Abstract
Background/Aim: The aim of this study was to evaluate whether the altered profile of adipocytokine and genetic fingerprint in NAFLD-associated metabolic syndrome “cluster” represents synergistic risk factors predicting onset of liver colorectal cancer metastases. Materials and Methods: A total of 165 colorectal cancer patients were enrolled, 56,3% were with metabolic syndrome/NAFLD. Serum samples were assayed for ADIPOQ, leptin and TNF-a levels by ELISA. ADIPOQ rs266729 C/G and TNF-308 A/G genotypes were analyzed in DNA isolated from whole blood. Results: Reduction in adiponectin levels and increase in leptin and TNF-α was shown in patients with liver metastases. This trend was influenced by BMI, MetS/NAFLD, and insulin resistance. ADIPOQ G rs266729 and TNF- 308 A allele are associated with obesity, MetS/NAFLD and insulin resistance. ADIPOQ CG/GG and GA/AA TNF-alpha genotypes confer susceptibility to liver metastases. Conclusion: Obesity and hepatic steatosis significantly favor the development of colorectal cancer liver metastases and the individual adipocytokines genetic profile may play an important predictive role.
Keywords: Adiponectin, leptin, TNF-α, colorectal cancer, metabolic syndrome, obesity, NAFLD
Currently, obesity and the related conditions, including metabolic syndrome (MetS), represent an important risk factor for colorectal cancer (CRC), which have led to an increased incidence for this type of cancer. Non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of the MetS, is an additional risk factor for liver metastasis of colorectal cancer (1-3). Moreover, given its highly favorable microenvironment for the onset of liver metastases, NAFLD represents a negative prognostic factor in patients with CRC (4). Visceral obesity is one of the factors that predispose to NAFLD and the accumulation of fat both at visceral and hepatic level leads to a dysfunction of the adipose tissue and in an imbalance of adipocytokines profile (5). The state of chronic inflammation caused by fat accumulation induces an increase in pro-inflammatory cytokines, such as tumor necrosis factor alfa (TNF-α), and a reduction of the adiponectin levels (ADIPOQ), an adipocytokine with anti-inflammatory action (6,7). An increased Body Mass Index (BMI), insulin resistance, and the presence of NAFLD, all features of MetS, sustain the inflammatory state, which is a fertile soil for the onset of cancer. In fact, MetS induces a state of insulin resistance and chronic inflammation with a consequent increase in the expression of the triad of pro-inflammatory cytokines TNF-α, Interleukin-1 (IL-1) and interleukin-6 (IL-6), associated with of a significant release of C-reactive protein (CRP) into the blood circulation (8-10). However, in obese subjects, adipocyte dysfunction is associated with a decreased ADIPOQ release into the circulation, with lack of insulin sensitization, and anti-inflammatory, anti-steatosic and antitumor actions (11). Regardless of other concomitant factors, the expansion of the adipocyte burden deprives patients with NAFLD of the anti-inflammatory and anti-fibrotic effect exerted by adiponectin whose levels are inversely correlated to the increase in adiposity (12,13). Another adipocytokine that is involved in the pathogenesis of NAFLD is Leptin. Leptin has a dual role in NAFLD (14). First, it plays a protective role against hepatic steatosis, especially at the early stages of the disease, and second, it exerts a non-beneficial effect by acting as a pro-inflammatory and fibrinogenic adipocytokine. Leptin exerts antisteatosic effects acting at the hepatocyte level (15,16). A state of leptin resistance appears if expansion of the visceral fat mass occurs (17). Accordingly, leptin no longer compensates for insulin resistance thus losing its antisteatosic action (18). Unhealthy eating habits and poor physical activity represent the two main environmental risk factors that predispose to obesity and related conditions (i.e. MetS, NAFLD and insulin resistance). Notably, environmental factors may act on a genetic predisposition, and interactions between environmental factors and inter-individual genetic variations could result in a more aggressive phenotype of the disease (19,20). ADIPOQ gene, which encodes for adiponectin, is located on chromosome 3q27 and is linked to a susceptibility locus for MetS. Circulating levels of ADIPOQ are influenced by genetic components (21). Among the variations of ADIPOQ gene, the SNP rs266729 found within the promoter region is significantly associated with ADIPOQ level and linked to susceptibility to cancer (22). ADOPOQ has been proposed as a determinant factor in the etiology of the MetS, because of its important regulatory action on insulin sensitivity and inflammation (23). Thus, the polymorphism in the ADIPOQ gene is believed to play a role in the pathogenesis of the MetS. Obesity and insulin resistance may be influenced by polymorphisms in Tumor necrosis factor α gene (TNFA) (24). The polymorphism TNFA-308 A/G in the gene promoter is associated with increased serum TNF-α levels and predisposes to insulin resistance (25,26). It has been shown that individuals who carry the 308A TNFA gene variant are at higher risk of developing obesity compared to controls and show significantly higher systolic arterial blood pressure and plasma insulin levels (27). This supports the hypothesis that TNFA gene could be involved in the pathogenesis of the metabolic syndrome (28). Also, genetic polymorphisms in the promoter region of TNFA gene are associated with different inflammatory and malignant conditions (29-31). In this study, we evaluated whether the altered profile of adipocytokines and the genetic fingerprint of the NAFLD-associated metabolic syndrome “cluster” represent synergistic risk factors for predicting the onset of liver colorectal cancer metastases.
Materials and Methods
Patients. In this study, from June 2014 to December 2017, 165 colorectal cancer patients were enrolled at IRCCS (National Cancer Research Institute) Giovanni Paolo II of Bari –Italy. In addition, a group of 50 healthy subjects was included. All patients diagnosed with colon cancer had positive colonoscopy, which was confirmed histologically. The clinical characteristics of patients (age, sex, therapeutic interventions, etc.) were obtained from medical records. At the time of enrollment, of the 165 patients, 62 (37.5%) were non-metastatic patients, 69 (41.8%) had liver metastases and 34 (20.6%) had lung metastasis. In all subjects, body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Waist circumference was measured in centimeter up to the umbilicus in the standing position after normal expiration. Metabolic syndrome (MetS) was also determined for all participants. Patients were considered to have MetS when they presented 3 or more of the joint statement criteria of the American Heart Association/National Heart Lung and Blood Institute (AHA/NHLBI) and the International Diabetes Federation (IDF). The presence of NAFLD in all participants independent from alcohol consumption was determined through radiological evidence of hepatic steatosis. In our series, 93 (56.3%) patients with metabolic syndrome were all associated with the presence of NAFLD. Insulin resistance was calculated according to the homeostasis model assessment-Insulin Resistance (HOMA IR). This score is used to define insulin resistance in research. HOMA-IR=(fasting glucose in mmol/l × fasting insulin)/22.5 OR (fasting glucose in mg/dl × fasting insulin)/405. HOMA-IR ≥2.50 indicates insulin resistance.
Blood collections. All blood samples of cancer patients were obtained preoperatively or prior to other therapeutic procedures. For all participants, venous blood samples were collected after an overnight fast, and serum samples were either used immediately for analysis of the biochemical profile (glucose, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL) cholesterol and triglycerides, ALT, AST, GGT, insulin, CRP), or were stored frozen at –20˚C for subsequent adipocytokine ELISA assay.
ADIPOQ, Leptin and TNF-a enzyme-linked immunoassay (ELISA). Serum samples from 165 CRC patients and 50 healthy donors were assayed for the levels of ADIPOQ, leptin and TNF-α by a sandwich ELISA assay (Quantikine Human ADIPOQ, Leptin and TNF-alfa Immunoassay; R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's recommendations. The absorbance of the solution produced was measured at 490 nm and was directly proportional to the amount of ADIPOQ, Leptin and TNF-alfa present in the sample. A standard curve was constructed by plotting the mean absorbance value measured for each standard versus its corresponding concentration.
Genotyping. Genotyping was performed after extraction of DNA from whole blood in EDTA using a standard QIAmp kit (QIAGEN Inc.) following the manufacturer’s recommendations. DNA was dissolved in (TE) buffer and quantified by measurement of optical density at 260 nm. To improve the genotyping quality and validation, all mutant and heterozygous samples were re-genotyped, and results were noted only for those samples that were reproducible and with no discrepancy.
ADIPOQ rs266729 polymorphism. For detection of the adiponectin rs266729 polymorphism, we performed a Tetra amplification refractory mutation system polymerase chain reaction (T-ARMS-PCR) as previously reported (22). Briefly, two external primers and two specific internal allele specific primers were used: external primers (Forward outer: 5’-GGA CTG TGG AGA TGA TAT CTG GGG GGC A-3’, Reverse outer: 5’-TGG CCT AGA AGC AGC CTG GAG AAC TGG A-3’), and allele specific internal primers (Forward inner (C allele): 5’-CTT GCA AGA ACC GGC TCA GAT CCT CCC- 3’, Reverse inner (G allele): 5’-GAG CTG TTC TAC TGC TAT TAG CTC TGC-3’). The final PCR mixture (20 μl), contained DNA (2 μl), 10 × PCR buffer (1.5 μl), 2 mM MgCl2 (1.5 μl), 10 mM dNTP (0.3 μl), 0.25 μl of each primer 1 U Taq DNA polymerase and water. The reaction cycle consisted of pre-denaturation at 95˚C for 2 min, denaturation at 95˚C for 20 sec, 35 cycles of annealing at 56˚C for 20 sec, extension at 72˚C for 40 sec and a final extension at 72˚C for 4 min for complete extension of all PCR fragments. The reaction was performed on a Thermal Cycler, BioRAD (Milan, Italy). The amplified DNA fragments were verified on a 2% agarose gel. Each study participant was classified into one of the three possible genotypes: homozygote C/C, heterozygote C/G or homozygote G/G.
Genotyping of TNFA -308A/G gene polymorphism. The genotyping was performed using the polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) technique. Amplification of the -308 TNFA gene polymorphic region was performed in a Thermal Cycler, BioRAD, using the following primers (Invitrogen Life Technologies, Carlsbad, CA, USA): 5’-AGG CAA TAG GTT TTG AGG GCC AT-3’ (Forward) and 5’-TCC TCC CTG CTC CGA TTC CG-3’ (Reverse). To introduce a restriction site into the wild-type nucleotide sequence during the amplification reaction, the forward primers for each TNFA gene polymorphism contain a single base-pair mismatch adjacent to the polymorphic site. The PCR was carried out in a final volume of 12.5 μl containing 0.1 μg/μl of DNA, 3 mM of each primer, 0.025 U/μl of Taq DNA polymerase, 1.25 μl of supplied buffer enzyme 1X, 2.5 mM of MgCl2, and 2.5 mM of each dNTP (Invitrogen Life Technologies, Carlsbad, CA, USA). Amplification conditions were as follows: initial denaturation at 94˚C for 4 min, 33 cycles of 94˚C for 30 sec, 60˚C for 30 sec and 72˚C for 30 sec, followed by 72˚C for 2 min for ending extension; resulting fragments of 107 bp was analyzed on a 6% polyacrylamide gel stained with silver nitrate (Invitrogen Life Technologies, Carlsbad, CA, USA). The amplified fragments of the -308 TNFA gene SNPs were incubated with 3 U NcoI restriction enzymes (New England BioLabs, Beverly, MA, USA), for 1 h at 37˚C. Finally, the digested PCR products were electrophoresed on 6% polyacrylamide gels and stained with silver nitrate for genotype identification. For the -308 SNP, fragments of 87 and 20 bp represent the wild-type genotype (G/G), fragments of 107, 87 and 20 bp represent the heterozygote genotype (G/A), and only one fragment of 107 bp represents the homozygote genotype (A/A).
Statistical analysis. For continuous variables, data were analyzed with the Mann-Whitney U-test, the unpaired Student’s t-test, ANOVA and Fischer’s exact test. Spearman’s correlation was used for correlation analysis between serum adipocytokine profiles. The allelic and genotypic frequencies were estimated by the Chi-square test and Fisher’s Exact test. Odds ratios (ORs) and 95% confidence intervals (95%CI) were calculated from the logistic model. p-Values ≤0.05 were considered statistically significant. All statistical analyses were performed by the Number Cruncher Statistical System-Power Analysis and Sample Size Software 2007 (NCSS-PASS, 329 North 1000 East Kaysville, UT, USA).
Results
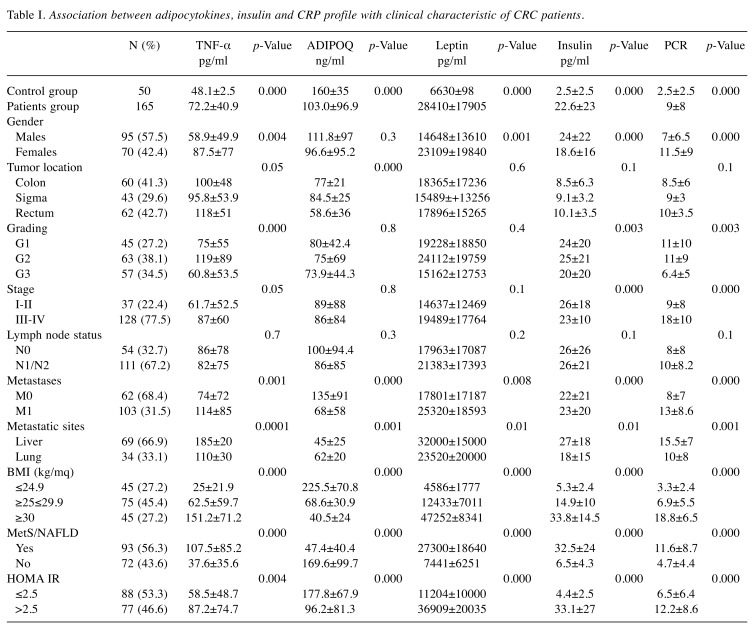
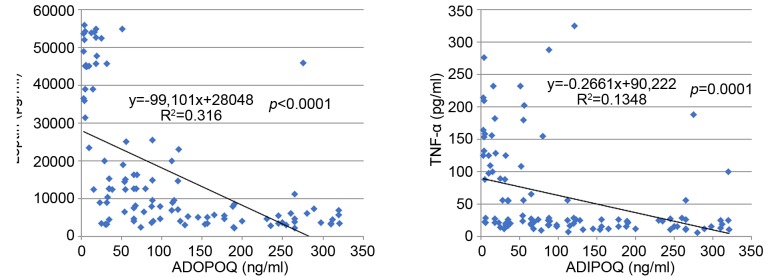
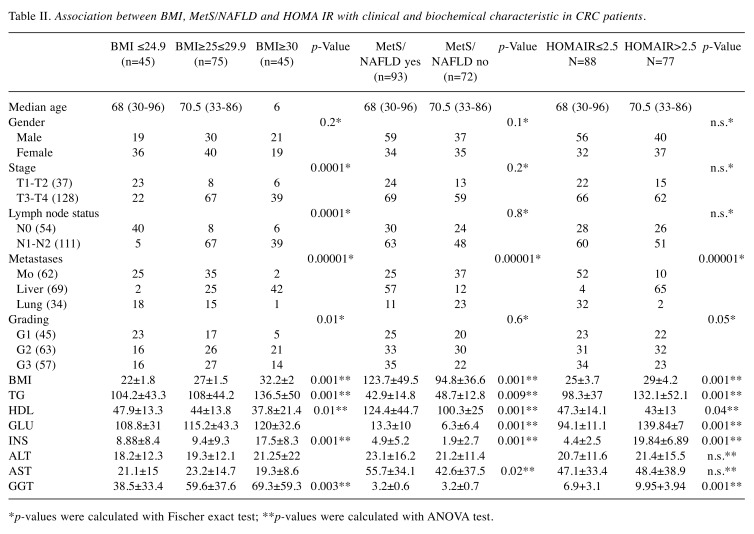
Association between adipocytokines, Insulin, and CRP profile with clinical characteristic of CRC patients. Serum levels of Adiponectin, Leptin, TNF-α, Insulin and CRP showed statistically significant differences (p<0.00001, t-test) between the control group and the patients, Table I. This suggests that these adipocytokines are actively involved in the promotion and progression of CRC. Serum levels of adiponectin were decreased compared to other adipocytokines, which instead were generally increased. In fact, a statistically significant inverse correlation was observed between adiponectin and the other adipocytokines (i.e. leptin and TNF-α) as shown in Figure 1. Also, in patients with liver metastases, a notable reduction in adiponectin levels in the circulation with a consequent increase in the values of leptin, insulin, TNF-α and CRP was observed. The data suggest that liver is implicated in the regulation of circulating levels of all these parameters. It is worth mentioning that BMI, MetS/NAFLD, and insulin resistance significantly influences serum levels of adipocytokines. Furthermore, patients with BMI >30, with MetS/NAFLD and a state of insulin resistance showed a greater risk of developing liver metastases (Table II). These findings suggest that the triple condition of BMI, MetS/NAFLD and HOMA IR may constitute a unique phenotype that exposes the patient to a favorable microenvironment for the development of liver metastases. Moreover, a condition of overweight/obesity is associated with the development of more advanced tumor stage (p<0.0001, Fisher’s exact test), poorly differentiated tumors (p=0.01, Fischer’s exact test), with lymph node involvement (p<0.0001, Fischer’s exact test), and with the presence of metastases (p<0.0001, Fischer’s exact test).
Table I. Association between adipocytokines, insulin and CRP profile with clinical characteristic of CRC patients.
Figure 1. Scatter plot showing an inverse correlation between serum levels of adiponectin with leptin and TNF-alpha.
Table II. Association between BMI, MetS/NAFLD and HOMA IR with clinical and biochemical characteristic in CRC patients.
*p-values were calculated with Fischer exact test; **p-values were calculated with ANOVA test.
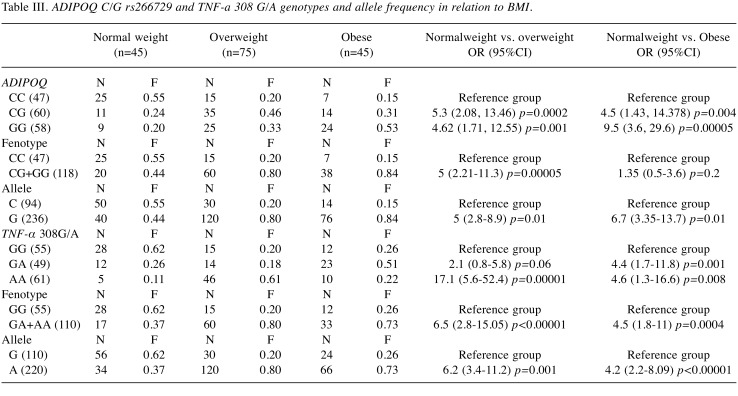
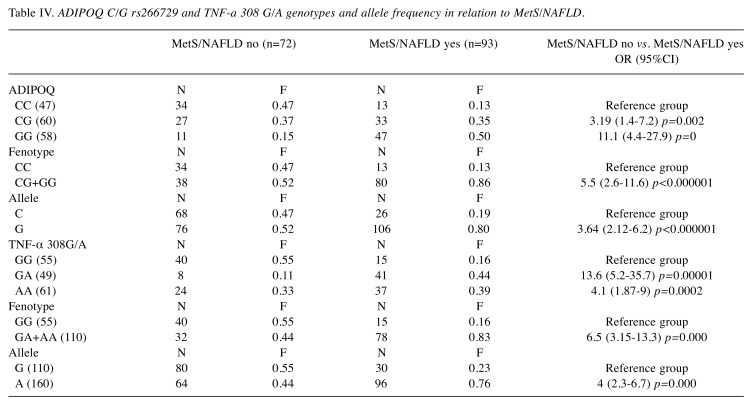
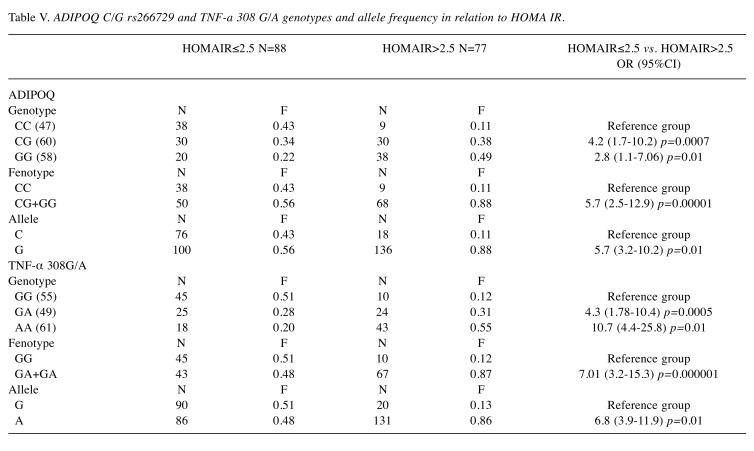
Association between Adiponectin and TNF-α genotypes with BMI, MetS/NAFLD and HOMA Index in CRC patients. To understand the serum patterns of adipocytokines, in the three groups of patients grouped by BMI, MetS/NAFLD and HOMA Index, the distribution of genotypes and alleles frequency of the ADIPOQ rs266729 C/G and TNFA 308 G/A polymorphism were investigated. We found that the presence of the ADIPOQ G allele were significantly higher in overweight (OR=5, p=0.01) and in obese patients (OR=6.7, p=0.01) than in normal weight patients suggesting that this genotype represents a risk factor for the development of obesity (Table III). Also, the presence of the G allele represents a significant risk factor for developing MetS (OR=3.64, p=0.0000001) and insulin resistance (OR=5.7, p=0.01) (Table IV and Table V). Moreover, analyzing the allelic distribution of TNFA polymorphisms, the presence of TNFA 308A allele was found to be significantly higher in overweight (OR=6.2, p=0.0001) and obese patients, (OR=4.2, p<0.00001). Also, the presence of the A allele represents a significant risk factor that predisposes to MetS/NAFLD and insulin resistance. Therefore, the genetic variability, the presence of the ADIPOQ G rs266729 and TNFA 308 A allele are associated with obesity, metabolic syndrome/NAFLD and insulin resistance. This genetic signature significantly influences the biochemical blood profile as reported below.
Table III. ADIPOQ C/G rs266729 and TNF-a 308 G/A genotypes and allele frequency in relation to BMI.
Table IV. ADIPOQ C/G rs266729 and TNF-a 308 G/A genotypes and allele frequency in relation to MetS/NAFLD.
Table V. ADIPOQ C/G rs266729 and TNF-a 308 G/A genotypes and allele frequency in relation to HOMA IR.
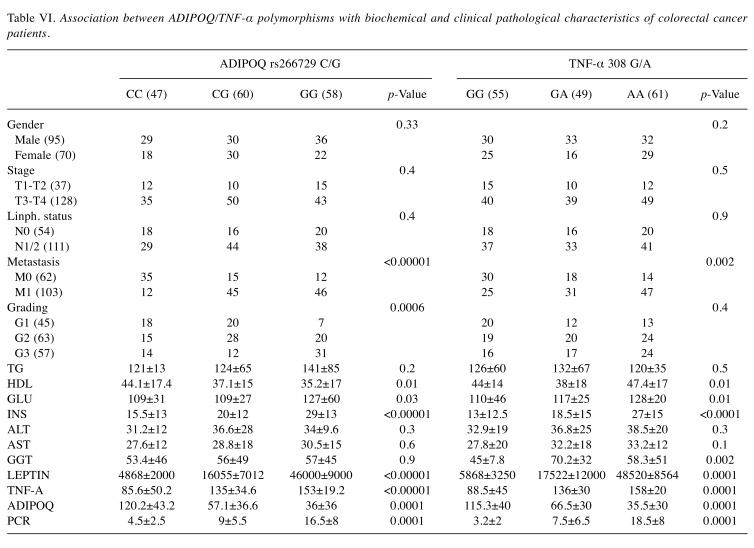
Association between ADIPOQ/TNFA polymorphisms with biochemical and clinical pathological characteristics of CRC patients. As shown in Table VI, the polymorphism 308 A/G in TNFA gene promoter is associated with increased serum TNF-α levels and predisposes to insulin resistance. In fact, this polymorphism is associated with increased blood levels of glucose and insulin (p=0.01, p<0.00001 respectively, ANOVA test). Furthermore, this polymorphism is associated with both high levels of GGT (p=0.002 and p<0.0001 respectively, ANOVA test) and to low levels of HDL (p=0.01 and 0.009 respectively, ANOVA test) which are two parameters indicative of metabolic syndrome and NAFLD. The rs266729 G/C ADIPOQ polymorphism is significantly associated with lower levels of circulating adiponectin (p<0.0001, ANOVA Test), and with increased insulin. Furthermore, these polymorphisms favor the establishment of an inflammatory microenvironment represented by elevated circulating levels of CRP, a well-established marker of inflammation. Moreover, leptin levels are also influenced by these polymorphisms. In fact, high levels of leptin are found in obese subjects, with insulin resistance and metabolic syndrome, suggesting that in these subjects a state of leptin resistance has been established. The development of leptin resistance is considered a hallmark of obesity. Moreover, these polymorphisms predispose to a more aggressive tumor phenotype with a poorly differentiated histotype, with involvement of lymph nodes and the presence of metastases.
Table VI. Association between ADIPOQ/TNF-α polymorphisms with biochemical and clinical pathological characteristics of colorectal cancer patients.
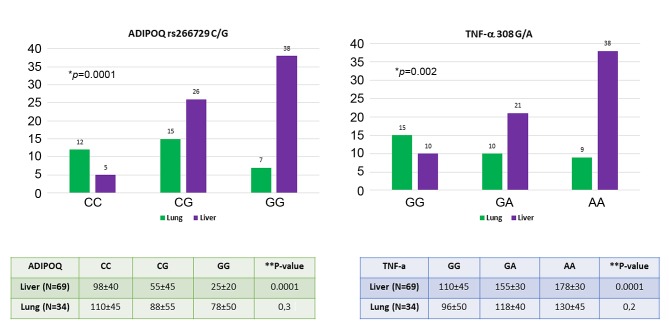
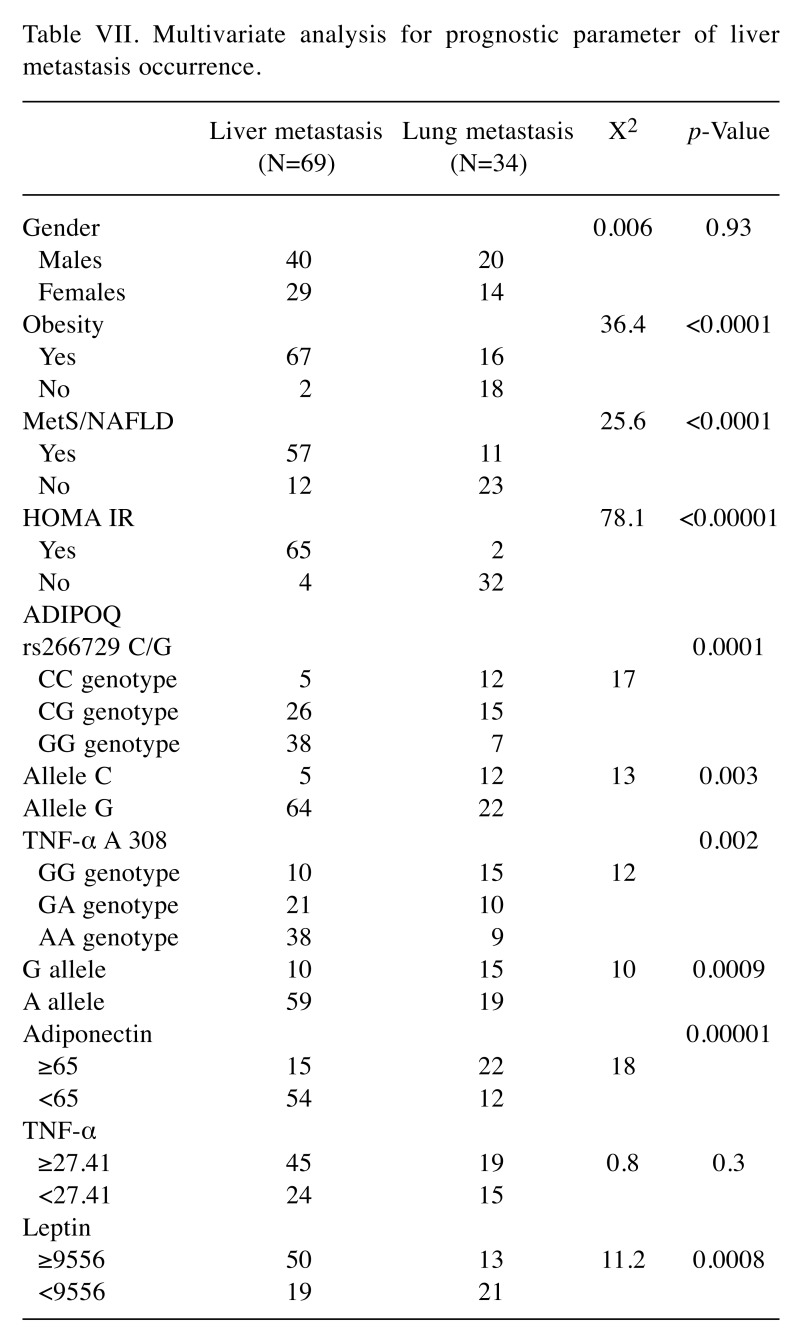
Association between ADIPOQ rs266729 C/G and TNFA 308 A/G polymorphisms with liver and lung metastasis. The genotypes of these polymorphisms in relation to hepatic and lung metastases are shown in Figure 2. Results in Figure 2 show that the presence of CG or GG genotype in the ADIPOQ gene and the presence of GA or AA genotype in the TNFA gene represent risk factor for the onset of liver metastases (p=0.0001 for ADIPOQ genotype, p=0.002 for TNFA genotype; Fischer’s exact test). These polymorphisms that affect the circulating levels of both adiponectin and TNF-α in these patients. In fact, there is a considerable decrease in adiponectin and an increase in TNF-α (particularly in patients harboring G allele in ADIPOQ gene and A allele in the TNFA gene) in CRC patients with liver metastases compared to those with lung metastases. Finally, Table VII shows a multiparametric analysis regarding the risk factors for the onset of liver metastases. Obesity, MetS/NAFLD, insulin resistance, the presence of the G allele in the adiponectin gene, low levels of adiponectin and high circulating levels of leptin and TNF-alpha generate a favorable microenvironment that predispose to development of liver metastases.
Figure 2. Association between genetic polymorphism and serum profile of adiponectin and TNF-alpha with liver and lung metastasis in colorectal cancer patients. *p-Value calculated with Fisher’s exact test; **p-value calculated with ANOVA test.
Table VII. Multivariate analysis for prognostic parameter of liver metastasis occurrence.
Discussion
Our study examined a more aggressive tumor phenotype in CRC patients with a greater risk of developing liver metastases. This phenotype is characterized by the presence of the following co-factors: BMI≥30, Homa IR≥2.5 and MetS/NAFLD. These patients generally show a decrease in serum levels of adiponectin and an increase in TNF-α and leptin circulating levels. This profile of circulating levels of adipocytokines is associated with a higher risk of developing liver metastases. Our findings corroborate the association of the adiponectin/leptin balance with the processes of mitogenesis, tumor growth and cell motility when adipose tissue dysfunction occurs (32,33). We additionally investigated the influence of genetic variability of ADIPOQ and TNFA gene to understand the serum patterns of adipocytokines. In this study, we found lower levels of adiponectin in patients harboring the G allele in ADIPOQ gene and higher levels of TNF-alfa in patients harboring A allele in TNFA gene. ADIPOQ G/G rs266729 and TNFA-308 A/A genotype predispose to obesity state, to MetS/NAFLD and to insulin resistance. Our data are in accordance with a report by Hsieh CJ and co-workers, who have shown that the GG rs266729 ADIPOQ genotype predisposes to NAFLD and that this genotype significantly lowers serum levels of adiponectin, a condition that may worsen liver steatosis (34). In addition, another study by Suriyaprom K et al., has shown that decreased blood concentration of adiponectin is associated with GG ADIPOQ rs266729 polymorphism and that this is significantly more frequent in patients with metabolic syndrome (35). Our study clearly showed that this genetic susceptibility influences the biochemical blood profile in our patients. In fact, these polymorphisms are significantly associated with increased blood levels of glucose, insulin, HDL and GGT, thus favoring insulin resistance, the onset of metabolic syndrome and NAFLD. Our data agree with what has been reported by Nascimento H et al., who demonstrated that adipokine gene SNPs are correlated with plasma levels of adipokine and lipid profile in pediatric obese patients (36). We reported that leptin levels are indeed influenced by ADIPOQ and TNFA polymorphisms, in fact, high levels of leptin are found in obese patients, with insulin resistance and MetS/NAFLD, indicating a consolidated state of leptin resistance. Leptin resistance is a hallmark of obesity (37). In obese individuals, elevated leptin acts as a pro-inflammatory adipokine and elevated circulating levels are associated with certain types of cancers (38). Leptin also stimulates the release of pro-inflammatory cytokines such as TNF-alpha, which in turn promotes inflammation and the overexpression of pro-angiogenic factors (i.e. VEGF and HIF-1α) with an increased risk for cancer development (39). Moreover, our study showed the establishment of a chronic inflammatory state sustained by high blood levels of CRP in patients with CRC. The association between central obesity, inflammation and insulin resistance, the three essential elements promoting NAFLD, led us to reinforce the hypothesis that NAFLD can be considered a hepatic manifestation of the metabolic syndrome, two conditions that share overlapping pathogenetic mechanisms (40,41). The primum movens of pathogenesis is believed to be the relationship between insulin resistance and obesity. In the early stages, triglycerides accumulate in the hepatocyte with alterations in lipid metabolism. A central role is played by visceral adipose tissue (42,43). High lipolytic activity of omental fat determines an increased flow of free fatty acids (FFA) that induce inflammatory pathways, leading to the development of both insulin and leptin resistance (44). In this scenario, adiponectin displays a down-regulatory effect in relation to weight gain, and it is possible that accumulation of visceral fat produces factors, such as TNF-α and leptin, that inhibit synthesis or secretion of adiponectin. The production of proinflammatory cytokines such as TNF-α at hepatic level and by macrophages of visceral adipose tissue appears to be an important mechanism (45,46). TNF-α represents one of the earliest events of liver injury in NAFLD. TNF-α may cause insulin resistance in adipose tissue with an increased release of FFA in the blood circulation with the creation of a self-perpetuating vicious circle. In the liver, both hemodynamic and micro environmental processes are involved in trapping and killing circulating tumor cells. A failure at any step of these processes may favor the colonization of circulating tumor cells in the liver. A growing body of evidence suggests that adipocytes in the tumor microenvironment play a crucial role in disease progression by providing fatty acids, pro-inflammatory cytokines and proteases (47,48). Since obesity is a well-recognized negative prognostic factor for colon cancer, increased adiposity may also have a negative effect in the treatment and survival of patients with CRC (49,50). Therefore, a more appropriate anticancer therapy should consider treating concomitant conditions including metabolic syndrome, dyslipidemia, and insulin resistance. A reduction in body weight is essential. A 10% reduction in weight in overweight subjects can normalize transaminases and increase insulin sensitivity. Aerobic physical exercise and nutritional modifications can improve metabolic syndrome and NAFLD (51,52). It is currently unclear whether obesity directly leads to metastatic disease via chronic systemic inflammation or whether obesity induces steatosis, which provides a fertile microenvironment for metastases. A combination of these factors is likely to occur. Indeed, our study showed that obesity and hepatic steatosis significantly favor the development of CRC liver metastases and that the individual genetic profile of adipocytokines may play an important predictive role.
Conflicts of Interest
The Authors have no conflicts of interest to declare regarding this study.
Authors’ Contributions
Rosa Divella: Author of Project and Principal Investigator; Antonella Daniele: anthropometric parameters; Raffaele De Luca: Patients recruitment; Antonio Mazzocca: Statistical analysis; Eustachio Ruggieri: Patients recruitment; Eufemia Savino: biochemical blood profile; Porzia Casamassima: biochemical blood profile; Michele Simone: select the patients; Carlo Sabbà: Liver ultrasound for assessment of steatosis; Angelo Paradiso: oncological evaluation of patients.
Acknowledgements
The Authors would like to thank all nursing staff of the abdomen surgery unit for support in recruiting patients enrolled in this study. This study was supported by the research project "Quality control on biological samples stored in Biobank: prospective analysis of genetic variability and identifications of serum biomarkers predisposing to the metabolic syndrome in a series of patients with colon of the colon" financed by the Italian Ministry of Health.
References
- 1.Kabat GC, Kim MY, Stefanick M, Ho GYF, Lane DS, Odegaard AO, Simon MS, Bea JW, Luo J, Wassertheil-Smoller S, Rohan TE. Metabolic obesity phenotypes and risk of colorectal cancer in postmenopausal women. Int J Cancer. 2018;143(3):543–551. doi: 10.1002/ijc.31345. PMID: 29488210. DOI: 10.1002/ijc.31345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goulart A, Varejão A, Nogueira F, Martins S, Mesquita-Rodrigues A, Sousa N, Leão P. The influence of metabolic syndrome in the outcomes of colorectal cancer patients. Diabetes Metab Syndr. 2017;11(S2):S867–S871. doi: 10.1016/j.dsx.2017.07.007. PMID: 28711516. DOI: 10.1016/j.dsx.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Ahn JS, Sinn DH, Min YW, Hong SN, Kim HS, Jung SH, Gu S, Rhee PL, Paik SW, Son HJ, Gwak GY. Non-alcoholic fatty liver diseases and risk of colorectal neoplasia. Aliment Pharmacol Ther. 2017;45(2):345–353. doi: 10.1111/apt.13866. PMID: 27859470. DOI: 10.1111/apt.13866. [DOI] [PubMed] [Google Scholar]
- 4.Kondo T, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kitagawa Y. The impact of hepatic fibrosis on the incidence of liver metastasis from colorectal cancer. Br J Cancer. 2016;115(1):34–39. doi: 10.1038/bjc.2016.155. PMID: 27280634. DOI: 10.1038/bjc.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divella R, Mazzocca A, Daniele A, Sabbà C, Paradiso A. Obesity, Nonalcoholic Fatty Liver Disease and Adipocytokines Network in Promotion of Cancer. Int J Biol Sci. 2019;15(3):610–616. doi: 10.7150/ijbs.29599. PMID: 30745847. DOI: 10.7150/ijbs.29599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220(2):T47–59. doi: 10.1530/JOE-13-0339. PMID: 24403378. DOI: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223. doi: 10.3390/ijms15046184. PMID: 24733068. DOI: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebert T, Gebhardt C, Scholz M, Wohland T, Schleinitz D, Fasshauer M, Blüher M, Stumvoll M, Kovacs P, Tönjes A. Relationship between 12 adipocytokines and distinct components of the metabolic syndrome. J Clin Endocrinol Metab. 2018;103(3):1015–1023. doi: 10.1210/jc.2017-02085. PMID: 29325128. DOI: 10.1210/jc.2017-02085. [DOI] [PubMed] [Google Scholar]
- 9.Sherling DH, Perumareddi P, Hennekens CH. Metabolic Syndrome. J Cardiovasc Pharmacol Ther. 2017;22(4):365–367. doi: 10.1177/1074248416686187. PMID: 28587579. DOI: 10.1177/1074248416686187. [DOI] [PubMed] [Google Scholar]
- 10.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14(3):232–244. doi: 10.1111/obr.12003. PMID: 23171381. DOI: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 11.Mather KJ, Goldberg RB. Clinical use of adiponectin as a marker of metabolic dysregulation. Best Pract Res Clin Endocrinol Metab. 2014;28(1):107–117. doi: 10.1016/j.beem.2013.06.008. PMID: 24417950. DOI: 10.1016/j.beem.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. 2017;18(8):pii E1649. doi: 10.3390/ijms18081649. PMID: 28758929. DOI: 10.3390/ijms18081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1062–1079. doi: 10.1016/j.metabol.2015.11.006. PMID: 26725002. DOI: 10.1016/j.metabol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF, Mantzoros CS. Circulating leptin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Diabetologia. 2016;59:30–43. doi: 10.1007/s00125-015-3769-3. PMID: 26407715. DOI: 10.1007/s00125-015-3769-3. [DOI] [PubMed] [Google Scholar]
- 15.Rotundo L, Persaud A, Feurdean M, Ahlawat S, Kim HS. The Association of leptin with severity of non-alcoholic fatty liver disease: A population-based study. Clin Mol Hepatol. 2018;24(4):392–401. doi: 10.3350/cmh.2018.0011. PMID: 30068065. DOI: 10.3350/cmh.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DI Maira G, Pastore M, Marra F. Liver fibrosis in the context of nonalcoholic steatohepatitis: the role of adipokines. Minerva Gastroenterol Dietol. 2018;64(1):39–50. doi: 10.23736/S1121-421X.17.02427-8. PMID: 28875689. DOI: 10.23736/S1121-421X.17.02427-8. [DOI] [PubMed] [Google Scholar]
- 17.Cernea S, Roiban AL, Both E, Huţanu A. Serum leptin and leptin resistance correlations with NAFLD in patients with type 2 diabetes. Diabetes Metab Res Rev. 2018;34(8):e3050. doi: 10.1002/dmrr.3050. PMID: 30052309. DOI: 10.1002/dmrr.3050. [DOI] [PubMed] [Google Scholar]
- 18.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. PMID: 23266767. DOI: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: causes, consequences, and solutions-but do we have the will. Fertil Steril. 2017;107(4):833–839. doi: 10.1016/j.fertnstert.2017.02.104. PMID: 28292617. DOI: 10.1016/j.fertnstert.2017.02.104. [DOI] [PubMed] [Google Scholar]
- 20.Sares-Jäske L, Knekt P, Lundqvist A, Heliövaara M, Männistö S. Dieting attempts modify the association between quality of diet and obesity. Nutr Res. 2017;45:63–72. doi: 10.1016/j.nutres.2017.08.001. PMID: 28967457. DOI: 10.1016/j.nutres.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Esfahani M, Movahedian A, Baranchi M, Goodarzi MT. Adiponectin: an adipokine with protective features against metabolic syndrome. Iran J Basic Med Sci. 2015;18(5):430–442. PMID: 26124928. [PMC free article] [PubMed] [Google Scholar]
- 22.Divella R, Daniele A, Mazzocca A, Abbate I, Casamassima P, Caliandro C, Ruggeri E, Naglieri E, Sabbà C, De Luca R. ADIPOQ rs266729 G/C gene polymorphism and plasmatic adipocytokines connect metabolic syndrome to colorectal cancer. J Cancer. 2017;8(6):1000–1008. doi: 10.7150/jca.17515. PMID: 28529612. DOI: 10.7150/jca.17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6(1):13–21. doi: 10.1111/j.1467-789X.2005.00159.x. PMID: 15655035. DOI: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 24.Wen PF, Wang XS, Zhang M, Cen H, Pan HF, Ye QL, Mao C, Ye DQ. Associations between TNF gene polymorphisms (-308 A/G, -238 A/G, -1031 C/T and -857 T/C) and genetic susceptibility to T1D: a meta-analysis. Endocrine. 2014;46(3):435–444. doi: 10.1007/s12020-014-0172-7. PMID: 24515539. DOI: 10.1007/s12020-014-0172-7. [DOI] [PubMed] [Google Scholar]
- 25.Gerasimova ON, Sigalovich EY, Dankovtseva EN, Nakonechnikov SN, Nikitin AG, Ivanova ZV, Masenko VP, Nosikov VV, Zateyshchikov DA. Carriage of a allele of polymorphic marker G(-238)A of TNF-alfa gene is associated with unfavorable prognosis in patients with chronic systolic heart failure. Kardiologiia. 2015;55(9):25–30. PMID: 28294921. [PubMed] [Google Scholar]
- 26.Beecham J, Hart A, Alexandre L, Hernon J, Kumar B, Lam S. Single nucleotide polymorphisms and post-operative complications following major gastrointestinal surgery: A systematic review and meta-analysis. J Gastrointest Surg. 2019 doi: 10.1007/s11605-019-04300-2. PMID: 31270721. DOI: 10.1007/s11605-019-04300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Yu K, Yang Z. Associations between TNF-α and interleukin gene polymorphisms with polycystic ovary syndrome risk: a systematic review and meta-analysis. J Assist Reprod Genet. 2015;32(4):625–634. doi: 10.1007/s10815-015-0449-7. PMID: 25690158. DOI: 10.1007/s10815-015-0449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobti RC, Kler R, Sharma YP, Talwar KK, Singh N. Risk of obesity and type 2 diabetes with tumor necrosis factor-α 308G/A gene polymorphism in metabolic syndrome and coronary artery disease subjects. Mol Cell Biochem. 2012;360(1-2):1–7. doi: 10.1007/s11010-011-0917-z. PMID: 22081334. DOI: 10.1007/s11010-011-0917-z. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q, Guo X, Wang Q, Zhao F. The association of TNF-308 (G/A) gene polymorphisms and hepatocellular carcinoma risk: a meta-analysis. Chin J Cancer Res. 2016;28(5):536–542. doi: 10.21147/j.issn.1000-9604.2016.05.09. PMID: 27877013. DOI: 10.21147/j.issn.1000-9604.2016.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Q, Lou GG, Liu YC, Qian L, Lv BD. The Tumor Necrosis Factor-α-308 and -238 polymorphisms and risk of hepatocellular carcinoma for Asian populations: A meta-analysis. Curr Ther Res Clin Exp. 2014;76:70–75. doi: 10.1016/j.curtheres.2014.04.001. PMID: 25352937. DOI: 10.1016/j.curtheres.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sookoian SC, González C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res Dec. 2005;13(12):2122–2131. doi: 10.1038/oby.2005.263. PMID: 16421346. DOI: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- 32.Parida S, Siddharth S, Sharma D. Adiponectin, obesity, and cancer: Clash of the Bigwigs in health and disease. Int J Mol Sci. 2019;20(10):pii E2519. doi: 10.3390/ijms20102519. PMID: 31121868. DOI: 10.3390/ijms20102519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modzelewska P, Chludzińska S, Lewko J, Reszeć J. The influence of leptin on the process of carcinogenesis. Contemp Oncol (Pozn) 2019;23(2):63–68. doi: 10.5114/wo.2019.85877. PMID: 31316286. DOI: 10.5114/wo.2019.85877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh CJ, Wang PW, Hu TH. Association of adiponectin gene polymorphism with nonalcoholic fatty liver disease in Taiwanese patients with type 2 diabetes. PLoS One. 2015;10(6):e0127521. doi: 10.1371/journal.pone.0127521. PMID: 26042596. DOI: 10.1371/journal.pone.0127521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suriyaprom K, Phonrat B, Tungtrongchitr R. Association of adiponectin gene -11377C>G polymorphism with adiponectin levels and the metabolic syndrome in Thais. Asia Pac J Clin Nutr. 2014;23(1):167–173. doi: 10.6133/apjcn.2014.23.1.01. PMID: 24561985. DOI: 10.6133/apjcn.2014.23.1.01. [DOI] [PubMed] [Google Scholar]
- 36.Nascimento H, Vieira E, Coimbra S, Catarino C, Costa E, Bronze-da-Rocha E, Rocha-Pereira P, Carvalho M, Ferreira Mansilha H, Rêgo C, Dos Santos R, Santos-Silva A, Belo L. Adipokine gene single-nucleotide polymorphisms in Portuguese obese adolescents: Associations with plasma concentrations of adiponectin, resistin, IL-6, IL-1β, and TNF-α. Child Obes. 2016;12(4):300–313. doi: 10.1089/chi.2015.0235. PMID: 27159547. DOI: 10.1089/chi.2015.0235. [DOI] [PubMed] [Google Scholar]
- 37.Andreoli MF, Donato J, Cakir I, Perello M. Leptin resensitisation: a reversion of leptin-resistant states. J Endocrinol. 2019;241(3):R81–R96. doi: 10.1530/JOE-18-0606. PMID: 30959481. DOI: 10.1530/JOE-18-0606. [DOI] [PubMed] [Google Scholar]
- 38.Ghadge AA, Khaire AA. Leptin as a predictive marker for metabolic syndrome. Cytokine. 121 doi: 10.1016/j.cyto.2019.154735. PMID: 31154250. DOI: 10.1016/j.cyto.2019.154735. [DOI] [PubMed] [Google Scholar]
- 39.Ghasemi A, Saeidi J, Azimi-Nejad M, Hashemy SI. Leptin-induced signaling pathways in cancer cell migration and invasion. Cell Oncol (Dordr) 2019;42(3):243–260. doi: 10.1007/s13402-019-00428-0. PMID: 30877623. DOI: 10.1007/s13402-019-00428-0. [DOI] [PubMed] [Google Scholar]
- 40.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. PMID: 26823198. DOI: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Karim MF, Al-Mahtab M, Rahman S, Debnath CR. Non-alcoholic fatty liver disease (NAFLD)--A Review. Mymensingh Med J. 2015;24(4):873–880. PMID: 26620035. [PubMed] [Google Scholar]
- 42.Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):637–653. doi: 10.1016/j.bpg.2014.07.008. PMID: 25194181. DOI: 10.1016/j.bpg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Lindenmeyer CC, McCullough AJ. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin Liver Dis. 2018;22(1):11–21. doi: 10.1016/j.cld.2017.08.003. PMID: 29128051. DOI: 10.1016/j.cld.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berk PD, Verna EC. Nonalcoholic Fatty Liver Disease: Lipids and Insulin Resistance. Clin Liver Dis. 2016;20(2):245–262. doi: 10.1016/j.cld.2015.10.007. PMID: 27063267. DOI: 10.1016/j.cld.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boutari C, Tziomalos K, Athyros VG. The adipokines in the pathogenesis and treatment of nonalcoholic fatty liver disease. Hippokratia. 2016;20(4):259–263. PMID: 29416297. [PMC free article] [PubMed] [Google Scholar]
- 46.Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF, Mantzoros CS. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia. 2016;59(1):30–43. doi: 10.1007/s00125-015-3769-3. PMID: 26407715. DOI: 10.1007/s00125-015-3769-3. [DOI] [PubMed] [Google Scholar]
- 47.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15(3):139–154. doi: 10.1038/s41574-018-0126-x. PMID: 30459447. DOI: 10.1038/s41574-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cozzo AJ, Fuller AM, Makowski L. Contribution of Adipose Tissue to Development of Cancer. Compr Physiol. 2017;8(1):237–282. doi: 10.1002/cphy.c170008. PMID: 29357128. DOI: 10.1002/cphy.c170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaukat A, Dostal A, Menk J, Church TR. BMI is a risk factor for colorectal cancer mortality. Dig Dis Sci. 2017;62(9):2511–2517. doi: 10.1007/s10620-017-4682-z. PMID: 28733869. DOI: 10.1007/s10620-017-4682-z. [DOI] [PubMed] [Google Scholar]
- 50.Simkens LH, Koopman M, Mol L, Veldhuis GJ, Ten Bokkel Huinink D, Muller EW, Derleyn VA, Teerenstra S, Punt CJ. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer. 2011;47(17):2560–2567. doi: 10.1016/j.ejca.2011.06.038. PMID: 21803570. DOI: 10.1016/j.ejca.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 51.George ES, Forsyth A, Itsiopoulos C, Nicoll AJ, Ryan M, Sood S, Roberts SK, Tierney AC. practical dietary recommendations for the prevention and management of nonalcoholic fatty liver disease in adults. Adv Nutr. 2018;9(1):30–40. doi: 10.1093/advances/nmx007. PMID: 29438460. DOI: 10.1093/advances/nmx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. doi: 10.1016/j.jhep.2017.05.016. PMID: 28545937. DOI: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]