Abstract
Morinda citrifolia (Rubiaceae) or Noni was previously reported to have leaf with broad therapeutic property whereas the fruit was rarely described as medicinal. Ironically, extensive research and review has been done on the fruit and little was known about the therapeutic activity of the leaf as a medicinal food. The aim of this study was to investigate the therapeutic effects of Morinda citrifolia (MC) ethanolic leaf extract on the hepatic structure and function in postmenopausal rats fed with thermoxidized palm oil (TPO) diet. Thirty eight female Sprague Dawley rats were divided into five groups: sham (Sham), ovariectomized (OVX), ovariectomized and treated with simvastatin 10 mg/kg (OVX+ST), ovariectomized and supplemented with low dose MC 500 mg/kg (OVX+MCLD), and ovariectomized and supplemented with high dose MC 1000 mg/kg (OVX+MCHD). All the ovariectomized groups were fed with TPO diet whereas the Sham group was fed with normal diet. Consumption of TPO diet in postmenopausal rats resulted in obesity, significantly elevated (P < 0.05) liver oxidative stress marker; malondialdehyde (MDA), diffuse microvesicular steatosis, and defective mitochondria. Treatment with MC leaf extract prevented hepatic steatosis by significantly increasing (P < 0.05) the liver antioxidant enzyme SOD and GPx, significantly increasing (P < 0.05) ALP, decreasing liver lipids infiltration, preventing mitochondrial damage, and overall maintaining the normal liver histology and ultrastructure. In conclusion, we provided detailed histological and ultrastructural evidence showing hepatoprotective effects of MC leaf extract through its antioxidant mechanism.
1. Introduction
Hepatic steatosis is a pathological condition that is prevalent in postmenopausal women due to loss of protective effects of oestrogen.Oestrogen deficiency that occurs following menopause causes metabolic changes, alteration in the body composition, and body fat distribution that leads to liver lipid infiltration [1]. Previous animal studies demonstrated that ovariectomy resulted in progressive fat accumulation in the liver [2]. Consumption of thermally oxidized oil or thermoxidized palm oil (TPO) diet by postmenopausal subjects appeared to accelerate the development of hepatic steatosis [2]. TPO is commonly present in daily food especially in fried cuisine and processed food [3]. The cooking oil is reused repeatedly in order to save costs. Chronic consumption of TPO is hazardous to health especially in elderly postmenopausal women because repeated heating of the oil at high temperature decreases the antioxidant content in the oil, increases lipid peroxidation, and generates free radicals-induced oxidative stress in the liver [4]. Previous animal studies showed that ingestion of food containing TPO resulted in elevated liver enzyme and microsteatosis changes in the liver [5]. Currently, there is no effective pharmacological treatment for this pathological condition except for the management of metabolic risk factors by using statins, weight loss, and exercise, but it is unrealistic as it is difficult to achieve or maintain [6]. The use of hormone replacement therapy (HRT) may be beneficial, but it is not recommended for hepatoprotection as it increases the risk of cardiovascular events [7]. Thus, novel therapeutic strategies and nutritive supplementation with functional food are needed to promote liver health.
Noni leaf is the leaf of Morinda citrifolia L. (Rubiaceae) or MC leaf which is an edible famine food and medicinal tropical plant originated from Southeast Asia, Australasia, Pacific Islands, and Hawaii [8]. MC is considered as a sacred plant as it was cited in the ancient text as “Ashyuka” which in Sanskrit means “longevity” (Neal, 1965). Previous review has reported that the leaf is the most commonly used part of the plants for treatment whereas the fruit is rarely described as medicinal [9]. The leaf is consumed as a raw vegetable by various culture around the world and is also cooked to promote postpartum health [10]. In Malaysia, MC is popularly known as mengkudu and is primarily grown for the use in Traditional Malay Medicine to treat a wide range of diseases such as beri beri, fever, cough, liver and kidney diseases, and internal bleeding [11]. MC leaf is rich in nutritient and was included in the World Health Organization (WHO) and Food and Agriculture Organization (FAO) food composition table for East Asia and the Pacific Islands [12]. It was reported to have a higher level of β-carotene compared to other green leafy vegetables and have successfully cured night blindness in children [13]. Rare phytoactive substances with health promoting potential isolated from the leaf includes dehydromethoxygaertheroside, dehydroepoxymethoxygaertheroside, borreiagenin [14], citrifoside, pheophorbide A, pyropheophorbide A, ursolic acid [15], and flavanoids [16]. Previously, we have reported that MC leaf extract possesses antiatherosclerotic effect through its anti-inflammatory activity in the aorta [17]. Since the liver is an indicator of vascular health by secreting and regulating various molecular cardiovascular disease (CVD) risk factors, we further investigated the mechanism of action of MC leaf by looking into the detailed histological and ultrastructural changes in the liver [18]. In this study, we investigated the basis of using MC leaf as a medicinal food in Traditional Malay Medicine to prevent liver disease by studying the effects of the leaf extract supplementation on the liver of postmenopausal rats fed with thermoxidized palm oil (TPO) diet. In particular, we studied the metabolic indicators (body weight, dietary intake, and 11βHSD1), liver function (transaminase level and antioxidant enzyme), and oxidative stress marker (MDA) with emphasis on the liver histological and ultrastructural findings. To the best of our knowledge, there is no other study done on the effect of MC on the ultrastructure of the liver.
2. Material and Methods
2.1. Preparation of Morinda citrifolia Ethanolic Leaf Extract
Morinda citrifolia ethanolic leaf extract in powder form was prepared by Professor Suhaila Mohamed from the Department of Bioscience, Universiti Putra Malaysia. Voucher specimen is available at the herbarium of the department. The extract was prepared by the following procedure as described by the manufacturer. Fresh Morinda citrifolia leaf were collected from Bukit Expo, Universiti Putra Malaysia, and was identified by a botanist. The leaves were washed and homogenized with water. Equal volume of 70% ethanol was then added, soaked for 3 hours, and filtered. The filtrate was put into rotary evaporator to remove the solvent. The resultant green paste was added with 20% starch to make it into powder form and dried in oven. The dried extract was packed in polythene bags with nitrogen purge. The extract was administered via oral gavage daily for three months at the doses of 500 mg/kg and 1000 mg/kg to the respective treatment groups [19].
2.2. Preparation of Thermoxidized Palm Oil Diet
Thermoxidized palm oil diet was custom prepared in our laboratory by formulating 5 times heated palm oil (15% w/w) with standard rat chow [20]. Fresh palm oil (Lam Soon Edible Oil, Malaysia) was thermally oxidized by heating it for five times through frying process [21]. Briefly, 2.5 litres of fresh palm oil was heated in a stainless-steel deep fryer until the temperature reached 180°C after which 1 kg of sweet potatoes were added and fried for 10 minutes. After the frying process, the palm oil was left to cool down to room temperature for 5 hours. The same oil was reused to fry the next batch of sweet potatoes without adding any fresh palm oil. The whole frying process was repeated four times to obtain 5 times heated palm oil (5HPO). 15% weight/weight of the prepared oil was mixed with ground standard rat chow (Gold Coin Sdn Bhd, Malaysia) and then stored in a tight container. The test diet was prepared fresh daily, weighed, and fed to the rats for 3 months.
2.3. Experimental Animals
Thirty eight healthy female Sprague Dawley rats (n = 38) aged 6 months old with body weight of 250-300 g were obtained from the Laboratory Animal Resource Unit, Universiti Kebangsaan Malaysia. The rats were housed in individual plastic cages at room temperature (27°C ± 2°C) with adequate ventilation and a 12-hour light-dark cycle in the Anatomy Department Animal House. All the experimental animals had ad libitum access to food (rat chow from Gold Coin, Selangor Malaysia) and tap water. All the animal handling procedures were in accordance with the institutional animal ethical guidelines with ethical approval number (UKMAEC approval number: FP/ANAT/2014/KHIN/24-SEPT./610-SEPT.-2014-JUNE-2016).
2.4. Study Design
The rats were acclimatized for one week and provided with standard rat chow and tap water. The rats were randomly divided into five groups. The first group underwent mock surgery by opening of the abdominal cavity and sewing it back to simulate surgical stress (Sham, n = 7) while the other four groups were ovariectomized (surgical removal of ovaries bilaterally) to produce oestrogen-deficient state. The second group was ovariectomized and fed with thermoxidized palm oil diet (OVX, n = 7). The third group was ovariectomized, fed with thermoxidized palm oil diet, and supplemented with oral simvastatin suspended in tap water at the dose of 10 mg/kg/day (OVX+ST, n = 8) [22]. The fourth group was ovariectomized, fed with thermoxidized palm oil diet, and supplemented with Morinda citrifolia low dose 500 mg/kg (OVX+MCLD, n = 8). The fifth group was ovariectomized, fed with thermoxidized palm oil diet, and supplemented with Morinda citrifolia high dose 1000 mg/kg (OVX+MCHD, n = 8) [19].
Bilateral ovariectomy was performed under anaesthesia using ventral approach [23]. Briefly, a lower abdomen midline skin incision was made; the ovary and part of the oviduct was identified, exteriorized, and removed. The same process was repeated to remove the contralateral ovary. The incision on the abdominal musculature was closed with 4/0 absorbable catgut suture (Merck, Germany) followed by closure of the skin incision using 4/0 nonabsorbable silk suture (Merck, Germany). Postoperatively, the rats were given antibiotic enrofloxacin (Baytril, Korea) intramuscularly, placed in a clean cage without wood shaving to avoid wound contamination, and strictly monitored postoperatively for behavioural changes. After three weeks of postoperative recovery period, all the ovariectomized rats were fed with thermoxidized palm oil diet and treated for three months. Physiological parameters such as body weight, food intake, and water intake were done to monitor the metabolic changes of the rats. At the end of the experimental period, the rats were sacrificed with diethyl ether (Sigma-Aldrich, Germany). The blood and liver tissues were collected. The success of ovariectomy was confirmed at necropsy by observation of marked atrophy of the uterine horns.
2.5. Serum Biochemical Analyses
Whole blood samples were collected via cardiac puncture, placed into plain tube, and sent immediately to Pathlab & Clinical Laboratory Sdn. Bhd., Malaysia, for serum analyses of liver function test (LFT). Serum AST, ALT, and ALP were measured using assay kits by colorimetric method according to the manufacturer's guidelines.
2.6. Liver Tissue Oxidative Stress Assessment
Immediately after sacrificing the rats, the liver tissues were dissected and stored at −80°C for detection of antioxidant enzymes. A part of the liver tissues were also excised and fixed for histological staining.
MDA level was measured using lipid peroxidation (MDA) colorimetric/fluorometric assay kit by BioVision, USA. 11β-Hydroxysteroid dehydrogenase enzyme type 1 (11βHSD1) was measured using ELISA kit for 11βHSD1 (Cloud-Clone Corp, USA). Tissue glutathione (GSH) was measured by using glutathione assay kit by Cayman Chemical Company, USA [24]; glutathione peroxidase (GPx) was measured using glutathione assay kit by Cayman Chemical Company, USA (Forstrom & Wheeler, 1990); catalase (CAT) was measured using catalase assay kit by Cayman Chemical Company, USA [25]; and superoxide dismutase (SOD) was measured using superoxide dismutase askay Kit by Cayman Chemical Company, USA [26]. All procedures were done according to the manufacturers' guidelines.
2.7. Histological Analyses and Histomorphometry
Immediately after removal, the liver tissues were fixed in 10% formalin for a week with a change in formalin solution to remove traces of blood from the tissue for histological staining. The samples were dehydrated and embedded in paraffin. Thin sections (5 μm) of the liver was cut and stained with haematoxylin and eosin stain to detect the presence of steatosis [27]. The tissues were also stained with Verhoeff van Gieson (VVG) stain to detect the presence of thinning and disruption of the elastic fibres [28].
In qualitative electron microscopy study, 1 mm3 sections of the liver tissues were obtained from two rats from each group. They were rinsed with 0.1 M phosphate-buffered saline (PBS), fixed with glutaraldehyde fixative, and stored at 4°C. The tissues were rinsed again with 0.1 M PBS followed by secondary fixation using 3% uranyl acetate and dehydration with series of ethanol. Infiltration process was done in propylene oxide and embedded in resin at 60°C for 24 hours. After the resin polymerized, the samples were sectioned with a glass knife and stained with toluidine blue stain for semithin section. The area of interests in the semithin tissue samples were identified. Ultrathin sections of the area of interests were obtained using a diamond knife. The samples were placed on the copper grid size of 200 networks. The results were viewed by two expert observers in a double-blinded fashion under transmission electron microscope Tecnai G2 model [29].
2.8. Statistical Analysis
All data were presented as mean ± standard error (SEM). Statistical significance level was set as P < 0.05. Normally distributed data were analysed by parametric test using analysis of variance (ANOVA) followed by post hoc Tukey. All statistical analyses were performed by using Statistical Package for Social Sciences (SPSS) software version 22 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Metabolic Function
Obese postmenopausal rat models were established two weeks after the ovariectomy. All the ovariectomized rats have body weight greater than the mean body weight plus one fold of standard deviation of the normal Sham operated group. Body weight of OVX (292 ± 5 g), OVX+ST (291 ± 8 g), OVX+MCLD (305 ± 11 g), and OVX+MCHD (294 ± 12 g) were significantly higher (P < 0.05) than the Sham group (249 ± 5 g). However, there were no significant differences (P > 0.05) in the body weight among all the ovariectomized groups. Food intake of OVX (16 ± 0.65 g/day), OVX+ST (16 ± 0.38 g/day), OVX+MCLD (15 ± 0.48 g/day), and OVX+MCHD (15 ± 0.65 g/day) were significantly higher (P < 0.05) than that of the Sham group (13 ± 0.34 g/day). OVX+ST (21.5 ± 0.68 ml/day) was shown to have significantly lower water intake compared to the Sham group (25.57 ± 0.90 ml/day). 11β-Hydroxysteroid dehydrogenase enzyme type 1 (11βHSD1) revealed no significant difference (P > 0.05) in all groups. The data is summarized in Table 1.
Table 1.
Physiological parameters and serum and liver tissue analysis of MCLE treatment.
| Variable | Sham | OVX | OVX+ST | OVX+MCLD | OVX+MCHD |
|---|---|---|---|---|---|
| Metabolic function | |||||
| Body weight (g) | 249 ± 5 | 292 ± 7∗ | 291 ± 8∗ | 305 ± 11∗ | 294 ± 12∗ |
| Food intake (g) | 12.86 ± 0.34 | 16.43 ± 0.65∗ | 15.5 ± 0.38∗ | 15.13 ± 0.48∗ | 15.00 ± 0.65∗ |
| Water intake (ml) | 25.57 ± 0.9 | 24.29 ± 1.23 | 21.5 ± 0.68∗ | 21.75 ± 1.22 | 22.5 ± 0.53 |
| 11-βHSD1 (ng/ml) | 31.9 ± 3.43 | 36.59 ± 0.42 | 37.06 ± 0.12 | 36.13 ± 0.92 | 33.82 ± 2.68 |
| Liver function | |||||
| Liver weight (g) | 7.43 ± 0.28 | 7.64 ± 0.37 | 6.69 ± 0.41 | 7.31 ± 0.34 | 6.69 ± 0.16 |
| AST (U/mL) | 128.6 ± 5.31 | 140.71 ± 17.626 | 156.13 ± 15.36 | 182.17 ± 20.56 | 172.13 ± 15.44 |
| ALT (U/mL) | 61.43 ± 6.36 | 0.71 ± 4.8217.3 | 54.13 ± 5.54 | 54.57 ± 3.08 | 63.43 ± 6.23 |
| ALP (U/mL) | 12.04 ± 1.06 | 1 ± 0.56 | 18.78 ± 1.69∗ | 13.76 ± 1.44 | 18.27 ± 2.03∗ |
| Oxidative indices | |||||
| MDA (nmol/mg) | 5.74 ± 0.48 | 7.54 ± 0.62∗ | 7.30 ± 0.33 | 8.31 ± 3.32∗ | 6.85 ± 0.31 |
| GSH (μm) | 26.27 ± 2.56 | 30.03 ± 1.52 | 30.46 ± 1.18 | 28.74 ± 1.68 | 34.31 ± 1.71 |
| GPx (nmol/mg) | 27.92 ± 1.78 | 27.28 ± 3.51 | 35.28 ± 28 | 32.35 ± 1.36 | 44.53 ± 2.50∗#+ |
| SOD (U/mg) | 0.064 ± 0.12 | 0.060 ± 0.08# | 0.078 ± 0.06 | 0.067 ± 0.12 | 0.103 ± 0.014 |
| CAT (nmol/mg) | 6.74 ± 0.53 | 7.16 ± 0.48 | 7.79 ± 0.59 | 7.27 ± 0.63 | 7.29 ± 0.64 |
Values are mean ± SEM, n = 7 (Sham, OVX), n = 8 (OVX+ST, OVX+MCLD, OVX+MCHD). ∗Significant difference from Sham, #significant difference from OVX, +significant difference from OVX+MCLD (P < 0.05).
3.2. Serum Biochemical Parameters (Liver Function)
No significant difference (P > 0.05) were noted in the liver weight in all groups. Serum markers of liver function showed no significant difference (P > 0.05) in the liver transaminase (AST and ALT) in all groups. Isolated rise of ALP (P < 0.05) were seen in OVX+ST (18.78 ± 1.69 U/mL) and OVX+MCHD (18.27 ± 2.03 U/mL). The data is summarized in Table 1.
3.3. Oxidative Stress Assessment
Consumption of thermoxidized palm oil diet were shown to significantly elevate (P < 0.05) the level of lipid peroxidation product malondialdehyde (MDA) in the untreated OVX group (7.54 ± 0.62 nmol/mg) and OVX+MCLD (8.31 ± 0.32 nmol/mg) as compared to the Sham group (5.74 ± 0.48 nmol/mg). The group supplemented with high dose MC (OVX+MCHD) showed significantly increased (P < 0.05) GPx level (44.53 ± 2.50 nmol/mg) compared to Sham (27.92 ± 1.78 nmol/mg), OVX (27.28 ± 3.51 nmol/mg), and OVX+MCLD (32.35 ± 1.36 nmol/mg). In addition, the OVX+MCHD group also showed significantly higher (P < 0.05) level of SOD (0.10 ± 0.01 U/mg) compared to the untreated OVX (0.05 ± 0.01 U/mg). However, no significant differences (P > 0.05) were observed in the level of GSH and CAT in all groups. The data is summarized in Table 1.
3.4. Liver Histopathological and Ultrastructural Assessment
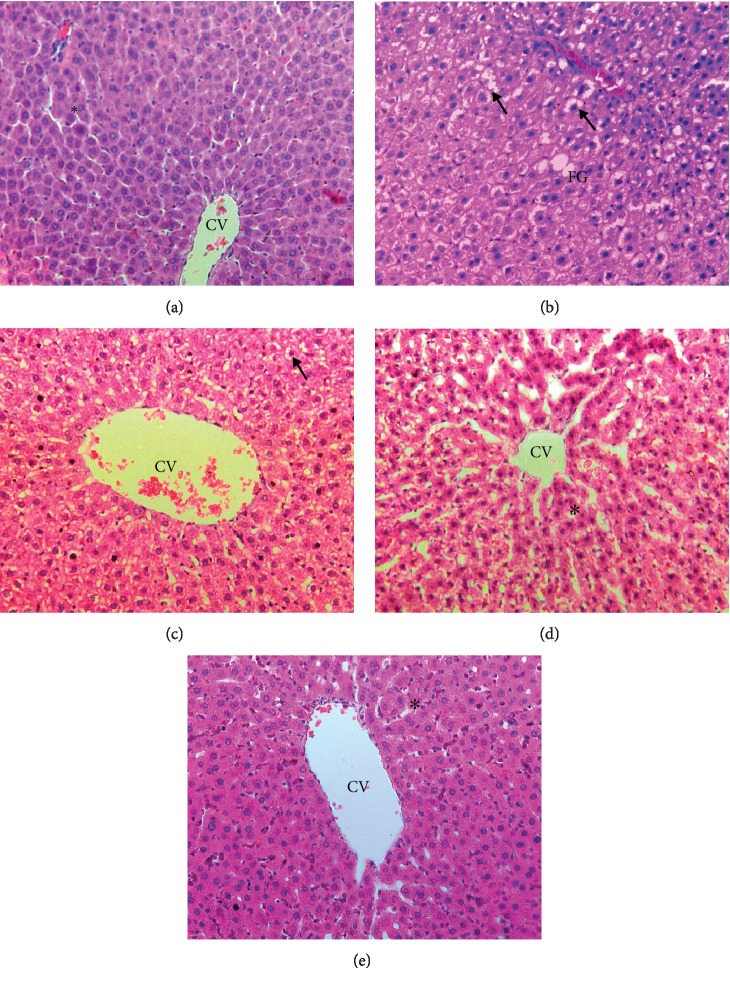
Histopathological evaluation of the liver showed normal hepatic architectures present in the Sham, OVX+MCLD, and OVX+MCHD where there were no signs of inflammation around the central vein and the surrounding sheets of hepatocytes (Figures 1(a), 1(d), and 1(e)). The untreated OVX group showed pathological features of diffuse microvesicular steatosis with features of hypercellularity, congestion, distortion of sinusoids, enlarged hepatocytes, and fat globules deposition (Figure 1(b)). OVX+ST also showed the presence of enlarged hepatocytes (Figure 1(c)).
Figure 1.
(a) Photomicrograph showing H&E-stained liver tissue of the Sham group. Normal sheets of hepatocytes (∗) were seen surrounding the central vein (CV). H&E staining 200x. (b) Photomicrograph showing H&E-stained liver tissue of the untreated OVX group. Note the presence of fat globules (FG) and enlarged hepatocytes with hypercellularity (arrow). H&E staining 200x. (c) Photomicrograph showing H&E-stained liver tissue of the ovariectomized group fed with TPO diet and treated with statin (OVX+ST) which also showed the presence of enlarged hepatocytes (arrow). H&E staining 200x. (d) Photomicrograph showing H&E-stained liver tissue of the ovariectomized group fed with TPO diet and treated with MC leaf 500 mg/kg (OVX+MCLD). Normal sheets of hepatocytes (∗) were seen surrounding the central vein (CV). H&E staining 200x. (e) Photomicrograph showing H&E-stained liver tissue of the ovariectomized group fed with TPO diet and treated with MC leaf 1000 mg/kg (OVX+MCHD). Normal sheets of hepatocytes (∗) were seen surrounding the central vein (CV). H&E staining 200x.
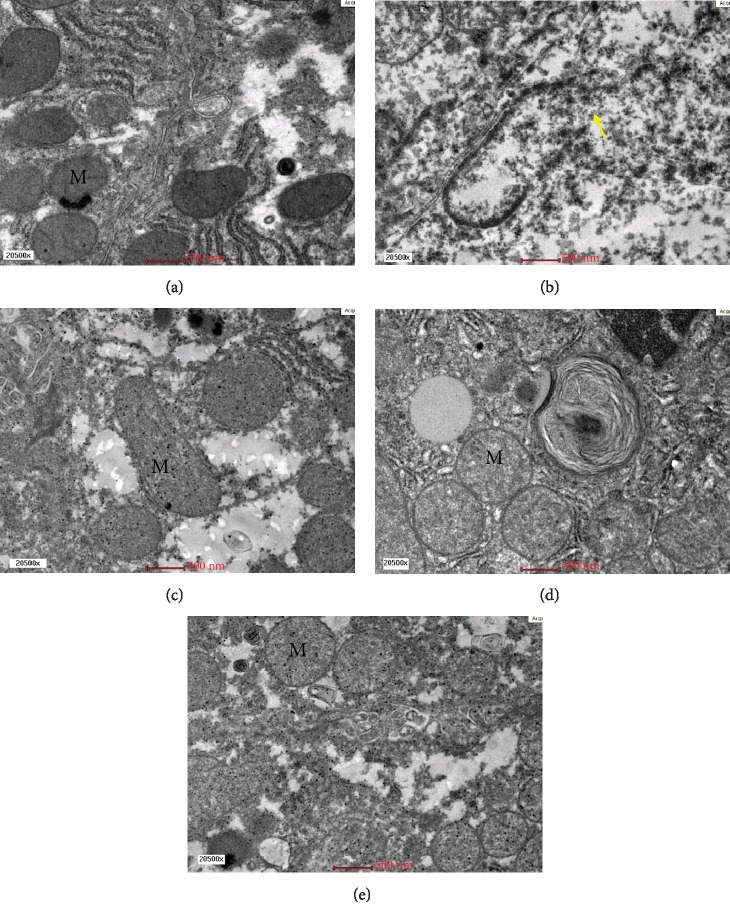
Qualitative electron microscopic findings revealed normal hepatocytes ultrastructure seen in the Sham group with the presence of normal organelles without necrotic cell, disintegrating cell, and apoptotic body. A few lipid droplets were present in a relatively normal distribution. The untreated OVX group showed pathological features of microvesicular steatosis evidenced by the presence of massive amounts of electron-dense lipid droplets deposition. The nucleus appeared relatively enlarged compared to the Sham group and foamy cytoplasm with dense granular deposits were observed (Figure 2(b)). OVX+ST also showed features of microvesicular steatosis as massive numbers of lipid droplets deposition were noted (Figure 2(c)).The OVX+MCLD and OVX+MCHD groups showed obviously less lipid droplets infiltration comparable to that of the Sham group (Figures 2(d) and 2(e)). Ultrastructural studies revealed normal mitochondria with cristae were present in the Sham group (Figure 3(a)). The untreated OVX group showed megamitochondria and ruptured mitochondria with cristolysis (Figure 3(b)). The OVX+ST also showed elongated mitochondria or megamitochondria (Figure 3(c)), whereas the groups treated with MC showed absence of mitochondrial damage (Figures 3(d) and 3(e)).
Figure 2.
(a) Electron micrograph showing the hepatocyte of the Sham group. Normal organelles were seen. EM 6000x. (b) Electron micrograph showing the hepatocyte of the untreated OVX group. Massive amounts of lipid droplets (LD) accumulation (circle) were present around the relatively enlarged nucleus. EM 6000x. (c) Electron micrograph showing the hepatocyte of the OVX rats fed with TPO diet and treated with statin (OVX+ST). Lipid droplet (LD) accumulation was seen surrounding the nucleus. EM 6000x. (d) Electron micrograph showing the hepatocyte of the OVX rats fed with TPO diet and treated with MC leaf 500 mg/kg. Relatively less lipid droplets were observed. EM 6000s. (e) Electron micrograph showing the hepatocyte of the OVX rats fed with TPO diet and treated with MC leaf 1000 mg/kg (OVX+MCHD). Limited amounts of lipid droplets were present. EM 6000x.
Figure 3.
(a) Electron micrograph showing the presence of normal mitochondria with cristae (M) in the hepatocyte of the Sham group. EM 20500x. (b) Electron micrograph showing megamitochondria with cristolysis and mitochondrial rupture (arrow) in the untreated OVX group. EM 20500x. (c) Electron micrograph showing megamitochondria with cristolysis and mitochondrial rupture (arrow) in the untreated OVX group. EM 20500x. (d) Electron micrograph showing normal mitochondria with cristae (M) in the hepatocyte of OVX+MCLD comparable to the Sham group. EM 20500x. (e) Electron micrograph showing normal mitochondria with cristae (M) in the hepatocyte of OVX+MCHD comparable to that of the normal Sham group. EM 20500x.
4. Discussion
Ovariectomized rats fed with thermoxidized palm oil (TPO) diet were used in this study as an experimental model of hepatic steatosis. Ovariectomized rat is an excellent animal model that represent postmenopausal oestrogen deficiency in human. The rats were fed with TPO diet to reflect the actual diet in human where most of our foods are cooked using palm oil especially fried cuisine and processed food [3]. In reality, elderly postmenopausal subjects exposed to TPO diet are more susceptible to develop hepatic steatosis due to loss of protective effects of oestrogen [2].
After 12 weeks of TPO feeding, all the ovariectomized rats developed hyperphagia and obesity. This metabolic change is due to the removal of catabolic actions of oestrogen which act upon central neuropeptidergic pathway that regulate feeding and energy expenditure in the hypothalamus [30]. In obese subjects, failure to downregulate 11β-HSD1 enzyme causes liver lipids infiltration [31]. However, in this study, we did not observe any significant difference in the level of 11β-HSD1 enzyme in all groups. According to this findings, we concluded that 11β-HSD1 did not play a role in the pathogenesis of hepatic steatosis in rat models.
Consumption of TPO in postmenopausal rats did not cause significant increase in liver transaminase which indicate that the liver is functioning optimally and there is no acute liver toxicity present. This findings were in contrast with previous study by [5]. The discrepancy occurs because longer duration of TPO feeding was used in that study. Isolated rise in ALP which were noted in the groups treated with statin (OVX+ST) and OVX+MCHD that could indicate increase in bone formation. Both statin and MC were reported to have significant antiosteoporotic activity by increasing the expression of ALP in vitro and increasing osteoclasts activity [32, 33].
Consumption of TPO in postmenopausal rats leads to oxidative stress in the liver. The untreated OVX group showed significantly higher lipid peroxidation product MDA. This result is in accordance with [34]. Repeated heating of palm oil at high temperature decreases the antioxidant content of the oil and changes its chemical composition through hydrolysis, oxidation, and polymerization [4]. Hydrolysis of the oil molecule produces free fatty acid (FFA) and secondary lipid peroxidation products such as aldehydes, ketones, and alcohols. Oxidation of lipids generates free radicals as fatty acid undergoes saturation and receives reactive oxygen species (ROS). ROS from the oil is absorbed into the food and subsequently into the GIT and blood circulation where it damages the lipids by initiating lipid peroxidation. The end product of lipid peroxidation is MDA which is highly mutagenic. In the liver, MDA causes inflammation [35] and oxidative stress leading to hepatic steatosis [36]. In our study, the group treated with low dose MC also showed significantly higher MDA level probably because the low dose was insufficient to promote therapeutic effects. However, high-dose MC showed lower MDA level nonsignificantly compared to the untreated group. Treatment with high-dose MC showed significantly higher antioxidant enzyme GPx and SOD in accordance with those reported by [37]. These findings proved that MC leaf extract protects the liver from oxidative stress by increasing the antioxidant enzyme, thus maintaining the oxidative balance in the liver. However, treatment with high-dose MC did not cause significant increase in CAT and GSH.
Oxidative stress is manifested as microvesicular steatosis visualized under H&E staining in the untreated OVX group. Microvesicular steatosis indicates the presence of severe mitochondrial dysfunction [38] due to a defect in mitochondrial β-oxidation [39]. In this study, TPO acts as a hepatotoxin that initiates lipid peroxidation causing histological changes such as distended hepatocytes, clear cytoplasm instead of pink, centrally located nucleus, and hepatocytes ballooning or enlarged hepatocytes. Hepatocytes ballooning is a histological hallmark of cellular injury and cytoskeletal damage [38]. Treatment with MC leaf minimalized all these histological damages.
Under electron microscopy, the most striking features found in the untreated OVX group include massive amounts of lipid droplets accumulation, foamy cytoplasm, matrix granulation, and ruptured mitochondria (Figures 2(b) and 2(c)). Megamitochondria and ruptured mitochondria indicate the presence of biochemical hepatic injury due to the disturbance in the mitochondrial electron transport chain and oxidative injury [40]. Megamitochondria also represents cellular adaptive response to oxidative damage. Elongated and enlarged mitochondria indicate the presence of metabolic abnormality [38]. Decreased protein synthesis in the mitochondria and impaired respiratory chain function lead to the appearance of mitochondrial matrix granules. Foamy cytoplasm was prominently seen in the untreated OVX group due to glycogen accumulation which occurs when lipids accumulate in the hepatocytes causing hepatocyte swelling, narrowing of sinusoidal lumen, sinusoidal damage, and decreased blood flow [29].
Treatment with statin in the absence of dyslipidemia appeared to cause massive accumulation of lipid droplets in the liver (Figure 2(c)) and megamitochondria (Figure 3(c)). Based on these findings, we do not support the use of statin as a primary prevention or prophylaxis of cardiovascular disease (CVD) as it causes liver lipid infiltration [41]. Treatment with MC did not cause lipid accumulation in the liver and mitochondrial damage (Figures 2(d) and 2(e)). These ultrastructural findings justified that MC leaf extract possesses hepatoprotective effects by preventing liver lipid accumulation, minimalized hepatocellular damage, and overall maintaining the normal histology of the liver.
Our findings are in contrast with previous reports stating that anthraquinones found in MC: morindin and rubiadin, are toxic and all MC products are screened for the presence of these compounds [8]. However, the toxic anthraquinones are only found in the root and bark where it is used as a colouring dye and not in the leaf [11]. Recent findings demonstrated that MC leaf extract showed no observable hepatotoxicity [42]. Phytoactive substances responsible for the antioxidant effects observed in this study are flavanoids (rutin, quercetin, and kaempferol) which act against lipid peroxidation, nitric oxide, and hydroxyl radicals [43]. Flavanoids found in MC also exert anti-inflammatory activity by inhibiting the release of proinflammatory cytokines such as TNF-α, IL-1β, and NO [44]. Ursolic acid also played a vital role in reversing hepatic steatosis and improving metabolic function by upregulating the hepatic peroxisome proliferator-activated receptor (PPAR-α) [45].
5. Conclusion
To date, our study is the first to our knowledge to rationalize the hepatoprotective effects of MC leaf extract against hepatic steatosis at ultrastructural level. Consumption of TPO diet in postmenopausal rats resulted in adverse metabolic changes such as obesity and hyperphagia, elevated lipid peroxidation product, MDA in the liver, and prominent pathological changes in the liver ultrastructure such as diffuse microvesicular steatosis with severe lipid droplet deposition and mitochondrial damage. Treatment with MC leaf extract resulted in elevated liver antioxidant enzymes, less lipid droplet deposition, and the normal liver histology and the ultrastructure was maintained. In conclusion, MC leaf extract prevents cellular hepatic injury through the antioxidant mechanism of flavanoids and ursolic acid.
Acknowledgments
This work was financially supported by Universiti Kebangsaan Malaysia fundamental grant (320007001), grant no. FF2014-368. We would like to thank Prof. Suhaila Mohamed for the supply of MC leaf extract, Low Kiat Cheong from the animal ethics committee, Nurjumiatun binti Hood from the electron microscopy unit, and staff of the Anatomy Department for sacrificing the rats on our behalf.
Data Availability
All the analysed data were presented in the thesis of Dr. Gloria Chong Chui Lin in the fulfilment of Master in Medical Science and are available at Universiti Kebangsaan Malaysia library. Correspondence should be addressed to miss_gloe@yahoo.com.
Disclosure
Part of the results in this study was presented as poster presentation in the 2nd International Conference on Advances in Medical Sciences (2nd ICAMS) 14–16 April 2015, Kuala Lumpur, Malaysia (abstract no. RS1000135).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Lavoie J. M., Pighon A. NAFLD, estrogens, and physical exercise: the animal model. Journal of Nutrition and Metabolism. 2012;2011:13. doi: 10.1155/2012/914938.914938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paquette A. M., Shinoda R. R., Lhoret P. D., Lavoie J. M. Time course of liver lipid infiltration in ovariectomized rats: impact of a high-fat diet. Maturitas. 2007;58(2):182–190. doi: 10.1016/j.maturitas.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Yagi K., Kiuchi K., Saito Y., et al. Use of a new methylene blue derivative for determination of lipid peroxides in foods. Biochemistry International. 1986;12(2):367–371. [PubMed] [Google Scholar]
- 4.Choe E., Min D. B. Chemistry of deep-fat frying oils. Journal of Food Science. 2007;72(5):R77–R86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 5.Jaarin K., Nor Aini U., Aishah M. A. S., Das S. Palm oil fat diet consumption and its effects on serum liver enzymes and microscopic changes in experimental rats. Pakistan Journal of Nutrition. 2015;14(9):575–580. doi: 10.3923/pjn.2015.575.580. [DOI] [Google Scholar]
- 6.Angulo P., Lindor K. D. Treatment of nonalcoholic fatty liver: present and emerging therapies. Seminar in Liver Disease. 2001;21(1):81–88. doi: 10.1055/s-2001-12931. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie J., Fisher B. M., Jaap A. J., Stanley A., Paterson K., Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clinical Endocrinology. 2006;65(1):40–44. doi: 10.1111/j.1365-2265.2006.02543.x. [DOI] [PubMed] [Google Scholar]
- 8.Nelson S. C., Elevitch C. R. Noni: The Complete Guide for Consumers and Growers. USA: Permanent Agriculture Resources; 2006. [Google Scholar]
- 9.McClatchey W. From Polynesian healers to health food stores: changing perspectives of Morinda citrifolia (Rubiaceae) Integrative Cancer Therapies. 2002;1:110–120. doi: 10.1177/1534735402001002002. [DOI] [PubMed] [Google Scholar]
- 10.Sabda S. 202 Khasiat Herba. Malaysia: Grup Buku Karangkraf; 2011. [Google Scholar]
- 11.Eland S. Indian mulberry – plant biographies. 2008. November 2014, http://www.plantlives.com/docs/M/Morinda_citrifolia.pdf.
- 12.Dignan C., Burlingame B., Kumar S., Aalsbersberg W. The Pacific Islands Food Composition Tables. 2nd. Rome: UN FAO; 2004. https://www.fao.org/docrep/007/y5432e00.htm. [Google Scholar]
- 13.Aalbersberg W. G. L., Hussein S., Sotheeswaran S., Parkinson S. Carotenoids in the leaves of Morinda citrifolia. Journal of Herbs, Spices and Medicinal Plants. 1993;2:51–54. doi: 10.1300/J044v02n01_07. [DOI] [Google Scholar]
- 14.Schripsema J., Caprini G. P., Dagnino D. Revision of structures of citrifolinin A, citrifolinoside, yopaaoside A, yopaaoside B, and morindacin, iridoids from Morinda citrifolia L. and Morinda coreia. Organic Letters. 2006;9:5337–5340. doi: 10.1021/ol0622108. [DOI] [PubMed] [Google Scholar]
- 15.Takashima J., Ikeda Y., Komiyama K., Hayashi M., Kishida A., Ohsaki A. New constituents from the leaves of Morinda citrifolia. Chemical and Pharmaceutical Bulletin. 2007;55(2):343–345. doi: 10.1248/cpb.55.343. [DOI] [PubMed] [Google Scholar]
- 16.Sang S., Cheng X., Zhu N., et al. Iridoid glycosides from the leaves of Morinda citrifolia. Journal of Natural Products. 2001;64(6):799–800. doi: 10.1021/np010011l. [DOI] [PubMed] [Google Scholar]
- 17.Chong C. L. G., Faizah O., Farida H. Vascular protective effects of Morinda citrifolia leaf extract on postmenopausal rats fed with thermoxidized palm oil diet: evidence at microscopic level. International Journal of Vascular Medicine. 2018;2018:10. doi: 10.1155/2018/6317434.6317434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chitturi S., Farrell G. C. Fatty liver now, diabetes and heart attack later? The liver as a barometer of metabolic health. Journal of Gastroenterology and Hepatology. 2007;22(7):967–969. doi: 10.1111/j.1440-1746.2007.04995.x. [DOI] [PubMed] [Google Scholar]
- 19.Mandukhail S. R., Aziz N., Gilani A. H. Studies on antidyslipidemic effects of Morinda citrifolia (Noni) fruit, leaves and root extracts. Lipids in Health and Disease. 2010;9(1, article 88) doi: 10.1186/1476-511x-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan K. X., Omar N. A., Low W. Y., et al. Reheated palm oil consumption and risk of atherosclerosis: evidence at ultrastructural level. Evidence-based Complementary and Alternative Medicine. 2012;2012:6. doi: 10.1155/2012/828170.828170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owu D. U., Osim E. E., Ebong P. E. Serum liver enzymes profile of Wistar rats following chronic consumption of fresh or oxidized palm oil diets. Acta Tropica. 1998;69:65–73. doi: 10.1016/s0001-706x(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 22.Birnbaum Y., Lin Y., Ye Y., Merla R., Perez-Polo J. R., Uretsky B. F. Pretreatment with high-dose statin, but not low-dose statin, ezetimibe, or the combination of low-dose statin and ezetimibe, limits infarct size in the rat. Journal of Cardiovascular Pharmacology and Therapeutics. 2008;13(1):72–79. doi: 10.1177/1074248407312839. [DOI] [PubMed] [Google Scholar]
- 23.Patki G., Allam F. H., Atrooz F., et al. Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female Wistar rats. PLoS One. 2013;8(9, article e74522) doi: 10.1371/journal.pone.0074522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foyer C. H., Lelandais M., Kunert K. J. Photooxidative stress in plants. Physiologia Plantarum. 1994;92:696–717. doi: 10.1111/j.1399-3054.1994.tb03042.x. [DOI] [Google Scholar]
- 25.Johansson L. H., Borg L. Spectrophotometric method for determination of catalase activity in small tissue samples. Analytical Biochemistry. 1988;174(1):331–336. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- 26.Maier C. M., Chan P. H. Book review: pole of superoxide dismutase in oxidative damage and neurodegenerative disorders. The Neuroscientist. 2002;8(4):323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- 27.Bancroft J. D., Harry C. C. Manual of Histological Techniques and their Diagnostic Application. 2nd. New York, NY, USA: Churchill Livingstone; 1994. [Google Scholar]
- 28.Drury R. A. B., Wallington E. A. Carleton’s Histological Technique. 5th. UK: Oxford University Press; 1980. [Google Scholar]
- 29.Ahishali E., Demir K., Ahishali B., et al. Electron microscopic findings in non-alcoholic fatty liver disease: is there a difference between hepatosteatosis and steatohepatitis? Journal of Gastroenterology and Hepatology. 2010;25:619–626. doi: 10.1111/j.1440-1746.2009.06142.x. [DOI] [PubMed] [Google Scholar]
- 30.Picard F., Deshaies Y., Lalonde J., et al. Effects of the estrogen antagonist EM-652.HCl on the energy balance and lipid metabolism in ovariectomized rats. International Journal of Obesity and Related Metabolic Disorders. 2000;4(7):830–840. doi: 10.1038/sj.ijo.0801240. [DOI] [PubMed] [Google Scholar]
- 31.Coyle F. M., Taylor N. F., Feakins R. Non-alcoholic fatty liver disease is associated with transcriptional dysregulation of 11β-hydoxysteroid dehydrogenase type 1 leading to excess intrahepatic glucocorticoid exposure. Endocrine Abstracts. 2008;15:p. 328. [Google Scholar]
- 32.Chen P. Y., Sun J. S., Tsuang Y. H., Chen M. H., Weng P. W., Lin F. H. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutrition Research. 2010;30(3):191–199. doi: 10.1016/j.nutres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Li N., Qin L. P., Han T., Wu Y. B., Zhang Q. Y. Inhibitory effects of Morinda officinalis extract on bone loss in ovariectomized rats. Molecules. 2009;14(6):2049–2061. doi: 10.3390/molecules14062049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falade A. O., Oboh G., Ademiluyi A. O., Odubanjo O. V. Consumption of thermally oxidized palm oil diets alters biochemical indices in rats. Beni-Suef University Journal of Basic and Applied Sciences. 2015;4(2):150–156. doi: 10.1016/j.bjbas.2015.05.009. [DOI] [Google Scholar]
- 35.Jaeschke H., Wang Y., Esani N. A. Reactive oxygen species activate the transcription factor NF-κB in the liver by induction of lipid peroxidation (abstract) Hepatology. 1996;24, article 238A [Google Scholar]
- 36.Letterson P., Fromenty B., Terris B. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. Journal of Hepatology. 1996;24(2):200–208. doi: 10.1016/s0168-8278(96)80030-4. [DOI] [PubMed] [Google Scholar]
- 37.Anitha T., Mohandass S. Anti-oxidant activity of Morinda citrifolia on lymphoma-bearing mice. Ancient Science of Life. 2006;26(1-2):p. 85. [PMC free article] [PubMed] [Google Scholar]
- 38.Tandra S., Yeh M. M., Brunt M. E., et al. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. Journal of Hepatology. 2011;55(3):654–659. doi: 10.1016/j.jhep.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fromenty B., Berson A., Pessayre D. Microvesicular steatosis and steatohepatitis: role of mitochondrial dysfunction and lipid peroxidation. Journal of Hepatology. 1997;26(1):13–22. doi: 10.1016/s0168-8278(97)82328-8. [DOI] [PubMed] [Google Scholar]
- 40.Le T. H., Caldwell S. H., Redick J. A. The zonal distribution of megamitochondria with crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2004;39(5):1423–1429. doi: 10.1002/hep.20202. [DOI] [PubMed] [Google Scholar]
- 41.Okuyama H., Langsioen P. H., Hamazaki T., et al. Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Review of Clinical Pharmacology. 2015;8(2):189–199. doi: 10.1586/17512433.2015.1011125. [DOI] [PubMed] [Google Scholar]
- 42.Shalan M. N. A. A., Mustapha N. M., Mohamed S. Chronic toxicity evaluation of Morinda citrifolia fruit and leaf in mice. Regulatory Toxicology Pharmacology. 2017;83:46–53. doi: 10.1016/j.yrtph.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Serafini M. R., Santos R. C., Guimarães A. G., et al. Morinda citrifolia Linn leaf extract possesses antioxidant activities and reduces nociceptive behavior and leukocyte migration. Journal of Medicinal Food. 2011;14(10):1159–1166. doi: 10.1089/jmf.2010.0254. [DOI] [PubMed] [Google Scholar]
- 44.Aurasorn S., Pattana S. Anti-inflammatory effect of Morinda citrifolia leaf extract on macrophage RAW 264.7 cells. ScienceAsia. 2015;41:5–11. doi: 10.2306/scienceasia1513-1874.2015.41.005. [DOI] [Google Scholar]
- 45.Li S., Meng F., Liao X., et al. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS One. 2014;9(1):1–13. doi: 10.1371/journal.pone.0086724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the analysed data were presented in the thesis of Dr. Gloria Chong Chui Lin in the fulfilment of Master in Medical Science and are available at Universiti Kebangsaan Malaysia library. Correspondence should be addressed to miss_gloe@yahoo.com.