Abstract
Aims
Current guidelines recommend initiating family screening for hypertrophic cardiomyopathy (HCM) after age 10 or 12 years unless early screening criteria are met. The aim was to evaluate if current screening guidelines miss early onset disease.
Methods and results
Children who underwent family screening for HCM before age 18 years were analysed. Major cardiac events (MaCEs) were defined as death, sudden cardiac death (SCD), or need for major cardiac interventions (myectomy, implantable cardioverter-defibrillator insertion, transplantation). Of 524 children screened, 331 were under 10 years of age, 9.9% had echocardiographic evidence of HCM, and 1.1% were symptomatic at first screening. The median (interquartile range) age at HCM onset was 8.9 (4.7–13.4) years, and at MaCE was 10.9 (8.5–14.3) years with a median time to MaCE from HCM onset of 1.5 (0.5–4.1) years. About 52.5% phenotype-positive children and 41% with MaCEs were <10 years old. Only 69% children with early HCM met early screening criteria. Cox regression identified male gender, family history of SCD, and pathogenic variants in MYH7/MYBPC3 as a predictor of early onset HCM and MaCEs.
Conclusion
A third of children not eligible for early screening by current guidelines had phenotype-positive HCM. MYH7 and MYBC3 mutation-positive patients were at highest risk for developing early HCM and experiencing an event or requiring a major intervention. Our findings suggest that younger family members should be considered for early clinical and genetic screening to identify the subset in need of closer monitoring and interventions.
Keywords: Hypertrophic cardiomyopathy, Family screening, Sudden cardiac death, Implantable cardioverter-defibrillator, Sarcomeric mutations
Introduction
The American College of Cardiology (ACC)/American Heart Association (AHA) consensus guidelines recommend 12 years as the starting age for family screening of first-degree relatives of affected probands with hypertrophic cardiomyopathy (HCM). Earlier screening is recommended only in cases with an early growth spurt, family history of sudden cardiac death (SCD), and prior to competitive sports participation [Class I recommendation, level of evidence (LOE)-C].1 The European Society of Cardiology (ESC) recommends that clinical and/or genetic screening be offered from age 10 years onwards (Class IIa, LOE-C) with earlier screening to be considered in families with malignant early onset disease, presence of cardiac symptoms or are involvement in demanding physical activity (Class IIb, LOE-C).2–4 Furthermore, relying on symptoms to initiate screening is challenging in children who are often unable to verbalize symptoms like angina, palpitations, or exercise intolerance. Setting 10–12 years as the minimum age cut-off for screening involves a risk of missing earlier onset disease. The primary goal of this study was to evaluate if the current screening guidelines miss early onset disease which can impact timely interventions aimed at preventing adverse outcomes.
Methods
This was a single centre, retrospective cohort study of children who underwent echocardiography prior to 18 years of age as part of family screening for primary HCM between 2000 and 2018. As a tertiary care referral centre, families from across the province were followed at our institution. First-degree relatives of HCM probands were screened clinically independent of age, although echocardiography was sometimes delayed till 3 years of age to avoid sedation for echocardiography. Screening included a clinic visit, electrocardiogram (ECG), echocardiogram, and genetic counselling for the purpose of cascade genetic testing. Hypertrophic cardiomyopathy probands and families with secondary HCM e.g. syndromic, neuromuscular, metabolic, or mitochondrial disease were excluded. The study was approved by the Institutional Research Ethics Board of the Hospital for Sick Children and the requirement for informed consent was waived.
The medical records were reviewed for demographics, family history, baseline clinical characteristics, symptoms, serial echocardiographic data, Holter results, genetic test results, and clinical outcomes. Phenotype-positive status was defined as maximal left ventricular (LV) posterior wall diameter or interventricular septal (IVS) diameter z-score >2 on two-dimensional echocardiography. Major cardiac events (MaCEs) were defined as a composite of death, SCD, resuscitated SCD due to ventricular fibrillation or ventricular tachycardia, or need for major cardiac interventions including surgical myectomy, implantable cardioverter-defibrillator (ICD) implantation, or cardiac transplantation.
Statistical analysis
Continuous variables were summarized as mean (± standard deviation) or median (interquartile range) as appropriate. Dichotomous and polytomous variables were summarized as frequencies and proportions. Children were presumed phenotype-negative between birth and the first record of them being phenotype-positive. Age-dependent freedom from phenotype-positive status and from a MaCE was evaluated using Kaplan–Meier estimates. Incidence rate of outcomes was calculated as the ratio of events per 100 patient-years of follow-up. We assessed the proportion of children who became phenotype-positive and/or experienced a MaCE who did not meet early screening criteria of symptoms or family history of SCD. Univariable Cox regression models were used to identify factors associated with HCM at <10 years and MaCEs. Genetic risk factors were dichotomized to by the presence or absence of a pathogenic or likely pathogenic (P/LP) variant, variant in a sarcomeric gene, and multiple P/LP variants. Factors significant in the multivariable model were retained and forward selection was applied to the other candidate factors significant at the univariable level. Forward selection of additional factors was based on minimizing Akaike’s information criteria (AIC). Models with clinical factors only were compared to models with clinical and genetic factors using the AIC, whereby a lower AIC indicates a better model. To quantify discriminative ability, the concordance statistic for Cox proportional hazard models was calculated.5
Results
Baseline characteristics
A total of 524 relatives (from 315 families) <18 years old at the time of screening echocardiography were eligible. The median number of at-risk children screened per family was 2 (range 1–6) (those screened due to a murmur were excluded). In total, 331 (63.2%) were screened before 10 years of age. Table 1 describes the baseline characteristics of the cohort. Of these, 52 (9.9%) were phenotype-positive at first evaluation and an additional 28 (5.4%) became phenotype-positive during follow-up. About 198 (37.8%) had a positive family history of SCD. Only 6 (1.1%) children were symptomatic at first screening. Genetic characteristics are described in Table 2. Overall 215 families were genotype-positive. Of these, 120 children from these families had clinical genetic testing of which 86 (71.7%) were genotype-positive (95 families declined genetic testing). Variants in MYH7 and MYBPC3 accounted for over 60% of genotype-positive cases.
Table 1.
Baseline characteristics (n = 524)
| Variables | N | Statistics |
|---|---|---|
| Demographic characteristics | ||
| Age at first echo (years) | 524 | 7.7 (3.7–12.0) |
| Gender | 524 | |
| Female | 235 (44.8%) | |
| Reported race | 157 | |
| Afro-Caribbean | 6 (3.8%) | |
| Caucasian | 131 (83.4%) | |
| Mixed | 7 (4.5%) | |
| Other | 13 (8.3%) | |
| Phenotype-positive at first screening | 524 | 52 (9.9%) |
| Symptomatic at screeninga | 523 | 6 (1.1%) |
| Screened before 12 years old | 524 | 392 (74.8%) |
| Phenotype-positive at last follow-up | 524 | 80 (15.3%) |
| Family history | ||
| Family history of sudden cardiac death | 524 | 198 (37.8%) |
| Family history of ICD | 524 | 150 (28.6%) |
| Youngest family member with SCD | 198 | |
| >60 years old | 11 (5.6%) | |
| 40–59 years old | 52 (26.3%) | |
| 25–39 years old | 74 (37.4%) | |
| <25 years old | 61 (30.8%) | |
| Closest affected family member | 522 | |
| First degree (mother/father/sibling) | 468 (89.7%) | |
| Second degree (grand-parent, uncle/aunt) | 47 (9.0%) | |
| Third or fourth degree | 7 (1.3%) |
Symptoms included: palpitations (1), chest pain (1), murmur (2), syncope (1), syncope, and palpitations (1).
Table 2.
Genetic characteristics (n = 524)
| Variables | N | Statistics |
|---|---|---|
| Paediatric relative with genetic testing | ||
| Subject had genetic testing | 524 | 120 (22.9%) |
| Variant identified | 120 | |
| No | 28 (23.3%) | |
| Pathogenic | 86 (71.7%) | |
| Variant of uncertain significance | 6 (5.0%) | |
| Affected gene | ||
| MYH7 | 86 | 27 (31.4%) |
| MYBPC3 | 86 | 43 (50.0%) |
| PRKAG2 + MYBPC3 | 86 | 1 (1.2%) |
| TPM1 | 86 | 8 (9.3%) |
| TNNI/TNNT | 86 | 3 (3.5%) |
| MYL2 | 86 | 2 (2.4%) |
| TMPO | 86 | 1 (1.2%) |
| Unknown/Missing | 86 | 1 (1.2%) |
| Proband with genetic testing | ||
| Variant identified in the proband | ||
| Pathogenic | 95 (23.5%) | |
| Variant of uncertain significance | 30 (7.4%) | |
| Affected gene | ||
| Unknown | 95 | 15 (15.8%) |
| MYH7 | 95 | 27 (28.4%) |
| MYBPC3 | 95 | 41 (43.1%) |
| TNN13 | 95 | 5 (5.3%) |
| TPM1 | 95 | 4 (4.2%) |
| TNNT2 | 95 | 2 (2.1%) |
| PRKAG2 | 95 | 1 (1.1%) |
Outcomes
Hypertrophic cardiomyopathy onset
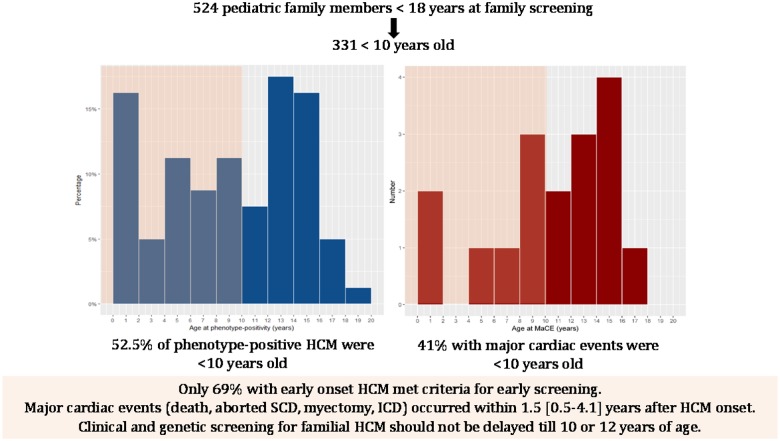
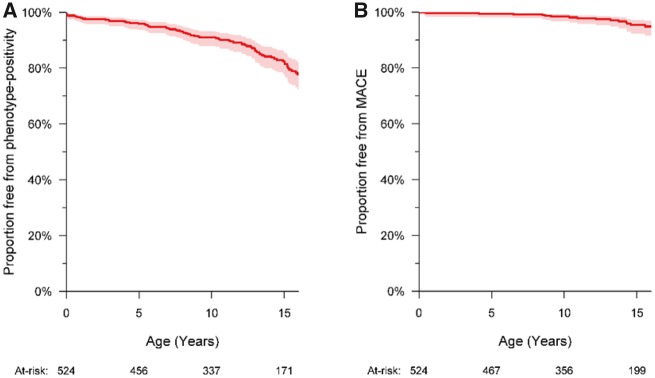
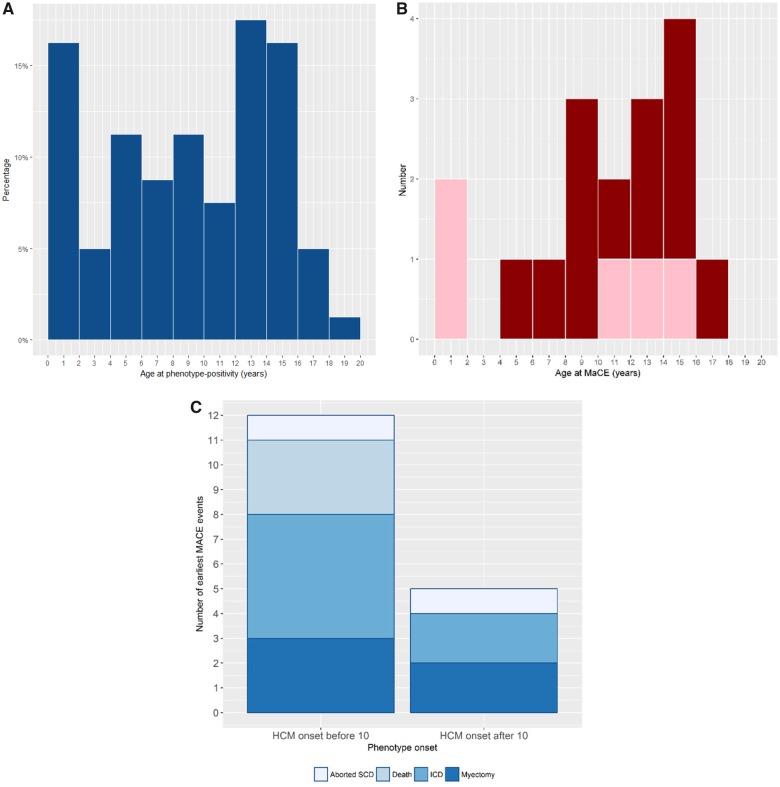
The median age at onset of HCM was 8.9 (4.7–13.4) years (Table 3). Freedom from phenotype-positive HCM at 10 years of age was 91.0% (88.0–93.8) (Figure 1) and freedom from moderate-severe hypertrophy defined as IVS z-score >5 was 95.2% (92.8–96.8). Of the 80 phenotype-positive children, 42 (52.5%) became phenotype-positive before 10 years of age (Figure 2A).
Table 3.
Outcomes (frequency and incidence rate) (n = 524)
| Variables | Statistics** (incidence rate) |
|---|---|
| Age at last echocardiogram (years) | 13.4 (8.6–16.6) |
| Length of follow-up (years) | 3.2 (0.0–6.4) |
| Maximal LV wall or IVS z-score >2 | 80 (3.62) |
| Primary outcome—earliest event | |
| Major cardiac event | 17 (0.77) |
| Death | 3 (0.14) |
| Heart transplant | 0 (0.00) |
| Aborted sudden cardiac death | 2 (0.09) |
| ICD implantation (all primary indication) | 7 (0.32) |
| Myectomy | 5 (0.23) |
| Other outcomes | |
| Abnormal blood pressure response on exercise test | 16 (0.73) |
| Non-sustained ventricular tachycardia on Holter | 7 (0.32) |
| Coronary unroofing | 3 (0.14) |
| Maximal LV wall or septal Z-score >5 | 49 (2.22) |
| Maximal LV wall or septal Z-score >10 | 13 (0.59) |
| LV outflow gradient >30 mmHg | 13 (0.59) |
| LV outflow gradient >50 mmHg | 10 (0.45) |
| LV outflow gradient >100 mmHg | 9 (0.41) |
Figure 1.
Freedom from hypertrophic cardiomyopathy and major cardiac event (n = 524). (A) Freedom from phenotype-positive hypertrophic cardiomyopathy (95% confidence interval) was 95.9% (93.7–97.3) at 5 years, 91.0% (88.0–93.3) at 10 years, 89.2% (85.9–91.8) at 12 years, and 82.4% (77.9–86.1) at 15 years. (B) Freedom from a major cardiac event was 99.4% (98.2–99.8) at 5 years, 98.4% (96.7–99.2) at 10 years, 97.8% (95.8–98.9), and 95.4% (92.3–97.3) at 15 years. MaCE, major cardiac event.
Figure 2.
Age distribution of hypertrophic cardiomyopathy onset and major cardiac event (n = 524). (A) Forty-two (52.5%) of the 80 phenotype-positive children became phenotype-positive before 10 years of age. (B) Seven (41%) of 17 children with a major cardiac event experienced it before age 10 years. Pink indicates death or aborted sudden cardiac death events and red indicates implantable cardioverter-defibrillator insertion and myectomy. (C) Major cardiac event by age of onset of hypertrophic cardiomyopathy. There were 21 events in 17 children with more events in those with hypertrophic cardiomyopathy before age 10 years. MaCE, major cardiac event; SCD, sudden cardiac death.
Major cardiac events
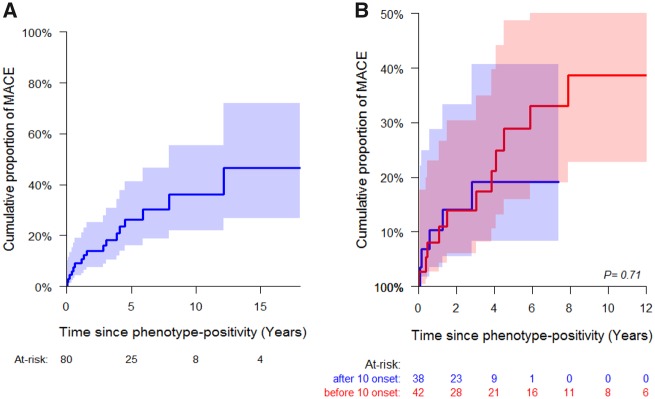
Seventeen (3.2%) children experienced a MaCE during follow-up (Table 4). Seven of 17 (41%) children experienced it before 10 years of age (Figure 2B). Freedom from a MaCE at 10 years of age was 98.4% (96.7–99.2) in the overall cohort. The median age at MaCE was 10.9 (8.5–14.3) years. There were a total of 21 events in 17 children. The first event included three deaths (two from heart failure and one SCD), two aborted SCD, five surgical myectomies, and seven ICDs for primary prevention (including two with appropriate shocks) (Figure 2C). With 2206 patient-years of follow-up, incidence rate of MaCE was 0.95 per 100 patient-years. All events occurred in phenotype-positive children. The median follow-up from first echocardiographic evaluation to MaCE was 2.8 (0.5–4.5) years, and from onset of HCM to MaCE was 1.5 (0.5–4.1) years. The cumulative proportion of MaCE in phenotype-positive children is shown in Figure 3A.
Table 4.
Major cardiac events in the overall cohort
| Age at diagnosis (years) | Age at MaCE (years) | Genetic test results (P/LP variant) | Major cardiac event | Cause of death/indication for ICD |
|---|---|---|---|---|
| 0 (foetal) | 0.4 | None | Death (cardiac) | Advanced heart failure |
| 0 (foetal) | 0.5 | MYBPC3 (homozygous) | Death (cardiac) | Advanced heart failure awaiting transplant |
| 0 (foetal) | 12.2 | MYH7 | ICD—primary prevention | Family history of SCD, severe septal hypertrophy, LGE on CMR |
| 1.1 | 4.1 | MYBPC3 | Surgical myectomy | Symptomatic with LVOT gradient >100 mmHg |
| 2.7 | 13.7 | MYH7 | Aborted SCD; ICD—secondary prevention | Secondary prevention |
| 4.2 | 8.7 | MYH7 | ICD—primary prevention | Family history of SCD, extreme septal hypertrophy, and abnormal BP response |
| 4.4 | 8.5 | MYBPC3 (compound heterozygote) | ICD—primary prevention | Family history of SCD, abnormal BP response (two appropriate shocks) |
| 5.4 | 6.5 | MYBPC3 | Surgical myectomy | Symptomatic with severe LVOTO on optimal medical therapy |
| 7 | 10.9 | MYBPC3 (compound heterozygote) | Death (SCD) | SCD |
| 7.6 | 15.6 | MYH7 | ICD—primary prevention | Family h/o SCD, severe septal hypertrophy, and palpitations |
| 8.8 | 10 | MYH7 | ICD—primary prevention | Extreme septal hypertrophy, abnormal BP response |
| 8.8 | 14.3 | None | Aborted SCD; ICD—secondary prevention | Secondary prevention |
| 9.1 | 9.1 | None | Surgical myectomy (+ primary prevention ICD 3 years later) | Family history of SCD, severe LVOTO, and NSVT |
| 11.5 | 14.3 | MYH7 | Surgical myectomy | Symptomatic with severe LVOTO on optimal medical therapy |
| 13 | 13.2 | MYBPC3 (compound heterozygote) | ICD—primary prevention | Severe septal hypertrophy, NSVT, and abnormal BP response (one appropriate shock) |
| 14.5 | 14.6 | None | Surgical myectomy (+ primary prevention ICD) | Severe LVOTO, NSVT, and abnormal BP response on exercise |
| 15.9 | 16.5 | TNNT2 | ICD—primary prevention | Unexplained syncope, severe septal hypertrophy, and abnormal ECG on exercise |
CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; LVOTO, LV outflow tract obstruction; MaCE, major cardiac event; NSVT, non-sustained ventricular tachycardia; P/LP, pathogenic or likely pathogenic.
Figure 3.
Major cardiac event in phenotype-positive children (n = 80). The x-axis shows the time between phenotype-positivity and major cardiac event in years. (A) The cumulative proportion (95% CI) of major cardiac event (A) at 5 years was 26.4% (58.7–83.9) in the overall cohort, and (B) at 10 years was 28.9% (15.9–48.7) in subjects with hypertrophic cardiomyopathy before 10 years of age and 19.1% (8.3–40.6) in subjects with hypertrophic cardiomyopathy after 10 years of age. MaCE, major cardiac event.
Major cardiac events in early onset hypertrophic cardiomyopathy
Thirteen of 17 (76.5%) children with a MaCE had early onset HCM. The cumulative proportion (95% confidence interval) of MaCEs at 5 years after HCM onset was 28.9% (15.9–48.7) in those who became phenotype-positive before 10 years of age and 19.1% (8.3–40.6) in those who became phenotype-positive after 10 years of age. For those who became phenotype-positive before 10 years of age, the median age at MaCE was 8.9 (5.9–11.2) years and median time from HCM onset to MaCE was 3.5 (0.9–4.9) years. For subjects who became phenotype-positive after 10 years of age, the median age at MaCE was 14.3 (14.27–14.59) years and the median time from HCM onset to MaCE was 0.6 (0.2–1.3) years (although the latter entails a shorter duration of follow-up) (Figure 3B). The freedom from death/aborted SCD was 99.6% (98.5–99.9) at 5 years, 99.6% (98.5–99.9) at 10 years, 99.3% (97.8–99.8) at 12 years, and 98.5% (96.2–99.4) at 15 years. The freedom from interventions (myectomy, ICD) was 100% at 5 years, 98.8% (97.1–99.5) at 10 years, 98.5% (96.7–99.3) at 12 years, and 96.9 (94.2–98.4) at 15 years. This suggests that childhood onset HCM can be progressive with a subset experiencing adverse events and major interventions early after diagnosis.
Eligibility for early screening
Overall, 201 (38.4%) children fulfilled criteria for early screening based on symptoms or family history of SCD. However, among the 42 children with HCM before 10 years of age, only 29 (69%) met criteria for early screening. The criteria in these 29 cases included symptoms in two and a family history of SCD in 28 cases (with one overlapping case). The freedom from HCM at 10 years of age was 84.3% (77.8–89.0) in subjects eligible for early screening compared to 94.9% (91.6–96.9) in subjects not eligible for early screening (P = 0.0003) (Figure 4). Also, of the seven children who had MaCE before 10 years of age, one (11.1%) was not eligible for early screening. This patient had MYH7 variant-positive HCM and received a primary prevention ICD for extreme septal hypertrophy and abnormal blood pressure response on exercise.
Figure 4.
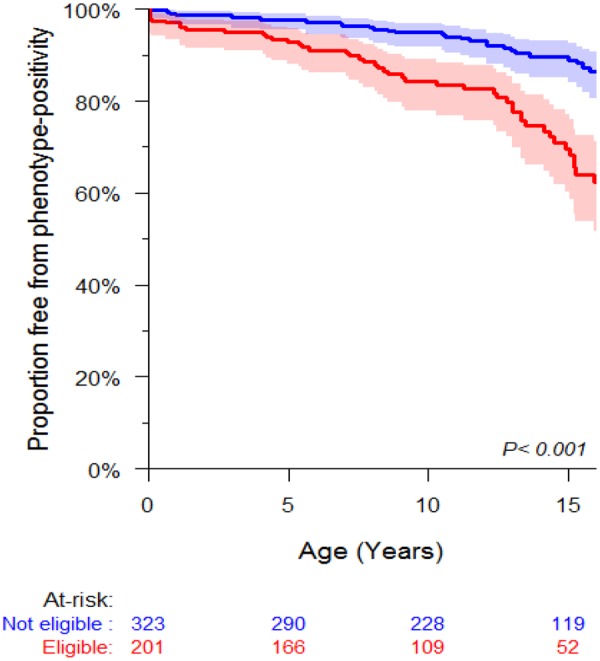
Freedom from hypertrophic cardiomyopathy stratified by eligibility for early screening. Freedom from hypertrophic cardiomyopathy in those eligible for early screening (red) was 92.8% (88.1–95.7) at 5 years, 84.3% (77.8–89.0) at 10 years, and 82.6% (75.7–87.7) at 12 years. Freedom from clinical hypertrophic cardiomyopathy in the remaining cohort (blue) was 97.8% (95.4–98.9) at 5 years, 94.9% (91.6–96.9) at 10 years, and 93.1% (89.3–95.6) at 12 years.
Factors associated with early hypertrophic cardiomyopathy and major cardiac events
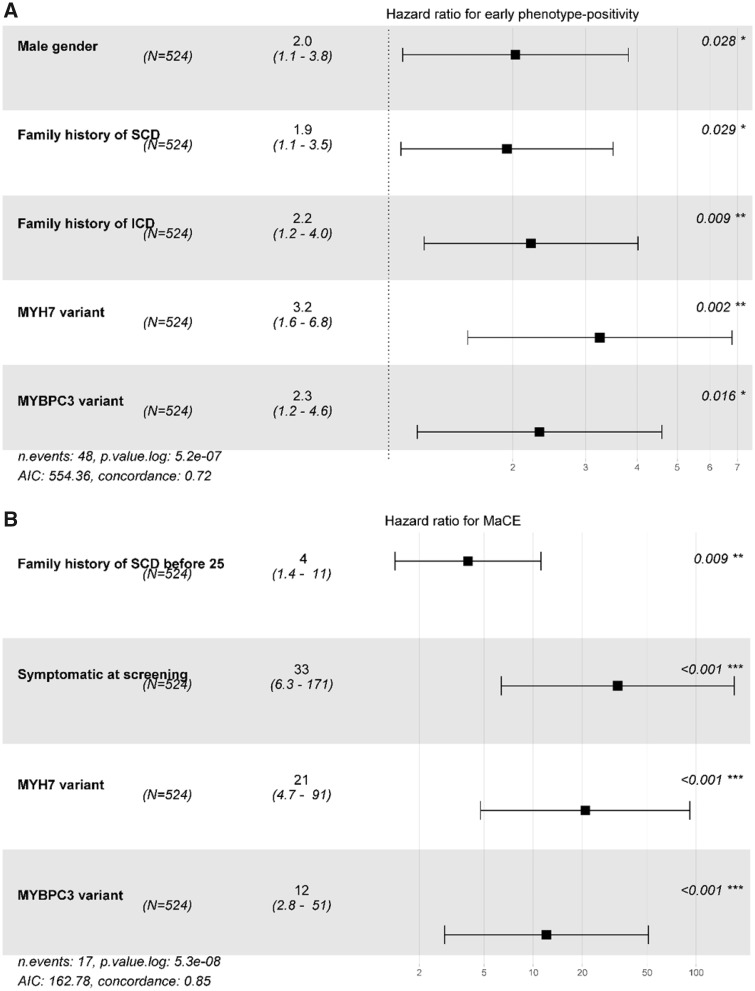
On multivariable Cox regression, male gender, family history of SCD/ICD, and P/LP variant in MYH7 or MYBPC3 in the child or family were associated with early onset HCM. Family history of SCD prior to 25 years of age, symptoms at screening and presence of a P/LP MYH7 or MYBPC3 variant were associated with early occurrence of a MaCE (Table 5 and Figure 5).
Table 5.
Factors associated with early onset hypertrophic cardiomyopathy and major cardiac event (n = 524)
| Univariable HR (95% CI) | P-value | Multivariable HR (95% CI) | P-value | |
|---|---|---|---|---|
| Early phenotype-positivity | ||||
| Male gender (ref = female) | 2.048 (1.099–3.816) | 0.024 | 2.028 (1.081–3.807) | 0.028 |
| Family history of SCD | 2.618 (1.474–4.650) | 0.001 | 1.935 (1.071–3.497) | 0.029 |
| Family history of SCD before age 25 years | 1.615 (0.756–3.452) | 0.22 | ||
| First-degree phenotype or genotype-positive relative | 1.695 (0.527–5.456) | 0.38 | ||
| Family history of ICD | 2.842 (1.612–5.010) | <0.001 | 2.212 (1.218–4.019) | 0.009 |
| Family history of transplant | 1.240 (0.385–43.990) | 0.72 | ||
| Symptomatic at screening | 4.130 (1.002–17.026) | 0.050 | — | — |
| P/LP MYH7 variant | 2.995 (1.526–5.877) | 0.001 | 3.247 (1.555–6.782) | 0.002 |
| P/LP MYBPC3 variant | 2.451 (1.331–4.513) | 0.004 | 2.319 (1.173–4.587) | 0.016 |
| P/LP variant in other gene | 0.385 (0.053–2.787) | 0.34 | — | — |
| P/LP variants in more than one gene | 3.234 (0.446–23.445) | 0.25 | — | — |
| MaCE a | ||||
| Male gender (ref = female) | 1.176 (0.448–3.090) | 0.74 | — | — |
| Family history of SCD | 3.516 (1.299–9.513) | 0.013 | 1.177 (0.325–4.260) | 0.80 |
| Family history of SCD before age 25 years | 5.829 (2.217–15.326) | <0.001 | 2.621 (0.687–9.990) | 0.158 |
| Family history of ICD | 3.497 (1.347–9.076) | 0.010 | 2.869 (0.903, 9.119) | 0.074 |
| Family history of transplant | 1.195 (0.158–9.018) | 0.86 | ||
| Symptomatic at screening | 15.400 (4.408–53.805) | <0.001 | 57.611 (9.549–347.595) | <0.001 |
| P/LP MYH7 variant | 6.068 (2.234–16.481) | <0.001 | 18.574 (4.183–82.475) | <0.001 |
| P/LP MYBPC3 variant | 2.884 (1.06–7.802) | 0.037 | 9.340 (2.123–41.190) | 0.003 |
| P/LP variant in other gene | 1.126 (0.149–8.491) | 0.91 | — | — |
Bold values significant on multivariable analysis.
CI, confidence interval; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; LP, likely pathogenic; MaCE, major cardiac event; P, Pathogenic; SCD, sudden cardiac death.
First degree affected relative and presence of multiple P/LP variants were not included in the models for MaCE because HR was unestimable as the events did not occur in one of the two groups.
Figure 5.
Factors associated with major cardiac event and early onset hypertrophic cardiomyopathy (n = 524). Forest plot of hazard ratios and 95% confidence intervals for multivariable model of freedom from (A) early onset hypertrophic cardiomyopathy and (B) major cardiac event. MaCE, major cardiac event.
Take home figure.
Occurrence of early onset hypertrophic cardiomyopathy (HCM) and early major cardiac events during screening of pediatric family members of a HCM proband.
Modelling
Factors associated with early onset HCM and MaCEs were included in the final model. Models with clinical factors only were compared to models with clinical and genetic factors using AIC whereby a lower AIC indicates a better model. For both outcomes, i.e. freedom from early onset HCM and freedom from early MaCEs, the combined clinical and genetic model had a lower AIC (i.e. performed better) than the model with only clinical factors (Table 6). The concordance statistic of the final combined Cox proportional hazard model for freedom from early onset HCM was 0.71 and for MaCE was 0.85 indicating good discriminative ability.
Table 6.
Clinical and genetic models
| Model | Factors included | AIC | Concordance |
|---|---|---|---|
| Early onset HCM | |||
| Clinical factors |
|
565 | 0.64 |
| Clinical + genetic factors |
|
556 | 0.71 |
| MaCE | |||
| Clinical factors |
|
182 | 0.72 |
| Clinical + genetic factors |
|
163 | 0.85 |
AIC, Akaike’s information criterion; MaCE, major cardiac event; SCD, sudden cardiac death.
Discussion
Our study has several important findings. First, 9.9% children screened for HCM were already phenotype-positive at first evaluation with MaCEs occurrence at a median follow-up of 1.5 (0.5–4.1) years from onset of HCM. 52.5% children with clinical HCM became phenotype-positive before 10 years of age and 41% MaCEs occurred in children before 10 years of age. The short interval to a MaCE suggests that many children already had a well-established phenotype by the time they were screened with a relatively rapid progression on follow-up. Second, a third of early HCM cases did not fulfil criteria for early screening like symptoms or family history of SCD. Missing data precluded our ability to include early onset HCM in the family in the screening criteria. Although the children who met early screening criteria had a lower freedom from early onset HCM and MaCEs, 31% children with early onset HCM did not meet eligibility for early screening and would potentially have been missed. Thirdly, we identified male gender, family history of SCD and presence of P/LP variants in MYH7 or MYBPC3 as predictors of early onset HCM and of MaCEs. Several published studies have reported an association of genetic factors with phenotype and outcomes in children and adults explaining some of the variability in disease onset both within and between families with variants in the same gene.2–4
As an autosomal dominant disorder, every first-degree relative from a HCM family regardless of age has a 50% chance of inheriting the disease. However, the guidelines for when to offer screening differ by age likely based on previous studies with an underrepresentation of children and pre-adolescents that reported that most HCM does not manifest until adolescence or adulthood.6–10 Our findings and the recent report by Norrish et al.11 which include large patient cohorts suggest that disease prevalence in children and pre-adolescents is not lower than in adolescents. They found that in 1198 consecutive children aged ≤18 years from 594 families who underwent serial evaluation, 72% of diagnoses were made in pre-adolescents with over a third of patients requiring medical, surgical or device therapy before 18 years of age. They concluded that the phenotype of familial HCM in childhood is varied and includes severe disease, suggesting that clinical screening should commence at a younger age. At the other end of the spectrum, Maurizi et al.12 recently published a report that showed that genotype-positive individuals who remain phenotype-negative by 18 years have a low frequency (10%) of phenotype conversion. Together, this suggests that disease prevalence and penetrance in children is not lower than in adolescents or even adults.
Secondly, the event rates in phenotype-positive children are not different than in adults. The risk of a SCD event in an adult diagnosed with clinical HCM is 1–2% per year.13 Our previous report on 98 genotype-positive probands with HCM <18 years old revealed a 10% event rate over a median follow-up of 1.4 years (0.4–3.0). These included 7% SCD events i.e. one SCD, three resuscitated SCD, and three appropriate shocks post primary prevention ICD.3 Another recent publication on outcomes in 446 children and adolescents with ICDs showed that appropriate shocks occurred in 15.7% of patients with primary prevention ICDs.14
Our study provided additional new insights compared to the Norrish study by evaluating the yield of clinical testing in patients who met early screening criteria vs. those who did not and also by evaluating the importance of early genetic screening to help predict early onset HCM. Despite good discriminative ability, the combined clinical and genetic models were not perfect in their predictions. This may be related to the availability of a genetic diagnosis in only a subset of the cohort since many families either decline genetic testing or remain genotype-elusive. This suggests that until better validated prediction models of early onset disease are available, clinical and genetic screening should not be delayed till 10 or 12 years of age since studies report effective coping with a positive result and no difference in quality of life between gene-positive children and peers.15,16 We recognize that there are costs associated with early screening. However, even if event rates are low, there are significant costs (emotional and financial) to families and to society associated with missed diagnoses and the preventable loss of a young life.17,18
Limitations
A retrospective study can entail a survivor bias and underestimate out of hospital deaths and early onset HCM since those who were not screened until 12 years of age may have had unrecognized disease prior to their first screening. The prediction model for early onset HCM and MaCEs was not validated in an independent external cohort. Also, a majority of events were cardiac interventions like myectomy and ICD which can involve practitioner bias.
In summary, the results of our study coupled with other recent evidence of the yield on clinical screening in young family members indicate that disease prevalence and penetrance as well as event rates are not lower in children compared to adolescents (and adults). Although annual event rate in children who develop HCM is low, given the potential for rapid progression, early detection of clinical HCM is important in order to facilitate close follow-up and timely interventions to prevent SCD, and to alleviate symptoms of worsening LV outflow obstruction before adverse events occur. In conclusion, younger family members should be considered for early clinical and genetic screening to identify the subset in need of close monitoring and interventions.
Funding
This work was supported by the Ted Rogers Centre for Heart Research and the Heart and Stroke Foundation Chair funds (to S.M.).
Conflict of interest: none declared.
See page 3682 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz487)
References
- 1. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW.. 2011 ACCF/AHA Guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary. Circulation 2011;124:2761–2796. [DOI] [PubMed] [Google Scholar]
- 2. Lopes LR, Brito D, Belo A, Cardim N.. Genetic characterization and genotype-phenotype associations in a large cohort of patients with hypertrophic cardiomyopathy—an ancillary study of the Portuguese registry of hypertrophic cardiomyopathy. Int J Cardiol 2019;278:173–179. [DOI] [PubMed] [Google Scholar]
- 3. Mathew J, Zahavich L, Lafreniere-Roula M, Wilson J, George K, Benson L, Bowdin S, Mital S.. Utility of genetics for risk stratification in pediatric hypertrophic cardiomyopathy. Clin Genet 2018;93:310–319. [DOI] [PubMed] [Google Scholar]
- 4. Sedaghat-Hamedani F, Kayvanpour E, Tugrul OF, Lai A, Amr A, Haas J, Proctor T, Ehlermann P, Jensen K, Katus HA, Meder B.. Clinical outcomes associated with sarcomere mutations in hypertrophic cardiomyopathy: a meta-analysis on 7675 individuals. Clin Res Cardiol 2018;107:30–41. [DOI] [PubMed] [Google Scholar]
- 5. Harrell F, Kerry LL, Daniel BM.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 6. Gray B, Ingles J, Semsarian C.. Natural history of genotype positive–phenotype negative patients with hypertrophic cardiomyopathy. Int J Cardiol 2011;152:258–259. [DOI] [PubMed] [Google Scholar]
- 7. Christiaans I, Birnie E, Bonsel GJ, Mannens MM, Michels M, Majoor-Krakauer D, Dooijes D, van Tintelen JP, van den Berg MP, Volders PG, Arens YH, van den Wijngaard A, Atsma DE, Helderman-van den Enden AT, Houweling AC, de Boer K, van der Smagt JJ, Hauer RN, Marcelis CL, Timmermans J, van Langen IM, Wilde AA.. Manifest disease, risk factors for sudden cardiac death, and cardiac events in a large nationwide cohort of predictively tested hypertrophic cardiomyopathy mutation carriers: determining the best cardiological screening strategy. Eur Heart J 2011;32:1161–1170. [DOI] [PubMed] [Google Scholar]
- 8. Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F.. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 2000;102:858–864. [DOI] [PubMed] [Google Scholar]
- 9. Vermeer AMC, Clur S-A, Blom NA, Wilde AAM, Christiaans I.. Penetrance of hypertrophic cardiomyopathy in children who are mutation positive. J Pediatrics 2017;188:91–95. [DOI] [PubMed] [Google Scholar]
- 10. Jensen Morten K, Havndrup O, Christiansen M, Andersen PS, Diness B, Axelsson A, Skovby F, Køber L, Bundgaard H.. Penetrance of hypertrophic cardiomyopathy in children and adolescents. Circulation 2013;127:48–54. [DOI] [PubMed] [Google Scholar]
- 11. Norrish G, Jager J, Field E, Quinn E, Fell H, Lord E, Cicerchia MN, Ochoa JP, Cervi E, Elliott PM, Kaski JP.. Yield of clinical screening for hypertrophic cardiomyopathy in child first-degree relatives: evidence for a change in paradigm. Circulation 2019. Epub ahead of print. doi:10.1161/CIRCULATIONAHA.118.038846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maurizi N, Michels M, Rowin EJ, Semsarian C, Girolami F, Tomberli B, Cecchi F, Maron MS, Olivotto I, Maron BJ.. Clinical course and significance of hypertrophic cardiomyopathy without left ventricular hypertrophy. Circulation 2019;139:830–833. [DOI] [PubMed] [Google Scholar]
- 13. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, Cirino AL, Fox JC, Lakdawala NK, Ware JS, Caleshu CA, Helms AS, Colan SD, Girolami F, Cecchi F, Seidman CE, Sajeev G, Signorovitch J, Green EM, Olivotto I.. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy. Circulation 2018;138:1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balaji S, DiLorenzo MP, Fish FA, Etheridge SP, Aziz PF, Russell MW, Tisma S, Pflaumer A, Sreeram N, Kubus P, Law IH, Kantoch MJ, Kertesz NJ, Strieper M, Erickson CC, Moore JP, Nakano SJ, Singh HR, Chang P, Cohen M, Fournier A, Ilina MV, Smith RT, Zimmerman F, Horndasch M, Li W, Batra A, Liberman L, Hamilton R, Janson CM, Sanatani S, Zeltser I, McDaniel G, Blaufox AD, Garnreiter JM, Katcoff H, Shah M.. Risk factors for lethal arrhythmic events in children and adolescents with hypertrophic cardiomyopathy and an implantable defibrillator: an international multi-center study. Heart Rhythm 2019. Epub ahead of print. doi:10.1016/j.hrthm.2019.04.040. [DOI] [PubMed] [Google Scholar]
- 15. Meulenkamp TM, Tibben A, Mollema ED, van Langen IM, Wiegman A, de Wert GM, de Beaufort ID, Wilde AAM, Smets E.. Predictive genetic testing for cardiovascular diseases: impact on carrier children. Am J Med Genet A 2008;146A:3136–3146. [DOI] [PubMed] [Google Scholar]
- 16. Smets EMA, Stam MMH, Meulenkamp TM, van Langen IM, Wilde AAM, Wiegman A, de Wert GM, Tibben A.. Health-related quality of life of children with a positive carrier status for inherited cardiovascular diseases. Am J Med Genet A 2008;146A:700–707. [DOI] [PubMed] [Google Scholar]
- 17. Graf J, Mühlhoff C, Doig GS, Reinartz S, Bode K, Dujardin R, Koch K-C, Roeb E, Janssens U.. Health care costs, long-term survival, and quality of life following intensive care unit admission after cardiac arrest. Crit Care 2008;12:R92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. You JJ, Woo A, Ko DT, Cameron DA, Mihailovic A, Krahn M.. Life expectancy gains and cost-effectiveness of implantable cardioverter/defibrillators for the primary prevention of sudden cardiac death in patients with hypertrophic cardiomyopathy. Am Heart J 2007;154:899–907. [DOI] [PubMed] [Google Scholar]