Abstract
Stress-induced hyperglycemia is common in critically ill patients, where elevated blood glucose and glycemic variability have been found to contribute to infection, slow wound healing, and short-term mortality. Early clinical studies demonstrated improvement in mortality and morbidity resulting from intensive insulin therapy targeting euglycemia. Follow-up clinical studies have shown mixed results suggesting that the risk of hypoglycemia may outweigh the benefits of aggressive glycemic control. None of the prior studies clarify whether euglycemic targets are in themselves harmful, or if the danger lies in the inadequacy of the available methods for achieving desired glycemic outcomes. In this paper, we use a recently developed simulation model of stress hyperglycemia to demonstrate that given an insulin protocol glycemic outcomes are specific to the patient population under consideration, and that there is a need to optimize insulin therapy at the population level. Next, we use the simulator to demonstrate that the performance of Adaptive Proportional Feedback (APF), a popular format for computerized insulin therapy, is sensitive to its parameters, especially to the parameters that govern the aggressiveness of adaptation. Finally, we propose a framework for simulation-based protocol optimization using an objective function that penalizes below-range deviations more heavily than comparable deviations above.
I. Introduction
Stress-induced hyperglycemia is a common occurrence in critically ill patients [1], regardless of health status (diabetic, pre-diabetic, or metabolically normal) prior to hospital admission. Elevated blood glucose (BG) and glycemic variability have been found to contribute to infection, slow wound healing, and short-term mortality [2], [3], [4], [5], [6], [7], [8]. In groundbreaking studies from 2001–2006, van den Berghe and colleagues reported improved outcomes for critically ill patients (particularly cardiac surgical patients) under Tight Glycemic Control (TGC) using a plasma glucose target range of 80–110 [9], [10], [11], and these results inspired many hospital Intensive Care Units (ICUs) to prescribe intensive insulin therapy with aggressive glucose targets. However, subsequent attempts to replicate improved outcomes via tight glycemic control have achieved mixed results. For example, van den Berghe et al. demonstrated no improvement in mortality rates and an increase in hypoglycemic events when TGC was applied to patients in a medical ICU [12]. More recent studies are even less encouraging. In particular, the NICE-SUGAR multicenter study found that the attempt to achieve a 81–108 mg/dl target range increases both 90 day mortality and hypoglycemic events, the latter by 13–fold [13]. Subsequently the American Association of Clinical Endocrinologists / American Diabetes Association (AACE/ADA), the Endocrine Society, and the American College of Physicians (ACP) have relaxed their guidelines for inpatient glycemic control, advocating a presumably safer target range of 140–180 mg/dl [14]. However, the current recommended targets are controversial [15], [16]. None of the prior studies clarify whether tight glycemic targets (e.g. BG 80–110 mg/dl) are in themselves harmful or if the danger lies in the inadequacy of the available methods for achieving and maintaining safe glycemic outcomes.
From a process control perspective, many factors may contribute to the variability of reported glycemic outcomes [14], [17], [18]. Ineffective care coordination can lead to improper implementation of an intensive insulin therapy protocol [17], [18]. Even if a protocol is implemented as intended, point-of-care device variability can affect outcomes, with errors from less than 3% to as high as 20% [14], [19], [20]. Additionally, the choice of protocol may affect the glycemic outcome for each patient. Commonly used paper-based protocols vary in target range, method of insulin delivery (intravenous and/or subcutaneous), time between measurements, practitioner adherence, nutrition support, and insulin amount prescribed for a specific blood glucose measurement or change in blood glucose over time. Thus, different protocols will have different outcomes, regardless of the institution or patient population [14], [20], [21]. For these reasons, it is not clear that simply shifting the BG target range to higher targets (e.g. 140–180 mg/dl) will result in safer outcomes for patients.
There is a clear need for modeling tools that facilitate the design of insulin therapy protocols that support the needs of specific patient populations. In vivo evaluation of alternative insulin therapy protocols (whether paper-based or computer-assisted) is expensive, time consuming, and potentially dangerous [21], [22], [23], [24], [25], and further large studies are unlikely in light of NICE-SUGAR. Moreover, it is generally infeasible to directly compare different insulin protocols in the same set of patients.
The general hypothesis of this work is that the challenges above can be addressed through (i) data-informed characterization of population-specific variability in patients’ insulin sensitivity parameters in a well validated simulation model of glucose metabolism; (ii) creation of a computer-based ICU BG Simulator centered around a virtual subject population that replicates the responses of the real patient population to different insulin therapies; and (iii) simulation-based in silico design optimization of TGC algorithms. Taken together, these steps provide an accurate means of evaluating, comparing, and optimizing insulin protocols that could improve TGC in specific patient populations. Relying on clinical data from the population in consideration, this methodology accounts for both the physiological characteristics of patients and the basic structure and operating constraints of the protocols themselves, leading to a systematic approach to the design of safe and effective insulin therapy protocols, ultimately allowing clinical researchers to re-examine the question of the appropriate target ranges for different patient populations.
II. Building a Population-Specific BG Simulator
The past several years have seen an increase in the availability and acceptance of validated in silico (i.e. simulated) patient populations [26], [27], [28], [29], [30], [31]. Simulation tools have proven tremendously useful in developing and evaluating advanced treatments for type 1 diabetes. Of particular note is the U.Va. / U. Padova Type 1 Simulator [29], [31], with 300 in silico patients, which has been accepted by the FDA as a platform for the evaluation of artificial pancreas algorithms prior to human subject trials, replacing the need for animal trials. The Type 1 Simulator was originally constructed from a comprehensive set of insulin and glucose physiology data measured from a large set of subjects [32], including individuals with healthy metabolism, prediabetes, and type 2 diabetes.
Simulation models of BG variability in critical care have been under development in a parallel line of research [33], [34], [35], beginning as an effort to explain the insulin needs of individual critically ill patients whose blood glucose levels rise and vary seemingly unpredictably. Efforts so far have been on the validation of simulators for comparing insulin infusion protocols such as seen in [36], [37].
A. An ICU BG Simulator
In this work we make use of a recently developed ICU BG Simulator [38], which is based on a reduced version of the oral glucose minimal model of [32] where the gut compartments of the model have been replaced with a model appropriate to distal enteral feeding. The effectiveness of the simulator derives from the fact that each associated in silico subject is a pairing of two elements: (i) a non-stressed in silico patient derived from the same data set used to develop the oral-glucose meal model [32] and (ii) a time-varying stress-action curve SA(t) ∈ [0,1] that accounts for stress-related variability in hepatic glucose production and the uptake of glucose by muscle and fat, as shown in Eqs. (1) and (2) below.
| (1) |
| (2) |
where EGP(t) refers to endogenous glucose production and Uid(t) refers to insulin dependent glucose uptake in the modeling framework of [32]. (Except for (i) the (1 – SA(t)) and .65 SA(t) factors above and (ii) the modification of the gut compartment model, the notation and parameterization of the model is identical that in [32].) Cloned in silico subjects for the ICU simulator are constructed from clinical data including BG samples, insulin infusion data, and feed rates from a representative sample of the population. Each in silico clone is constructed using a two step process. First, we identify a closest match from the base (non-stressed) populations of in silico subjects (i.e. subjects without diabetes, subjects with pre-diabetes, and subjects with type 2 diabetes), picking the closest unstressed subject whose BG trace lies strictly below the historical data for the same set insulin infusions and feed rates. Next, we infer an appropriate stress action curve that, when superimposed onto the best (unstressed) match, reproduces the same set of BG values as in the historical data. (This is done numerically, taking advantage of the fact that simulated BG increases monotonically with increasing stress action.) From the in silico clones, it is possible to create an in silico population that is representative of the patient population at hand by “mixing and matching” the corresponding parameters of the underlying meal-model subjects and the SA curves [38].
The ICU BG Simulator allows varying inputs of enteral feedings, intravenous feedings, and intravenous insulin protocols. Identical populations of ICU in silico patients can be simulated, and the results compared under different treatment conditions, e.g. varying degrees of BG measurement error, BG sampling frequency and timing, BG thresholds for insulin dosing or IV glucose hypoglycemia rescue, and human error in protocol implementation. Such models can be compared after the fact and prospectively to real ICU patients.
B. Validation
We have used two distinct data sets to validate the simulator and its ability to adapt to different patient populations: (i) data from burn ICU patients (BURN) and (ii) mixed medical/surgical ICU patients from New Zealand (NZ). For the BURN dataset over 11,000 hours of data were obtained from 154 burn patients, whose mean age was 34 and who were 86% male; one-third died. They were treated with a simple sliding-scale type of insulin protocol aimed at a BG target of 80–110 mg/dl. The NZ data was over 2100 hours in length from 12 of 20 patients in a published data set [39]; mean age was 67 and 50% were male; none died. The NZ protocol (SPRINT [33]), which also aimed at a BG target of 80–110 mg/dl, consisted of lookup tables for both feeding and insulin rates.
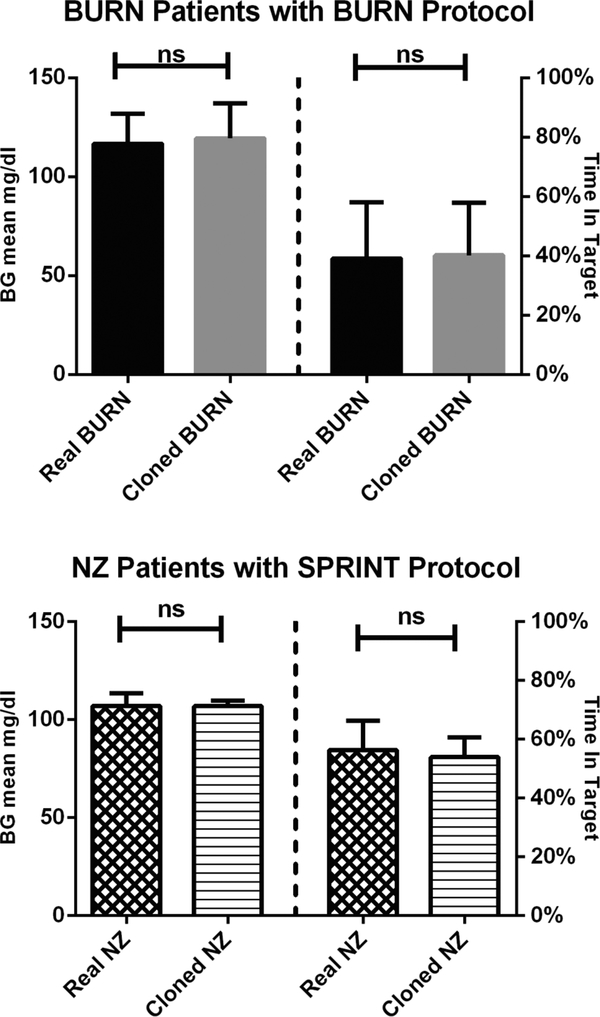
Using data from the burn victims, we created 212 cloned BURN in silico patients using the two-step procedure described above. (Since some of the 154 burn patients had very long hospital stays we were able to generate 212 distinct segments of stress action from the available data.) As shown in the top panel of Fig. 1, the cloned BURN patients were then re-run through the ICU BG Simulator using the same insulin therapy protocol that the real burn victims experienced, with no significant differences observed in mean BG or percent time in the 80–110 mg/dl target range. The bottom panel of Fig. 1 shows the outcome of replicating the process of validation for 12 “cloned” NZ in silico patients that were generated from 12 patients undergoing the SPRINT glycemic control strategy, again with no significant differences. Both comparisons are of the “cloned” subjects run within the simulator against the corresponding historical data.
Fig. 1.
Simulations of “cloned” Burn and NZ patients using their respective insulin protocols, demonstrating the ability of the in silico model to replicate the glycemic outcomes of the original populations. Wilcoxon rank sum test; p < 0.05 considered significant.
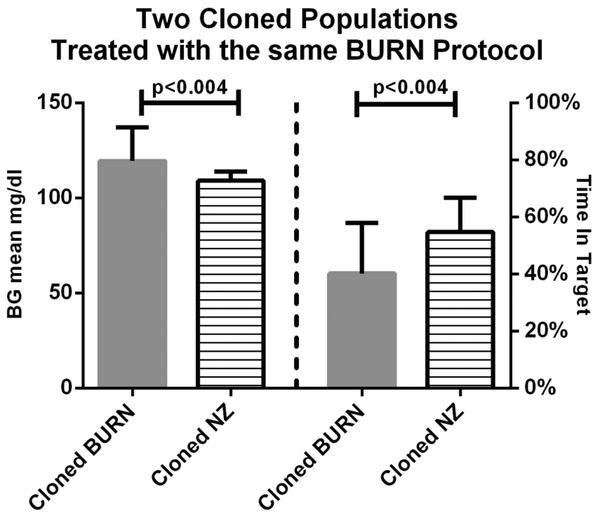
Next, we used the ICU BG Simulator to evaluate glycemic outcomes for both in silico patient populations (BURN and NZ) using the BURN treatment protocol. Significant differences were observed in mean BG and percent time in the target 80–110 mg/dl range. Fig. 2 demonstrates that BURN and NZ patient populations are indeed distinct, supporting the assertion that one protocol does not treat all populations the same.
Fig. 2.
Comparison of glycemic outcomes of BURN in silico patients vs. NZ in silico patients simulated using the Burn insulin protocol. Even though the NZ population is much smaller, BG means and percents of time in target BG range are significantly different, suggesting that the populations have different characteristics with respect to controllability of BG with the same insulin protocol. Wilcoxon rank sum test; p < 0.05 considered significant.
III. Insulin Protocols and Feedback Control: Process Thresholds vs. Target Ranges
As there are many different kinds of intensive care units (medical, surgical, pediatric, etc.) responsible for distinct patient populations, many units have crafted their own insulin protocols to match their specific clinical needs. As a result a large number of different protocols are available in the literature [40]. Many “paper-based” insulin protocols take the form of a “sliding scale” where insulin actions are obtained from decision trees and a look-up tables [41], [42]. Generally, from the actions specified by any given protocol (including the conditions that initiate the protocol itself) it is possible to infer a target BG control range [BGtarget,lo,BGtarget,hi].
Because the critical care setting is a complex environment with many demands placed on clinic staff, protocol compliance is a major issue, leading to the emergence of computerized insulin protocols [43], [44], [45], [46], [47]. Computerized insulin protocols are able to avoid the rough “discretization” of sliding-scale paper-based protocol (insulin delivery can be computed precisely in response to the patient’s BG), and the integration of these systems into the hospital’s information system allows for automated reminders to check in on their patient’s therapy. While some computerized insulin protocols are now commercially available [48], [49], it is still a challenge to configure these protocols to meet the needs of the patient populations being treated.
A. Adaptive Proportional Feedback (APF)
Adaptive Proportional Feedback (APF) is a popular format for computerized insulin delivery [48], [45], [40]. To illustrate the challenges of optimizing this type of insulin therapy, we describe a generic APF protocol below, where the rate of insulin delivery is adjusted as new blood glucose samples are taken every hour:
| (3) |
where (i) ID(n) is the rate of insulin delivery (U/hr) computed from the n-th sample of blood glucose BG(n), (ii) β0 (mg/dl) is a fixed intercept parameter, and (iii) K(n) (U/hr)/(mg/dl) is a multiplier parameter whose value is updated at every blood glucose sample according to
| (4) |
where βlo and βhi are low and high BG adaptation thresholds, and κ is the increment for multiplier adjustments when BG lies outside of [βlo, βhi].
An intensive care unit that is considering adopting an APF strategy would have to be able to choose appropriate values for the fixed parameters β0, κ,βlo,βhi, along with the initial multiplier K(0). In this regard, the literature [48], [40] suggests nominal values for the intercept β0, increment κ, and the initial multiplier K(0), and the low and high BG adaptation thresholds βlo and βhi are usually set to be the endpoints of the desired target blood glucose range, [BGtarget,lo,BGtarget,hi]. Without a validated simulation model, the unit adopting these nominal parameters would have no way of knowing in advance whether they will actually achieve clinical objectives.
B. Simulation Experiments with Different APF Settings
To assess the sensitivity of APF insulin therapy to the parameters β0, κ,βlo,βhi, we used the ICU BG Simulator of Section II-A with 100 in silico burn victims to measure glycemic outcomes for 108 distinct APF “designs”. To accentuate the impact of APF design parameters, we ran the simulator assuming zero errors in BG measurement. The 108 designs include combinations of nine distinct [βlo, βhi] adaptation windows, six distinct multiplier increments values κ, and two distinct BG intercept values β0. Due to space limitations, it is not possible to present summary statistics for all 108 designs; rather, we focus on the variability of glycemic outcomes associated with different [βlo, βhi] adaptation windows. Table I presents the percentage time actually spent in each of the nine [βlo, βhi] windows as a function of the six-by-two combinations of multiplier increments values κ and BG intercept values β0 Specifically, the first column of data presents the minimum percentage time spent in [βlo, βhi] (along with the corresponding κ and β0), the second column presents the maximum percentage time spent in [βlo, βhi], and the third column presents the maximum time spent in [βlo, βhi] across all 108 designs.
TABLE I.
Preclinical in silico results for 108 APF parameter settings
| Threshold parameters βlo, βhi | Min mean % time in [βlo, βhi] %, | Max mean % time in [βlo, βhi] %, | Design that maximizes mean % time in [βlo, βhi] %, |
|---|---|---|---|
| 110, 130 | 36.12 (100, 0.005) | 47.51 (100, 0.03) | 47.51 (100, 0.03, 110, 130) |
| 120, 140 | 36.95 (100, 0.005) | 44.34 (100, 0.03) | 44.34 (100, 0.03, 120, 140) |
| 130, 150 | 35.00 (100, 0.005) | 39.62 (100, 0.025) | 39.62(100, 0.025, 130, 150) |
| 140, 160 | 31.53 (70, 0.03) | 35.11 (100, 0.015) | 35.11 (100, 0.015, 140, 160) |
| 150, 170 | 26.91 (70, 0.03) | 30.23 (100, 0.02) | 30.23 (100, 0.02, 150, 170) |
| 110, 140 | 48.76 (100, 0.005) | 61.26 (100, 0.03) | 61.55 (100, 0.03, 110, 130) |
| 120, 150 | 48.78 (100, 0.005) | 57.34 (100, 0.03) | 57.34 (100, 0.03, 120, 150) |
| 130, 160 | 45.66 (100, 0.005) | 51.38 (100, 0.03) | 51.38 (100, 0.03, 130, 160) |
| 140, 170 | 41.42 (100, 0.005) | 45.43 (100, 0.025) | 45.43 (100, 0.025,140, 170) |
From Table I it is clear that glycemic outcomes from APF insulin therapy are sensitive to the choice of β0,κ,βlo,βhi For example, for BG target ranges that are close to euglycemia with βlo and βhi set to be equal to the endpoints of the target range (e.g. BGtarget,lo = βlo = 110 and BGtarget,hi = βhi = 130), the mean percentage time in range can vary from 36.12% to 47.51% depending on the value of the intercept and multiplier increment parameters, β0 and κ, respectively. It appears that aggressive multiplier increments κ (greater than default .01) and higher intercepts β0 (100 rather than 70 mg/dl) are associated with maximizing percent time in range. As a final observation, note that when the goal is to maximize the percentage time in [110, 140], it is actually best to use βιo = 110 and βhi = 130. Since there is only one example like this it seems that the guideline for picking βιo and βhi to be equal to the endpoints of the desired target range is a good recommendation, at least for the coarse sampling of the design space presented here.
IV. Towards a Simulation-Based Protocol Optimization Methodology
The debate about the safety and efficacy of tight glycemic control so far has been framed mainly in terms of identifying an appropriate target range of BG values. Both the proponents and detractors of tight control around euglycemic values seem to acknowledge the inevitability of occasional hypoglycemia [20]. Interestingly, without any claims on the robustness of the insulin protocols that have been tested, it is unclear whether euglycemic targets are inherently dangerous, or whether it is simply the case that better insulin protocols need to be developed that are more sensitive to the risk of hypoglycemia. Thus, for the insulin protocols in use today, it is difficult to tell whether the clinical specification of a control range is truly a reflection of physiological need, or whether the target range is a reflection of the inability to prevent hypoglycemic excursions. Toward the goal of resolving this conflict in the design of new insulin protocols, we propose the following simulation-based protocol optimization methodology.
A. A Range-Specific Asymmetric Cost Function
Following [50], [51], [52], there is a natural asymmetry of disutility associated with BG excursions below and above euglycemia. While hyperglycemia is associated with infection and slow wound healing, insulin overdose resulting in hypoglycemia presents an acute short term risk that must be avoided. To capture this asymmetry we introduce a cost function designed to attribute equal cost (disutility) J to the endpoints of the desired BG target range [BGtarget,lo,BGtarget,hi]:
| (5) |
with c chosen so that J(BGtarget,lo) = J(BGtarget,hi) = 1. In terms of the “shape” of J, note that J(BG) = 0 when . Also, J penalizes BG excursions below BGtarget,lo much more heavily than comparable excursions above BGtarget,hi. A cost-optimal insulin protocol would be one that minimizes the expected value of
| (6) |
across the whole population of representative in silico clones.
B. Example: Best of 108 APF Designs
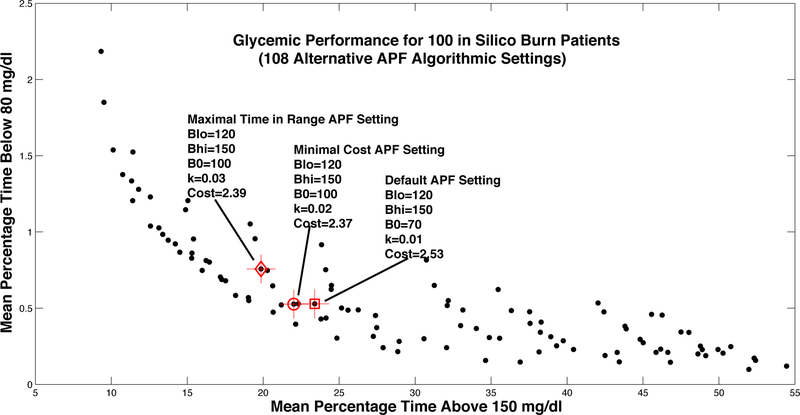
Here we assume that the target range for glycemic control has been determined by a clinical team to be [120, 150], i.e. BGtarget,lo = 120 and BGtarget,hi = 150. With these endpoints in mind, we computed mean cost for each in silico burn victim in the ICU BG Simulator, and then computed average mean cost across the entire population. This assessment of average cost was computed for each of the 108 APF designs considered in Section III-B. The cost-optimal design (the best of the 108) is highlighted in Fig. 3, along with the nominal APF design (with βlo = BGtarget,lo and βhi = BGtarget,hi) and the design that maximizes the percentage time actually spent in [BGtarget,lo,BGtarget,hi]. Each dot in Fig. 3 shows the mean percentage time spent above 150 mg/dl plotted against mean percentage time below 80 mg/dl for one of the 108 designs.
Fig. 3.
Glycemic outcomes from in silico evaluation of 108 different settings of the generic APF protocol, indicating both the default setting and an improved design with a lower percentage of time spent above 150 mg/dl. (Crosses represent standard errors for sample average of each metric.)
The simulation results demonstrate that there is a tradeoff between mean percentage time above 150 mg/dl and mean percentage time below 80 mg/dl, with a clear Pareto frontier in terms of these two outcomes. Interestingly, the three highlighted designs (each one targeting [120, 150]) are all close to each other, both in terms of (i) the percentage time above and below 150 and 80 md/dl, respectively, and (ii) APF parameter settings. The cost-optimal APF design, which achieves an average cost of 2.37, appears to be slightly better than the default APF setting in terms of percentage time above 150 mg/dl. The design that actually maximizes the percentage time in [120, 150] achieves even lower percentage time above 150 mg/dl, but it does so at the expense of greater percentage time below 80 mg/dl. Interestingly, all three of these designs have βlo = BGtarget,lo = 120 mg/dl and βhi = BGtarget,hi = 150 mg/dl.
V. Conclusions and Future Directions
In this paper we have addressed the need for modeling tools that facilitate the design, assessment, and evaluation of insulin therapy protocols that support the needs of specific patient populations. Population-specific simulation can play a key role in preclinical evaluation of alternative insulin therapy protocols whether they are paper-based or computer-assisted. We have presented a methodological framework that includes (i) data-informed characterization of the population-specific variability in patients’ insulin sensitivity parameters in a well validated simulation model of glucose metabolism; (ii) creation of a computer-based ICU BG Simulator centered around a virtual subject population that replicates the responses of the real patient population to different insulin therapies; and (iii) simulator-based optimization of tight glycemic control algorithms, which can be extended to account for noisy measurements and to more fully explore the design space (e.g. using response surface methods). The results presented here illustrate the potential of this approach, supporting future research into radically new inpatient glycemic management strategies including (i) closed-loop systems akin to the type 1 artificial pancreas that take advantage of next-generation continuous glucose monitoring devices and (ii) modular glycemic management strategies in which insulin and feed rates are managed in a coordinated fashion for optimal glycemic control.
Acknowledgments
This work was sponsored in part by the National Institutes of Health (1R21EB018052–01, T15LM009462, and R01DK082805). This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- [1].McCowen KC, Malhotra A, and Bistrian BR, “Stress-induced hyperglycemia,” Critical Care Clinics, vol. 17, no. 1, pp. 107–124, 2001. [DOI] [PubMed] [Google Scholar]
- [2].Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, and Desai M, “Association of hyperglycemia with increased mortality after severe burn injury,” Journal of Trauma and Acute Care Surgery, vol. 51, no. 3, pp. 540–544, 2001. [DOI] [PubMed] [Google Scholar]
- [3].Krinsley JS, “Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients,” Mayo Clinic Proceedings, vol. 78, pp. 1471–1478, 2003. [DOI] [PubMed] [Google Scholar]
- [4].Butler SO, Btaiche IF, and Alaniz C, “Relationship between hyperglycemia and infection in critically ill patients,” Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, vol. 25, no. 7, pp. 963–976, 2005. [DOI] [PubMed] [Google Scholar]
- [5].Cheung NW, Napier B, Zaccaria C, and Fletcher JP, “Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition,” Dia Care, vol. 28, no. 10, pp. 2367–237’, 2005. [DOI] [PubMed] [Google Scholar]
- [6].Gale SC, Sicoutris C, Reilly PM, Schwab CW, and Gracias VH, “Poor glycemic control is associated with increased mortality in critically ill trauma patients,” American Surgeon, vol. 73, no. 5, pp. 454–460, 2007. [DOI] [PubMed] [Google Scholar]
- [7].Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, and Bailey M, “Hypoglycemia and outcome in critically ill patients,” Mayo Clinic Proceedings, vol. 85, no. 3, pp. 217–224, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bochicchio GV, Sung J, Joshi M, Bochicchio K, Johnson SB, Meyer W, and Scalea TM, “Persistent hyperglycemia is predictive of outcome in critically ill trauma patients,” J Trauma, vol. 58, no. 5, pp. 921–924, 2005. [DOI] [PubMed] [Google Scholar]
- [9].Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, and Bouillon R, “Intensive insulin therapy in critically ill patients,” New England Journal of Medicine, vol. 345, no. 19, pp. 1359–1367, 2001. [DOI] [PubMed] [Google Scholar]
- [10].Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, Bouillon R, and Schetz M, “Intensive insulin therapy in mixed medical/surgical intensive care units benefit versus harm,” Diabetes, vol. 55, no. 11, pp. 3151–3159, 2006. [DOI] [PubMed] [Google Scholar]
- [11].Pittas AG, Siegel RD, and Lau J, “Insulin therapy for critically ill hospitalized patients: A meta-analysis of randomized controlled trials,” Arch Intern Med, vol. 164, no. 18, pp. 2005–2011, 2004. [DOI] [PubMed] [Google Scholar]
- [12].Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, and Bouillon R, “Intensive insulin therapy in the medical icu,” New England Journal of Medicine, vol. 354, no. 5, pp. 449–461, 2006. [DOI] [PubMed] [Google Scholar]
- [13].Investigators TN-SS, “Intensive versus conventional glucose control in critically ill patients,” New England Journal of Medicine, vol. 360, no. 13, pp. 1283–1297, 2009. [DOI] [PubMed] [Google Scholar]
- [14].Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, and Umpierrez GE, “American association of clinical endocrinologists and american diabetes association consensus statement on inpatient glycemic control,” Diabetes Care, vol. 32, no. 6, pp. 1119–1131, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kansagara D, Fu R, Freeman M, Wolf F, and Helfand M, “Intensive insulin therapy in hospitalized patients: a systematic review,” Ann. Intern. Med, vol. 154, no. 4, pp. 268–282, 2011. [DOI] [PubMed] [Google Scholar]
- [16].Lanspa MJ, Hirshberg EL, Phillips GD, Holmen J, Stoddard G, and Orme J, “Moderate glucose control is associated with increased mortality compared to tight glucose control in critically ill nondiabetics,” 2013, vol. 143, no. 5, pp. 1226–1234, Chest [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anger KE and Szumita PM, “Barriers to glucose control in the intensive care unit,” Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, vol. 26, no. 2, pp. 214–228, 2006. [DOI] [PubMed] [Google Scholar]
- [18].Aragon D, “Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control,” Am J Crit Care, vol. 15, no. 4, pp. 370–377, 2006. [PubMed] [Google Scholar]
- [19].Chase JG, Shaw GM, Wong XW, Lotz Lin J, and Hann CE, “Model-based glycaemic control in critical care—a review of the state of the possible,” Biomedical Signal Processing and Control, vol. 1, no. 1, pp. 3–21, 2006. [Google Scholar]
- [20].Mesotten D and Van den Berghe G, “Glycemic targets and approaches to management of the patient with critical illness,” Curren Diab Rep, vol. 12, no. 1, pp. 101–107, 2012. [DOI] [PubMed] [Google Scholar]
- [21].Blaha J, Kopecky P, Matias M, Hovorka R, Kunstyr J, Kotulak T, Lips M, Rubes D, Stritesky M, Lindner J, Semrad M, and Haluzik M, “Comparison of three protocols for tight glycemic control in cardiac surgery patients,” Dia Care, vol. 32, no. 5, pp. 757–761, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chase JG, Shaw G, Le Compte A, Lonergan T, Willacy M, Wong X-W, Lin J, Lotz T, L. D D, and Hann C, “Implementation and evaluation of the sprint protocol for tight glycaemic control in critically ill patients: a clinical practice change,” Critical Care, vol. 12, no. 2, p. R49, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chase JG, Shaw GM, Lotz T, Le Compte A, Wong J, Lin J, Lonergan T, Willacy M, and Hann CE, “Model-based insulin and nutrition administration for tight glycaemic control in critical care,” Current Drug Delivery, vol. 4, no. 4, pp. 283–296, 2007. [DOI] [PubMed] [Google Scholar]
- [24].Preiser J-C, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, and Chioléro R, “A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the glucontrol study,” Intensive Care Medicine, vol. 35, no. 10, pp. 1738–1748, 2009. [DOI] [PubMed] [Google Scholar]
- [25].De La Rosa GDC, Donado JH, Restrepo AH, Quintero AM, González LG, Saldarriaga NE, Bedoya M, Toro JM, Velásquez JB, and Valencia JC, “Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial,” Critical Care, vol. 12, no. 5, p. R120, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rostami-Hodjegan A and Tucker G, “In silico simulations to assess the in vivo consequences of in vitro metabolic drug-drug interactions,” Drug Discovery Today: Technologies, vol. 1, no. 4, pp. 441–448, 2004. [DOI] [PubMed] [Google Scholar]
- [27].Rostami-Hodjegan A and Tucker GT, “Simulation and prediction of in vivo drug metabolism in human populations from in vitro data,” Nature Reviews Drug Discovery, vol. 6, no. 2, pp. 140–148, 2007. [DOI] [PubMed] [Google Scholar]
- [28].Michelson S, Sehgal A, and Friedrich C, “In silico prediction of clinical efficacy,” Current opinion in biotechnology, vol. 17, no. 6, pp. 666–670, 2006. [DOI] [PubMed] [Google Scholar]
- [29].Kovatchev B, Breton M, Dalla Man C, and Cobelli C, “In Silico preclinical trials: A proof of concept in closed-loop control of type 1 diabetes,” J Diab Sci and Tech, vol. 3, pp. 44–55, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patek SD, Bequette BW, Breton M, Buckingham BA, Dassau E, Doyle III FJ, Lum J, Magni L, and Zisser H, “In Silico Preclinical Trials: Methodology and Engineering Guide to Closed-Loop Control in Type 1 Diabetes Mellitus,” J Diab Sci and Tech, vol. 3, pp. 269–282, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dalla Man C, Micheletto F, Lv D, Breton M, Kovatchev B, and Cobelli C, “The uva/padova type 1 diabetes simulator: New features,” J Diabetes Sci Technol, vol. 8, no. 1, pp. 26–34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dalla Man C, Rizza RA, and Cobelli C, “Meal simulation model of the glucose-insulin system,” IEEE Trans Biomedical Eng, vol. 54, no. 10, pp. 1740–1749, 2007. [DOI] [PubMed] [Google Scholar]
- [33].Chase JG, Shaw GM, Lin J, Doran CV, Hann C, Robertson MB, Browne PM, Lotz T, Wake GC, and Broughton B, “Adaptive bolus-based targeted glucose regulation of hyperglycaemia in critical care,” Med Eng Phys, vol. 27, no. 1, pp. 1–11, 2005. [DOI] [PubMed] [Google Scholar]
- [34].Hovorka R, Chassin LJ, Ellmerer M, Plank J, and Wilinska ME, “A simulation model of glucose regulation in the critically ill,” Physiol Meas, vol. 29, no. 8, pp. 959–978, 2008. [DOI] [PubMed] [Google Scholar]
- [35].Herpe TV, Espinoza M, Haverbeke N, Moor BD, and Van den Berghe G, “Glycemia prediction in critically ill patients using an adaptive modeling approach,” J Diabetes Sci Technol, vol. 1, no. 3, pp. 348–356, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chase JG, Suhaimi F, Penning S, Preiser J-C, Le Compte AJ, Lin J, Pretty CG, Shaw GM, Moorhead KT, and Desaive T, “Validation of a model-based virtual trials method for tight glycemic control in intensive care,” Biomed Eng Online, vol. 9, p. 84, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wilinska ME, Blaha J, Chassin LJ, Cordingley JJ, Dormand NC, Ellmerer M, Haluzik M, Plank J, Vlasselaers D, Wouters PJ, and Hovorka R, “Evaluating glycemic control algorithms by computer simulations,” Diabetes Technol Ther, vol. 13, no. 7, pp. 713–722,2011. [DOI] [PubMed] [Google Scholar]
- [38].Ortiz EA, “Simulating glycemic variability in critically ill burn patients,” M.S. Thesis in Systems and Information Engineering, University of Virginia, 2012. [Google Scholar]
- [39].Chase JG, Le Compte A, Shaw GM, Blakemore A, Wong J, Lin J, and Hann CE, “A benchmark data set for model-based glycemic control in critical care,” J Diabetes Sci Technol, vol. 2, no. 4, pp. 584–594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Steil GM, Deiss D, Shih J, Buckingham B, Weinzimer S, and Agus MS, “Intensive care unit insulin delivery algorithms: Why so many? how to choose?” J Diabetes Sci Technol, vol. 3, no. 1, pp. 125–140, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].PA PAG, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, Lee SL, Dziura JD, and Inzucchi SE, “Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit,” Diabetes Care, vol. 27, no. 2, pp. 461–467, 2004. [DOI] [PubMed] [Google Scholar]
- [42].Trence DL, Kelly JL, and Hirsch IB, “The rationale and management of hyperglycemia for in-patients with cardiovascular disease: time for change,” J Clin Endocrinol Metab, vol. 88, no. 6, pp. 2430–2437, 2003. [DOI] [PubMed] [Google Scholar]
- [43].Rood E, Bosman RJ, Van Der Spoel JI, TAYLOR P, and Zandstra DF, “Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation,” J Am Med Inform Assoc, vol. 12, no. 2, pp. 172–180, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cao CGL, Ozdas A, and Slagle J, “Utilization of computerized order entry protocols in the icu for glucose management,” in Proceedings IEA2006 World Congress on Ergonomics, 2006. [Google Scholar]
- [45].Boord JB, Sharifi M, Greevy RA, Griffent MR, Lee VK, Webb TA, May ME, Waitman LR, May AK, and Miller RA, “Computer-based insulin infusion protocol improves glycemia control over manual protocol,” J Am Med Inform Assoc, vol. 14, no. 3, pp. 278–287, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Van Herpe T, Mesotten D, Wouters PJ, Herbots J, Voets E, Buyens J, De Moor B, and Van den Berghe G, “LOGIC-insulin algorithm-guided versus nurse-directed blood glucose control during critical illness: the LOGIC-1 single-center, randomized, controlled clinical trial.” Diabetes Care, vol. 36, no. 2, pp. 188–194, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rattan R and Nasraway SA, “The Future is Now: Software-Guided Intensive Insulin Therapy in the Critically Ill,” Journal of Diabetes Science and Technology, vol. 7, no. 2, pp. 548–554, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Davidson P, Steed R, and Bode B, “Glucommander a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation,” Diabetes Care, vol. 28, no. 10, pp. 2418–2423, 2005. [DOI] [PubMed] [Google Scholar]
- [49].Juneja R, Roudebush CP, Nasraway SA, Golas AA, Jacobi J, Carroll J, Nelson D, Abad VJ, and Flanders SJ, “Computerized intensive insulin dosing can mitigate hypoglycemia and achieve tight glycemic control when glucose measurement is performed frequently and on time,” Critical Care, vol. 13, no. 5, p. R163, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kovatchev B, Straume M, Cox D, and Farhy L, “Risk analysis of blood glucose data: a quantitative approach to optimizing the control of insulin dependent diabetes,” J Theor Med, vol. 3, pp. 1–10, 2001. [Google Scholar]
- [51].Farhy LS, Ortiz EA, Kovatchev BP, Mora AG, Wolf SE, and Wade CE, “Average daily risk range as a measure of glycemic risk is associated with mortality in the intensive care unit: A retrospective study in a burn intensive care unit,” J Diabetes Sci Technol, vol. 5, no. 5, pp. 1087–1098, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Patek SD, Breton M, Vereshchetin P, Jiang B, and Kovatchev BP, “Model-Based Control of Type 1 Diabetes in “Risk Space”,” in IFAC World Congress, 2014. [Google Scholar]