Increases in NAc CP-AMPA Receptors: Implications for Enhanced Cue-Induced Motivation and Food Addiction. Neuropsychopharmacology, 2016. 41(13): p. 2977–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacQueen J Some methods for classification and analysis of multivariate observations. in Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Statistics. 1967. Berkeley, Calif: University of California Press. [Google Scholar]
- 20.Pickens CL, et al. , Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. Psychopharmacology, 2012. 3: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu L, et al. , Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl), 2004. 176(1): p. 101–8. [DOI] [PubMed] [Google Scholar]
- 22.Deroche V, Le Moal M, and Piazza PV, Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci, 1999. 11(8): p. 2731–6. [DOI] [PubMed] [Google Scholar]
- 23.Chen YW, et al. , Effect of cafeteria diet history on cue-, pellet-priming-, and stress-induced reinstatement of food seeking in female rats. PLoS One, 2014. 9(7): p. e102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball KT, et al. , Chronic restraint stress during withdrawal increases vulnerability to drug priming-induced cocaine seeking via a dopamine D1-like receptor-mediated mechanism. Drug Alcohol Depend, 2018. 187: p. 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inbar D, et al. , Chronic calorie-dense diet drives differences in motivated food seeking between obesity-prone and resistant mice. Addict Biol, 2019: p. e12753. [DOI] [PubMed]
- 26.Oginsky MF, et al. , Enhanced cocaine-induced locomotor sensitization and intrinsic excitability of NAc medium spiny neurons in adult but not in adolescent rats susceptible to diet-induced obesity. Psychopharmacology (Berl), 2016. 233(5): p. 773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayanaswami V, et al. , Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes (Lond), 2013. 37(8): p. 1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burokas A, et al. , Extinction and reinstatement of an operant responding maintained by food in different models of obesity. Addict Biol, 2018. 23(2): p. 544–555. [DOI] [PubMed] [Google Scholar]
- 29.Gearhardt AN, et al. , Neural correlates of food addiction. Archives of General Psychiatry, 2011. 68(8): p. 808–16. Epub 2011 Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkow ND, Wise RA, and Baler R, The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci, 2017. 18(12): p. 741–752. [DOI] [PubMed] [Google Scholar]
- 31.Stice E, et al. , The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev, 2013. 37(9 Pt A): p. 2047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy EG, Choi E, and Pratt WE, Nucleus accumbens dopamine and mu-opioid receptors modulate the reinstatement of food-seeking behavior by food-associated cues. Behav Brain Res, 2011. 219(2): p. 265–72. [DOI] [PubMed] [Google Scholar]
- 33.Ball KT, Combs T, and Beyer D, Opposing roles for dopamine D1- and D2-like receptors in discrete cue-induced reinstatement of food seeking. Behavioural Brain Research, 2011. 222(2): p. 390–393. [DOI] [PubMed] [Google Scholar]
- 34.Nair SG, et al. , Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology, 2011. 36(2): p. 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfarr S, et al. , Choice for Drug or Natural Reward Engages Largely Overlapping Neuronal Ensembles in the Infralimbic Prefrontal Cortex. J Neurosci, 2018. 38(14): p. 3507–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frascella J, et al. , Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint? Ann N Y Acad Sci, 2010. 1187: p. 294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, et al. , Food and drug reward: overlapping circuits in human obesity and addiction. Current Topics in Behavioral Neurosciences, 2012. 11: p. 1–24. [DOI] [PubMed] [Google Scholar]
- 38.Kenny PJ, Common cellular and molecular mechanisms in obesity and drug addiction. Nature Reviews Neuroscience, 2011. 12(11): p. 638–51. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 39.Nair SG, et al. , The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Progress in Neurobiology, 2009. 89(1): p. 18–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters J, Kalivas PW, and Quirk GJ, Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem, 2009. 16(5): p. 279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasseter HC, et al. , Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci, 2010. 3: p. 101–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Oever MC, et al. , Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neuroscience and Biobehavioral Reviews, 2010. 35(2): p. 276–84. [DOI] [PubMed] [Google Scholar]
- 43.Feltenstein MW and See RE, The neurocircuitry of addiction: an overview. Br J Pharmacol, 2008. 154(2): p. 261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart J, de Wit H, and Eikelboom R, Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review, 1984. 91(2): p. 251–68. [PubMed] [Google Scholar]
- 45.Mueller D and Stewart J, Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res, 2000. 115(1): p. 39–47. [DOI] [PubMed] [Google Scholar]