Abstract
In the present study we sought to investigate interactions between hypothalamic nitric oxide (NO) and ghrelin signaling on food intake and energy substrate utilization as measured by the respiratory exchange ratio (RER). Guide cannulae were unilaterally implanted in either the arcuate (ArcN) or paraventricular (PVN) nuclei of male Sprague-Dawley rats. Animals were pretreated with either subcutaneous (2.5-10 mg/kg/ml) or central (0-100 pmol) N-nitro-L-Arginine methyl ester (L-NAME) followed by 50 pmol of ghrelin administered into either the ArcN or PVN. Both L-NAME and ghrelin were microinjected at the onset of the active cycle and food intake and RER were measured 2 hours postinjection. RER was measured as the ratio of the volume of carbon dioxide expelled relative to the volume of oxygen consumed (VCO2/VO2) using an open-circuit indirect calorimeter. Our results demonstrated that peripheral and central L-NAME pretreatment dose-dependently attenuated ghrelin induced increases in food intake and RER in either the ArcN or PVN. In fact the 100 pmol dose largely reversed the metabolic effects of ghrelin in both anatomical regions. These findings suggest that ghrelin enhancement of food intake and carbohydrate oxidation in the rat ArcN and PVN is NO-dependent.
Keywords: Arcuate Nucleus, Paraventricular Nucleus, L-NAME, Metabolism, Appetite, Hypothalamus
1. Introduction
Ghrelin, named for its potent effect on growth hormone (GH) release, is a 28-amino acid peptide cleaved from preproghrelin and encoded by the GHRL gene. The peptide was first identified in the rat (Rattus norvigecus) stomach by Kojima et. al. (1999) as the endogenous ligand for the growth hormone secretagogue receptor 1a (GHSR1a), encoded by the GHRS gene. The predominantly active form of ghrelin requires acylation by ghrelin-O-acyl transferase (GOAT) (Müller et. al. 2015; Gutierrez et al., 2008; Romero et al., 2010; Mohan and Unniappan, 2013). GOAT is conserved across a variety of vertebrate, including mice (Mus musculus), rats (Rattus norvegicus), domestic chickens (Gallus domesticus) and goldfish (Carassius auratus) (Gutierrez et al., 2008; Seim et al., 2016; Blanco et al., 2017). Furthermore it appears that a truncated form of GHSR1a, GHSR1b, is also cleaved from the same gene and has been reported to reduce cell surface GHSR1a availability via heterodimerization (Chow et al., 2012). It has been demonstrated across various species that ghrelin is a critical signal in energy homeostasis (Müller et al., 2015; Kojima et al., 2016; Kaiya et al., 2013; Currie et al., 2005; Brockway et al., 2016; Abtahi et al., 2016; Shousha et al., 2005; Unniappan et al., 2002).
Mammals, teleosts and bullfrog larvae present potent orexigenic effects from ghrelin administration (Currie et al., 2005, Asakawa et al., 2001; Kamegai et. al., 2001; Kang et al. 2011; Shimizu et al., 2014; Unniappan et al., 2002), though it is generally anorexigenic, but less consistently, in avians (Kaiya et al., 2013; Tachibana and Tsutsui, 2016; Zendehdel and Hassanpour, 2014). Ghrelin administration into the arcuate (ArcN) and paraventricular (PVN) nuclei of the hypothalamus in rodents reliably enhances food intake and the respiratory exchange ratio (RER) (Abtahi et al., 2016; Currie et al., 2005). On the other hand, intracerebroventricular (ICV) microinjection of rat ghrelin attenuates food intake and RER in brooding chickens (Geelissen et al., 2006). Shousha et al., (2005) reported that in the Japanese Quail (Coturnix japonica), ghrelin elicits an increase in food intake at low doses and attenuates it at higher doses. Despite these opposing roles in ingestive behavior between rodents and avians, fasting increases plasma ghrelin in both groups of animals (Kaiya et al., 2013; Tachibana and Tsutsui, 2016). Concurrently, food intake lowers plasma ghrelin in both mammals (Vester Boler et al., 2012) and avians (Kaiya et al., 2007). Recent work by Goymann et al., (2017) reported that acylated plasma ghrelin predicts lipid content in garden warblers (Sylvia borin) and that unacylated ghrelin administration increases migratory restlessness behavior, suggesting that the differences in ghrelin mediation of energy homeostasis between avians and other animals may be due to migratory behavior. Although ghrelin is highly conserved across vertebrate (Seim et al., 2016), it is necessary to investigate the potential circuits that may contribute to species similarities and differences in ghrelin modulation of energy balance.
Nitric oxide (NO) is a gaseous transmitter critical for appetitive signaling within the mammalian hypothalamus (Morley et al., 2011; Gaskin et al., 2003). In mice, ICV pretreatment with N-nitro-L-Arginine methyl ester (L-NAME), a nitric oxide synthase (NOS) inhibitor, attenuates ghrelin enhancement of food intake (Gaskin et al., 2003) and NOS knockout (KO) abolishes ghrelin's appetitive effects (Morley et al., 2011). In addition, peripheral L-NAME reportedly decreases NOS mRNA expression in both the ArcN and PVN (Huai-Zhen and Xiao-Tang, 2000). These behavioral studies are supported by in vitro data illustrating that nitric oxide synthase is necessary for ghrelin induced GH release (Rodriguez-Pacheco et al., 2008; Grey and Chang, 2012).
In rodents, the mRNA for GHSR1a and NOS are both expressed in the ArcN and PVN (Zigman et al., 2006; Ng et al., 1999) and NOS is necessary for ghrelin enhancement of food intake (Gaskin et al., 2003). Ghrelin exerts its effects on energy homeostasis via GHSR1a expressed on neuropeptide Y (NPY)/agouti related peptide (AgRP) neurons in the ArcN, including Y1 receptors (Kohno et al., 2003; Currie et al., 2005; Abtahi et al., 2016), which are co-localized with NOS expressing neurons (Fetissov et al., 2003). This suggests that NOS may play a critical role in ghrelin mediation of both appetite and energy substrate utilization as measured by RER. Arcuate NPY/AgRP neurons have excitatory projections to the PVN and are implicated in the enhancement of food intake (Fenselau et al., 2017). Hypothalamic ghrelin shifts energy metabolism from lipid to enhanced carbohydrate oxidation, an effect attenuated by 5-hydroxytryptamine (5-HT), urocortin I (UcnI) and glucagon-like peptide 1 agonism (Currie et al., 2010; Currie et al., 2011a; Abtahi et al., 2016). In the present study, we examined the interaction of ghrelin and NO signaling in the expression of appetitive behavior and energy metabolism in male Sprague-Dawley rats since the integrated hypothalamic control of these two systems has yet to be investigated. Our findings demonstrated that subthreshold L-NAME microinjection into the ArcN and PVN attenuated ghrelin enhancement of food intake and RER, suggesting that ghrelin's orexigenic and metabolic effects are NO-dependent.
2. Materials and methods
2.1. Animals
Adult male Sprague-Dawley rats (N=64) were purchased from Envigo Laboratories and weighed between 285-315 g at the time of surgery. Rodents were individually housed in polypropylene cages with ad libitum access to standard rodent chow pellets (Purina) and water. The animal colony room was maintained on a 12 h light/dark cycle (lights off at 1500 h) and at a temperature of 22 ± 2°C. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Reed College.
2.2. Apparatus
Oxygen consumption (O2) and carbon dioxide (CO2) production were measured using an Oxymax Comprehensive Lab Animal Monitoring System (CLAMS) open-circuit indirect calorimeter (Columbus Instruments, Columbus, OH). Detectors measured O2 and CO2 sequentially across each test chamber. The flow rate was set at 2 litres/min. Concentrations of the gases were recorded in ml/kg body weight/min. RER was calculated as the volume of CO2 produced (VCO2) divided by the volume of O2 consumed (VO2). The analysers were calibrated prior to each test using primary gas standards of high purity (Praxair, Vancouver, WA).
2.3. Stereotactic Surgery
Rats were anesthetized with ketamine (100 mg/kg IP; Henry Schein, Melville, NY) and xylazine (5 mg/kg IP; Sigma-Aldrich, St. Louis, MO) and placed in a Kopf stereotaxic frame with the incisor bar set 3.5 mm below the interaural line. Stereotaxic coordinates for the guide cannula relative to bregma were: ArcN, posterior 2.3 mm, lateral ±0.2 mm, and ventral 5.7 mm; PVN, posterior –1.8 mm, lateral ±0.3 mm, and ventral –4.5 mm (Paxinos & Watson, 2014). Unilateral guide cannulae (22-gauge; Plastics One, Roanoke, VA) were implanted 4 mm dorsal to the target to prevent tissue damage. Implants were secured with acrylic cement and stainless steel anchor screws. A 28-gauge stainless-steel inner stylet maintained cannula patency. Behavioral and metabolic testing began following a postoperative recovery period of two weeks.
2.4. Design and Procedure
In behavioral testing, an initial group of rats with cannulae aimed at the ArcN received subcutaneous (SC) injections of L-NAME (0, 2.5 and 10 mg/kg) paired with 50 pmol of ghrelin or vehicle administered directly into the ArcN. Injections were administered at the onset of the nocturnal cycle and food intake was measured 2 h postinjection. Under control conditions two consecutive vehicle injections of sterile saline were infused. All rats received each dose of L-NAME, paired with ghrelin or vehicle, in randomized order. At least 4 days separated successive testing. In other rats, cannulae were aimed at the PVN, and similar doses of SC L-NAME were paired with PVN ghrelin or vehicle microinjection to determine the impact of SC L-NAME treatment on PVN ghrelin-stimulated eating. Subsequently, to examine the effect of direct ArcN L-NAME on eating induced by ArcN ghrelin, additional rats were administered 0, 10, or 100 pmol of L-NAME into the ArcN, paired with 50 pmol of ghrelin or vehicle, also directly injected into this nucleus. And finally, similar manipulations were employed with PVN-implanted animals wherein L-NAME was injected directly into the PVN paired with PVN ghrelin.
Identical injection procedures and treatments were followed for metabolic testing using separate groups of rats. Again, systemic or ArcN L-NAME was co-administered with ArcN ghrelin. RER was measured over a 2-h period and testing began immediately after the rats were placed into the apparatus. Food and water were not available during this time. In additional rats, PVN L-NAME was paired with PVN ghrelin.
Ghrelin (Research Biopeptides, San Diego, CA) and L-NAME (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile saline. All central injections were administered in a volume of 0.2 μl into the ArcN and PVN using a microinjector extending 4 mm beyond the permanent guide cannula. Subcutaneous L-NAME was injected in at doses of 2.5-10 mg/kg and in a volume of 1.0 ml/kg paired with a fixed dose of 50 pmol ghrelin into the ArcN or PVN. For direct ArcN or PVN investigations, L-NAME was administered at 0, 10 and 100 pmol paired with a 50 pmol of ghrelin.
2.5. Histological Anaylsis
At the conclusion of testing, cannulae placements were confirmed via histological examination. To assist in cannulae verification, prior to extractions animals received a 100 mg/kg dose of sodium pentobarbital (Sigma-Aldrich, St. Louis, MO) and were microinjected with 0.2 μL of black ink. Sections were examined by light microscopy and viewed relative to the stereotaxic atlas of Paxinos and Watson (2014). All rats reported here were found to have injector tracks extending into the ArcN and PVN. Figure 1 illustrates representative histological images of implants targeting the ArcN and PVN.
Figure 1.
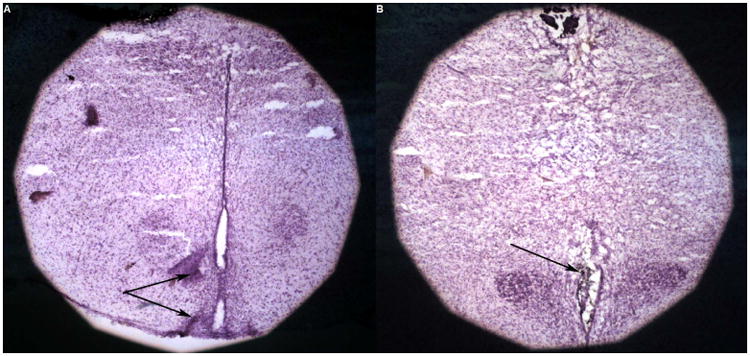
Histological confirmation of injection sites targeting the ArcN (A) and PVN (B) of the hypothalamus. The black arrows indicate black ink deposits injected into each structure.
2.6 Statistics
Data were analyzed by separate two-way analyses of variance (ANOVA) for repeated measures. The software employed was Statistica 13 Academic (Quest Software, Tulsa, OK). Comparisons between group means were evaluated using post hoc Tukey tests. The criterion for statistical significance was p<0.05.
3. Results
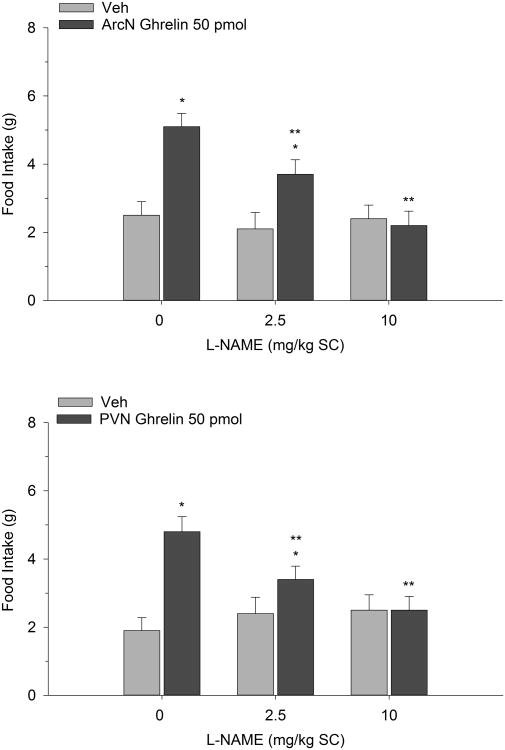
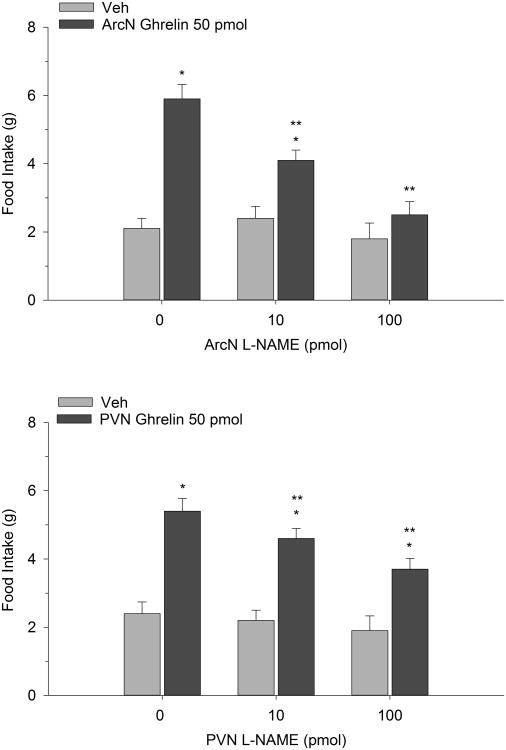
Figure 2 illustrates the effect of ghrelin co-administered with systemic L-NAME on 2-h food intake measured during the early nocturnal period. Two-way repeated measures ANOVA indicated a significant interaction (F(2,12)=10.61, p<0.002). Ghrelin (50 pmol) reliably increased food intake compared to vehicle control when administered into the ArcN. Pretreatment with L-NAME (10 and 100 mg/kg), injected SC, dose-dependently suppressed the orexigenic action of the peptide. In fact the highest dose of 100 mg/kg completely reversed ghrelin's effect. It is noteworthy that both L-NAME doses, when co-administered with saline, did not significantly alter intake. Similarly, PVN ghrelin treatment potentiated dark onset eating and this effect was dose-dependently blocked by SC L-NAME (F(2,14)=5.72, p<0.01). Again, when these same L-NAME doses were administered with Veh, we found them to be subthreshold. In separate tests, the impact of direct ArcN and PVN L-NAME treatment on ghrelin-stimulated eating was investigated. While PVN L-NAME effectively attenuated ghrelin's orexigenic effect (F(2,16)=8.04, p<0.003) ArcN L-NAME completely reversed ghrelin's effect on food intake, particularly at the 100 pmol dose (F(2,14)=8.16, p<0.004). The findings are depicted in Figure 3.
Figure 2.
Food intake after co-administration of L-NAME and ghrelin. L-NAME was injected SC and ghrelin was delivered directly into the ArcN (n=7) or into the PVN (n=8). Values represent mean intakes (±S.E.M.) measured over the initial 2 h of the nocturnal cycle. *p<0.05 compared to saline vehicle (Veh); **p<0.05 compared to ghrelin.
Figure 3.
Effect of ArcN and PVN L-NAME on the eating-stimulant action of ArcN (n=8) and PVN (n=9) ghrelin. Values are represented as mean intakes (±S.E.M.) over 2 h. *p<0.05 compared to Veh; **p<0.05 compared to ghrelin.
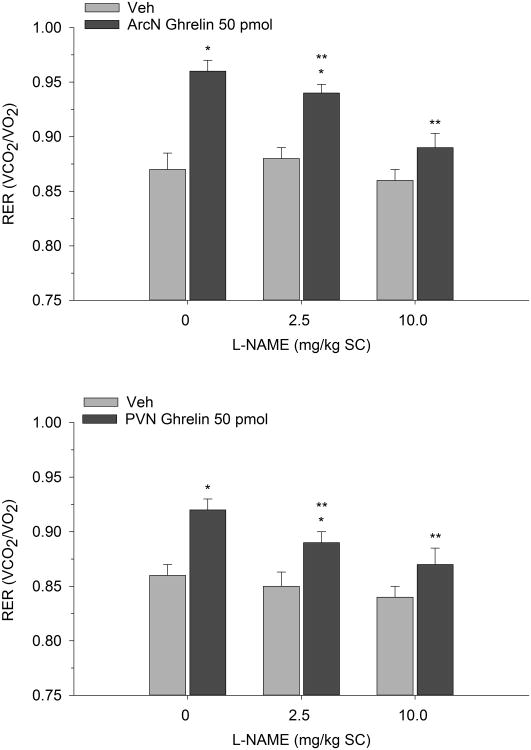
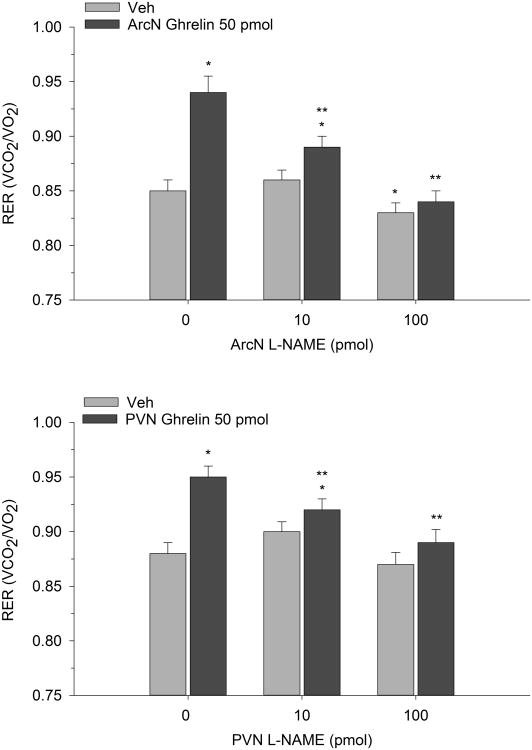
In 2-h metabolic testing, we used a similar injection protocol and paired systemic or hypothalamic L-NAME with ghrelin to investigate the effects on RER. Two-way repeated measures ANOVA indicated that ArcN ghrelin increased RER indicating a shift toward enhanced carbohydrate oxidation (F(2,14)=10.49, p<0.001). While systemic L-NAME alone had no effect on RER, co-administration with ArcN ghrelin reversed ghrelin's metabolic action. Similar observations were found when subcutaneous L-NAME was paired with PVN ghrelin (F(2,12)=8.90, p<0.004) (see Figure 4). Finally direct ArcN or PVN L-NAME treatment also completely reversed the metabolic action of ArcN and PVN ghrelin (F(2,16)=7.86, p<0.005) and F(2,14)=6.06, p<0.02) respectively). That is, while local administration of ghrelin into either the ArcN or PVN again increased RER, as predicted, co-administration with L-NAME directly into either hypothalamic nucleus suppressed ghrelin's ability to promote carbohydrate oxidation as shown in Figure 5.
Figure 4.
Mean (±S.E.M.) RER values following subcutaneously administered L-NAME paired with ArcN (n=8) and PVN (n=7) ghrelin. RER was assessed over a 2-h period. *p<0.05 compared to Veh; **p<0.05 compared to ghrelin.
Figure 5.
Mean (±S.E.M.) RER values in rats co-administered ArcN or PVN L-NAME with ArcN (n=9) or PVN (n=8) ghrelin. RER values are represented at 2 h postinjection. *p<0.05 compared to Veh; **p<0.05 compared to ghrelin.
4. Discussion
In the present study, both ArcN and PVN ghrelin treatment increased food intake and promoted carbohydrate oxidation via increases in RER. L-NAME, in turn, potently blocked each of these effects. In fact, systemic and direct ArcN or PVN L-NAME administration reliably suppressed ghrelin-stimulated eating and increases in RER. The inhibitory effects on ghrelin were dose-dependent and specifically observed during the early period of the animal's active cycle, a time point at which ghrelin produces its most robust effects on food intake and carbohydrate metabolism (Currie et al., 2005; Currie et al., 2011a). Overall these findings suggest that the orexigenic and metabolic actions of the gastric peptide, specifically within the ArcN and PVN, are NO-dependent.
Prior work in our lab has demonstrated that ghrelin reliably and dose-dependently increases food intake as well as RER (Currie et al., 2005; Currie et al., 2011a Abtahi et al., 2016). Furthermore, ghrelin interacts with the orexigenic peptide NPY to dose-dependently enhance food intake and RER in the ArcN and PVN (Currie et al., 2005). We have also demonstrated that the anorexigenic transmitters, 5-HT (Currie et al., 2010) and GLP-1 (Abtahi et al., 2016) antagonize ghrelin in the ArcN and PVN, along with PVN UcnI (Currie et al., 2001; Currie et al., 2011a). The results of the current study additionally suggest that NO signaling modulates the appetitive and metabolic effects of hypothalamic ghrelin.
Increasing evidence suggests that NO acts as a neuromodulator of the endocrine system and that it exerts a physiological role in regulation of food intake. NO is synthesized by nitric oxide synthase (NOS) from arginine (Bode-Böger et al., 2007) and recent evidence implicates the peptide in metabolic control within the hypothalamus (Liu and Xue, 2017). In particular, daily administration of 500 μg l-arginine into the lateral ventricles of rats for four days enhanced time to exhaustion and total workload, while 200 μg of L-NAME over four days decreased time to exhaustion and total workload (Liu and Xue, 2017). The same experiment further reported that l-arginine robustly increased NOS mRNA expression in the PVN, and to a lesser degree in the dorsomedial hypothalamus (DMH), with no effect of L-NAME. Furthermore, it has been demonstrated that NO donor sodium nitroprusside dose-dependently increases proopiomelanocortin (POMC) mRNA in mice (Wellhauser et al., 2016).
Several studies have demonstrated that hypothalamic NOS is altered by the orexigenic peptides ghrelin and NPY and decreased by the anorexigenic peptide leptin (Fetissov et al., 2003; Gaskin et al., 2003; Rodriguez-Pacheco et al., 2008; Morley et al., 2011; Joffin et. al., 2011; Bellefontaine et al., 2014). First order appetitive regulation in the mammalian hypothalamus is the result of competition between anorexigenic POMC/cocaine and amphetamine regulated transcript (CART) neurons and orexigenic NPY/AgRP neurons in the ArcN (Mason et al., 2014; Müller et al., 2015), both of which express GHSR1a, leptin receptors, and NOS (Zigman et al., 2006; Fetissov et al., 2003; Wellhauser et al., 2016, Ng et al., 1999; Yu et al., 2017; Ghamari-Langroudi et al., 2011). These neurons have inhibitory projections on each other (Delporte 2013; Mason et al., 2014; Müller et al., 2015) as well as projections to α-melanocyte stimulating hormone (α-MSH)/melanocortin 4 receptor (MC4R) neurons in the PVN (Fenselau et al., 2017; Mason et al., 2014; Müller et al., 2015). Although one report suggests that NO induction via sodium nitroprusside inhibits GHSR1a expressing neurons in the ArcN in vitro (Riediger et al., 2006), the same drug has been found to increase POMC mRNA expression in the ArcN (Wellhauser et al., 2016). It appears that the in vitro findings may have been due to an increase in activity of anorexigenic neurons in the same nuclei. In addition, we found that the highest dose of L-NAME reversed ghrelin's effect in the ArcN, but not the PVN, most likely due to the ArcN having greater GHSR1a expression compared to the PVN in rodents (Zigman et al., 2006).
Furthermore, work in avians and teleosts suggests that NO's integral role in ghrelin signaling is not limited to ingestive and metabolic behavior. In teleosts, nitric oxide is necessary for ghrelin elicitation of luteinizing hormone (LH) and GH (Grey and Chang, 2012; Grey and Chang, 2013), while in avians nitric oxide regulates food intake when manipulated both peripherally and centrally (Khan et al., 2007; Shousha et al., 2005; Hassanpour et al., 2015), with recent work suggesting that the uniquely anorexigenic effects of ghrelin in avians may be due to it playing a key role in lipid storage and migratory behavior (Goymann et al., 2017). This contrasts with the orexigenic effect of ghrelin and ghrelin induced carbohydrate oxidation in the rat as we have demonstrated in the present report.
Our lab has also reported in the past that L-NAME dose-dependently elicits an anorexigenic effect in the dorsal (DRN) and median (MRN) raphe nuclei and attenuates the orexigenic effect of 5-HT1A agonist 8-OH-DPAT in these nuclei as well (Currie et al., 2011b), suggesting that NO regulation of appetitive behavior extends beyond the hypothalamus. Moreover, we recently demonstrated that pairing PVN ghrelin with MRN 8-OH-DPAT resulted in a greater orexigenic effect than either manipulation alone (Wauson et al., 2015). In the same series of experiments we found that MRN 8-OH-DPAT attenuated the anxiogenic effect of PVN ghrelin as measured by an elevated plus maze, suggesting that 5-HT and ghrelin interact in the regulation of stress activation. As a result, hypothalamic and midbrain NO may impact mammalian anxiety-like behavior, and is a topic of further investigation in our lab. Nitric oxide may also play a critical role in reward and drug dependence, with recent work finding that NO in the nucleus accumbens is necessary for cue induced reinstatement in cocaine-dependent rats (Smith et al., 2017). Similarly, methamphetamine enhances NOS expression in the nucleus accumbens shell, which is attenuated by access to an exercise wheel (Engelmann et al., 2014), suggesting that NO signaling may connect metabolic and reward circuitry.
In conclusion, increasing evidence suggests that NO may be an evolutionarily conserved endocrine modulator that is critical for homeostatic and hormonal neurophysiology and may extend to more complex behaviors such as stress and reward in mammals and migration in avians. Correspondingly, our results demonstrate that NO is necessary for ghrelin enhancement of appetitive motivation and RER in the ArcN and PVN. Future research could be directed at investigating the appetitive and metabolic impact of other manipulations of the NO axis such as NO scavengers and donors as well as more selective inhibitors.
Highlights.
Peripheral administration of L-NAME dose-dependently attenuates ghrelin enhancement of food intake in the ArcN and PVN
ArcN and PVN injections of L-NAME dose-dependently attenuate ghrelin enhancement of food intake.
Both peripheral and central administration of L-NAME dose-dependently attenuate ghrelin enhancement of respiratory exchange ratio in the ArcN and PVN.
Acknowledgments
Supported by grants from the NIH (NIH 070496-01A1) and the M.J. Murdock Charitable Trust (Life Sciences) to PJC.
Abbreviations
- L-NAME
N-nitro-L-Arginine methyl ester
- ArcN
arcuate nucleus
- PVN
paraventricular nucleus
- GHSR1a
growth hormone secretagogue receptor 1a
- GOAT
ghrelin-O-acyl-transferase
- RER
respiratory exchange ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abtahi S, VanderJagt HL, Currie PJ. The glucagon-like peptide-1 analog exendin-4 antagonizes the effect of acyl ghrelin on the respiratory exchange ratio. NeuroReport. 2016;27:992–996. doi: 10.1097/WNR.0000000000000650. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Bellefontaine N, Chachlaki K, Parkash J, Vanacker C, Colledge W, d'Anglemont de Tassigny X, Garthwaite J, Bouret SG, Prevot V. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. Journal of Clinical Investigation. 2014;124:2550–2559. doi: 10.1172/JCI65928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Gómez-Boronat M, Alonso-Gómez ÁL, Yufa R, Unniappan S, Delgado MJ, Valenciano AI. Characterization of Ghrelin O-Acyltransferase (GOAT) in goldfish (Carassius auratus) PLOS ONE. 2017;12:e0171874. doi: 10.1371/journal.pone.0171874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode-böger S, Scalera F, Ignarro L. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacology & Therapeutics. 2007;114:295–306. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Brockway ET, Krater KR, Selva JA, Wauson SER, Currie PJ. Impact of [d-Lys3]-GHRP-6 and feeding status on hypothalamic ghrelin-induced stress activation. Peptides. 2016;79:95–102. doi: 10.1016/j.peptides.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Chow KBS, Sun J, Chu KM, Cheung WT, Cheng CHK, Wise H. The truncated ghrelin receptor polypeptide (GHS-R1b) is localized in the endoplasmic reticulum where it forms heterodimers with ghrelin receptors (GHS-R1a) to attenuate their cell surface expression. Mol Cell Endocrinol. 2012;348:247–254. doi: 10.1016/j.mce.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Mirza A, Fuld R, Park D, Vasseli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. AJP: Regulatory, Integrative and Comparative Physiology. 2005;289:R353–R358. doi: 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Coiro CD, Duenas R, Guss JL, Mirza A, Tal N. Urocortin I inhibits the effects of ghrelin and neuropeptide Y on feeding and energy substrate utilization. Brain research. 2011a;1385:127–134. doi: 10.1016/j.brainres.2011.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV, Bishop C, Coiro CD, Koob GF, Rivier J, Vale W. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain research. 2001;916:222–228. doi: 10.1016/s0006-8993(01)02851-7. [DOI] [PubMed] [Google Scholar]
- Currie PJ, John CS, Nicholson ML, Chapman CD, Loera KE. Hypothalamic paraventricular 5-hydroxytryptamine inhibits the effects of ghrelin on eating and energy substrate utilization. Pharmacology Biochemistry and Behavior. 2010;97:152–155. doi: 10.1016/j.pbb.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie PJ, Mirza A, Dono LM, John CS, Wall DG. Anorexigenic action of nitric oxide synthase inhibition in the raphe nuclei. NeuroReport. 2011b;22:696–699. doi: 10.1097/WNR.0b013e32834a3dab. [DOI] [PubMed] [Google Scholar]
- Delporte C. Structure and Physiological Actions of Ghrelin. Scientifica. 2013;2013:1–25. doi: 10.1155/2013/518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Structure and Function. 2014;219:657–672. doi: 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenselau H, Campbell JN, Verstegen AMJ, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, Mandelblat-Cerf Y, Livneh Y, Lowell BB. A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nature Neuroscience. 2016;20:42–51. doi: 10.1038/nn.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetissov SO, Xu ZQ, Byrne LC, Hassani H, Ernfors P, Hökfelt T. Neuropeptide Y targets in the hypothalamus: nitric oxide synthesizing neurones express Y1 receptor. Journal of neuroendocrinology. 2003;15:754–760. doi: 10.1046/j.1365-2826.2003.01051.x. [DOI] [PubMed] [Google Scholar]
- Gaskin FS, Farr SA, Banks WA, Kumar VB, Morley JE. Ghrelin-induced feeding is dependent on nitric oxide. Peptides. 2003;24:913–918. doi: 10.1016/s0196-9781(03)00160-8. [DOI] [PubMed] [Google Scholar]
- Geelissen SM, Swennen Q, Geyten SV, Kühn ER, Kaiya H, Kangawa K, Decuypere E, Buyse J, Darras VM. Peripheral ghrelin reduces food intake and respiratory quotient in chicken. Domestic Animal Endocrinology. 2006;30:108–116. doi: 10.1016/j.domaniend.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proceedings of the National Academy of Sciences. 2011;108:355–360. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Lupi S, Kaiya H, Cardinale M, Fusani L. Ghrelin affects stopover decisions and food intake in a long-distance migrant. Proceedings of the National Academy of Sciences. 2017;114:1946–1951. doi: 10.1073/pnas.1619565114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey CL, Chang JP. Nitric oxide signaling in ghrelin-induced LH release from goldfish pituitary cells. General and Comparative Endocrinology. 2013;183:7–13. doi: 10.1016/j.ygcen.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Grey CL, Chang JP. Ghrelin-induced growth hormone release from goldfish pituitary cells is nitric oxide dependent. General and Comparative Endocrinology. 2012;179:152–158. doi: 10.1016/j.ygcen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proceedings of the National Academy of Sciences. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour S, Zendehdel M, Babapour V, Charkhkar S. Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. British Poultry Science. 2015;56:443–451. doi: 10.1080/00071668.2015.1059407. [DOI] [PubMed] [Google Scholar]
- Joffin N, Niang F, Forest C, Jaubert AM. Is there NO help for leptin? Biochimie. 2012;94:2104–2110. doi: 10.1016/j.biochi.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Kaiya H, Kangawa K, Miyazato M. Update on ghrelin biology in birds. General and Comparative Endocrinology. 2013;190:170–175. doi: 10.1016/j.ygcen.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Kaiya H, Saito ES, Tachibana T, Furuse M, Kangawa K. Changes in ghrelin levels of plasma and proventriculus and ghrelin mRNA of proventriculus in fasted and refed layer chicks. Domestic Animal Endocrinology. 2007;32:247–259. doi: 10.1016/j.domaniend.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- Kang KS, Yahashi S, Matsuda K. Central and peripheral effects of ghrelin on energy balance, food intake and lipid metabolism in teleost fish. Peptides. 2011;32:2242–2247. doi: 10.1016/j.peptides.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Khan MSI, Tachibana T, Hasebe Y, Masuda N, Ueda H. Peripheral or central administration of nitric oxide synthase inhibitor affects feeding behavior in chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2007;148:458–462. doi: 10.1016/j.cbpa.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-y-containing neurons in the rat arcuate nucleus ca2+ signaling via protein kinase a and n-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hamamoto A, Sato T. Ghrelin O-acyltransferase (GOAT), a specific enzyme that modifies ghrelin with a medium-chain fatty acid. Journal of Biochemistry. 2016;160:189–194. doi: 10.1093/jb/mvw046. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Liu H, Xue J. Involvement of hypothalamic nitric oxide signaling in the modulation of a rat's exercise capacity. NeuroReport. 2017;28:408–413. doi: 10.1097/WNR.0000000000000763. [DOI] [PubMed] [Google Scholar]
- Mason BL, Wang Q, Zigman JM. The Central Nervous System Sites Mediating the Orexigenic Actions of Ghrelin. Annual Review of Physiology. 2014;76:519–533. doi: 10.1146/annurev-physiol-021113-170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA. Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience. 2011;198:95–111. doi: 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Sell RL, Hileman SM, Banks WA. Nitric oxide is a central component in neuropeptide regulation of appetite. Peptides. 2011;32:776–780. doi: 10.1016/j.peptides.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D'Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LHT, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Molecular Metabolism. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Ng YK, Xue YD, Wong PTH. Different Distributions of Nitric Oxide Synthase-Containing Neurons in the Mouse and Rat Hypothalamus. Nitric Oxide. 1999;3:383–392. doi: 10.1006/niox.1999.0247. [DOI] [PubMed] [Google Scholar]
- Mohan H, Unniappan S. Discovery of ghrelin o-acyltransferase. Endocrine Development. 2013;25:16–24. doi: 10.1159/000346039. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 7th. Academic Press; New York, NY: 2014. [Google Scholar]
- Riediger T, Giannini P, Erguven E, Lutz T. Nitric oxide directly inhibits ghrelin-activated neurons of the arcuate nucleus. Brain Research. 2006;1125:37–45. doi: 10.1016/j.brainres.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pacheco F, Luque RM, Tena-Sempere M, Malagón MM, Castaño JP. Ghrelin Induces Growth Hormone Secretion Via a Nitric Oxide /cGMP Signalling Pathway. Journal of Neuroendocrinology. 2008;20:406–412. doi: 10.1111/j.1365-2826.2008.01645.x. [DOI] [PubMed] [Google Scholar]
- Romero A, Kirchner H, Heppner K, Pfluger PT, Tschop MH, Nogueiras R. GOAT: the master switch for the ghrelin system? European Journal of Endocrinology. 2010;163:1–8. doi: 10.1530/EJE-10-0099. [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Fan XT. Ketamine and L-NAME inhibit NOS and somatostatin mRNA expression induced by altitude hypoxia in the rat hypothalamus. Sheng Li Xue Bao [Acta Physiologica Sinica] 52:119–122. [PubMed] [Google Scholar]
- Seim I, Jeffery PL, Thomas PB, Walpole CM, Maugham M, Fung JNT, Yap PY, O'Keeffe AJ, Lai J, Whiteside EJ, Herington AC, Chopin LK. Multi-species sequence comparison reveals conservation of ghrelin gene-derived splice variants encoding a truncated ghrelin peptide. Endocrine. 2016;52:609–617. doi: 10.1007/s12020-015-0848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Kaiya H, Matsuda K. Stimulatory effect of ghrelin on food intake in bullfrog larvae. Peptides. 2014;51:74–79. doi: 10.1016/j.peptides.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Shousha S, Nakahara K, Kojima M, Miyazato M, Hosoda H, Kangawa K, Murakami N. Different effects of peripheral and central ghrelin on regulation of food intake in the Japanese quail. General and Comparative Endocrinology. 2005;141:178–183. doi: 10.1016/j.ygcen.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C, Stankeviciute NM, Smith RJ, Allen NP, Lorang MR, Griffin WC, Boger HA, Kalivas PW. Accumbens nNOS Interneurons Regulate Cocaine Relapse. The Journal of Neuroscience. 2017;37:742–756. doi: 10.1523/JNEUROSCI.2673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T, Tsutsui K. Neuropeptide Control of Feeding Behavior in Birds and Its Difference with Mammals. Frontiers in Neuroscience. 2016;10:1–13. doi: 10.3389/fnins.2016.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unniappan S, Lin X, Cervini L, Rivier J, Kaiya H, Kangawa K, Peter RE. Goldfish Ghrelin: Molecular Characterization of the Complementary Deoxyribonucleic Acid, Partial Gene Structure and Evidence for Its Stimulatory Role in Food Intake. Endocrinology. 2002;143:4143–4146. doi: 10.1210/en.2002-220644. [DOI] [PubMed] [Google Scholar]
- Vester Boler BM, Faber TA, Bauer LL, Swanson KS, Smiley S, Bechtel PJ, Fahey GC. Acute satiety response of mammalian, avian and fish proteins in dogs. British Journal of Nutrition. 2012;107:146–154. doi: 10.1017/S0007114511002261. [DOI] [PubMed] [Google Scholar]
- Wauson SER, Sarkodie K, Schuette LM, Currie PJ. Midbrain raphe 5-HT1A receptor activation alters the effects of ghrelin on appetite and performance in the elevated plus maze. Journal of Psychopharmacology. 2015;29:836–844. doi: 10.1177/0269881115581981. [DOI] [PubMed] [Google Scholar]
- Wellhauser L, Chalmers JA, Belsham DD. Nitric Oxide Exerts Basal and Insulin-Dependent Anorexigenic Actions in POMC Hypothalamic Neurons. Molecular Endocrinology. 2016;30:402–416. doi: 10.1210/me.2015-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CH, Chu SC, Chen PN, Hsieh YS, Kuo DY. Participation of ghrelin signalling in the reciprocal regulation of hypothalamic NPY/POMC-mediated appetite control in amphetamine-treated rats. Appetite. 2017;113:30–40. doi: 10.1016/j.appet.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Zendehdel M, Hassanpour S. Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. The Journal of Physiological Sciences. 2014;64:383–391. doi: 10.1007/s12576-014-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of Ghrelin Receptor mRNA in the Rat and the Mouse Brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]