Abstract
Background/Aim: This study examined the in vitro effects of the bile duct cancer drug PRIMA-1MET on cholangiocarcinoma (CCA) cell growth to determine its potential usefulness in CCA therapy. Materials and Methods: The effect of this drug on the expression of senescent markers (p16INK4A and p21) and the phosphorylation of p53 was investigated, as was the association between senescent markers and the patients’ clinicopathological data. Results: PRIMA-1MET inhibited CCA cell growth with the half maximal-inhibitory concentration (IC50) values of 21.9-40.8 μM. PRIMA-1MET induced phospho-p53, p16INK4A and p21 triggering cellular senescence and apoptosis. High expressions of p16INK4A and p21 were associated with a high survival rate of patients with CCA. Conclusion: PRIMA-1MET may potentially be an alternative anticancer agent that might lead to a better prognosis in patients with CCA.
Keywords: Cellular senescence, PRIMA-1MET, apoptosis, cholangiocarcinoma
Cholangiocarcinoma (CCA) is described as a malignant tumor arising from the bile duct epithelia (1). Although rare in Western countries, it has high incidences in Asia, with the highest rate occurring in the Northeast Thailand (2). In these countries, CCA develops along the biliary trees after repeated infection with liver fluke (Opisthorchis viverrini) followed by chronic inflammation. This is defined as a major risk factor of CCA (3-5). This cancer is resistant to common chemotherapy, leading to a poor prognosis, with low 5-year survival and high mortality rates. Most patients receive treatment when the disease is already advanced, when surgical resection, which is prospectively curative for early-stage disease, is usually ineffective (6,7). Therefore, the development of targeted cancer therapies leading to the replacement of conventional cancer chemotherapy could be a better strategy for CCA treatment.
Recently, targeting the cellular senescence pathway became a new strategy for cancer therapy (8). Cellular senescence is an irreversible growth arrest in response to various stresses by activating p53-dependent and -independent pathways (9). Currently, increased levels of lysosomal β-galactosidase activity and tumor-suppressor proteins such as p16INK4A and p21 are commonly used as biomarkers of senescence (10,11). Moreover, cellular senescence also acts as a barrier to tumorigenesis. Environmental stresses can force cancer cells to become responsive to cellular senescence via the activation of the p53-dependent and -independent pathways. The accumulation of p16INK4A and p21 represents a good prognostic factor for cancer treatment (12). This leads us to focus more on using pro-senescent agents for CCA treatment. PRIMA-1MET is a methylated derivative and structural analog of PRIMA-1 (p53 re-activation and induction of massive apoptosis). PRIMA-1MET has been reported to restore p53 activity leading to the induction of cellular senescence and apoptosis (13). PRIMA-1MET has been approved for treatment of acute myeloid leukemia and other hematological malignancies, and cancer of the lung, prostate, colorectum, ovary, esophagus, and breast in several different countries (14-20). However, the effect of PRIMA-1MET on CCA is still unknown. In the present study, we aimed to investigate whether PRIMA-1MET inhibits CCA progression and has any value for the therapy of CCA.
Materials and Methods
Human CCA tissues. Human CCA tissues were collected from patients with CCA who were admitted to Srinagarind Hospital, Khon Kaen University, and were kept at the Specimen Bank, Cholangiocarcinoma Research Institute, Faculty of Medicine, Khon Kaen University. The study protocol was approved by the Ethics Committee for Human Research, Khon Kaen University (HE571283 and HE611390).
Human CCA cell lines. Three human CCA cell lines which possess mutant TP53 gene (21) namely poorly differentiated adenocarcinoma KKU-100 cells and well-differentiated adenocarcinoma KKU-213 and KKU-G023 cells were obtained from the tumor tissues of patients with CCA established at the Cholangiocarcinoma Research Institute Khon Kaen University. Human CCA cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) augmented with 10% heat-inactivated fetal bovine serum (FBS; Gibco/BRL, Grand Island, NY, USA), 2 mg/ml sodium bicarbonate and 1% antibiotic solution (Life Technologies Inc., Grand Island, NY, USA). All cell lines were cultured at 37˚C in a humidified incubator maintained with an atmosphere of 5% CO2.
Cell viability assay. CCA cells (2×103) suspended in 100 μl media were seeded in 96-well plates in triplicate. Cells were treated with different concentrations of PRIMA-1MET ranging from 1.563 to 100 μM for 48 h. A cell viability assay was performed using sulforhodamine B (SRB; Sigma-Aldrich, St. Louis, MO, USA) as previously described (22). Absorbance was measured at 540 nm using a microplate reader (Tecan Austria GmbH, Grodig, Austria). Two cell lines with low sensitive and highly sensitive to PRIMA-1MET were selected to further molecular analyses.
Western blotting. CCA cells (1×105 cells/ml) were seeded in 6-well plates and left to adhere on plates overnight prior to start of treatment with PRIMA-1MET at 3.125 to 50 μM for 24 h. CCA cells were lysed with radioimmuno-precipitation assay buffer. The amount of protein was quantified using the Pierce BCA™ Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). Forty micrograms of protein were electrophoresed using a 4-12% (w/v) sodium dodecyl sulfate–polyacrylamide gel before transfer to a polyvinylidene fluoride membrane (Whatman, Dassel, Germany). The membrane was incubated with 5% skim milk in tris-buffered saline (TBS) to block nonspecific binding at room temperature for 1 h then incubated with primary antibodies including anti-cyclin dependent kinase inhibitor 2A (CDKN2A/p16INK4A), anti-p21, anti-phospho-p53 (Ser15), anti-p53 (Abcam, Cambridge, MA, USA), anti-BCL2-associated X (BAX) (BD Biosciences, San Jose, CA, USA), anti-B-cell lymphoma 2 (BCL2) (Cell Signaling Technology, Danvers, MA, USA) at 4˚C overnight. After rinsing with 0.1% Tween-20 in TBS, the membrane was incubated with horseradish peroxidase-conjugated rabbit or mouse IgG at room temperature for 1 h. After rinsing the membrane with Tween-20 in TBS, immunoreactive materials were developed by Amersham™ECL™Prime Western Blotting Detection Reagent (GE Healthcare Life Science, Buckinghamshire, UK) for chemiluminescent detection. β-Actin was used as an internal loading control. Intensity of each protein bands was semi-quantitatively assessed by densitometry using ImageJ software 1.48v (Wayne Rasband, USA). Each experiment was performed in triplicate.
Senescence associated β-galactosidase staining. CCA cell lines (200 cell/ml) were seeded in 12-well plates and allowed to adhere overnight. Cells were then treated with DMEM as a control or 3.0 μM of PRIMA-1MET and incubated at 37˚C for 24 h. After that, the treated cells were continued in DMEM for 9 days post-treatment. Senescence-associated β-galactosidase staining was performed using senescence-associated β-galactosidase staining kit (Cell Signaling Technology). The cells were checked under a microscope (×200 magnification) for the development of blue color.
Flow cytometric analysis. CCA cell lines (1×105 cells/ml) were seeded in a 6-well plate and treated with different concentrations of PRIMA-1MET ranging from 12.5 to 100 μM for 24 h. After suspending using trypsin they were washed twice with cold phosphate-buffered saline. Each sample was resuspended in 100 μl of incubation buffer, 2 μl of annexin-V-fluorescein and propidium iodide, and incubated on ice for 15 min in the dark. Each sample was mixed with 400 μl of incubation buffer and put into a BD Falcon tube (BD Biosciences, Tewsbury, MA, USA). Apoptosis cells were detected using a flow cytometer (BD FACSCanto™II; BD Biosciences, San Jose, CA, USA).
Immunohistochemical analysis. Immunohistochemical analysis was performed to investigate the expression pattern of p16INK4A and p21 in human CCA tissues using the standard protocol (23). Antigen retrieval was performed using a pressure cooker with 1x Tris-EDTA+0.05% Tween20. Rabbit anti-CDKN2A/p16INK4A (dilution 1:100) and Rabbit anti-p21 (dilution 1:50) were used as primary antibodies to react antigens in the tissue sections at 4˚C overnight. After washing, the tissue sections were incubated with a horse radish peroxidase-conjugated secondary antibody (DakoEnvision+System-HRP Labelled Polymer Anti-Rabbit antibody; Dako, Santa Clara, CA, USA) for 1 h at room temperature. The stained tissue sections were reviewed under a light microscope. Tumor tissues were given a score according to the intensity of nuclear staining (negative=0, weak=1, moderate=2, and strong=3) and percentage of stained cells. The staining score was calculated by multiplying the intensity and frequency for each case. The expression of p16INK4A and p21 proteins was categorized into low and high expression groups using the mean of p16INK4A and p21 scores as a cut-off value.
Statistical analysis. Statistical analysis was performed using the Statistical Package for the Social Science; SPSS software v.16 (SPSS, Chicago, IL, USA). The correlation between the expression of p16INK4A and p21 with the clinicopathological data of CCA patients was analyzed by Fisher’s exact test. Survival analysis was performed using Kaplan–Meier and the log-rank test. Protein expression levels and growth were compared using mean±S.D. of treated and untreated cells by Student t-test. The results were considered to be significant at p-values of less than 0.05.
Results
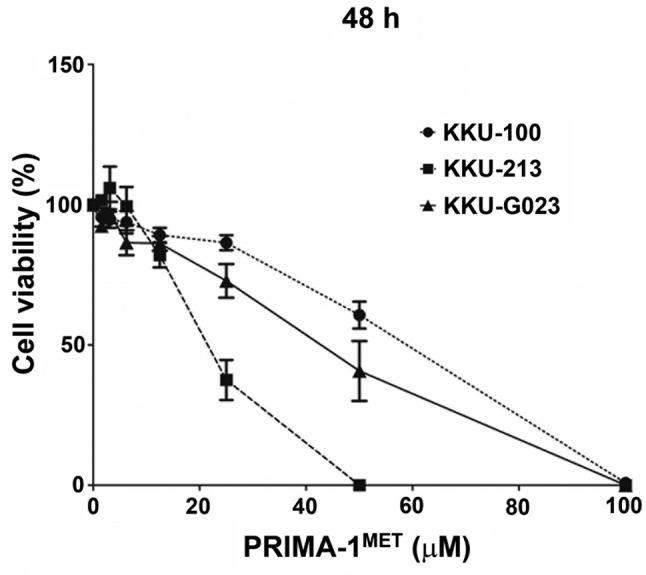
PRIMA-1MET inhibits CCA cell proliferation. The cytotoxic effect of PRIMA-1MET on the growth of the human CCA cell lines KKU-100, KKU-213 and KKU-G023 was determined using an SRB assay. The three CCA cell lines were treated with PRIMA-1MET for 48 h at different concentrations (0-100 μM). The results show that PRIMA-1MET effectively inhibited CCA cell proliferation in a dose-dependent manner (Figure 1). Our results show that at the end of 48 h, PRIMA-1MET inhibited KKU-100, KKU-213 and KKU-G023 with average the half maximal-inhibitory concentration values of 58.5±5.4, 21.9±3.4 and 44.9±11.0 μM, respectively. Two cell lines with low sensitive (KKU-100) and highly sensitive (KKU-213) to PRIMA-1MET were selected for further molecular analyses.
Figure 1. Cytotoxic effect of PRIMA-1MET on cholangiocarcinoma (CCA) cell growth. PRIMA-1MET treated CCA cell lines showed a decrease in cell proliferation at 48 h. Data are shown as mean±SD obtained from three independent experiments.
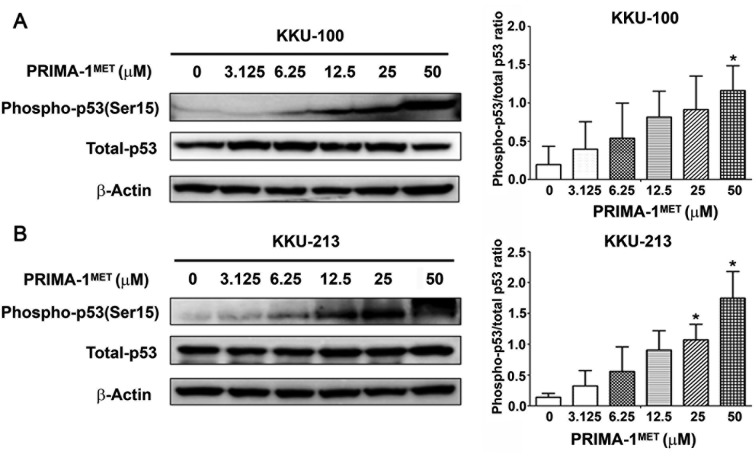
Reactivation of p53 by PRIMA-1MET. Two cell lines, KKU-100 (low sensitivity) and KKU213 (highly sensitivity) were treated with PRIMA-1MET at different concentrations (0-50 μM) for 24 h. Western blot analysis demonstrated that the phospho-p53 (Ser15)/total p53 ratio was markedly increased in KKU-100 at 50 μM (Figure 2A) and in KKU-213 at 25 and 50 μM (Figure 2B) cells as compared with untreated cells.
Figure 2. Reactivation of p53 by PRIMA-1MET in KKU-100 and KKU-213 cells. Western blot analysis showed phospho-p53(Ser15) protein expression in KKU-100 (A) and KKU-213 (B) after PRIMA-1MET treatment. The relative level of phospho-p53(Ser15) protein expression was quantified for PRIMA-1MET-treated cholangiocarcinoma cells. The phospho-p53(Ser15)/total-p53 ratio was analyzed. Data represent the mean±SD of protein band intensities taken from three independent experiments. These were normalized to the intensity of β-actin. *Significantly different from untreated cells at p<0.05.
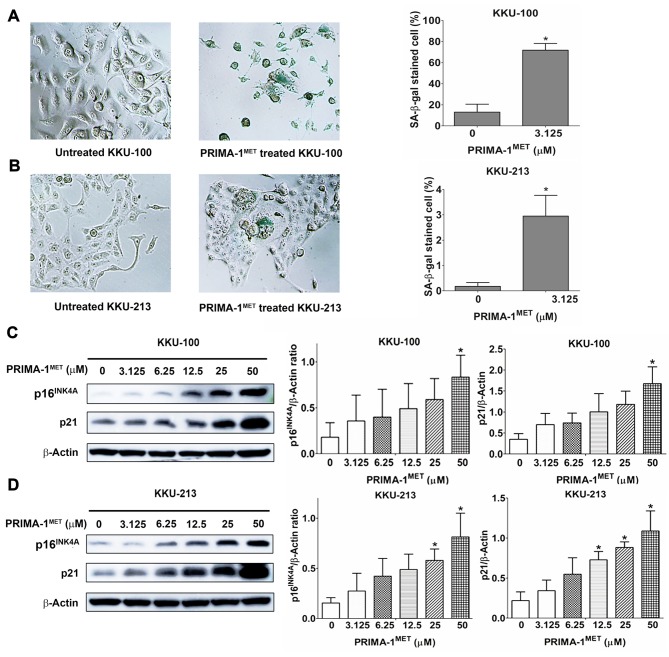
Induction of cellular senescence by PRIMA-1MET in CCA cell lines. Two cell lines, KKU-100 and KKU213 treated with 3.125 μM of PRIMA-1MET exhibited a senescent phenotype as visualized under a light microscope, including enlarged size, flattened cell shape, induced nuclear granularity and increased volume of cytoplasm (Figure 3). PRIMA-1MET treatment of cells at 3.125 μM showed that the proportion of cells with positive staining for senescence-associated β-galactosidase was significantly higher than that of the untreated cells for both KKU-100 (Figure 3A) and KKU-213 (Figure 3B) cells. KKU-100 and KKU-213 cells were treated with PRIMA-1MET for 24 h and western blotting was performed to evaluate the expression levels of p16INK4A and p21 proteins (Figure 3C and D, left panel). Our results showed that p16INK4A expression was significantly increased in PRIMA-1MET treated KKU-100 cells at 50 μM and KKU-213 cells at 25 and 50 μM when compared with the untreated cells. Similarly, p21 expression was significantly increased in PRIMA-1MET treated KKU-100 at 50 μM and KKU-213 cells at 12.5, 25 and 50 μM when compared with the untreated cells (Figure 3C and D, right panel).
Figure 3. The induction of cellular senescence by PRIMA-1MET in KKU-100 and KKU-213 cells. A and B: The left panel shows senescence associated (SA)-β-galactosidase staining in KKU-100 (A) and KKU-213 (B) for 9 days after the initiation of treatment with 3.125 μM PRIMA-1MET (original magnification 100×). The right panel shows quantification of the mean±SD proportion of SA-β-gal-positive CCA cells taken from three independent experiments. C and D: The left panel shows western blot analysis of p16INK4A and p21 protein expression in KKU-100 (C) and KKU-213 (D) cells after PRIMA-1MET treatment. The right panel shows quantification of the mean±SD protein band intensity taken from three independent experiments. These were normalized to the intensity of β-actin. *Significantly different from untreated cells at p<0.05.
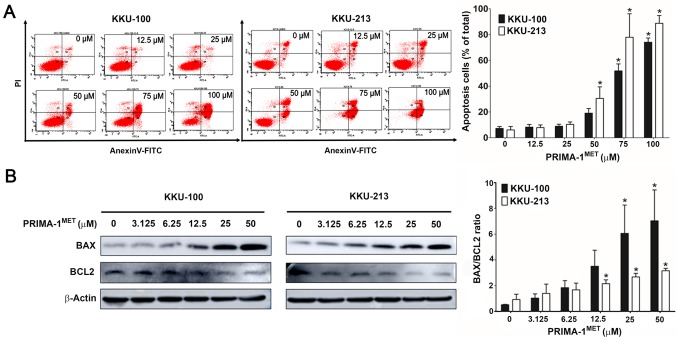
PRIMA-1MET induced apoptosis of CCA cells. KKU-100 and KKU-213 cells were treated with PRIMA-1MET at different concentrations (0-100 μM) for 24 h, they were then evaluated by flow cytometry using annexin-V and propidium iodide staining. The results showed that the annexin V-positive cells were significantly increased in KKU-100 at 75 and 50 μM and KKU-213 cells at 50, 75 and 100 μM treated with PRIMA-1MET in a dose-dependent manner when compared with untreated cells (Figure 4A). Western blotting was performed to evaluate the expression levels of BAX and BCL2 proteins in KKU-100 and KKU-213 cells treated with PRIMA-1MET for 24 h (Figure 4B, left panel). Our results showed that the BAX/BCL2 ratio was significantly increased in PRIMA-1MET-treated KKU-100 at 25 and 50 μM and KKU-213 cells at 12.5, 25 and 50 μM (Figure 4B, right panel) when compared with the untreated cells.
Figure 4. Effects of PRIMA-1MET on the induction of apoptosis in KKU-100 and KKU-213 cells. KKU-100 and KKU-213 cells were treated with PRIMA-1MET for 24 h. Annexin-V and PI staining was analyzed by flow cytometry and expression of apoptosis-associated proteins was quantified. A: Representative histograms from flow cytometry are shown in the left panels, with data the percentage of annexin-V and propidium iodine (PI)-positive CCA cells with and without PRIMA-1MET treatment in the right panel. B: The left panel shows western blot analysis of BCL2-associated X(BAX) and B-cell lymphoma 2 (BCL2) expression in CCA cell lines after PRIMA-1MET treatment for 24 h. The BAX/BCL2 ratio was analyzed. Theright panel shows the mean±SD protein band intensity from three independent experiments. The data were normalized to the intensity of β-actin. Significantly different from untreated cells at p<0.05.
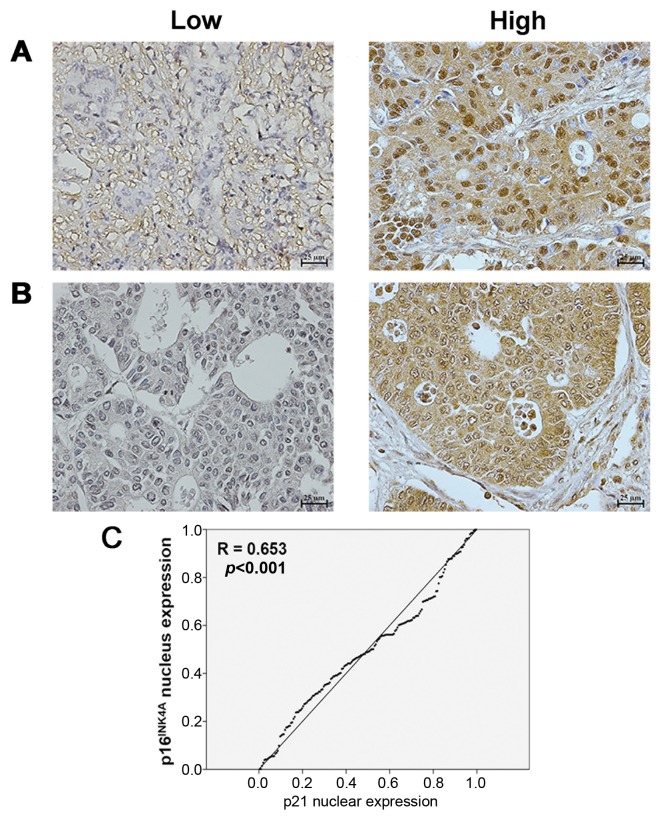
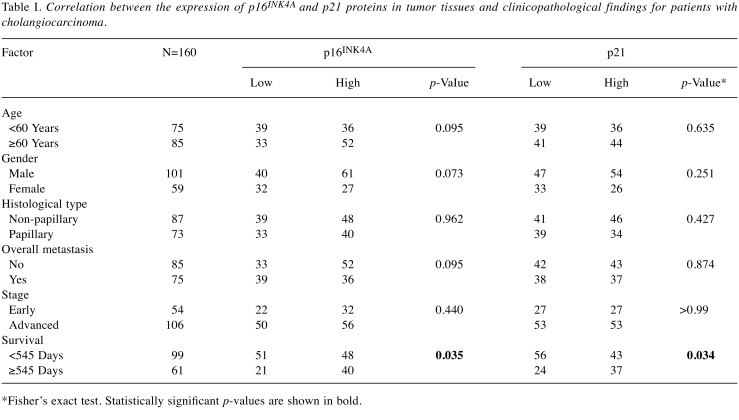
Expression of p16INK4A and p21 protein in human CCA tissues. Of the CCA tissues from 160 patients with intrahepatic CCA, 101 (63%) were from males and 59 (37%) from females. The age of the patients ranged from 36 to 79 years (median age=60 years). For all 160 CCA cases, positive staining of p16INK4A and p21 proteins was seen in the nuclei of cells in the tissue sections. Exemplary images of staining of cancerous tissues are shown in Figure 5A and B. p16INK4A expression was found to be low in 45% (72/160) and high in 55% (88/160) of cases. Similarly, p21 expression was found to be low in 39% (62/160) and high in 61% (98/160) of cases.
Figure 5. Immunohistochemical staining scored as low (left) and high (right) p16INK4A (A) and p21 (B) protein expression in 160 cases of human cholangiocarcinoma tissues. Magnification was ×200. C: Bivariate analysis revealed that p16INK4A protein expression was significantly positively correlated with p21.
Correlation of p16INK4A and p21 protein scores with clinicopathological features of patients with CCA. As shown in Table I, Fisher’s exact test demonstrated that high p16INK4A and p21 expression was associated with longer patient survival (p=0.035 and p=0.034, respectively). Age, gender, histological type, overall metastasis and lymph node metastasis did not significantly differ between these two groups. The immunohistochemical scores for p16INK4A and p21 proteins were analyzed for their association using bivariate analysis. The results revealed that p16INK4A protein was positively correlated with p21 (p<0.001) (Figure 5C).
Table I. Correlation between the expression of p16INK4A and p21 proteins in tumor tissues and clinicopathological findings for patients with cholangiocarcinoma.
*Fisher’s exact test. Statistically significant p-values are shown in bold.
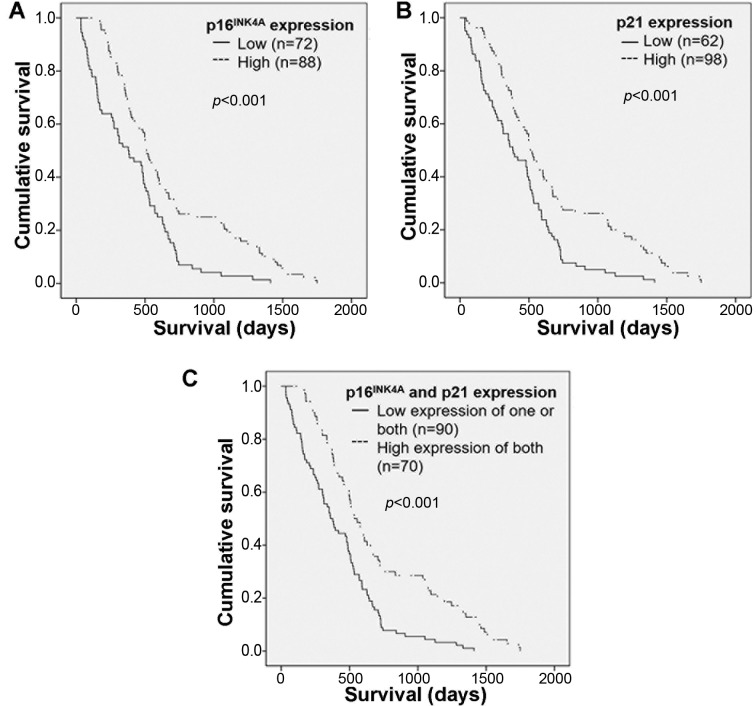
Log-rank analysis was carried out to determine the cumulative survival rate of patients with CCA according to expression of p16INK4A or p21. Individuals with high expression had a significantly longer survival rate than those with low expression (p<0.001) (Figure 6A and B). Moreover, patients with CCA who had high expression of both of p16INK4A and p21 had significantly longer survival than those with low expression of only one or of both proteins (p<0.001) (Figure 6C).
Figure 6. Cumulative survival rate of patients with cholangiocarcinoma according to expression of p16INK4A and p21 alone (A and B) and in combination (C). Patients with high expression of either protein alone had a significantly longer survival than did those with low expression. Patients who had high expression of both of p16INK4A and p21 had significantly longer survival than those with low expression of one or both proteins.
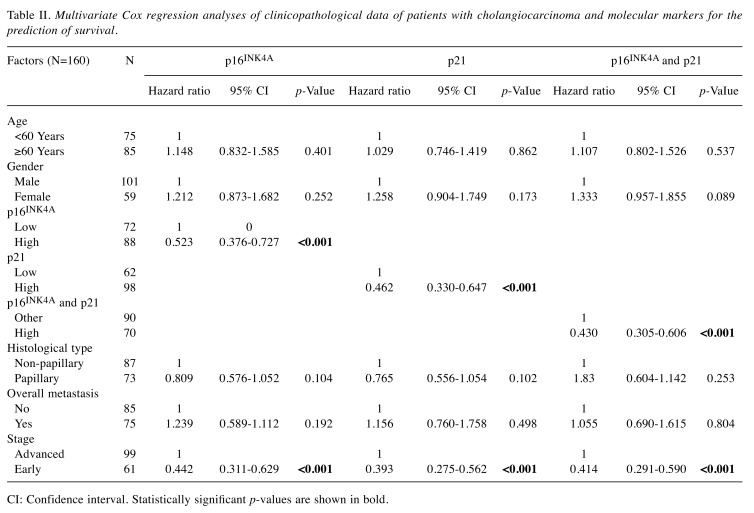
Multivariate Cox proportional hazards regression analyses were adjusted for the effects of age, gender, histological type, overall metastasis, stage, as well as p16INK4A and p21 scores (Table II). The results showed that both p16INK4A and p21 scores were independent prognostic factors, with higher levels conferring statistically significant protection against CCA (both p<0.001). Patients who had high p16INK4A and p21 expression had a 1.9- and 2.1-fold reduced risk for CCA, respectively. The effects of the combination of p16INK4A and p21 proteins with the clinicopathological data of CCA patients were adjusted and also analyzed using multivariate Cox proportional hazard regression method. High expression of both p16INK4A and p21 remained a significant predictor for CCA patients (p<0.001). The high expression of p16INK4A, and of p21, in CCA tissues of patients with early-stage disease was confirmed to be independently prognostic for patients with CCA as shown in Table II.
Table II. Multivariate Cox regression analyses of clinicopathological data of patients with cholangiocarcinoma and molecular markers for the prediction of survival.
CI: Confidence interval. Statistically significant p-values are shown in bold.
Discussion
Pro-senescent drugs can serve as a new weapon to inhibit tumor cell growth. Approximately 50% of all human cancers bear a p53 mutation, including CCA (24), resulting in resistance to conventional chemotherapy (25). Anticancer drugs such as PRIMA-1MET have been shown to restore expression of p53, leading to the induction of cellular senescence and apoptosis, in both in vivo and in vivo studies (26-29). We demonstrated that PRIMA-1MET significantly inhibited cell proliferation of CCA cell lines. PRIMA-1MET restored p53 activity, demonstrating an increase in the phospho-p53 (Ser15) level in both cell lines. This phosphorylation activated the p53 signaling pathway, leading to the induction of cellular senescence through an increase in senescence-associated β-galactosidase activity. p16INK4A and p21 protein levels were also associated with an increase in the BAX/BCL2 ratio and apoptosis. Our results are consistent with previous studies showing the effect of PRIMA-MET on other types of cancer (14,16-20,30).
Cellular senescence is an irreversible arrest of growth that acts as a barrier to tumorigenesis by activating both p53-dependent and p53-independent pathways. We found that high expression of the senescent markers p16INK4A and p21 in CCA tissues was significantly associated with longer patient survival compared to low expression. Our results are consistent with previous studies demonstrating that the p16INK4A and p21 proteins can possibly serve as good prognostic markers for predicting overall survival in many types of cancer, such as adenocarcinoma, gastric cancer, Hodgkin lymphoma, and non-small cell lung cancer (10,12,31,32).
Taken together, the findings of our study suggest that patients with CCA who have a high expression of p16INK4A and p21 might be predicted as belonging to a good prognostic group. The induction of cellular senescence is a therapeutic strategy for inhibiting the growth of CCA cells. Our results reveal that PRIMA-1MET might be a potential anticancer drug for CCA treatment by the induction of cellular senescence and apoptosis. Currently, recruitment is underway for three clinical trials with the objective of testing the safety and efficacy of PRIMA-1MET treatment in advanced esophageal carcinoma (NCT02999893), high-grade serous ovarian cancer (NCT02098343), and mutant p53 hematological myeloid malignant disease (NCT03072043) (https://clinicaltrials.gov). Our results suggest that PRIMA-1MET is an attractive candidate for further investigation as an anticancer agent in clinical trials for patients with CCA.
Conflicts of Interest
The Authors declare that no competing interest exists in regard to this study.
Authors’ Contributions
NN and WL designed the study. AT collected patient tissues. PS evaluated the clinicopathological data of patients. CP and YK performed experiments and result analysis. CP wrote the article. NN read, edited and approved the final version of article.
Acknowledgements
This work was supported by a grant from a Mid-Career Grant (RSA5980012) of Thailand Research Fund to NN, the Faculty of Medicine, Khon Kaen University, for its support by a grant fund for the M.Sc. program, to CP (IN61322), a grant from Khon Kaen University to NN, and a grant from CASCAP Program to NN. We would like to acknowledge Prof. Trevor N. Petney, for editing the MS via Publication Clinic KKU, Thailand.
References
- 1.Blechacz B. Cholangiocarcinoma: Current knowledge and new developments. Gut Liver. 2017;11(1):13–26. doi: 10.5009/gnl15568. PMID: 27928095. DOI: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sripa B, Pairojkul C. Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol. 2008;24(3):349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. PMID: 18408464. DOI: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyson GL, El-Serag HB. Risk factors for cholangio-carcinoma. Hepatology. 2011;54(1):173–184. doi: 10.1002/hep.24351. PMID: 21488076. DOI: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haswell-Elkins MR, Sithithaworn P, Elkins D. Opisthorchis viverrini and cholangiocarcinoma in Northeast Thailand. Parasitol Today. 1992;8(3):86–89. doi: 10.1016/0169-4758(92)90241-s. PMID: 15463578. [DOI] [PubMed] [Google Scholar]
- 5.Elkins DB, Mairiang E, Sithithaworn P, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, Haswell-Elkins MR. Cross-sectional patterns of hepatobiliary abnormalities and possible precursor conditions of cholangiocarcinoma associated with Opisthorchis viverrini infection in humans. Am J Trop Med Hyg. 1996;55(3):295–301. doi: 10.4269/ajtmh.1996.55.295. PMID: 8842118. DOI: 10.4269/ajtmh.1996.55.295. [DOI] [PubMed] [Google Scholar]
- 6.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12(1):131–150. doi: 10.1016/j.cld.2007.11.003. PMID: 18242501. DOI: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366(9493):1303–1314. doi: 10.1016/S0140-6736(05)67530-7. PMID: 16214602. DOI: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 8.Acosta JC, Gil J. Senescence: A new weapon for cancer therapy. Trends Cell Biol. 2012;22(4):211–219. doi: 10.1016/j.tcb.2011.11.006. PMID: 22245068. DOI: 10.1016/j.tcb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. PMID: 23140366. DOI: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calio A, Zamo A, Ponzoni M, Zanolin ME, Ferreri AJ, Pedron S, Montagna L, Parolini C, Fraifeld VE, Wolfson M, Yanai H, Pizzolo G, Doglioni C, Vinante F, Chilosi M. Cellular senescence markers p16INK4A and p21CIP1/WAF are predictors of Hodgkin lymphoma outcome. Clin Cancer Res. 2015;21(22):5164–5172. doi: 10.1158/1078-0432.CCR-15-0508. PMID: 26199387. DOI: 10.1158/1078-0432.CCR-15-0508. [DOI] [PubMed] [Google Scholar]
- 11.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4(12):1798–1806. doi: 10.1038/nprot.2009.191. PMID: 20010931. DOI: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed S, Yasufuku K, Hiroshima K, Nakajima T, Yoshida S, Suzuki M, Sekine Y, Shibuya K, Iizasa T, Farouk A, Fujisawa T. Prognostic implications of cell cycle-related proteins in primary resectable pathologic N2 nonsmall cell lung cancer. Cancer. 2007;109(12):2506–2514. doi: 10.1002/cncr.22651. PMID: 17487846. DOI: 10.1002/cncr.22651. [DOI] [PubMed] [Google Scholar]
- 13.Lambert JM, Gorzov P, Veprintsev DB, Soderqvist M, Segerback D, Bergman J, Fersht AR, Hainaut P, Wiman KG, Bykov VJ. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell. 2009;15(5):376–388. doi: 10.1016/j.ccr.2009.03.003. PMID: 19411067. DOI: 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Ali D, Jonsson-Videsater K, Deneberg S, Bengtzen S, Nahi H, Paul C, Lehmann S. APR-246 exhibits anti-leukemic activity and synergism with conventional chemotherapeutic drugs in acute myeloid leukemia cells. Eur J Haematol. 2011;86(3):206–215. doi: 10.1111/j.1600-0609.2010.01557.x. PMID: 21114538. DOI: 10.1111/j.1600-0609.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- 15.Bykov VJ, Zache N, Stridh H, Westman J, Bergman J, Selivanova G, Wiman KG. PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene. 2005;24(21):3484–3491. doi: 10.1038/sj.onc.1208419. PMID: 15735745. DOI: 10.1038/sj.onc.1208419. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann S, Bykov VJ, Ali D, Andren O, Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A, Paul C, Wiman KG, Andersson PO. Targeting p53 in vivo: A first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30(29):3633–3639. doi: 10.1200/JCO.2011.40.7783. PMID: 22965953. DOI: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 17.Li XL, Zhou J, Chan ZL, Chooi JY, Chen ZR, Chng WJ. PRIMA-1MET (APR-246) inhibits growth of colorectal cancer cells with different p53 status through distinct mechanisms. Oncotarget. 2015;6(34):36689–36699. doi: 10.18632/oncotarget.5385. PMID: 26452133. DOI: 10.18632/oncotarget.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu DS, Read M, Cullinane C, Azar WJ, Fennell CM, Montgomery KG, Haupt S, Haupt Y, Wiman KG, Duong CP, Clemons NJ, Phillips WA. APR-246 potently inhibits tumour growth and overcomes chemoresistance in preclinical models of oesophageal adenocarcinoma. Gut. 2015;64(10):1506–1516. doi: 10.1136/gutjnl-2015-309770. PMID: 26187504. DOI: 10.1136/gutjnl-2015-309770. [DOI] [PubMed] [Google Scholar]
- 19.Mohell N, Alfredsson J, Fransson A, Uustalu M, Bystrom S, Gullbo J, Hallberg A, Bykov VJ, Bjorklund U, Wiman KG. APR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis. 2015;6:e1794. doi: 10.1038/cddis.2015.143. PMID: 26086967. DOI: 10.1038/cddis.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Synnott NC, Bauer MR, Madden S, Murray A, Klinger R, O'Donovan N, O'Connor D, Gallagher WM, Crown J, Fersht AR, Duffy MJ. Mutant p53 as a therapeutic target for the treatment of triple-negative breast cancer: Preclinical investigation with the anti-p53 drug, PK11007. Cancer Lett. 2018;414:99–106. doi: 10.1016/j.canlet.2017.09.053. PMID: 29069577 DOI: 10.1016/j.canlet.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 21.Saensa-Ard S, Leuangwattanawanit S, Senggunprai L, Namwat N, Kongpetch S, Chamgramol Y, Loilome W, Khansaard W, Jusakul A, Prawan A, Pairojkul C, Khantikeo N, Yongvanit P, Kukongviriyapan V. Establishment of cholangiocarcinoma cell lines from patients in the endemic area of liver fluke infection in Thailand. Tumour Biol. 2017;39(11):1010428317725925. doi: 10.1177/1010428317725925. PMID: 29110582. DOI: 10.1177/1010428317725925. [DOI] [PubMed] [Google Scholar]
- 22.Namwat N, Amimanan P, Loilome W, Jearanaikoon P, Sripa B, Bhudhisawasdi V, Tassaneeyakul W. Characterization of 5-fluorouracil-resistant cholangiocarcinoma cell lines. Chemotherapy. 2008;54(5):343–351. doi: 10.1159/000151541. PMID: 29110582. DOI: 10.1177/1010428317725925. [DOI] [PubMed] [Google Scholar]
- 23.Detre S, Saclani Jotti G, Dowsett M. A "quickscore" method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–878. doi: 10.1136/jcp.48.9.876. PMID: 7490328. DOI: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jusakul A, Kongpetch S, Teh BT. Genetics of Opisthorchis viverrini-related cholangiocarcinoma. Curr Opin Gastroenterol. 2015;31(3):258–263. doi: 10.1097/MOG.0000000000000162. PMID: 25693006. DOI: 10.1097/MOG.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 25.Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8(5):8921–8946. doi: 10.18632/oncotarget.13475. PMID: 27888811. DOI: 10.18632/oncotarget.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert JM, Moshfegh A, Hainaut P, Wiman KG, Bykov VJ. Mutant p53 reactivation by PRIMA-1MET induces multiple signaling pathways converging on apoptosis. Oncogene. 2010;29(9):1329–1338. doi: 10.1038/onc.2009.425. PMID: 19946333. DOI: 10.1038/onc.2009.425. [DOI] [PubMed] [Google Scholar]
- 27.Rokaeus N, Klein G, Wiman KG, Szekely L, Mattsson K. Prima-1(met) induces nucleolar accumulation of mutant p53 and PML nuclear body-associated proteins. Oncogene. 2007;26(7):982–992. doi: 10.1038/sj.onc.1209858. PMID: 16909106. DOI: 10.1038/sj.onc.1209858. [DOI] [PubMed] [Google Scholar]
- 28.Zandi R, Selivanova G, Christensen CL, Gerds TA, Willumsen BM, Poulsen HS. PRIMA-1MET/APR-246 induces apoptosis and tumor growth delay in small cell lung cancer expressing mutant p53. Clin Cancer Res. 2011;17(9):2830–2841. doi: 10.1158/1078-0432.CCR-10-3168. PMID: 21415220. DOI: 10.1158/1078-0432.CCR-10-3168. [DOI] [PubMed] [Google Scholar]
- 29.Zache N, Lambert JM, Wiman KG, Bykov VJ. PRIMA-1MET inhibits growth of mouse tumors carrying mutant p53. Cell Oncol. 2008;30(5):411–418. doi: 10.3233/CLO-2008-0440. PMID: 18791272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bykov VJ, Issaeva N, Zache N, Shilov A, Hultcrantz M, Bergman J, Selivanova G, Wiman KG. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem. 2005;280(34):30384–30391. doi: 10.1074/jbc.M501664200. PMID: 15998635. DOI: 10.1074/jbc.M501664200. [DOI] [PubMed] [Google Scholar]
- 31.Alfsen GC, Reed W, Sandstad B, Kristensen GB, Abeler VM. The prognostic impact of cyclin dependent kinase inhibitors p21WAF1, p27KIP1, and p16INK4/MTS1 in adenocarcinomas of the uterine cervix: An immunohistochemical evaluation of expression patterns in population-based material from 142 patients with international federation of gynecology and obstetrics stage I and II adenocarcinoma. Cancer. 2003;98(9):1880–1889. doi: 10.1002/cncr.11727. PMID: 14584070. DOI: 10.1002/cncr.11727. [DOI] [PubMed] [Google Scholar]
- 32.Seo YH, Joo YE, Choi SK, Rew JS, Park CS, Kim SJ. Prognostic significance of p21 and p53 expression in gastric cancer. Korean J Intern Med. 2003;18(2):98–103. doi: 10.3904/kjim.2003.18.2.98. PMID: 12872447. DOI: 10.3904/kjim.2003.18.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]