Abstract
Liver ischemia/reperfusion (IR) injury is a common phenomenon after liver resection and transplantation, which often results in liver graft dysfunction such as delayed graft function and primary nonfunction. The mammalian target of rapamycin (mTOR) is an evolutionarily highly conserved serine/threonine protein kinase, which coordinates cell growth and metabolism through sensing environmental inputs under physiological or pathological conditions, involved in the pathophysiological process of IR injury. In this review, we mainly present current evidence of the beneficial role of mTOR in modulating inflammation and autophagy under liver IR to provide some evidence for the potential therapies for liver IR injury.
1. Introduction
Liver resection and transplantation are the most effective approaches for liver cancer and other end-stage liver diseases. However, liver ischemia/reperfusion (IR) injury is a common complication after liver surgery, which is characterized by aggravated hepatocellular damage in the ischemic liver after the restoration of blood flow [1]. Additionally, abdominal trauma, myocardial ischemia, stroke, and hemorrhagic shock can also cause insufficient liver blood flow, resulting in liver IR injury after reperfusion. Liver IR injury can be divided into warm IR injury and cold IR injury, based on different ischemia conditions. The warm IR injury develops during liver surgery and various forms of shock and trauma, while the cold IR injury occurs during liver transplantation [2]. The severity of the injury ranges from moderate serum aminotransferase level increase to postoperative liver failure after liver resection or to delayed graft function and even primary nonfunction after liver transplantation [3]. Thus, it is of vital importance to investigate the underlying mechanisms and search for possible interventions to protect the liver from IR injury.
Various factors are involved in the pathophysiological process of liver IR injury, including active oxygen species (ROS) overproduction, excessive inflammatory response (redundant inflammatory cytokine release and activation of complement system), the overactivation of autophagy and endoplasmic reticulum stress (ERS), and mitochondrial dysfunction [2]. Among all these factors, inflammation and autophagy are two critical ones. Mammalian target of rapamycin (mTOR) is a critical regulator of cell growth and metabolism that senses and integrates various signals under physiological and pathological conditions, playing critical roles in regulating liver IR injury [4–9].
In this review, we will focus on the role of mTOR signaling in regulating inflammation and autophagy processes in liver IR injury, highlighting the protective role of mTOR signaling and providing some evidence for the potential therapies for liver IR injury.
2. mTOR Signaling Pathway
The mammalian target of rapamycin (mTOR) is an evolutionarily highly conserved serine/threonine protein kinase that plays a vital role in regulating mRNA translation, metabolism, and protein turnover [10]. And its dysfunction relates to autoimmune diseases, cancer, obesity, and senescence [11]. mTOR combines with several proteins to constitute two distinct complexes, named mTOR complexes 1 (mTORC1) and 2 (mTORC2). mTORC1 is composed of five components: mTOR, regulatory protein associated with mTOR (Raptor), mammalian lethal with Sec13 protein 8 (mLST8 or GßL), proline-rich Akt substrate of 40 kDa (PRAS40), and DEP domain containing mTOR interacting protein (DEPTOR). mTORC2 is composed of mTOR, rapamycin insensitive companion of mTOR (Rictor), mLST8, DEPTOR, and the regulatory subunits mSin1 and Protor1/2 [10]. mTORC1 integrates stimuli from intracellular and extracellular cues, such as growth factors, energy status, amino acids, stress, and oxygen, and is sensitive to rapamycin. mTORC1 plays a crucial role in controlling protein, lipid, nucleotide, and glucose metabolism, autophagy, energy metabolism, lysosome biogenesis, cell survival, and cytoskeletal organization [12]. mTORC2 is insensitive to nutrients and acute rapamycin treatment but sensitive to growth factors [12], which regulate cell cytoskeletal remodeling, cell migration, glucose metabolism, ion transport, and cell survival [10]. Moreover, mTORC2 can phosphorylate and activate Akt (on S473), a major effector of the insulin/PI3K pathway, which is essential for the activation of mTORC1 [10]. Besides, mTORC2 can also be phosphorylated and activated by Akt in the subunit of mSin1 (on T86) [13]. Since mTORC1 is the better characterized and well-studied mTOR complex and exerts major regulatory function on various fundamental cell processes, we will mainly focus on mTORC1 in this review.
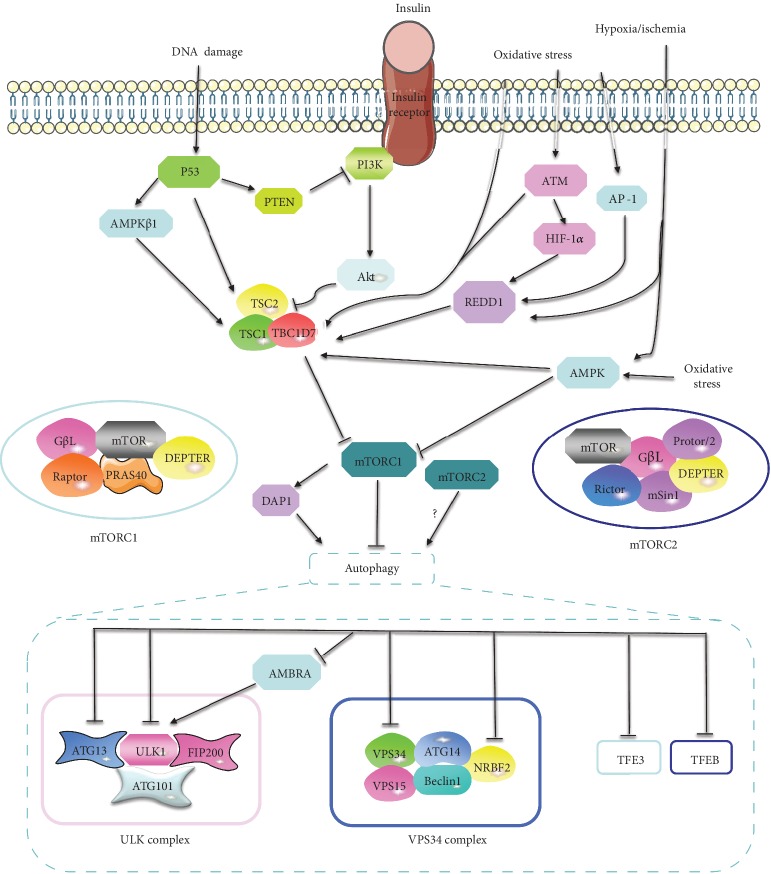
mTORC1 integrates upstream signaling molecules such as growth factors (insulin), epidermal growth factor (EGF), amino acids, energy, stress, and mitogens via multiple signaling pathways [14]. There exist four major upstream signaling pathways of mTORC1, including the insulin/phosphatidylinositol-3 kinase/protein kinase B (insulin/PI3K/Akt) signaling pathway, EGF/Ras/Raf/mitogen activated protein kinase (EGF/Ras/Raf/Mek/Erk) signaling pathway, Wnt/glycogen synthase kinase-3β (Wnt/GSK-3β) signaling pathway, and adenosine monophosphate-activated protein kinase (AMPK) signaling pathway [12, 15]. All of these four axes are converged at least partially on tuberous sclerosis complex (TSC), which is composed of TSC1, TSC2, and TBC1D7 and functions as a GTPase activating protein (GAP) of the Ras homolog enriched in brain (Rheb) GTPase. The GTP-bound form of Rheb directly binds and activates mTORC1 activity. As a GAP of Rheb, TSC converts GTP-Rheb into its inactive GDP-bound form to inhibit the activity of mTORC1 [10] (Figure 1).
Figure 1.
The mTOR signaling pathway is involved in liver IR injury and plays crucial roles in autophagy. Growth factors such as insulin activates mTORC1 through the PI3K/Akt/mTORC1 pathway. However, the activation of the AMPK signaling pathway will lead to the inhibition of mTORC1 through activating TSC complex. Hypoxia/ischemia, oxidative stress, and DNA damage are mechanisms commonly involved in liver IR injury. The decrease of ATP induced by hypoxia/ischemia activates AMPK, which inhibits mTORC1 through activating TSC or suppressing mTOR directly. Additionally, hypoxia/ischemia also activates REDD1, which promotes the TSC-mediated suppression of mTOR. Oxidative stress induces the activation of ATM, which inhibits mTORC1 through activating TSC directly or through phosphorylating HIF1α, resulting in induction of REDD1, causing the activation of TSC. Besides, oxidative stress promotes the activation of AP-1, which transcriptionally upregulates the expression of REDD1. Finally, DNA damage inhibits mTOR through inducing PTEN, AMPKβ1, and TSC, which are targeted by p53. mTOR signaling plays a crucial and complex role in autophagy. In the initial phase of autophagy, mTORC1 inhibits ULK1 complex (ULK1/Atg13/ATG101/FIP200) via directly phosphorylating ULK1 and ATG13. Besides, mTORC1 can also inhibit ULK complex through phosphorylating and suppressing AMBRA, which enhances the activity and stability of ULK1. Additionally, mTORC1 represses the initial of autophagy also through inhibiting VPS34 complex (VPS34/VPS15/Beclin1/ATG14/NRBF2) by directly phosphorylating ATG14 and NRBF2. In the elongation/closure phase, mTORC1 suppresses autophagic and lysosomal biogenesis through phosphorylating TFEB and TFE3 to modulate their nuclear-cytoplasmic shuttling. Moreover, mTORC1 can also augment autophagy through phosphorylating DAP1, which acts as a buffering mechanism that counterbalances the autophagic flux and prevents its overactivation. Additionally, mTORC2 also participated in the induction of autophagy through an unclear mechanism.
3. The mTOR Signaling and Liver IR
3.1. Inhibition of mTOR Signaling in Liver IR
The inhibition of mTOR signaling during liver IR has been shown in many studies [5, 6, 9]. Hypoxia/ischemia, oxidative stress, and DNA damage are commonly involved in liver IR injury [16], which suppress mTOR through various molecular pathways.
Under conditions of hypoxia/ischemia, liver AMPK is activated in a very short window of time (about 2 min) to respond to the increased intracellular AMP/ATP and/or ADP/ATP ratio [17, 18]. Activated AMPK suppresses mTORC1 by phosphorylating TSC (on S1345) to amplify the inhibitory activity of TSC to mTORC1 [19]. Besides, AMPK directly phosphorylates Raptor (on S792), a component of mTORC1, leading to the allosteric inhibition of mTORC1 [19]. In addition, hypoxia/ischemia inhibits mTORC1 also through mediating regulated in DNA damage and development 1 (REDD1) in hepatocytes [20]. In response to hypoxia/ischemia, the expression of REDD1 is transcriptionally upregulated [21]. REDD1 converges on TSC and promotes TSC-mediated suppression of mTORC1 through mediating 14-3-3 protein shuttling from TSC to REDD1 [15].
Oxidative stress, known as redox balance dysregulation and overformation of ROS, also exerts inhibitory effects on mTORC1 [22–28]. Antioxidants such as N-acetylcysteine [29] and hydrogen sulfide [8, 30] can effectively restore the activity of mTORC1, which is repressed by oxidative stress in organ IR injury, including the liver. The mechanisms behind may be as follows: ROS can activate TSC to suppress the activation of mTORC1 [31]. Besides, ROS also inhibits mTORC1 through activating cytoplasmic ataxia telangiectasia mutated (ATM) [22, 25]. Activated ATM further activates TSC [22] or phosphorylates HIF1α, leading to the activation of REDD1 [32], resulting in the inhibition of mTORC1. Additionally, H2O2-induced ROS burst can induce the activation of activator protein-1 (AP-1), which transcriptionally regulates the activation of REDD1 in hepatocytes [33], leading to the suppression of mTORC1. Moreover, ROS can inhibit mTORC1 through activating AMPK as well [24, 34].
Finally, the DNA damage will lead to the activation of p53, which causes the activation of TSC2, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), and β1 subunits of the AMPK (AMPKβ1), resulting in the suppression of mTORC1 [35] (Figure 1).
3.2. The Beneficial Effects of mTOR Signaling in Liver IR Injury
The beneficial effects of mTOR in IR have been observed in the heart [36–43], brain [44–47], intestine [48, 49], and kidney [50]. Similarly, the protective function of mTOR signaling in liver IR injury has been revealed in some studies (Table 1). Bortezomib [4], melatonin [5], geniposide [7], NaHS [8], and agomir-miR-494 [9] administration attenuated liver IR injury through activating mTOR signaling. Additionally, genetic overexpression of liver mTOR directly significantly reduces liver inflammation and apoptosis induced by IR [6].
Table 1.
The effect of mTOR in liver IR injury.
| Study | Effect of mTOR | Animal model | Interventions | “Side effects” of intervention |
|---|---|---|---|---|
| Bejaoui et al. [4] | Protective | Obese Zucker rats | Bortezomib (100 nmol/L) addition to Institut George Lopez- (IGL-) 1 preservation solution | Enhances the activity of AMPK [4]. Attenuates inflammatory processes through YKL-40 [143] and NF-κB [144, 145] inhibition. Activates endothelial nitric oxide synthase (eNOS) [146] |
| Kang et al. [5] | Protective | C57BL/6 mice | Melatonin (10 mg/kg, i.p.) 15 min prior to ischemia and again before reperfusion | Inhibits oxidative stress. Improve the endothelial function. Restores mitochondrial function. Suppresses TLR and JNK pathways [94]. Activates RISK, SAFE, ERK1/2, PKB, PKC, JAK/STAT3, SIRT1/SIRT3, AMPKα, and Notch1/Mfn2 pathways [122] |
| Li et al. [6] | Protective | Alb-TSC1−/− and Alb-mTOR−/− transgenic mice | Overexpression and knockdown of liver mTOR | None |
| Rong et al. [7] | Protective | Sprague-Dawley (SD) rats | Geniposide (5, 10, and 20 mg/kg, i.p.) 30 minutes before ischemia | Inhibits oxidative stress through activating heme oxygenase-1 (HO-1) [147]. Prevents apoptosis via improving mitochondrial dysfunction and activating glucagon-like peptide-1 receptor (GLP-1R) [148] |
| Shimada et al. [8] | Protective | C57BL/6J mice | NaHS (1 mg/kg, i.v.) 10 min before reperfusion | Inhibits lipid peroxidation and inflammation reactions. Upregulates intracellular antioxidant and antiapoptotic signaling pathways. Inhibits mitochondrial permeability transition pore (mPTP) opening, reduces cell apoptosis, and activates Akt/GSK3β signaling [149] |
| Su et al. [9] | Protective | Sprague-Dawley (SD) rats | agomir-miR-494 (20 μL of 500 pmol/d, 7 d, i.p.) prior to ischemia | Upregulates hypoxia-inducible factor-1 alpha (HIF-1α) and HO-1 [150]. Inhibits proapoptotic protein PTEN, ROCK1, and CaMKIIδ [151] |
| Sheng et al. [140] | Detrimental | Sprague-Dawley (SD) rats | Berberine pretreatment (100 mg/kg/d, 2 weeks) | Reduces oxidative stress, inflammation response, endoplasmic reticulum stress (ERS), and apoptosis via activating silent information regulator 1 (SIRT1) signaling [152] and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway [153]. Suppresses inducible nitric oxide synthesis [154] |
| Rao et al. [141] | Detrimental | C57BL/6 mice | 1.5% isoflurane with 25% oxygen balanced with nitrogen before ischemia | Induces HO-1 expression [155]. Preserves mitochondrial oxidative capacity [156]. Enhances the expression of guanosine triphosphate cyclohydrolase- (GTPCH-) 1 and eNOS [157]. Induces the generation of transforming growth factor-β1 (TGF-β1) [158] |
| Zhu et al. [142] | Detrimental | C57BL/6 mice | Rapamycin (1 mg/kg, i.p.) 1 hour prior to ischemia | Inhibits ERS [142]. Activates mTORC2/Akt pathway [138]. Recruits natural killer T (NKT) cells to IR region [159]. Activates JAK/STAT pathway, ERK, and eNOS [160] |
| Zhu et al. [138] | Detrimental | C57BL/6 mice | Rapamycin (1-5 mg/kg, i.p.) 1 hour prior to ischemia | Same as above |
i.p.: intraperitoneal injection; i.v.: intravenous injection.
In this review, we focused on the impact of mTOR signaling on inflammatory response and autophagy to discuss the beneficial effect of mTOR signaling on liver IR injury.
4. mTOR Attenuates Inflammation Response in Liver IR Injury
An excessive inflammatory response is recognized as a key mechanism of liver IR injury. Inflammatory networks, including inflammatory cells and humoral factors, play a vital role in liver IR injury [51]. Kupffer cells (KCs), neutrophils, CD4+ T lymphocytes, and natural killer T (NKT) cells are the main cellular participants. Complement factors, cytokines, and chemokines are the main humoral factors. Additionally, sinusoidal endothelial cells (SECs) and hepatocytes are also important participants and play critical roles in the inflammatory response during the liver IR process, leading to hepatocellular damage [52].
The mTOR signaling is appreciated to be a potent activator of the immune response, as its role in regulating cellular metabolism which is closely related to the proliferation and activation of immune cells, including neutrophils, mast cells, macrophages, dendritic cells (DCs), T lymphocytes, and B lymphocytes [53, 54]. However, an increasing body of evidence has emerged indicating that mTOR plays a pivotal anti-inflammatory role in liver IR injury. Myeloid mTOR activation through PTEN deficiency leads to the suppression of liver immune activation and protected livers from IR injury [55]. Additionally, mTOR-deficient mice showed greater expression levels of inflammation-related genes such as MCP-1, TNF-α, and IL-6 than wild-type (WT) mice after liver IR via negatively modulating NF-κB [6]. Besides, the opposite effect was seen in TSC1-deficient (mTOR-activated) mice, which showed a weaker inflammation response to liver IR injury than WT mice [6]. However, the mechanism of mTOR signaling in regulating the inflammatory response in liver IR injury largely remains unclear. In this section, we will discuss the regulatory role of mTOR signaling on inflammatory cells and humoral factors in liver IR injury (Figure 2).
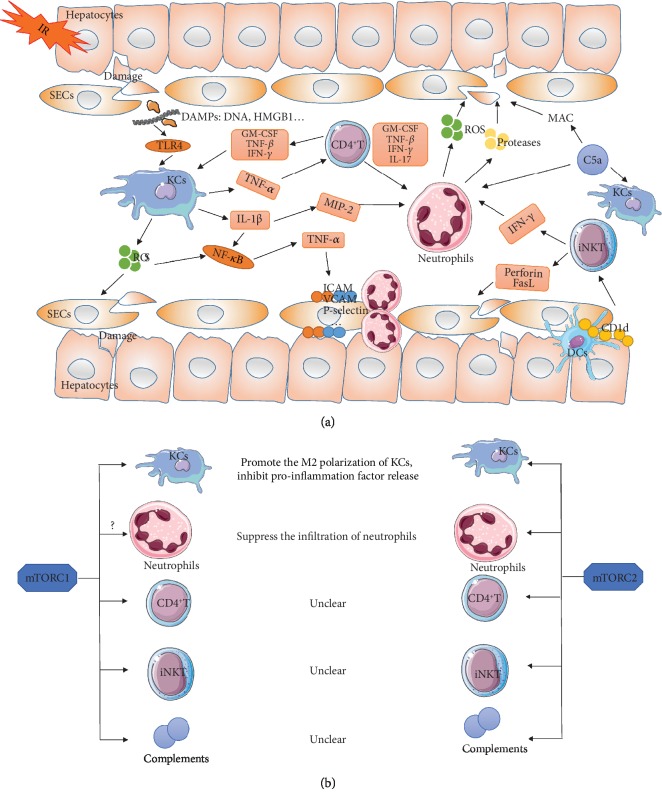
Figure 2.
(a) Schematic diagram of the inflammatory response during liver IR injury. Liver IR injury induces the damage of hepatocytes and SECs, leading to the release of DAMPs, resulting in the activation of KCs. Activated KCs release ROS and proinflammatory molecules (TNF-α, IL-1β), leading to the injury of hepatocytes and SECs and the activation of neutrophils and CD4+ T lymphocytes. The activation of CD4+ T lymphocytes amplifies the activation of KCs and neutrophils through releasing GM-CSF, TNF-β, and INF-γ. Activated neutrophils lead to the damages of hepatocytes and SECs through the release of ROS and proteases. iNKT cells are activated through interacting with CD1d, expressing on hepatocytes and APC within the liver. Activated NKT cells damage the liver directly through secreting perforin and FasL and through activating neutrophils. The complement system is activated in IR injury, which induced cell lysis via the formation of MAC or through activating KCs and neutrophils. (b) The impact of mTOR signaling on inflammatory response in liver IR injury. During liver IR injury, both mTORC1 and mTORC2 promote the M2 polarization of KCs (macrophages) and inhibit the release of proinflammation factors. Besides, mTORC2 also suppresses the infiltration of neutrophils during liver IR injury. Additionally, mTORC1 may play a role in inhibiting neutrophil infiltration through negatively regulating ICAM-1 expression in SECs. However, the role of mTOR signaling on CD4+ T lymphocytes, iNKT, and the complement system in liver IR injury remains unclear.
4.1. Kupffer Cells (KCs)
KCs, the liver-resident macrophages, play a key role in initiating and propagating inflammatory response of liver IR injury. In the early stage of reperfusion (within 2 h), KCs are activated by damage-associated molecular patterns (DAMPs), such as high-mobility group box 1 protein (HMGB1) and DNA fragments, through activating Toll-like receptor 4 (TLR4) [56]. On activation, KCs release ROS and proinflammatory molecules, including IL-1β and TNF-α. ROS induces oxidative damages to proteins, enzymes, nucleic acids, cytoskeleton, and lipid, leading to mitochondrial dysfunction and lipid peroxidation, contributing to injury of hepatocytes and SECs, resulting in both apoptotic and necrotic cell death [57]. ROS can also activate NF-κB, which upregulates the expression of proinflammatory cytokines, including TNF-α [58]. IL-1β activates NF-κB and macrophage inflammatory protein-2 (MIP-2), leading to the aggregation and adhesion of neutrophils [59]. TNF-α recruits and activates neutrophils and CD4+ T lymphocytes to the site of injury [60].
Numbers of studies have revealed the impact of mTOR signaling on KCs (or macrophages). The inhibition of mTORC1 increases inflammation and promotes the recruitment of inflammatory macrophages by enhancing NF-κB activity [61]. The Akt/mTOR signaling pathway can convert proinflammatory M1 macrophage into the anti-inflammatory M2 type through regulating the expression and phosphorylation of Acly [62]. Acly is a key enzyme in Ac-CoA synthesis, which increases the production of Ac-CoA in M2 macrophages and leads to the activation of M2 macrophages, resulting in the suppression of inflammation [62]. Besides, the Akt/mTOR signaling pathway can also integrate metabolic signals to support the activation of M2 macrophage [62]. However, researchers also found that increased activity of mTORC1 by ablating TSC1 promoted M1 macrophage polarization and suppressed M2 macrophage polarization [63]. The controversial results may be due to the fact that macrophage polarization is also regulated by environmental cues. And the different environmental and metabolic cues sensed by mTOR signaling influence macrophage polarization in some complex and unknown manners [64]. In the context of liver IR injury, astaxanthin activated the Akt/mTOR/HIF-1α signaling pathway in KCs, reducing the production of ROS and the expression of inflammatory cytokines, attenuating liver IR injury [65]. Similarly, activation of mTORC1 induced by PTEN deficiency promotes the M2 polarization of macrophages and increases the production of IL-10, decreasing the release of TNF-α, IL-6, and IL-12 when responding to TLR stimulation in liver IR injury [66]. Additionally, the deficiency of Rictor, a core component of mTORC2, increases the infiltration of macrophage/neutrophil and the release of cytokine/chemokine during liver IR injury [67], indicating an important role of mTORC2 on suppressing KCs.
4.2. Neutrophils
The activation of neutrophils is the major cause of injury in the late phase of liver IR (between 6 and 24 h after reperfusion) [68]. As described above, during the first 2 h of reperfusion, KCs activate and release ROS and proinflammatory cytokines, including TNF-α, which upregulates intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and P-selectin on the surface of SECs and hepatocytes, leading to the accumulation of neutrophils in the sinusoidal space and causing microcirculatory disturbances [69]. Additionally, neutrophils migrate toward the site of injury through extravasation and chemotaxis [70]. The accumulation of neutrophils in the site of injury leads to hepatocellular damages through degranulation with release of a large amount of proteases and ROS [71]. Additionally, neutrophils propagate the inflammatory response by recruiting other members of the immune system [72].
The mTOR signaling plays critical roles in the proliferation and activation of neutrophils [53, 54]. Rapamycin promotes the infiltration of neutrophils through inducing the expression of ICAM-1 via the activation of NF-κB in endothelial cells, indicating that mTORC1 can inhibit the migration of neutrophils [73]. Besides, in the liver [67] and kidney [74] IR injury, mTORC2 suppresses the infiltration of neutrophils and attenuates organ IR injury. However, the role and mechanism of mTOR signaling (especially mTORC1) on regulating neutrophils in IR injury remain unclear, and further studies are needed to investigate.
4.3. CD4+ T Lymphocytes
CD4+ T lymphocytes are important cellular participants of inflammation response in liver IR injury, which plays a critical role in promoting liver IR injury [75–77]. As described above, the activation of KCs can activate CD4+ T lymphocytes through releasing TNF-α. Activated CD4+ T lymphocytes release granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-β, and INF-γ, which in turn amplify the activation of KCs and promote the recruitment of neutrophils into the liver sinusoids [78, 79]. What is more, CD4+ T lymphocytes can also recruit neutrophils through producing IL-17 [80].
mTOR signaling is crucial for the differentiation of CD4+ T lymphocytes [81]. However, the role and mechanism of mTOR signaling on regulating CD4+ T lymphocytes in liver IR injury remain unclear; further investigations are needed to reveal.
4.4. Natural Killer T (NKT) Cells
Natural killer T (NKT) cells are a kind of markedly enriched nonconventional T cells in the liver, accounting for up to 30% of the intrahepatic lymphocytes [82]. NKT cells are divided into two subtypes: Type I (invariant, iNKT) and Type II; iNKT accounts for the majority [83]. The high abundance of iNKT cells in the liver and their rapid response (within hours) to activation suggest that they might play a role in liver IR injury [83, 84]. iNKT cells are recruited to the postischemic liver and are activated through interacting with CD1d antigen-presenting molecules, which express on hepatocytes and antigen-presenting cells (APC) within the liver. Activated iNKT cells damage the liver directly through secreting perforin and FasL and indirectly through activating neutrophils by the production of IFN-γ [85, 86]. Reducing the recruitment and cytokine production of iNKT cells ameliorates liver IR injury [87, 88]. Contrary to iNKT cells, Type II NKT cells have an anti-inflammatory effect [89].
Several studies have demonstrated the importance of mTOR in iNKT cells. mTOR signaling plays a critical role for both early and late stages of iNKT cell development [90]. However, the role and mechanism of mTOR signaling in mediating iNKT cells in liver IR injury remain unknown; further investigations are thus needed to explore.
4.5. Complement System
Apart from the immune cells, cytokines, and chemokines mentioned above, the complement system serves as an important participant of the inflammatory response in liver IR injury. The complement system consists of about 30 soluble and membrane-bound proteins, which are a well-acknowledged mediator of inflammation. Studies have shown that IR activated the complement system through the classical, the alternative, or the mannose-binding lectin (MBL) pathways [91]. On activation, the complement system induces cell lysis via the formation of membrane attack complexes (MAC). Additionally, the activated complement factor, C5a, can also activate KCs and recruit and activate neutrophils [92, 93], leading to liver damage. Additionally, complement system inhibitors, including C5a receptor (C5aR) antagonist, C5a monoclonal antibodies, C1 inhibitor, cobra venom factor (CVF), and soluble complement receptor type 1 (sCR1), have been shown to be effective in attenuating liver IR injury [94].
Researches revealed that the complement system linked tightly with mTOR signaling [95]. In the CD4+ T lymphocytes, C3a activates the C3aR on lysosomes, causing low-level mTOR activation, and C3a binding to C3aR on the cell surface results in sustained mTOR activity. Besides, C3b also associated with mTOR [95]. On the other hand, the activation of mTOR signaling significantly suppresses LPS-induced C5aR expression in macrophages [96]. However, the role of mTOR signaling in mediating the complement system in liver IR injury remains unknown; further investigations are thus needed to explore (Figure 2).
Besides, a recent study found that mTOR signaling played roles in maintaining the activity of CD4+Foxp3+ regulatory T cells (Tregs), which is capable of modulating other immune cells and suppressing the inflammatory response, indicating a novel mechanism of the anti-inflammatory role of mTOR in IR injury [97].
5. mTOR Inhibits Excessive Autophagy in Liver IR Injury
5.1. Definition of Autophagy
Autophagy is an evolutionarily highly conserved self-degradative process that targets intracellular components to lysosomes for degradation and recycling to maintaining cellular homeostasis [98]. There are four recognized types of autophagy: macroautophagy, microautophagy, chaperone-mediated autophagy (CMA), and noncanonical autophagy [99]. Here, we will focus on macroautophagy, which will be henceforth referred to as autophagy.
In nutrient-rich conditions, autophagy holds at a low level to maintain intracellular homeostasis through the removal of long-lived and malformed proteins and damaged organelles, called basal autophagy [100]. The activity of cellular autophagy can be markedly upregulated by starvation, hypoxia, energy depletion, ERS, infection, and other stimuli, which are called induced autophagy [101]. Upon induction, small double-membrane vesicles, called autophagosomes, sequester proteins, damaged organelles, and exogenous pathogens. And then, the outer membrane of autophagosomes fuses with lysosomes to form autolysosomes, in which the cargos and inner membrane of autophagosomes were degraded into biological active macromolecules (amino acids, nucleotide, free fatty acids, etc.) and be recycled for the synthesis of protein and ATP [102]. Furthermore, autophagy also plays a pivotal role in cellular homeostasis by regulating the turnover of mitochondria [103], ER [104], peroxisomes [105], and lipid [106] through selective forms. Nevertheless, excessive autophagy, leading to excessive degradation of essential proteins and organelles, can also induce a programmed cell death, called Type II programmed cell death [107]. Additionally, there also exist crosstalks between autophagy and other cell death mechanisms, including apoptosis and necrosis [108].
5.2. Autophagy and Liver IR Injury
An increasing body of evidence has emerged indicating that autophagy plays pivotal roles in IR injury of the heart [109], liver [3], brain [110], kidney [111], and lung [112], whereas its role remains controversial in these organs. Recent studies indicated that autophagy acts as a double-edged sword in either a beneficial or a detrimental way in ischemia and reperfusion phases, respectively. Autophagy acts as a compensatory mechanism to counterbalance ATP deprivation in the stage of ischemia, while sustained and excessive activation of autophagy during reperfusion phage results in cell death [113]. It was proven by multiple types of research in heart [114], liver [115], and brain [116] IR injuries. Additionally, a recent study found in the model of hypoxia/reoxygenation of adipose-derived mesenchymal stem cells (ADMSC) that the autophagy of ADMSC activated in the initial hypoxia period and markedly enhanced in the phase of reoxygenation. Interestingly, in the hypoxia phase, apelin upregulated protective autophagy through activating the AMPK/mTOR/ULK1 pathway. In the reoxygenation period, apelin suppressed excessive autophagy through Akt/Bcl2/Beclin1 signaling [117]. Similarly, berberine exerts protective effects in IR injury both through activating autophagy [118] and suppressing excessive autophagy [119]. The dual modulative effects of apelin and berberine keep autophagy activity at a moderate level to be protective for cell survival.
Accumulative evidence has shown that autophagy was hepatoprotective in liver IR injury [120–124]. However, some studies suggested that autophagy was deleterious in liver IR injury [5, 115, 125, 126]. The different results may be attributed to the various magnitudes of autophagy, owing to the different types of IR mode (cold/warm or partial/global IR) and the different liver conditions (lean/fatty), which lead to the different levels of autophagy activation. The controversial results may also owe to the “side effects” (except for regulating autophagy) of interventions adopted in the researches (Table 2).
Table 2.
The relationship between autophagy and liver IR injury.
| Study | Effect of autophagy | Animal model Liver type |
IR mode (ischemia/reperfusion time) | Autophagy change in IR | Interventions (effect on autophagy) | “Side effects” of intervention |
|---|---|---|---|---|---|---|
| Lee et al. [121] | Protective | BALB/c Lean |
Warm 75% (45 min/2, 3, 6, 12, and 24 h) | Increase | Everolimus I (1 mg/kg each time, i.p.) 24 h before and immediately after reperfusion (+) | Reduces inflammation and apoptosis [121]. Reduces HO-1 expression and increases iNOS level [161]. |
| Liu et al. [123] | Protective | SD Lean |
Warm 75% (1 h/1 h, 6 h) | Increase | Baicalein (100 mg/kg, i.p.), 1 h prior to ischemia (+) | Activates HO-1 [123], PTEN/Akt/NO pathway [162]. Inhibits NF-κB [163] and MAPK pathway [164]. |
| Khader et al. [120] | Protective | C57BL/6 Lean |
Warm 70% (1 h/12 h) | Increase | SRT1720 (20 mg/kg, i.v.) before reperfusion (+) | Stimulates the mitochondrial biogenesis. Reduces oxidative stress and inflammation [165]. |
| Yang et al. [124] | Protective | C57BL/6 Lean/fatty |
Warm 75% (1 h/0.5, 1.5, 3, 6, 12, and 24 h) | Increase | Tri-iodothyronine (0.002 mg, i.p.) precondition (+) | Reduces oxidative stress, apoptosis, and inflammation. Activates MEK/ERK/mTORC1 pathway [124]. |
| Zhao et al. [166] | Protective | C57BL/6 Lean/fatty |
Warm 75% (1 h/20 min) | Increase | Calpain inhibitor III (10 mg/kg, i.p.) 6 h prior to ischemia (+) | Inhibits the degradation of structural proteins. Suppresses apoptosis. Alters Ca2+ handling [167]. |
| Li et al. [168] | Detrimental | C57BL/6 Lean |
Warm 75% (1.5 h/2, 6, 12, and 24 h) | Increase | miR-17 agomir or antagomir (10 nM) 24 h prior to ischemia (+) | Inhibits PTEN [169], STAT3 [168], and death receptor 6 (DR6) [170]. |
| Kang et al. [5] | Detrimental | C57BL/6 Lean |
Warm 75% (1 h/1, 5, and 24 h) | Increase | Melatonin (10 mg/kg, i.p.) 15 min prior to ischemia and again immediately before reperfusion (-) | Inhibits oxidative stress. Improves the endothelial function. Restores mitochondrial function. Suppresses TLR and JNK pathways [94]. Activates RISK, SAFE, ERK1/2, PKB, PKC, JAK/STAT3, Sirt1/Sirt3, AMPKα, and Notch1/Mfn2 pathways [122]. |
| Gotoh et al. [115] | Detrimental | Wistar Lean |
Cold 100% (24 h/15 min) | Increase | Wortmannin (100 nM) or LY294002 (10 μM) was added to the UW solution in the in situ perfusion and during storage, respectively (-) | Inhibits PI3K/Akt pathway [115]. |
| Shen et al. [126] | Detrimental | BALB/c Lean |
Warm 75% (45 min/4, 8, and 16 h) | Increase | Ethyl pyruvate (20 mg/kg, 40 mg/kg, and 80 mg/kg, i.v.) 1 h prior to ischemia (-) | Inhibits HMGB1/TLR/NF-κB pathway [171]. Suppresses oxidative stress [172] and apoptosis [126]. |
| Gupta et al. [125] | Detrimental | C57BL/6 Fatty |
Warm 75% (20 min/24 h) | Increase | Ex4 (20 μg/kg i.v.) 2 h prior to ischemia and immediately after surgery (-) | Activates Nrf2 [173], Akt/eNOS [174], and HMGB1 [175] pathways. |
| Yun et al. [176] | Protective | C57BL/6 Lean |
Warm 75% (1 h/1, 4, and 24 h) | Decrease | Hemin (30 mg/kg) 16 h and 3 h prior to ischemia; carbon monoxide-releasing molecule-2 (20 mg/kg, i.p.) immediately before reperfusion (+) | Activates HO-1 [176], nuclear factor-erythroid 2-related factor 2 (Nrf2) [177]. Suppresses NF-κB p65 nuclear translocation [177] and calpain-2 [176]. |
| Kim et al. [178] | Protective | C57BL/6 Lean |
Cold 100% (45 min/2 and 4 h) | Decrease | Carbamazepine (25 mg/kg, i.p.), overnight before IR (+) | Inhibits MPT Ca2+ overload. Suppresses calpain-2 [178]. |
| Zaouali et al. [139] | Protective | Zucker Fatty |
Cold 100% (24 h/2 h) | Decrease | Melatonin and trimetazidine were added to the UW solution during graft storage for 24h (+) | Same as above. |
| Minor et al. [179] | Protective | Wistar Fatty |
Cold 100% (20 h/1.5 h and 2 h) | Decrease | Hypothermic reconditioning during the last 90 minutes of preservation (+) | Inhibits oxidative stress [179]. Enhances mitochondrial function [180]. |
i.p.: intraperitoneal injection; i.v.: intravenous injection.
5.3. mTOR Signaling and Autophagy
Autophagy can be regulated by the Bcl-2 signaling pathway, mTOR signaling pathway, MAPK signaling pathway, and p53 signaling pathway [127]. Among them, autophagy is mainly negatively regulated by the mTOR signaling pathway [128].
Under physiology conditions, growth factors such as insulin or EGF activate mTORC1 through insulin/PI3K/Akt/mTORC1 and EGF/Ras/Raf/Mek/Erk/mTORC1 axis, respectively [15]. Activated mTOR signaling exerts a potent inhibitory effect on multiple phases of autophagy [129]. In the initiation phase, mTORC1 suppresses autophagy by inhibiting ULK complex (ULK1/Atg13/ATG101/FIP200), a kinase complex indispensable to initiate autophagy [130] via directly phosphorylating ULK1 (on S317) and ATG13 [131]. Besides, mTORC1 can also inhibit ULK complex through phosphorylating and suppressing Beclin 1-regulated autophagy protein 1 (AMBRA) [132], which enhances the activity and stability of ULK1. Additionally, mTORC1 represses the initial of autophagy also through inhibiting another crucial complex for autophagy induction, vacuolar protein sorting 34 (VPS34) complex (VPS34/VPS15/Beclin1/ATG14/NRBF2), by directly phosphorylating ATG14 and nuclear receptor binding factor 2 (NRBF2) (on S133 and S120) [129]. In the elongation/closure phase, mTORC1 suppresses autophagic and lysosomal biogenesis through phosphorylating TFEB (on S211) and TFE3 (on S321) to modulate their nuclear-cytoplasmic shuttling [129] (Figure 1). Moreover, mTORC1 can also augment autophagy through phosphorylating death-associated protein 1 (DAP1), which acts as a buffering mechanism that counterbalances the autophagic flux and prevents its overactivation [133]. Additionally, researchers found that the activation of mTORC2 also participated in the induction of autophagy [134] (Figure 1).
As shown above, in conditions of hypoxia/ischemia, oxidative stress and DNA damage induced by liver IR, AMPK, REDD1, and ATM will be activated and lead to the suppression of mTOR signaling, resulting in the activation/overactivation of autophagy. Since the indispensable role of mTOR signaling on autophagy, numerous researches focused on mTOR signaling for regulating autophagy to protect against liver IR injury. Researches have shown that melatonin [5] and microRNA-101 [135] attenuated liver IR injury by suppressing autophagy through activating mTOR signaling. However, researches have also shown that activation of autophagy through mTOR inhibitor rapamycin [136–138] and everolimus [121] has been shown to protect against liver IR injury. In addition, a recent study showed that inhibition of mTORC2 by Rictor deficiency aggravated liver IR injury through suppressing autophagy [67]. The paradox result may be attributed to the double-edged effect of autophagy in liver IR injury that the moderate level of autophagy mitigated liver IR injury in ischemia, while the excessive level of autophagy aggravated liver IR injury in reperfusion. This explains the finding that in moderate and advanced steatotic liver that autophagy was impaired, melatonin combined with trimetazidine elevated liver autophagy, rather than inhibited, and improved liver IR injury [139].
These findings indicated an intricate function of autophagy in liver IR injury and a complexity effect of the mTOR pathway in regulating autophagy. We believe that moderate regulation of autophagy through modulating the PI3K/Akt/mTORC1 pathway or mTORC1/mTORC2 balancing may serve as a potential strategy for attenuating liver IR injury.
6. Conclusions
Liver IR injury is a clinical phenomenon in various settings including liver resection and transplantation, which is a major cause of morbidity and mortality in liver surgeries and limits the use of grafts available for transplantation.
Liver IR injury is typified by the excessive inflammatory response, which involves a complex interaction network between the inflammatory cells and humoral factors, leading to liver dysfunction and cell injury. Although mTOR signaling is a potent proinflammatory regulator on the growth and differentiation of multiple inflammatory cells, in the context of liver IR injury, it seems to play a significant role in anti-inflammation through regulating KCs and neutrophils. However, the mechanism of mTOR signaling on anti-inflammation still remains unclear, especially on the regulation of CD4+ T lymphocytes, NKT cells, and complement systems in the context of liver IR injury.
Additionally, significant changes of autophagy in hepatocytes are observed in liver IR injury; enhancing autophagy under ischemia conditions can promote survival, whereas excessive and long-term augmentation of autophagy during reperfusion may promote cell death. mTOR signaling plays a complexity effect in regulating autophagy. Keeping autophagy at a moderate level during liver IR through modulating the PI3K/Akt/mTORC1 pathway or mTORC1/mTORC2 balancing may serve as a potential strategy for attenuating liver IR injury. A comprehensive study and an illuminating evaluation of the mTOR pathway are thus needed before clinical usage of the autophagy regulator in liver IR patients.
However, in contrast to the beneficial effect of mTOR mentioned above, some studies have shown that berberine precondition [140], isoflurane precondition [141], and rapamycin dealing [138, 142], which associated with inhibition of mTOR signaling, also showed protective effects on liver IR injury, indicating a detrimental role of mTOR signaling in liver IR injury (Table 1). The controversial results may be due to the “side effects” (except for regulating mTOR) of the interventions and the different levels of autophagy in the liver IR models adopted in these researches (Table 1). Besides, Li et al. utilized complementary genetic models with gain or loss of function of mTOR signaling in the liver and demonstrated the beneficial effect of mTOR in liver IR injury [6]. Thus, we hold the idea that mTOR signaling plays a protective role in liver IR injury.
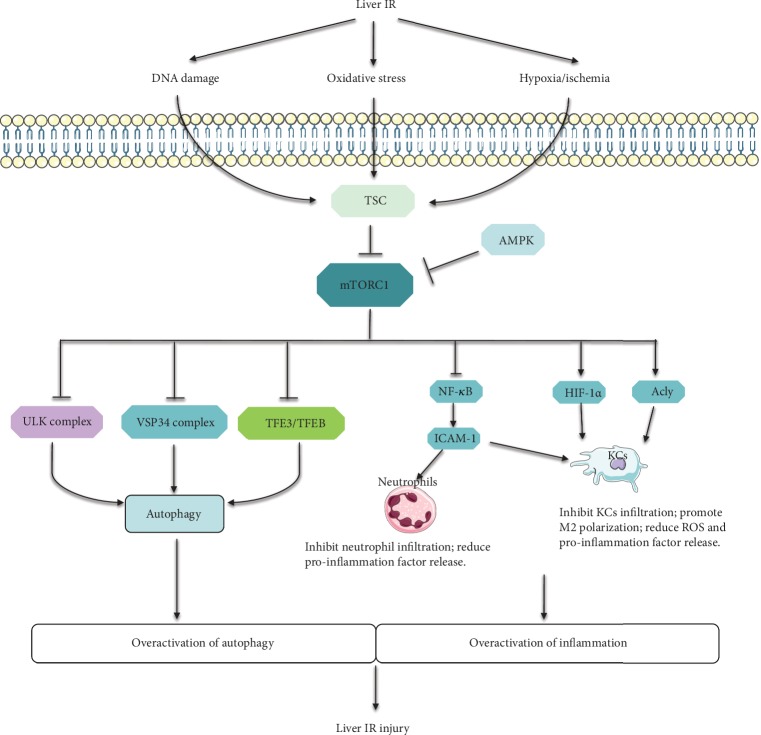
In a word, the impact of mTOR signaling on the inflammatory response and autophagy provides an attractive therapeutic target for liver IR injury (Figure 3).
Figure 3.
The summary of the protective role of mTOR in liver IR injury: involvement of inflammation and autophagy. During liver IR injury, IR-induced hypoxia/ischemia, oxidative stress, and DNA damage suppress mTORC1 through activating TSC or AMPK via multiple signaling pathways. The repression of mTORC1 leads to the overactivation of autophagy through activating ULK complex, VSP34 complex, and TFE3/TFEB. Additionally, the inhibition of mTORC1 promotes the infiltration of neutrophils and KCs through the NF-κB/ICAM-1 axis. Besides, mTORC1 suppression also reduces the M2 polarization and promotes ROS and proinflammation factor release through inhibiting Acly and HIF-1α, resulting in the overactivation of inflammation. The overactivation of autophagy and inflammation leads to the liver IR injury finally.
Acknowledgments
This study was supported by the Natural Science Foundation of China (No. 81570568 to Jiliang Wang and No. 81602419 to Huili Li).
Contributor Information
Huili Li, Email: huili_li@hust.edu.cn.
Jiliang Wang, Email: jiliang_wang@hust.edu.cn.
Conflicts of Interest
All authors have declared no competing interest exists.
References
- 1.van Riel W. G., van Golen R., Reiniers M. J., Heger M., van Gulik T. How much ischemia can the liver tolerate during resection? Hepatobiliary Surgery and Nutrition. 2016;5(1):58–71. doi: 10.3978/j.issn.2304-3881.2015.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J., Li R. J., Lv G. Y., Liu H. Q. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. European Review for Medical and Pharmacological Sciences. 2015;19(11):2036–2047. [PubMed] [Google Scholar]
- 3.Cursio R., Colosetti P., Gugenheim J. Autophagy and liver ischemia-reperfusion injury. BioMed Research International. 2015;2015:16. doi: 10.1155/2015/417590.417590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bejaoui M., Zaouali M. A., Folch-Puy E., et al. Bortezomib enhances fatty liver preservation in Institut George Lopez‐1 solution through adenosine monophosphate activated protein kinase and Akt/mTOR pathways. Journal of Pharmacy and Pharmacology. 2014;66(1):62–72. doi: 10.1111/jphp.12154. [DOI] [PubMed] [Google Scholar]
- 5.Kang J. W., Cho H. I., Lee S. M. Melatonin inhibits mTOR-dependent autophagy during liver ischemia/reperfusion. Cellular Physiology and Biochemistry. 2014;33(1):23–36. doi: 10.1159/000356647. [DOI] [PubMed] [Google Scholar]
- 6.Li Z., Zhang J., Mulholland M., Zhang W. mTOR activation protects liver from ischemia/reperfusion-induced injury through NF-κB pathway. The FASEB Journal. 2017;31(7):3018–3026. doi: 10.1096/fj.201601278R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rong Y. P., Huang H. T., Liu J. S., Wei L. Protective effects of geniposide on hepatic ischemia/reperfusion injury. Transplantation Proceedings. 2017;49(6):1455–1460. doi: 10.1016/j.transproceed.2017.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Shimada S., Fukai M., Wakayama K., et al. Hydrogen sulfide augments survival signals in warm ischemia and reperfusion of the mouse liver. Surgery Today. 2015;45(7):892–903. doi: 10.1007/s00595-014-1064-4. [DOI] [PubMed] [Google Scholar]
- 9.Su S., Luo D., Liu X., et al. miR-494up-regulates the PI3K/Akt pathway via targetting PTEN and attenuates hepatic ischemia/reperfusion injury in a rat model. Bioscience Reports. 2017;37(5):p. BSR20170798. doi: 10.1042/BSR20170798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxton R. A., Sabatini D. M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Annals of the New York Academy of Sciences. 2015;1346(1):33–44. doi: 10.1111/nyas.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laplante M., Sabatini D. M. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G., Murashige D. S., Humphrey S. J., James D. E. A positive feedback loop between Akt and mTORC2 via SIN1 phosphorylation. Cell Reports. 2015;12(6):937–943. doi: 10.1016/j.celrep.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Soliman G. The role of mechanistic target of rapamycin (mTOR) complexes signaling in the immune responses. Nutrients. 2013;5(6):2231–2257. doi: 10.3390/nu5062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang K., Fingar D. C. Growing knowledge of the mTOR signaling network. Seminars in Cell & Developmental Biology. 2014;36:79–90. doi: 10.1016/j.semcdb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gracia-Sancho J., Casillas-Ramirez A., Peralta C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: a 2015 update. Clinical Science. 2015;129(4):345–362. doi: 10.1042/CS20150223. [DOI] [PubMed] [Google Scholar]
- 17.Hardie D. G., Schaffer B. E., Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends in Cell Biology. 2016;26(3):190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majd S., Power J. H. T., Chataway T. K., Grantham H. J. M. A comparison of LKB1/AMPK/mTOR metabolic axis response to global ischaemia in brain, heart, liver and kidney in a rat model of cardiac arrest. BMC Cell Biology. 2018;19(1):p. 7. doi: 10.1186/s12860-018-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwinn D. M., Shackelford D. B., Egan D. F., et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff N. C., Vega-Rubin-de-Celis S., Xie X. J., Castrillon D. H., Kabbani W., Brugarolas J. Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Molecular and Cellular Biology. 2011;31(9):1870–1884. doi: 10.1128/MCB.01393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoshani T., Faerman A., Mett I., et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Molecular and Cellular Biology. 2002;22(7):2283–2293. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander A., Cai S. L., Kim J., et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proceedings of the National Academy of Sciences. 2010;107(9):4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byun Y. J., Kim S. K., Kim Y. M., Chae G. T., Jeong S. W., Lee S. B. Hydrogen peroxide induces autophagic cell death in C6 glioma cells via BNIP3-mediated suppression of the mTOR pathway. Neuroscience Letters. 2009;461(2):131–135. doi: 10.1016/j.neulet.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Chen L., Xu B., Liu L., et al. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKα leading to apoptosis of neuronal cells. Laboratory Investigation. 2010;90(5):762–773. doi: 10.1038/labinvest.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Z., Kozlov S., Lavin M. F., Person M. D., Paull T. T. ATM activation by oxidative stress. Science. 2010;330(6003):517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 26.Seo G., Kim S. K., Byun Y. J., et al. Hydrogen peroxide induces Beclin 1-independent autophagic cell death by suppressing the mTOR pathway via promoting the ubiquitination and degradation of Rheb in GSH-depleted RAW 264.7 cells. Free Radical Research. 2011;45(4):389–399. doi: 10.3109/10715762.2010.535530. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L., Kimball S. R., Jefferson L. S., Shenberger J. S. Hydrogen peroxide impairs insulin-stimulated assembly of mTORC1. Free Radical Biology and Medicine. 2009;46(11):1500–1509. doi: 10.1016/j.freeradbiomed.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q., Liu C., Liu W., et al. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicological Sciences. 2015;143(1):81–96. doi: 10.1093/toxsci/kfu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S., Wang C., Yan F., et al. N-Acetylcysteine attenuates diabetic myocardial ischemia reperfusion injury through inhibiting excessive autophagy. Mediators of Inflammation. 2017;2017:10. doi: 10.1155/2017/9257291.9257291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie L., Yu S., Yang K., Li C., Liang Y. Hydrogen sulfide inhibits autophagic neuronal cell death by reducing oxidative stress in spinal cord ischemia reperfusion injury. Oxidative Medicine and Cellular Longevity. 2017;2017:15. doi: 10.1155/2017/8640284.8640284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Kim J., Alexander A., et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nature Cell Biology. 2013;15(10):1186–1196. doi: 10.1038/ncb2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cam H., Easton J. B., High A., Houghton P. J. mTORC1 Signaling under Hypoxic Conditions Is Controlled by ATM-Dependent Phosphorylation of HIF-1α. Molecular Cell. 2010;40(4):509–520. doi: 10.1016/j.molcel.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho S. S., Kim K. M., Yang J. H., et al. Induction of REDD1 via AP-1 prevents oxidative stress-mediated injury in hepatocytes. Free Radical Biology and Medicine. 2018;124:221–231. doi: 10.1016/j.freeradbiomed.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Emerling B. M., Weinberg F., Snyder C., et al. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radical Biology and Medicine. 2009;46(10):1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Z., Hu W., de Stanchina E., et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Research. 2007;67(7):3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 36.Aoyagi T., Higa J. K., Aoyagi H., Yorichika N., Shimada B. K., Matsui T. Cardiac mTOR rescues the detrimental effects of diet-induced obesity in the heart after ischemia-reperfusion. American Journal of Physiology: Heart and Circulatory Physiology. 2015;308(12):H1530–H1539. doi: 10.1152/ajpheart.00008.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aoyagi T., Kusakari Y., Xiao C. Y., et al. Cardiac mTOR protects the heart against ischemia-reperfusion injury. American Journal of Physiology: Heart and Circulatory Physiology. 2012;303(1):H75–H85. doi: 10.1152/ajpheart.00241.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan G., Yu J., Asare P. F., et al. Danshensu alleviates cardiac ischaemia/reperfusion injury by inhibiting autophagy and apoptosis via activation of mTOR signalling. Journal of Cellular and Molecular Medicine. 2016;20(10):1908–1919. doi: 10.1111/jcmm.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glazer H. P., Osipov R. M., Clements R. T., Sellke F. W., Bianchi C. Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling. Cell Cycle. 2009;8(11):1738–1746. doi: 10.4161/cc.8.11.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Land S. C., Tee A. R. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. Journal of Biological Chemistry. 2007;282(28):20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 41.Schenkel P. C., Tavares A. M. V., Fernandes R. O., et al. Time course of hydrogen peroxide–thioredoxin balance and its influence on the intracellular signalling in myocardial infarction. Experimental Physiology. 2012;97(6):741–749. doi: 10.1113/expphysiol.2012.064832. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M., Sun D., Li S., et al. Lin28a protects against cardiac ischaemia/reperfusion injury in diabetic mice through the insulin‐PI3K‐mTOR pathway. Journal of Cellular and Molecular Medicine. 2015;19(6):1174–1182. doi: 10.1111/jcmm.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y., Fang H., Lin S., et al. Qiliqiangxin protects against cardiac ischemia-reperfusion injury via activation of the mTOR pathway. Cellular Physiology and Biochemistry. 2015;37(2):454–464. doi: 10.1159/000430368. [DOI] [PubMed] [Google Scholar]
- 44.Chi O. Z., Mellender S. J., Barsoum S., Liu X., Damito S., Weiss H. R. Effects of rapamycin pretreatment on blood-brain barrier disruption in cerebral ischemia-reperfusion. Neuroscience Letters. 2016;620:132–136. doi: 10.1016/j.neulet.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 45.Fu L., Huang L., Cao C., Yin Q., Liu J. Inhibition of AMP-activated protein kinase alleviates focal cerebral ischemia injury in mice: interference with mTOR and autophagy. Brain Res. 2016;1650:103–111. doi: 10.1016/j.brainres.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 46.Xie L., Sun F., Wang J., et al. mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. The Journal of Immunology. 2014;192(12):6009–6019. doi: 10.4049/jimmunol.1303492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X., Hei C., Liu P., et al. Inhibition of mTOR pathway by rapamycin reduces brain damage in rats subjected to transient forebrain ischemia. International Journal of Biological Sciences. 2015;11(12):1424–1435. doi: 10.7150/ijbs.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ban K., Kozar R. A. Protective role of p70S6K in intestinal ischemia/reperfusion injury in mice. PLoS One. 2012;7(7):p. e41584. doi: 10.1371/journal.pone.0041584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H., Cui Z., Luo G., et al. Ghrelin attenuates intestinal ischemia/reperfusion injury in mice by activating the mTOR signaling pathway. International Journal of Molecular Medicine. 2013;32(4):851–859. doi: 10.3892/ijmm.2013.1452. [DOI] [PubMed] [Google Scholar]
- 50.Fantus D., Rogers N. M., Grahammer F., Huber T. B., Thomson A. W. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nature Reviews Nephrology. 2016;12(10):587–609. doi: 10.1038/nrneph.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;284(1):G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 52.Abu-Amara M., Yang S. Y., Tapuria N., Fuller B., Davidson B., Seifalian A. Liver ischemia/reperfusion injury: Processes in inflammatory networks—A review. Liver Transplantation. 2010;16(9):1016–1032. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 53.Cobbold S. P. The mTOR pathway and integrating immune regulation. Immunology. 2013;140(4):391–398. doi: 10.1111/imm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powell J. D., Pollizzi K. N., Heikamp E. B., Horton M. R. Regulation of immune responses by mTOR. Annual Review of Immunology. 2012;30(1):39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue S., Rao J., Zhu J., et al. Myeloid PTEN deficiency protects livers from ischemia reperfusion injury by facilitating M2 macrophage differentiation. The Journal of Immunology. 2014;192(11):5343–5353. doi: 10.4049/jimmunol.1400280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Q., Liu Y., Shi Y., Zheng M., He J., Chen Z. The role of intracellular high-mobility group box 1 in the early activation of Kupffer cells and the development of Con A-induced acute liver failure. Immunobiology. 2013;218(10):1284–1292. doi: 10.1016/j.imbio.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chemico-Biological Interactions. 1991;79(2):115–136. doi: 10.1016/0009-2797(91)90077-K. [DOI] [PubMed] [Google Scholar]
- 58.Sanlioglu S., Williams C. M., Samavati L., et al. Lipopolysaccharide Induces Rac1-dependent Reactive Oxygen Species Formation and Coordinates Tumor Necrosis Factor-α Secretion through IKK Regulation of NF-κB. Journal of Biological Chemistry. 2001;276(32):30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- 59.Welborn B. M., III, Moldawer L. L., Seeger J. M., Minter R. M., Huber T. S. Role of endogenous interleukin-10 in local and distant organ injury after visceral ischemia-reperfusion. Shock. 2003;20(1):35–40. doi: 10.1097/01.SHK.0000071062.67193.b6. [DOI] [PubMed] [Google Scholar]
- 60.Hanschen M., Zahler S., Krombach F., Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86(5):710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida T., Mett I., Bhunia A. K., et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nature Medicine. 2010;16(7):767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Covarrubias A. J., Aksoylar H. I., Yu J., et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife. 2016;5 doi: 10.7554/eLife.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu L., Yang T., Li L., et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nature Communications. 2014;5(1) doi: 10.1038/ncomms5696. [DOI] [PubMed] [Google Scholar]
- 64.Weichhart T., Hengstschlager M., Linke M. Regulation of innate immune cell function by mTOR. Nature Reviews Immunology. 2015;15(10):599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S., Takahara T., Fujino M., et al. Astaxanthin prevents ischemia-reperfusion injury of the steatotic liver in mice. PLoS One. 2017;12(11):p. e0187810. doi: 10.1371/journal.pone.0187810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamo N., Ke B., Busuttil R. W., Kupiec-Weglinski J. W. PTEN‐mediated akt/β‐Catenin/foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology. 2013;57(1):289–298. doi: 10.1002/hep.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu D., Zhu J., Jeong S., et al. Rictor deficiency aggravates hepatic ischemia/reperfusion injury in mice by suppressing autophagy and regulating MAPK signaling. Cellular Physiology and Biochemistry. 2018;45(6):2199–2212. doi: 10.1159/000488165. [DOI] [PubMed] [Google Scholar]
- 68.Elias-Miro M., Jimenez-Castro M. B., Rodes J., Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radical Research. 2013;47(8):555–568. doi: 10.3109/10715762.2013.811721. [DOI] [PubMed] [Google Scholar]
- 69.Vollmar B., Glasz J., Leiderer R., Post S., Menger M. D. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. The American journal of pathology. 1994;145(6):1421–1431. [PMC free article] [PubMed] [Google Scholar]
- 70.de Oliveira T. H. C., Marques P. E., Proost P., Teixeira M. M. M. Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Laboratory Investigation. 2018;98(1, article BFlabinvest201790):51–62. doi: 10.1038/labinvest.2017.90. [DOI] [PubMed] [Google Scholar]
- 71.Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2006;290(6):G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 72.Quesnelle K. M., Bystrom P. V., Toledo-Pereyra L. H. Molecular responses to ischemia and reperfusion in the liver. Archives of Toxicology. 2015;89(5):651–657. doi: 10.1007/s00204-014-1437-x. [DOI] [PubMed] [Google Scholar]
- 73.Minhajuddin M., Fazal F., Bijli K. M., Amin M. R., Rahman A. Inhibition of Mammalian Target of Rapamycin Potentiates Thrombin-Induced Intercellular Adhesion Molecule-1 Expression by Accelerating and Stabilizing NF-κB Activation in Endothelial Cells. The Journal of Immunology. 2005;174(9):5823–5829. doi: 10.4049/jimmunol.174.9.5823. [DOI] [PubMed] [Google Scholar]
- 74.Dai H., Watson A. R., Fantus D., Peng L., Thomson A. W., Rogers N. M. Rictor deficiency in dendritic cells exacerbates acute kidney injury. Kidney International. 2018;94(5):951–963. doi: 10.1016/j.kint.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pommey S., Lu B., McRae J., et al. Liver grafts from CD39‐overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology. 2013;57(4):1597–1606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 76.Reifart J., Rentsch M., Mende K., et al. Modulating CD4+ T cell migration in the postischemic liver: hepatic stellate cells as new therapeutic target? Transplantation. 2015;99(1):41–47. doi: 10.1097/TP.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 77.Shen X., Wang Y., Gao F., et al. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50(5):1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caldwell C. C., Tschoep J., Lentsch A. B. Lymphocyte function during hepatic ischemia/reperfusion injury. Journal of Leukocyte Biology. 2007;82(3):457–464. doi: 10.1189/jlb.0107062. [DOI] [PubMed] [Google Scholar]
- 79.Casillas-Ramírez A., Mosbah I. B., Ramalho F., Roselló-Catafau J., Peralta C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sciences. 2006;79(20):1881–1894. doi: 10.1016/j.lfs.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 80.Caldwell C. C., Okaya T., Martignoni A., Husted T., Schuster R., Lentsch A. B. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005;289(5):G969–G976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]
- 81.Delgoffe G. M., Pollizzi K. N., Waickman A. T., et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature Immunology. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richards J. A., Wigmore S. J., Anderton S. M., Howie S. E. M. NKT cells are important mediators of hepatic ischemia-reperfusion injury. Transplant Immunology. 2017;45:15–21. doi: 10.1016/j.trim.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zimmerman M., Martin A., Yee J., Schiller J., Hong J. Natural killer T cells in liver ischemia-reperfusion injury. Journal of Clinical Medicine. 2017;6(4):p. 41. doi: 10.3390/jcm6040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lappas C. M., Day Y. J., Marshall M. A., Engelhard V. H., Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. The Journal of Experimental Medicine. 2006;203(12):2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuboki S., Sakai N., Tschöp J., Edwards M. J., Lentsch A. B., Caldwell C. C. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296(5):G1054–G1059. doi: 10.1152/ajpgi.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L., Huang L., Sung S. S. J., et al. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. The Journal of Immunology. 2007;178(9):5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 87.Shimamura K., Kawamura H., Nagura T., et al. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cellular Immunology. 2005;234(1):31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 88.Zhu H., Zhang Q., Chen G. CXCR6 deficiency ameliorates ischemia-reperfusion injury by reducing the recruitment and cytokine production of hepatic NKT cells in a mouse model of non-alcoholic fatty liver disease. International Immunopharmacology. 2019;72:224–234. doi: 10.1016/j.intimp.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 89.Halder R. C., Aguilera C., Maricic I., Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. Journal of Clinical Investigation. 2007;117(8):2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang W., Gorentla B., Zhong X. P., Shin J. mTOR and its tight regulation for iNKT cell development and effector function. Molecular Immunology. 2015;68(2):536–545. doi: 10.1016/j.molimm.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Montalvo-Jave E. E., Escalante-Tattersfield T., Ortega-Salgado J. A., Piña E., Geller D. A. Factors in the pathophysiology of the liver ischemia-reperfusion injury. Journal of Surgical Research. 2008;147(1):153–159. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jaeschke H., Farhood A., Bautista A. P., Spolarics Z., Spitzer J. J. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. American Journal of Physiology. 1993;264(4):G801–G809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 93.Woodruff T. M., Arumugam T. V., Shiels I. A., Reid R. C., Fairlie D. P., Taylor S. M. Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. Journal of Surgical Research. 2004;116(1):81–90. doi: 10.1016/j.jss.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Arumugam T. V., Shiels I. A., Woodruff T. M., Granger D. N., Taylor S. M. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21(5):401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 95.Kolev M., Kemper C. Keeping it all going-complement meets metabolism. Frontiers in Immunology. 2017;8:p. 1. doi: 10.3389/fimmu.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bisht K., Wegiel B., Tampe J., et al. Biliverdin modulates the expression of C5aR in response to endotoxin in part via mTOR signaling. Biochemical and Biophysical Research Communications. 2014;449(1):94–99. doi: 10.1016/j.bbrc.2014.04.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen G., Dong Z., Liu H., et al. mTOR signaling regulates protective activity of transferred CD4+Foxp3+ T cells in repair of acute kidney injury. The Journal of Immunology. 2016;197(10):3917–3926. doi: 10.4049/jimmunol.1601251. [DOI] [PubMed] [Google Scholar]
- 98.Yang Z., Klionsky D. J. Eaten alive: a history of macroautophagy. Nature Cell Biology. 2010;12(9):814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mizushima N., Yoshimori T., Ohsumi Y. The role of Atg proteins in autophagosome formation. Annual Review of Cell and Developmental Biology. 2011;27(1):107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 100.Kuma A., Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Seminars in Cell & Developmental Biology. 2010;21(7):683–690. doi: 10.1016/j.semcdb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 101.Kuma A., Hatano M., Matsui M., et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 102.White E., Mehnert J. M., Chan C. S. Autophagy, metabolism, and cancer. Clinical Cancer Research. 2015;21(22):5037–5046. doi: 10.1158/1078-0432.CCR-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim I., Rodriguez-Enriquez S., Lemasters J. J. Selective degradation of mitochondria by mitophagy. Archives of Biochemistry and Biophysics. 2007;462(2):245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khaminets A., Heinrich T., Mari M., et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522(7556):354–358. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 105.Farre J. C., Manjithaya R., Mathewson R. D., Subramani S. PpAtg30 tags peroxisomes for turnover by selective autophagy. Developmental Cell. 2008;14(3):365–376. doi: 10.1016/j.devcel.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh R., Kaushik S., Wang Y., et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baehrecke E. H. Autophagic programmed cell death in Drosophila. Cell Death Differ. 2003;10(9):940–945. doi: 10.1038/sj.cdd.4401280. [DOI] [PubMed] [Google Scholar]
- 108.Khandia R., Dadar M., Munjal A., et al. A comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: current knowledge and prospects for disease prevention, novel drug design, and therapy. Cells. 2019;8(7):p. 674. doi: 10.3390/cells8070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma S., Wang Y., Chen Y., Cao F. The role of the autophagy in myocardial ischemia/reperfusion injury. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2015;1852(2):271–276. doi: 10.1016/j.bbadis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y. C., Zhang S., du T. Y., Wang B., Sun X. Q. Hyperbaric oxygen preconditioning reduces ischemia-reperfusion injury by stimulating autophagy in neurocyte. Brain Research. 2010;1323:149–151. doi: 10.1016/j.brainres.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 111.Decuypere J. P., Ceulemans L. J., Agostinis P., et al. Autophagy and the kidney: implications for ischemia-reperfusion injury and therapy. American Journal of Kidney Diseases. 2015;66(4):699–709. doi: 10.1053/j.ajkd.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 112.Ryter S. W., Choi A. M. K. Autophagy in the lung. Proceedings of the American Thoracic Society. 2010;7(1):13–21. doi: 10.1513/pats.200909-101JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daniels L. J., Varma U., Annandale M., Chan E., Mellor K. M., Delbridge L. M. D. Myocardial energy stress, autophagy induction, and cardiomyocyte functional responses. Antioxidants & Redox Signaling. 2019;31(6):472–486. doi: 10.1089/ars.2018.7650. [DOI] [PubMed] [Google Scholar]
- 114.Matsui Y., Takagi H., Qu X., et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circulation Research. 2007;100(6):914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 115.Gotoh K., Lu Z., Morita M., et al. Participation of autophagy in the initiation of graft dysfunction after rat liver transplantation. Autophagy. 2009;5(3):351–360. doi: 10.4161/auto.5.3.7650. [DOI] [PubMed] [Google Scholar]
- 116.Liu W., Shang G., Yang S., et al. Electroacupuncture protects against ischemic stroke by reducing autophagosome formation and inhibiting autophagy through the mTORC1-ULK1 complex-Beclin1 pathway. International Journal of Molecular Medicine. 2016;37(2):309–318. doi: 10.3892/ijmm.2015.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liang D., Han D., Fan W., et al. Therapeutic efficacy of apelin on transplanted mesenchymal stem cells in hindlimb ischemic mice via regulation of autophagy. Scientific Reports. 2016;6(1, article BFsrep21914) doi: 10.1038/srep21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin Y., Sheng M., Weng Y., et al. Berberine protects against ischemia/reperfusion injury after orthotopic liver transplantation via activating Sirt1/FoxO3α induced autophagy. Biochemical and Biophysical Research Communications. 2017;483(2):885–891. doi: 10.1016/j.bbrc.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 119.Huang Z., Han Z., Ye B., et al. Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. European Journal of Pharmacology. 2015;762:1–10. doi: 10.1016/j.ejphar.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 120.Khader A., Yang W. L., Godwin A., et al. Sirtuin 1 stimulation attenuates ischemic liver injury and enhances mitochondrial recovery and autophagy. Critical Care Medicine. 2016;44(8):e651–e663. doi: 10.1097/CCM.0000000000001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee S. C., Kim K. H., Kim O. H., Lee S. K., Kim S. J. Activation of Autophagy by Everolimus Confers Hepatoprotection Against Ischemia–Reperfusion Injury. American Journal of Transplantation. 2016;16(7):2042–2054. doi: 10.1111/ajt.13729. [DOI] [PubMed] [Google Scholar]
- 122.Liu A., Fang H., Wei W., Dirsch O., Dahmen U. Ischemic preconditioning protects against liver ischemia/reperfusion injury via heme oxygenase-1-mediated autophagy. Critical Care Medicine. 2014;42(12):e762–e771. doi: 10.1097/CCM.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 123.Liu A., Huang L., Guo E., et al. Baicalein pretreatment reduces liver ischemia/reperfusion injury via induction of autophagy in rats. Scientific Reports. 2016;6(1) doi: 10.1038/srep25042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang J., Wang Y., Sui M., Liu F., Fu Z., Wang Q. X. Tri-iodothyronine preconditioning protects against liver ischemia reperfusion injury through the regulation of autophagy by the MEK/ERK/mTORC1 axis. Biochemical and Biophysical Research Communications. 2015;467(4):704–710. doi: 10.1016/j.bbrc.2015.10.080. [DOI] [PubMed] [Google Scholar]
- 125.Gupta N. A., Kolachala V. L., Jiang R., et al. Mitigation of autophagy ameliorates hepatocellular damage following ischemia-reperfusion injury in murine steatotic liver. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2014;307(11):G1088–G1099. doi: 10.1152/ajpgi.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shen M., Lu J., Dai W., et al. Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy. Mediators of Inflammation. 2013;2013:12. doi: 10.1155/2013/461536.461536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Swampillai A. L., Salomoni P., Short S. C. The role of autophagy in clinical practice. Clinical Oncology (Royal College of Radiologists) 2012;24(6):387–395. doi: 10.1016/j.clon.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 128.Kim Y. C., Guan K. L. mTOR: a pharmacologic target for autophagy regulation. The Journal of clinical investigation. 2015;125(1):25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu Z., Yang C., Iyaswamy A., et al. Balancing mTOR signaling and autophagy in the treatment of Parkinson's disease. International Journal of Molecular Sciences. 2019;20(3):p. 728. doi: 10.3390/ijms20030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hosokawa N., Hara T., Kaizuka T., et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Molecular Biology of the Cell. 2009;20(7):1981–1991. doi: 10.1091/mbc.e08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jung C. H., Jun C. B., Ro S. H., et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Molecular Biology of the Cell. 2009;20(7):1992–2003. doi: 10.1091/mbc.e08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nazio F., Strappazzon F., Antonioli M., et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nature Cell Biology. 2013;15(4):406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- 133.Koren I., Reem E., Kimchi A. DAP1, a novel substrate of mTOR, negatively regulates autophagy. Current Biology. 2010;20(12):1093–1098. doi: 10.1016/j.cub.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 134.Gurusamy N., Lekli I., Mukherjee S., et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovascular Research. 2010;86(1):103–112. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song H., du C., Wang X., Zhang J., Shen Z. MicroRNA-101 inhibits autophagy to alleviate liver ischemia/reperfusion injury via regulating the mTOR signaling pathway. International Journal of Molecular Medicine. 2019;43(3):1331–1342. doi: 10.3892/ijmm.2019.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tan R., Tian H., Yang B., et al. Autophagy and Akt in the protective effect of erythropoietin helix B surface peptide against hepatic ischaemia/reperfusion injury in mice. Scientific Reports. 2018;8(1):p. 14703. doi: 10.1038/s41598-018-33028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang D., Ma Y., Li Z., et al. The role of AKT1 and autophagy in the protective effect of hydrogen sulphide against hepatic ischemia/reperfusion injury in mice. Autophagy. 2012;8(6):954–962. doi: 10.4161/auto.19927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu J., Lu T., Yue S., et al. Rapamycin protection of livers from ischemia and reperfusion injury is dependent on both autophagy induction and mammalian target of rapamycin complex 2-Akt activation. Transplantation. 2015;99(1):48–55. doi: 10.1097/TP.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zaouali M. A., Boncompagni E., Reiter R. J., et al. AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: a role for melatonin and trimetazidine cocktail. Journal of Pineal Research. 2013;55(1):65–78. doi: 10.1111/jpi.12051. [DOI] [PubMed] [Google Scholar]
- 140.Sheng M., Zhou Y., Yu W., Weng Y., Xu R., du H. Protective effect of Berberine pretreatment in hepatic ischemia/reperfusion injury of rat. Transplantation Proceedings. 2015;47(2):275–282. doi: 10.1016/j.transproceed.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 141.Rao Z., Pan X., Zhang H., et al. Isoflurane preconditioning alleviated murine liver ischemia and reperfusion injury by restoring AMPK/mTOR-mediated autophagy. Anesthesia and Analgesia. 2017;125(4):1355–1363. doi: 10.1213/ANE.0000000000002385. [DOI] [PubMed] [Google Scholar]
- 142.Zhu J., Hua X., Li D., Zhang J., Xia Q. Rapamycin attenuates mouse liver ischemia and reperfusion injury by inhibiting endoplasmic reticulum stress. Transplantation Proceedings. 2015;47(6):1646–1652. doi: 10.1016/j.transproceed.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 143.Tiriveedhi V., Upadhya G. A., Busch R. A., et al. Protective role of bortezomib in steatotic liver ischemia/reperfusion injury through abrogation of MMP activation and YKL-40 expression. Transplant Immunology. 2014;30(2-3):93–98. doi: 10.1016/j.trim.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen F. T., Yang C. M., Yang C. H. The protective effects of the proteasome inhibitor bortezomib (velcade) on ischemia-reperfusion injury in the rat retina. PLoS One. 2013;8(5):p. e64262. doi: 10.1371/journal.pone.0064262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ramachandran S., Liaw J. M., Jia J., et al. Ischemia-reperfusion injury in rat steatotic liver is dependent on NFκB P65 activation. Transplant Immunology. 2012;26(4):201–206. doi: 10.1016/j.trim.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zaouali M. A., Bardag-Gorce F., Carbonell T., et al. Proteasome inhibitors protect the steatotic and non-steatotic liver graft against cold ischemia reperfusion injury. Experimental and Molecular Pathology. 2013;94(2):352–359. doi: 10.1016/j.yexmp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 147.Kim J., Kim H. Y., Lee S. M. Protective effects of geniposide and genipin against hepatic ischemia/reperfusion injury in mice. Biomolecules and Therapeutics. 2013;21(2):132–137. doi: 10.4062/biomolther.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang Y. Q., Chang G. L., Wang Y., Zhang D. Y., Cao L., Liu J. Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells: improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway. Cellular Physiology and Biochemistry. 2016;39(1):407–421. doi: 10.1159/000445634. [DOI] [PubMed] [Google Scholar]
- 149.Wu D., Wang J., Li H., Xue M., Ji A., Li Y. Role of hydrogen sulfide in ischemia-reperfusion injury. Oxidative Medicine and Cellular Longevity. 2015;2015:16. doi: 10.1155/2015/186908.186908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun G., Zhou Y., Li H., et al. Over-expression of microRNA-494 up-regulates hypoxia-inducible factor-1 alpha expression via PI3K/Akt pathway and protects against hypoxia-induced apoptosis. Journal of Biomedical Science. 2013;20(1):p. 100. doi: 10.1186/1423-0127-20-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang X., Zhang X., Ren X. P., et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122(13):1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu L., Li Q., Yu B., et al. Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: role of silent information regulator 1. Oxidative Medicine and Cellular Longevity. 2016;2016:16. doi: 10.1155/2016/1689602.1689602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao G. L., Yu L. M., Gao W. L., et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacologica Sinica. 2016;37(3):354–367. doi: 10.1038/aps.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gu L., Li N., Yu W., et al. Berberine reduces rat intestinal tight junction injury induced by ischemia-reperfusion associated with the suppression of inducible nitric oxide synthesis. The American Journal of Chinese Medicine. 2013;41(06):1297–1312. doi: 10.1142/S0192415X13500870. [DOI] [PubMed] [Google Scholar]
- 155.Schmidt R., Tritschler E., Hoetzel A., et al. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Annals of Surgery. 2007;245(6):931–942. doi: 10.1097/01.sla.0000256891.45790.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Collange O., Charles A. L., Noll E., et al. Isoflurane anesthesia preserves liver and lung mitochondrial oxidative capacity after gut ischemia-reperfusion. Anesthesia & Analgesia. 2011;113(6):1438–1441. doi: 10.1213/ANE.0b013e3182367a10. [DOI] [PubMed] [Google Scholar]
- 157.Baotic I., Weihrauch D., Procknow J., et al. Isoflurane favorably modulates guanosine triphosphate cyclohydrolase-1 and endothelial nitric oxide synthase during myocardial ischemia and reperfusion injury in rats. Anesthesiology. 2015;123(3):582–589. doi: 10.1097/ALN.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim M., Park S. W., Kim M., D’Agati V. D., Lee H. T. Isoflurane post-conditioning protects against intestinal ischemia-reperfusion injury and multiorgan dysfunction via transforming growth Factor-β1 generation. Annals of Surgery. 2012;255(3):492–503. doi: 10.1097/SLA.0b013e3182441767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang C., Zheng L., Li L., et al. Rapamycin protects kidney against ischemia reperfusion injury through recruitment of NKT cells. Journal of Translational Medicine. 2014;12(1):p. 224. doi: 10.1186/s12967-014-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Das A., Salloum F. N., Durrant D., Ockaili R., Kukreja R. C. Rapamycin protects against myocardial ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. Journal of Molecular and Cellular Cardiology. 2012;53(6):858–869. doi: 10.1016/j.yjmcc.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kezic A., Thaiss F., Becker J. U., Tsui T. Y., Bajcetic M. Effects of everolimus on oxidative stress in kidney model of ischemia/reperfusion injury. American Journal of Nephrology. 2013;37(4):291–301. doi: 10.1159/000348496. [DOI] [PubMed] [Google Scholar]
- 162.Li J., Chang W. T., Li C. Q., et al. Baicalein preventive treatment confers optimal cardioprotection by PTEN/Akt/NO activation. American Journal of Chinese Medicine. 2017;45(05):987–1001. doi: 10.1142/S0192415X17500525. [DOI] [PubMed] [Google Scholar]
- 163.Liu A., Huang L., Fan H., et al. Baicalein pretreatment protects against liver ischemia/reperfusion injury via inhibition of NF-κB pathway in mice. International Immunopharmacology. 2015;24(1):72–79. doi: 10.1016/j.intimp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 164.Lai C. C., Huang P. H., Yang A. H., et al. Baicalein attenuates lung injury induced by myocardial ischemia and reperfusion. American Journal of Chinese Medicine. 2017;45(04):791–811. doi: 10.1142/S0192415X17500422. [DOI] [PubMed] [Google Scholar]
- 165.Han D., Wang J., Ma S., Chen Y., Cao F. SIRT1 as a promising novel therapeutic target for myocardial ischemia reperfusion injury and cardiometabolic disease. Current Drug Targets. 2017;18(15):1746–1753. doi: 10.2174/1389450116666150630110529. [DOI] [PubMed] [Google Scholar]
- 166.Zhao Q., Guo Z., Deng W., et al. Calpain 2-mediated autophagy defect increases susceptibility of fatty livers to ischemia-reperfusion injury. Cell Death & Disease. 2016;7(4):p. e2186. doi: 10.1038/cddis.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Inserte J., Hernando V., Garcia-Dorado D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovascular Research. 2012;96(1):23–31. doi: 10.1093/cvr/cvs232. [DOI] [PubMed] [Google Scholar]
- 168.Li S., Zhang J., Wang Z., et al. MicroRNA‐17 regulates autophagy to promote hepatic ischemia/reperfusion injury via suppression of signal transductions and activation of transcription‐3 expression. Liver Transplantation. 2016;22(12):1697–1709. doi: 10.1002/lt.24606. [DOI] [PubMed] [Google Scholar]
- 169.Shi J., Bei Y., Kong X., et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics. 2017;7(3):664–676. doi: 10.7150/thno.15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hao J., Wei Q., Mei S., et al. Induction of microRNA-17-5p by p53 protects against renal ischemia-reperfusion injury by targeting death receptor 6. Kidney International. 2017;91(1):106–118. doi: 10.1016/j.kint.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jun J. H., Song J. W., Shin E. J., Kwak Y. L., Choi N., Shim J. K. Ethyl pyruvate is renoprotective against ischemia-reperfusion injury under hyperglycemia. Journal of Thoracic and Cardiovascular Surgery. 2018;155(4):1650–1658. doi: 10.1016/j.jtcvs.2017.10.069. [DOI] [PubMed] [Google Scholar]
- 172.Caglayan E. K., Caglayan K., Göcmen A. Y., et al. Protective effect of ethyl pyruvate on ischemia-reperfusion injury in rat ovary: biochemical and histopathological evaluation. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2014;182:154–159. doi: 10.1016/j.ejogrb.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 173.Zhen Y. Y., Yang C. C., Hung C. C., et al. Extendin-4 protects kidney from acute ischemia-reperfusion injury through upregulation of NRF2 signaling. American Journal of Translational Research. 2017;9(11):4756–4771. [PMC free article] [PubMed] [Google Scholar]
- 174.Chien C. T., Jou M. J., Cheng T. Y., Yang C. H., Yu T. Y., Li P. C. Exendin-4-loaded PLGA microspheres relieve cerebral ischemia/reperfusion injury and neurologic deficits through long-lasting bioactivity-mediated phosphorylated Akt/eNOS signaling in rats. Journal of Cerebral Blood Flow and Metabolism. 2015;35(11):1790–1803. doi: 10.1038/jcbfm.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu G., Zhang Y., Jiang H., Hu X. Exendin-4 attenuates myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein expression. Cardiology Journal. 2013;20(6):600–604. doi: 10.5603/CJ.2013.0159. [DOI] [PubMed] [Google Scholar]
- 176.Yun N., Cho H. I., Lee S. M. Impaired autophagy contributes to hepatocellular damage during ischemia/reperfusion: heme oxygenase-1 as a possible regulator. Free Radical Biology and Medicine. 2014;68:168–177. doi: 10.1016/j.freeradbiomed.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 177.Chi X., Guo N., Yao W., et al. Induction of heme oxygenase-1 by hemin protects lung against orthotopic autologous liver transplantation-induced acute lung injury in rats. Journal of Translational Medicine. 2016;14(1) doi: 10.1186/s12967-016-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim J. S., Wang J. H., Biel T. G., et al. Carbamazepine suppresses calpain-mediated autophagy impairment after ischemia/reperfusion in mouse livers. Toxicology and Applied Pharmacology. 2013;273(3):600–610. doi: 10.1016/j.taap.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Minor T., Stegemann J., Hirner A., Koetting M. Impaired autophagic clearance after cold preservation of fatty livers correlates with tissue necrosis upon reperfusion and is reversed by hypothermic reconditioning. Liver Transplantation. 2009;15(7):798–805. doi: 10.1002/lt.21751. [DOI] [PubMed] [Google Scholar]
- 180.Minor T., Paul A. Hypothermic reconditioning in organ transplantation. Current Opinion in Organ Transplantation. 2013;18(2):161–167. doi: 10.1097/MOT.0b013e32835e29de. [DOI] [PubMed] [Google Scholar]