Abstract
Objective
To investigate the potential active compounds and underlying mechanisms of Paeonia lactiflora Pall. (PLP) on the treatment of Alzheimer's disease (AD) based on network pharmacology.
Methods
The active components of PLP were collected from Traditional Chinese Medicine System Pharmacology (TCMSP) database, and their possible target proteins were predicted using TCMSP, SwissTargetPrediction, and STITCH databases. The putative AD-related target proteins were identified from Therapeutic Target Database (TTD), GeneCards, and MalaCards database. The compound-target-disease network interactions were established to obtain the key targets about PLP acting on AD by network topology analysis. Then, the function annotation and signaling pathways of key targets were performed by GO and KEGG enrichment analysis using DAVID tools. Finally, the binding capacity between active ingredients and key targets was validated by molecular docking using SystemsDock tools.
Results
There were 7 active compounds involving in 151 predicted targets identified in PLP. Besides, a total of 160 AD-related targets were identified. Among these targets, 30 shared targets of PLP and AD were acquired. After topological analysis of the PLP potential target-AD target network, 33 key targets that were highly responsible for the therapeutic effects of PLP on AD were obtained. Further GO and KEGG enrichment analysis showed that these key targets were significantly involved in multiple biological processes and pathways which participated in cell apoptosis and inflammatory response and maintained the function of neurons to accomplish the anti-AD activity. The molecular docking analysis verified that the 7 active compounds had definite affinity with the key targets.
Conclusions
The ameliorative effects of PLP on AD were predicted to be associated with regulating neural cell apoptosis, inflammatory response, and neurotrophy via various pathways such as PI3K-Akt signaling pathway, MAPK signaling pathway, and neurotrophin signaling pathway.
1. Introduction
Alzheimer's disease (AD) is one of the commonest neurodegenerative diseases with high incidence and intricate pathogenesis. Unfortunately, there is no efficacious treatment options for AD patients. AD contributes to about two-thirds of dementia cases and affects more than 5 million people in US [1]. The prevalence of AD is everincreasing with years, and AD has been the sixth primary cause of death in the US, although the AD-induced death emerges on average 8.5 years [2]. The clinical features of AD contain memory loss and cognitive impairment at early stage, subsequent topographical difficulties, and alongside loss of confidence, judgement and attention. As the condition progresses, cognitive deficiency becomes deteriorative and widespread so as to interfere with activities of daily living and bring poor quality of life to the patients and their families [3]. However, the current mainstream treatments for AD such as acetylcholinesterase (AChE) inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists show limited efficacy. The major pathogenic events leading to AD are attributed to the accumulation of insoluble amyloid-β (Aβ) to form senile plaque and aggregation of microtubule protein tau in neurofibrillary tangles (NFTs) in neurons [4]. Recently, the clinical trials showed that new drugs targeting Aβ or tau failed to improve cognitive ability and clinical outcomes of AD patients, and thus they were discontinued. It suggested that the efficacy of single-target drug was limited and hard to meet clinical needs [5–7]. It is important and necessary to develop novel drugs with multitargets for AD treatment.
Traditional Chinese Medicines (TCMs) has been used for the treatment of dementia for hundreds of years in China. According to the theory of TCM, AD pertains to long-lasting nutrition deficiency in the brain as a result of obstruction of blood flow and disturbance of “Qi” motion in liver [8]. Paeonia lactiflora Pall. (PLP) is a well-known and widely used herbal medicine that can nourish blood and regulate the liver. Modern pharmacological studies disclosed that formulae containing PLP had evident activities in reducing tau aggregation and ameliorating cognition deficits [9–11]. The aqueous and ethanol extracts of PLP exhibited strong inhibition on AChE activity as indicated by IC50 values at 20 and 8 μg/ml, which was even stronger than Salvia miltiorrhiza Bge. and Polygonum multijiorum Thunb., implying great potential for AD treatment [12]. Although paeoniflorin was reported to be one of the active ingredients in PLP which could improve dementia through neuroprotection and anti-inflammation, the other active compounds in PLP and possible multitarget mechanisms were seldom reported [13, 14].
Network pharmacology is an emerging approach for drug discovery. Up to date, this approach has been successfully employed to elucidate the multitarget effects of TCM, which is consistent with the holistic perspective of TCM theory. The use of network pharmacology in the research of TCM integrates phytochemistry, pharmacology, and bioinformatics; effectively bridges the gap between western medicine and traditional medicine; and also greatly facilitates mechanistic studies on the synergistic actions of TCM [15]. In the current study, the active compounds and underlying mechanisms of PLP acting on AD were comprehensively investigated using the network pharmacology approach. The compounds in PLP and their putative target proteins were identified from the public databases. The biological processes and underlying pathways associated with PLP acting on AD were obtained by enrichment analysis. The combination between the active ingredients in PLP and the key targets was evaluated by simulative molecular docking as well. The present study elucidated the potential active compounds and underlying mechanisms of PLP acting on AD and provided theoretic evidence for the multi-ingredient and multitarget effects of PLP, suggesting the feasibility of developing PLP or its active compounds as alternative therapy for AD.
2. Materials and Methods
2.1. Identification of Chemical Ingredients in PLP
The chemical ingredients in PLP were identified from Traditional Chinese Medicine System Pharmacology (TCMSP, http://lsp.nwu.edu.cn/browse.php) database [16]. The database provides comprehensive information about ingredients in herbs including chemical structure, oral bioavailability, Caco-2 intestinal epithelial permeability, half-life, drug likeness, drug targets, and their association with diseases and interaction network, etc. The pharmacokinetic properties including absorption, distribution, metabolism, and excretion (ADME) are important contributors for bioactivities of drug. In this study, three ADME-related parameters including oral bioavailability (OB) ≥30%, half-life (HL) ≥4, and drug likeness (DL) ≥0.18 were employed to identify the potential active ingredients in PLP. As suggested by TCMSP, the compounds with OB ≥30% and HL ≥4 have good absorption and slow metabolism after oral administration. The compounds with DL ≥0.18 were chemically suitable for drug development.
2.2. Prediction of Compound-Related Targets
The compound-related targets were predicted depending on chemical similarities and pharmacophore models via TCMSP, SwissTargetPrediction (http://www.swisstargetprediction.ch/), and STITCH (http://stitch.embl.de/) databases. The probability value of each target marked in SwissTargetPrediction database was used to give a rank list for the targets and evaluate the accuracy of the predictions, whose probability value ≥0.5 was collected in our present study [17]. Besides, confidence score marked in STITCH database provided a reference to define a set of high-confidence interactions between compounds and protein modules [18]. The target proteins with confidence score ≥7 were identified as compound-related targets.
2.3. Identification of AD-Related Targets
The known AD-related targets were collected from three databases including TTD (Therapeutic Target Database, https://db.idrblab.org/ttd/), GeneCards (https://www.genecards.org/), and MalaCards (https://www.malacards.org/pages/info).
All the targets obtained above were standardized as gene names and UniProt IDs by searching from UniprotKB (https://www.uniprot.org/) database with “Homo sapiens” species [19].
2.4. Network Construction and Topological Analysis
The compound-target network of PLP and disease-target network of AD, as well as PLP potential target-AD target interaction network, was constructed by Cytoscape v3.7.0 software which is a useful tool for analysis and visualization of the biological network. The topological analysis was performed by the network analyzer module of Cytoscape software. Three topological parameters including degree centrality (DC), betweenness centrality (BC), and closeness centrality (CC) were used to estimate the central properties of the nodes in the network. In the PLP potential target-AD target interaction network, DC ≥ median DC, BC ≥ median BC, and CC ≥ median CC were employed to screen the key targets of PLP acting on AD.
2.5. GO and KEGG Pathway Enrichment Analysis
DAVID (https://david.ncifcrf.gov/) is an online biological knowledgebase and an analytic tool to extract biological information about gene functional classification, functional annotation, and enriched pathways [20]. Gene Ontology (GO) analysis including biological process, cell component, and molecular function, as well as KEGG pathway enrichment analysis, were performed using DAVID database. GO terms with Bonferroni value <0.05 and KEGG pathways with P value <0.05 were considered to have significance.
2.6. Validation of the Binding Capacity between Active Ingredients and Key Targets by Molecular Docking
Molecular docking is a useful method for drug targets and drug screening research by mimicking the interactions between compounds and proteins to predict their binding capacity and affinity based on their structures. SystemsDock (http://systemsdock.unit.oist.jp/), a web server for assessing protein-ligand binding property, permits high-precision docking simulation and molecular pathway map for comprehensive characterization of ligand selectivity and interpretation of ligand action on a complex molecular network [21]. The crystal structure of the key targets were obtained from PDB (Protein Data Bank) database, and then the molecular docking results between active ingredients in PLP and key targets were analyzed using systemsDock to validate their binding properties. Docking score is a parameter that is the predicted binding affinity to each of target proteins. Docking score at 5.52 is set as the cutoff to classify the good binding capacity between a compound and protein [21].
3. Results
3.1. Compound-Target Network of PLP
Total 85 compounds were identified in PLP including terpenoids, glycosides, flavonoids, oleum volatile, and phenols. According to the characteristics of oral bioavailability, half-life, and drug likeness of the compounds, 7 compounds were screened out as the potential active ingredients including β-sitosterol, kaempferol, lactiflorin, mairin, paeoniflorigenone, paeoniflorin, and palbinone, which are listed in Table 1. The characteristics of the 85 compounds in PLP are shown in Stable 1.
Table 1.
The characteristics of active ingredients in PLP.
| Compounds | Molecular formula | Structure | Molecular weight | OB (%) | HL | DL |
|---|---|---|---|---|---|---|
| β-sitosterol | C29H50O |

|
414.79 | 36.91 | 5.36 | 0.75 |
|
| ||||||
| Kaempferol | C15H10O6 |

|
286.25 | 41.88 | 14.74 | 0.24 |
|
| ||||||
| Lactiflorin | C23H26O10 |
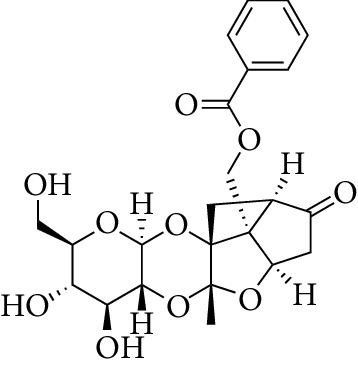
|
462.49 | 49.12 | 7.26 | 0.8 |
|
| ||||||
| Mairin | C30H48O3 |
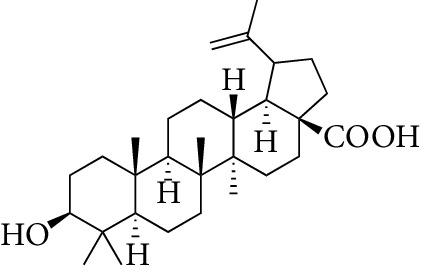
|
456.78 | 55.38 | 8.87 | 0.78 |
|
| ||||||
| Paeoniflorigenone | C17H18O6 |

|
318.35 | 87.59 | 7.45 | 0.37 |
|
| ||||||
| Paeoniflorin | C23H28O11 |

|
480.51 | 53.87 | 13.88 | 0.79 |
|
| ||||||
| Palbinone | C22H30O4 |

|
358.52 | 43.56 | 4.34 | 0.53 |
From the TCMSP, SwissTargetPrediction, and STITCH databases, a total of 151 compound-related targets were identified. Among these targets, there were 57 targets involved in β-sitosterol, 58 targets involved in kaempferol, 5 targets related to lactiflorin, 24 targets related to mairin, 18 targets related to paeoniflorin, 3 targets involved in palbinone, and only one target connected to paeoniflorigenone. These seven compounds also had some shared bioactive targets and connection networks. The targets of each compound are listed in Stable 2, and the compound-target network of PLP is shown in Figure 1(a).
Figure 1.
(a) Compound-target network of PLP. Red rectangle nodes represent potential active compounds in PLP, while blue rectangle nodes represent potential targets of PLP. (b)–(d) GO enrichment analysis for potential targets of PLP (count number ≥15). (e) KEGG enrichment analysis for potential targets of PLP (count number ≥15).
To clarify the characteristics of compound-related targets on molecular function and pathway level, GO function and KEGG pathway enrichment analysis were performed (Figures 1(b)–1(e)). Most of these potential targets existed on the plasma membrane with molecular function of protein binding. Specifically, these targets were involved in biological processes such as oxidation-reduction process, regulation of transcription, response to drug, signal transduction, and inflammatory response. Furthermore, the results of KEGG enrichment analysis demonstrated that there were totally 101 pathways (P value <0.05) affected by the active ingredients of PLP. The top 10 (count number ≥15) enriched pathways contained metabolic pathways, pathways in cancer, hepatitis B, tuberculosis, neuroactive ligand-receptor interaction, nonalcoholic fatty liver disease, toxoplasmosis, steroid hormone biosynthesis, influenza A, and calcium signaling pathway.
3.2. Disease-Target Network of AD
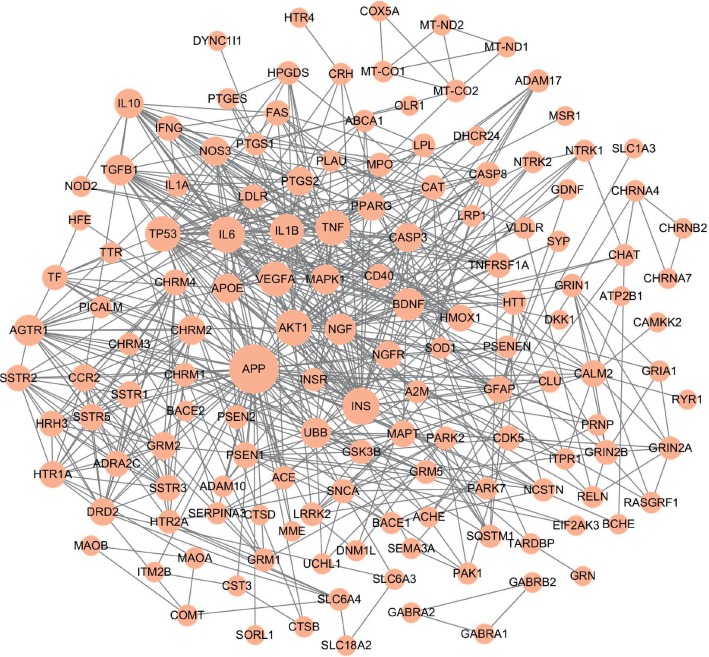
From the TTD, GeneCards, and MalaCards databases, a total of 160 AD-related targets were identified (Stable 3), among which 142 targets showed high interactions (confidence score ≥7) in the protein-protein interactions map generated by the STRING database. The disease-target network of AD was constructed and consisted of 142 nodes (targets with high interactions) and 626 link edges. The central attributes of each node were evaluated by topology analysis. In Figure 2, the size of nodes was proportional to degree centrality. Notably, amyloid precursor protein (APP, degree = 56) which played an essential role in the pathogenesis of AD brain was identified as the most important protein in this disease-target network. In addition, according to both degree and betweenness centrality, INS, AKT1, IL6, TP53, BDNF, and NGF were also recognized as important AD targets.
Figure 2.
Disease-target network of AD. Pink ellipse nodes represent AD-related targets, and the size of nodes is proportional to degree centrality by topology analysis.
3.3. PLP Potential Target-AD Target Network
To identify the relationship between the PLP potential targets and AD-related targets, their targets were analyzed, and thus 30 shared targets of PLP and AD were acquired. The interaction network of PLP potential target-AD target was established and shown in Figure 3(a). There were 260 targets and 1634 link edges which had high interactions with confidence score ≥7 in the STRING database. Based on criteria of DC ≥ median DC, BC ≥ median BC, and CC ≥ median CC, 33 key targets of PLP acting on AD were obtained (Table 2) and further used to construct the interaction network consisting of 33 nodes and 254 link edges (Figure 3(b)).
Figure 3.
(a) PLP potential target-AD target network. (b) Network of the 33 key targets. Blue ellipse nodes stand for PLP potential targets, pink ellipse nodes represent AD-related targets, and red ellipse nodes represent the shared targets of PLP and AD. The size of nodes is proportional to degree centrality by topology analysis. (c) GO enrichment analysis for 33 key targets. (d) KEGG enrichment analysis for 33 key targets.
Table 2.
The 33 key targets of PLP acting on AD.
| Target | Name | Degree | Betweenness centrality | Closeness centrality |
|---|---|---|---|---|
| APP | Amyloid precursor protein | 81 | 0.192 | 0.517 |
| TP53 | Tumor protein P53 | 54 | 0.096 | 0.507 |
| AKT1 | RAC-alpha serine/threonine-protein kinase | 54 | 0.039 | 0.483 |
| MAPK8 | Mitogen-activated protein kinase 8 | 51 | 0.042 | 0.489 |
| IL6 | Interleukin 6 | 50 | 0.035 | 0.485 |
| INS | Insulin | 47 | 0.060 | 0.490 |
| TNF | Tumor necrosis factor | 46 | 0.022 | 0.473 |
| MAPK1 | Mitogen-activated protein kinase 1 | 45 | 0.036 | 0.481 |
| JUN | Transcription factor AP1 | 44 | 0.026 | 0.482 |
| VEGFA | Vascular endothelial growth factor A | 43 | 0.029 | 0.475 |
| APOE | Apolipoprotein E | 38 | 0.040 | 0.458 |
| IL8 | Interleukin 8 | 38 | 0.028 | 0.444 |
| BDNF | Brain-derived neurotrophic factor | 37 | 0.039 | 0.465 |
| CASP3 | Caspase 3 | 35 | 0.023 | 0.461 |
| IL1B | Interleukin-1β | 35 | 0.016 | 0.443 |
| RELA | Transcription factor p65 | 33 | 0.012 | 0.456 |
| UBB | Ubiquitin B | 32 | 0.032 | 0.441 |
| HSP90A | Heat shock protein HSP 90 | 32 | 0.031 | 0.431 |
| NOS3 | Endothelial nitric oxide synthase | 30 | 0.018 | 0.449 |
| AGTR1 | Type-1 angiotensin II receptor | 29 | 0.016 | 0.437 |
| PTGS2 | Prostaglandin G/H synthase 2 | 29 | 0.026 | 0.435 |
| NGF | Nerve growth factor | 28 | 0.013 | 0.443 |
| PPARG | Peroxisome proliferator activated receptor γ | 28 | 0.013 | 0.434 |
| AR | Androgen receptor | 25 | 0.026 | 0.421 |
| SP1 | Sp1 transcription factor | 24 | 0.014 | 0.424 |
| CASP8 | Caspase 8 | 22 | 0.007 | 0.436 |
| PSEN1 | Presenilin 1 | 22 | 0.015 | 0.427 |
| CDK5 | Cyclin-dependent kinase 5 | 22 | 0.013 | 0.424 |
| NGFR | Nerve growth factor receptor | 21 | 0.011 | 0.440 |
| CAT | Catalase | 21 | 0.020 | 0.423 |
| MAPT | Microtubule associated protein tau | 20 | 0.010 | 0.422 |
| LDLR | Low-density lipoprotein receptor | 20 | 0.020 | 0.417 |
| GSK3B | Glycogen synthase kinase-3β | 17 | 0.006 | 0.433 |
These key targets were primarily distributed in cellular components such as cytosol and cytoplasm and mainly involved in biological processes such as regulation of transcription, regulation of apoptotic process, regulation of gene expression, inflammatory response, and regulation of nitric oxide biosynthetic process via molecular function of protein binding by GO enrichment analysis (Figure 3(c)).
The KEGG enrichment analysis was carried out to further explore the underlying mechanisms of PLP on AD. The representative top 10 pathways based on the number of enriched genes as well as fold changes and P value are shown in Figure 3(d). These key targets were closely related to PI3K-Akt signaling pathway, MAPK signaling pathway, neurotrophin signaling pathway, TNF signaling pathway, toll-like receptor signaling pathway, etc., which participated in cell apoptosis and inflammatory response and maintained the function of neurons to accomplish the anti-AD activity of PLP.
3.4. The Binding Capacity between Active Compounds and Key Targets by Molecular Docking
To further verify the binding capacity between active compounds and key targets, molecular docking through systemsDock was performed. The docking results are shown in Figure 4 based on the docking score which was a negative logarithm of the experimental dissociation/inhibition constant, ranging from 0 to 10 that represented weak to strong binding. The docking scores showed that the active compounds of PLP, especially β-sitosterol and mairin, had good binding activity to AD putative targets included in the key targets, indicating the specific action proteins of PLP for AD treatment.
Figure 4.
Heat map of binding capacity between the active compounds and key targets by molecular docking. The heat map was depicted based on docking scores.
4. Discussion
According to the theory of TCM, long-term nutrition deficiency in the brain as a result of blood stasis and “Qi” stagnation in liver is one of the pathogenic reasons for AD. PLP is a well-known herbal medicine with a function of nourishing and regulating blood and is commonly used to improve nutrition deficiency in the brain. Among the large numbers of formulae for treatment of dementia, PLP is one of the most widely used herbs and usually works as a monarch herb in a prescription [22]. The present study aimed to explore the potential active compounds and underlying mechanisms of PLP acting on AD and provided theoretic evidence for developing PLP or its active compounds as alternative therapy for AD. The representative compounds and potential mechanisms of PLP on AD treatment are depicted in Figure 5. In the current study, there were 7 compounds including β-sitosterol, kaempferol, lactiflorin, mairin, paeoniflorigenone, paeoniflorin, and palbinone identified as the potential active ingredients of PLP, of which the biological activities against AD were reported previously. For example, kaempferol, that is, a natural acetylcholinesterase inhibitor could delay the loss of climbing ability, ameliorate memory deficiency, and reduce oxidative stress and neuroinflammation both in the ovariectomized rat model and transgenic Drosophila model of AD [23–25]. Mairin, also named as betulinic acid, that is, a pentacyclic triterpenoid, could prevent Aβ/streptozotocin-induced spatial and passive avoidance memory deficits and reduce Aβ fibril plaques in the hippocampus region of the AD rat model through protecting microcirculation, alleviating inflammation, and upregulating BDNF expression [26, 27]. Paeoniflorin is reported to improve memory deficits, attenuate amyloidogenesis, prevent Aβ-induced astrocytes and microglia activation, and suppress inflammatory responses in the transgenic AD model [14]. β-sitosterol belongs to the group of phytosterols which are active ingredients existing in a diversity of natural plants. It is reported that β-sitosterol bound to the active sites of AChE and BChE as an inhibitor by molecular docking and exhibited an IC50 value of 55 and 50 μg/ml against these two enzyme activities [28]. In addition, administration of β-sitosterol at 10 mg/kg body weight/day demonstrated gradual improvement in memory deficiency and motor coordination in the transgenic AD model [28]. Therefore, the above active components indicate the effectiveness and diversity of chemical ingredients in PLP for treating AD.
Figure 5.
Representative compounds and potential mechanisms of PLP on AD treatment. The green triangle stands for representative compounds in PLP, and the red rectangle stands for the compound-related targets.
The results of PLP potential target-AD target network analysis acquired 33 key targets of PLP acting on AD. These key targets were mainly involved in biological processes such as regulation of transcription, regulation of apoptotic process, regulation of gene expression, inflammatory response, and regulation of nitric oxide biosynthetic process via molecular function of protein binding. The predicted results in our current study were consistent with some previous publications. It is reported that β-sitosterol attenuated Aβ-induced neural cell apoptosis and inflammation through downregulating iNOS expression and NF-κB, p38, and ERK activation [29]. Kaempferol inhibited inflammatory response and iNOS expression as well as NO generation through suppressing NF-κB, p38, JNK, and AKT phosphorylation [30]. Mairin promoted M2 phenotype microglial polarization and prevented M1 polarization through calmodulin-dependent protein kinase kinase β/AMPK activation, specifically decreased iNOS and TNF-α expression when the BV2 cells were treated with lipopolysaccharide (LPS) [31]. Further molecular docking assay in this study showed that β-sitosterol and mairin were conferred strong binding activity with MAPK1 (ERK2), MAPK8 (JNK1), NOS3, and TNF-α, while kaempferol had strong combination with AKT1 and TNF-α.
As observed in the results of KEGG pathway enrichment analysis, the key targets of PLP acting on AD were mainly related to PI3K-Akt signaling pathway, MAPK signaling pathway, neurotrophin signaling pathway, TNF signaling pathway, and so on. AD is an intricate neurodegenerative disease that the underlying mechanisms have not been clearly elucidated. It is recognized that the pathogenesis of AD is associated with various biological processes such as synaptic loss, specific neurotransmitters reduction, neuroinflammation, and neuronal death [32]. The neural cell death induced by aggregated Aβ plaque plays a crucial role in the pathogenesis of AD. The PI3K-Akt pathway participates in cell survival and death, particularly exhibiting beneficial effect on cell survival and inhibitory effect on cell apoptosis once Akt is activated [33]. In addition, PI3K-Akt pathway is involved in the initiation of the autophagic process which is a major intracellular machinery for degrading misfolded proteins and damaged organelles and has been reported to be involved in the pathogenesis of AD through acting on its downstream mTOR complex [34]. Notably, ubiquitin-proteasome system (UPS) is another major intracellular abnormal protein degradation system in eukaryotic cells that is likely associated with the etiology of AD. Ubiquitin possesses the function of labeling and binding to the proteins for degradation such as APP and γ-secretase activating protein that contributes to etiology of AD [35, 36]. It is found that aberrant form of this protein originating from misreading of UBB gene was accumulated in brain tissues of AD patients [37]. The molecular docking assay in this study showed that β-sitosterol, mairin, and palbinone presumably bound to UBB with strong affinity, implying the possible target of PLP on AD treatment.
MAPK pathway, the downstream signaling of multiple pathways, participated in synapse plasticity, neural cell survival, cell apoptosis, and neuroinflammation. A lot of studies have demonstrated that the crucial proteins of the MAPK signaling pathway such as ERK1, JNK, and p38 were all elevated in AD animal models, thus targeting these proteins could reduce Aβ production, tau phosphorylation, neuroinflammation, and synaptic loss, as well as slowed down the degeneration of cognitive function [38–40]. For example, Neurotropin® alleviated the accumulation of Aβ plaques and Aβ-induced neural cell death via suppression of HIF-1α, p-ERK1/2, p-JNK, and p-p38 in APP/PS1 mice [41]. Therefore, targeting MAPK signaling pathway-related proteins was a promising strategy for AD treatment.
BDNF and NGF are two of the pivotal factors involved in neurotrophin signaling pathway and play important roles in cholinergic synapse and synaptic plasticity. BDNF contributes to the development of hippocampal structure and function, while NGF promotes the function of cerebrum cholinergic neurons and prohibits neural cell death [42]. It is found that BDNF levels in peripheral blood and in cerebrospinal fluid as well as in hippocampus and neocortex of AD patients were significantly decreased compared with controls. However, NGF level in blood did not show evident change, but NGF levels in cerebrospinal fluid and hippocampus and neocortex of AD patients were significantly increased, suggesting that aberrations of neurotrophic factors were involved in the etiology and pathogenesis of AD [43]. Targeting neurotrophin signaling pathway to restore neural function was potential strategy for AD treatment [44]. The results of network analysis in this study also demonstrated that BDNF was one of the key targets related to the treatment of PLP for AD, and kaempferol had strong binding activity with BDNF by molecular docking.
In view of the pathological complexity of disease, herbal medicine can act on various molecules and targets to exert systematic actions on the disease. Network pharmacology is a powerful method to study the synergistic actions and underlying mechanisms of traditional medicines. However, it is a predicting method based on database analysis to find the possible mechanisms of drugs. Further biological studies are warranted to verify the above findings.
5. Conclusions
In conclusion, the present study discovered compound-target-disease interactions and possible mechanisms of PLP on AD treatment using network pharmacology strategy and predicted that β-sitosterol, kaempferol, lactiflorin, mairin, paeoniflorigenone, paeoniflorin, and palbinone were potential active ingredients of PLP, which possibly prevented AD via inhibiting neural cell apoptosis, inflammatory response, and promoting neurotrophy. It provided the theoretic elucidation of the ameliorative effect of PLP against AD and might facilitate the development of PLP or its active compounds as alternative therapy for AD.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (grant no. 81574038), Project of Traditional Chinese Medicine Bureau of Guangdong Province (no. 20191286), Shenzhen Basic Discipline Layout Project (nos. JCYJ20170412161254416 and JCYJ20170413161352000), and Shenzhen Sanming Project of Medicine and Health (no. SZSM201612049).
Contributor Information
Min Ma, Email: tmamin@jnu.edu.cn.
Zhengzhi Wu, Email: szwzz001@163.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
There are no conflicts of interest to declare.
Authors' Contributions
ZW, MM, and MC designed the study and supervised the project. QZ and LL performed the network pharmacology analysis and prepared the manuscript. YJ and ZC collected the data. LD, MC, MM, and ZW all made contributions to the revision of manuscript. All authors read and approved the final manuscript.
Supplementary Materials
The characteristics of 85 compounds in PLP are shown in Supplementary Table 1. The predicted targets of β-sitosterol, kaempferol, lactiflorin, mairin, paeoniflorin, palbinone, and paeoniflorigenone are listed in Supplementary Table 2. The acknowledged targets of Alzheimer disease are displayed in Supplementary Table 3.
References
- 1.Corriveau R. A., Koroshetz W. J., Gladman J. T., et al. Alzheimer’s disease-related dementias summit 2016: national research priorities. Neurology. 2017;89(23):2381–2391. doi: 10.1212/wnl.0000000000004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2018;14(3):367–429. doi: 10.1016/j.jalz.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Lane C. A., Hardy J., Schott J. M. Alzheimer’s disease. European Journal of Neurology. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Q., Siu W., Li L., et al. Autophagy in Alzheimer’s disease and promising modulatory effects of herbal medicine. Experimental Gerontology. 2019;119:100–110. doi: 10.1016/j.exger.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Doody R. S., Thomas R. G., Farlow M., et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. New England Journal of Medicine. 2014;370(4):311–321. doi: 10.1056/nejmoa1312889. [DOI] [PubMed] [Google Scholar]
- 6.Salloway S., Sperling R., Fox N. C., et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. New England Journal of Medicine. 2014;370(4):322–333. doi: 10.1056/nejmoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panza F., Solfrizzi V., Seripa D., et al. Tau-based therapeutics for Alzheimer’s disease: active and passive immunotherapy. Immunotherapy. 2016;8(9):1119–1134. doi: 10.2217/imt-2016-0019. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z., Gu J., Xiu J., et al. Traditional Chinese medicine for senile dementia. Evidence-Based Complementary and Alternative Medicine. 2012;2012:13. doi: 10.1155/2012/692621.692621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen I. C., Lin T.-H., Hsieh Y.-H., et al. Formulated Chinese medicine Shaoyao-Gancao-Tang reduces tau aggregation and exerts neuroprotection through anti-oxidation and anti-inflammation. Oxidative Medicine and Cellular Longevity. 2018;2018:16. doi: 10.1155/2018/9595741.9595741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y., Hu Z.-Y., Yuan H., et al. Danggui-Shaoyao-San improves learning and memory in female SAMP8 via modulation of estradiol. Evidence-Based Complementary and Alternative Medicine. 2014;2014:9. doi: 10.1155/2014/327294.327294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan Z., Liu J., Chen L., et al. Danggui-Shaoyao-San ameliorates cognition deficits and attenuates oxidative stress-related neuronal apoptosis in d-galactose-induced senescent mice. Journal of Ethnopharmacology. 2012;141(1):386–395. doi: 10.1016/j.jep.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 12.Lin H. Q., Ho M. T., Lau L. S., Wong K. K., Shaw P. C., Wan D. C. C. Anti-acetylcholinesterase activities of traditional Chinese medicine for treating Alzheimer’s disease. Chemico-Biological Interactions. 2008;175(1–3):352–354. doi: 10.1016/j.cbi.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Ma X.-H., Duan W.-J., Mo Y.-S., et al. Neuroprotective effect of paeoniflorin on okadaic acid-induced tau hyperphosphorylation via calpain/Akt/GSK-3β pathway in SH-SY5Y cells. Brain Research. 2018;1690:1–11. doi: 10.1016/j.brainres.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H.-R., Peng J.-H., Cheng X.-B., Shi B.-Z., Zhang M.-Y., Xu R.-X. Paeoniflorin atttenuates amyloidogenesis and the inflammatory responses in a transgenic mouse model of Alzheimer’s disease. Neurochemical Research. 2015;40(8):1583–1592. doi: 10.1007/s11064-015-1632-z. [DOI] [PubMed] [Google Scholar]
- 15.Yuan H., Ma Q., Cui H., et al. How can synergism of traditional medicines benefit from network pharmacology? Molecules. 2017;22(7):1135–1153. doi: 10.3390/molecules22071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ru J., Peng L., Jinan W., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gfeller D., Grosdidier A., Wirth M., Daina A., Michielin O., Zoete V. Swisstargetprediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Research. 2014;42(W1):W32–W38. doi: 10.1093/nar/gku293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn M., Szklarczyk D., Franceschini A., von Mering C., Jensen L. J., Bork P. STITCH 3: zooming in on protein-chemical interactions. Nucleic Acids Research. 2012;40(D1):D876–D880. doi: 10.1093/nar/gkr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Research. 2017;46(5):D158–D169. doi: 10.1093/nar/gky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang D. W., Sherman B. T., Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 21.Hsin K.-Y., Matsuoka Y., Asai Y., et al. SystemsDock: a web server for network pharmacology-based prediction and analysis. Nucleic Acids Research. 2016;44(W1):W507–W513. doi: 10.1093/nar/gkw335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May B. H., Lu C., Bennett L., Hügel H. M., Xue C. C. L. Evaluating the traditional Chinese literature for herbal formulae and individual herbs used for age-related dementia and memory impairment. Biogerontology. 2012;13(3):299–312. doi: 10.1007/s10522-012-9375-6. [DOI] [PubMed] [Google Scholar]
- 23.Bahrani H., Mohamad J., Paydar M., Rothan H. Isolation and characterisation of acetylcholinesterase inhibitors from Aquilaria subintegra for the treatment of Alzheimer’s disease (AD) Current Alzheimer Research. 2014;11(2):206–214. doi: 10.2174/1567205011666140130151344. [DOI] [PubMed] [Google Scholar]
- 24.Babaei P., Jafari A., Kouhestani S. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regeneration Research. 2018;13(10):1827–1832. doi: 10.4103/1673-5374.238714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beg T., Jyoti S., Naz F., et al. Protective effect of kaempferol on the transgenic Drosophila model of Alzheimer’s disease. CNS & Neurological Disorders—Drug Targets. 2018;17(6):421–429. doi: 10.2174/1871527317666180508123050. [DOI] [PubMed] [Google Scholar]
- 26.Navabi S. P., Sarkaki A., Mansouri E., Badavi M., Ghadiri A., Farbood Y. The effects of betulinic acid on neurobehavioral activity, electrophysiology and histological changes in an animal model of the Alzheimer’s disease. Behavioural Brain Research. 2018;337:99–106. doi: 10.1016/j.bbr.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Sarkaki A., Farbood Y., Badavi M., et al. The protective effect of betulinic acid on microvascular responsivity and protein expression in Alzheimer disease induced by cerebral micro-injection of beta-amyloid and streptozotocin. Microcirculation. 2018;25(8) doi: 10.1111/micc.12503.e12503 [DOI] [PubMed] [Google Scholar]
- 28.Ayaz M., Junaid M., Ullah F., et al. Anti-Alzheimer’s studies on beta-sitosterol isolated from polygonum hydropiper L. Frontiers in Pharmacology. 2017;8:697. doi: 10.3389/fphar.2017.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S., Youn K., Jun M. Major compounds of red ginseng oil attenuate Aβ25-35-induced neuronal apoptosis and inflammation by modulating MAPK/NF-κB pathway. Food & Function. 2018;9(8):4122–4134. doi: 10.1039/c8fo00795k. [DOI] [PubMed] [Google Scholar]
- 30.Park S., Sapkota K., Kim S., Kim H., Kim S. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. British Journal of Pharmacology. 2011;164(3):1008–1025. doi: 10.1111/j.1476-5381.2011.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C., Zhang C., Zhou H., et al. Inhibitory effects of betulinic acid on LPS-induced neuroinflammation involve M2 microglial polarization via CaMKKbeta-dependent AMPK activation. Frontiers in Molecular Neuroscience. 2018;11:98. doi: 10.3389/fnmol.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masters C. L., Bateman R, Blennow K, Rowe C. C, Sperling R. A, Cummings J. L. Alzheimer’s disease. Nature Reviews Disease Primers. 2015;1(1):p. 15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 33.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochemical Journal. 2008;415(3):333–344. doi: 10.1042/bj20081056. [DOI] [PubMed] [Google Scholar]
- 34.Guo F., Liu X., Cai H., Le W. Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathology. 2018;28(1):3–13. doi: 10.1111/bpa.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu J., Li J.-G., Hoffman N. E., Madesh M., Praticò D. Degradation of gamma secretase activating protein by the ubiquitin-proteasome pathway. Journal of Neurochemistry. 2015;133(3):432–439. doi: 10.1111/jnc.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong L., Huang H.-C., Jiang Z.-F. Relationship between amyloid-beta and the ubiquitin-proteasome system in Alzheimer’s disease. Neurological Research. 2014;36(3):276–282. doi: 10.1179/1743132813y.0000000288. [DOI] [PubMed] [Google Scholar]
- 37.Munari F., Bortot A., Assfalg M., D’Onofrio M. Alzheimer’s disease-associated ubiquitin mutant Ubb + 1: properties of the carboxy-terminal domain and its influence on biomolecular interactions. International Journal of Biological Macromolecules. 2018;108:24–31. doi: 10.1016/j.ijbiomac.2017.11.121. [DOI] [PubMed] [Google Scholar]
- 38.Feld M., Krawczyk M. C., Sol Fustiñana M., et al. Decrease of ERK/MAPK overactivation in prefrontal cortex reverses early memory deficit in a mouse model of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2014;40(1):69–82. doi: 10.3233/jad-131076. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Q., Wang M., Du Y., et al. Inhibition of c-Jun N-terminal kinase activation reverses Alzheimer disease phenotypes in APPswe/PS1dE9 mice. Annals of Neurology. 2015;77(4):637–654. doi: 10.1002/ana.24361. [DOI] [PubMed] [Google Scholar]
- 40.Lee J. K., Kim N.-J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules. 2017;22(8):1287–1309. doi: 10.3390/molecules22081287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang W.-L., Zhao D.-Q., Wang F., et al. Neurotropin alleviates hippocampal neuron damage through a HIF-1α/MAPK pathway. CNS Neuroscience & Therapeutics. 2017;23(5):428–437. doi: 10.1111/cns.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iulita M. F., Bistué Millón M. B., Pentz R., et al. Differential deregulation of NGF and BDNF neurotrophins in a transgenic rat model of Alzheimer’s disease. Neurobiology of Disease. 2017;108:307–323. doi: 10.1016/j.nbd.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Du Y., Wu H.-T., Qin X.-Y., et al. Postmortem brain, cerebrospinal fluid, and blood neurotrophic factor levels in Alzheimer’s disease: a systematic review and meta-analysis. Journal of Molecular Neuroscience. 2018;65(3):289–300. doi: 10.1007/s12031-018-1100-8. [DOI] [PubMed] [Google Scholar]
- 44.Choi S. H., Bylykbashi E., Chatila Z. K., et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018;361(6406) doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The characteristics of 85 compounds in PLP are shown in Supplementary Table 1. The predicted targets of β-sitosterol, kaempferol, lactiflorin, mairin, paeoniflorin, palbinone, and paeoniflorigenone are listed in Supplementary Table 2. The acknowledged targets of Alzheimer disease are displayed in Supplementary Table 3.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.