Abstract
BACKGROUND
We previously reported prolonged progression-free survival and marginally prolonged overall survival among postmenopausal patients with hormone receptor–positive metastatic breast cancer who had been randomly assigned to receive the aromatase inhibitor anastrozole plus the selective estrogen-receptor down-regulator fulvestrant, as compared with anastrozole alone, as first-line therapy. We now report final survival outcomes.
METHODS
We randomly assigned patients to receive either anastrozole or fulvestrant plus anastrozole. Randomization was stratified according to adjuvant tamoxifen use. Analysis of survival was performed by means of two-sided stratified log-rank tests and Cox regression. Efficacy and safety were compared between the two groups, both overall and in subgroups.
RESULTS
Of 707 patients who had undergone randomization, 694 had data available for analysis. The combination-therapy group had 247 deaths among 349 women (71%) and a median overall survival of 49.8 months, as compared with 261 deaths among 345 women (76%) and a median overall survival of 42.0 months in the anastrozole-alone group, a significant difference (hazard ratio for death, 0.82; 95% confidence interval [CI], 0.69 to 0.98; P = 0.03 by the log-rank test). In a subgroup analysis of the two strata, overall survival among women who had not received tamoxifen previously was longer with the combination therapy than with anastrozole alone (median, 52.2 months and 40.3 months, respectively; hazard ratio, 0.73; 95% CI, 0.58 to 0.92); among women who had received tamoxifen previously, overall survival was similar in the two groups (median, 48.2 months and 43.5 months, respectively; hazard ratio, 0.97; 95% CI, 0.74 to 1.27) (P = 0.09 for interaction). The incidence of long-term toxic effects of grade 3 to 5 was similar in the two groups. Approximately 45% of the patients in the anastrozole-alone group crossed over to receive fulvestrant.
CONCLUSIONS
The addition of fulvestrant to anastrozole was associated with increased long-term survival as compared with anastrozole alone, despite substantial crossover to fulvestrant after progression during therapy with anastrozole alone. The results suggest that the benefit was particularly notable in patients without previous exposure to adjuvant endocrine therapy. (Funded by the National Cancer Institute and AstraZeneca; ClinicalTrials.gov number, .)
Metastatic hormone-receptor–positive breast cancer is considered to be incurable. Although some patients have many years of disease control with a third-generation aromatase inhibitor such as anastrozole, the median survival is only 41.3 months.1
We hypothesized that adding the selective estrogen-receptor down-regulator fulvestrant to anastrozole therapy would be more effective than treatment with anastrozole alone, given that one of the resistance mechanisms to anastrozole is chronic stimulation of estrogen receptors by low levels of estradiol. As we previously reported, the combination of fulvestrant and anastrozole prolonged progression-free survival (median, 15.0 months with the combination therapy vs. 13.5 months with anastrozole alone; hazard ratio for progression or death, 0.80; P = 0.007) in a prospective, randomized clinical trial (S0226).1 The incidence of severe toxic effects was similar in the two groups, and almost all the patients were able to receive treatment. Furthermore, at a median follow-up of 3 years, we observed that the median overall survival was 47.7 months with the combination therapy and 41.3 months with anastrozole alone (hazard ratio, 0.81; 95% confidence interval [CI], 0.65 to 1.00; P = 0.05).1 We now report updated trial outcomes at a median follow-up of 7 years in patients who did not have disease progression, and we discuss the effect of combination therapy (anastrozole plus fulvestrant) as compared with anastrozole alone on overall survival in various subgroups defined on the basis of clinical characteristics.
Methods
Trial Oversight
We conducted this investigator-initiated, multi-center, randomized, open-label trial (S0226) to compare the efficacy of the addition of fulvestrant to anastrozole therapy with that of anastrozole therapy alone (followed by use of fulvestrant in patients who were not in visceral crisis) in patients with metastatic breast cancer. The trial was designed and conducted, and the data were analyzed, by the Southwest Oncology Group Cooperative Group, which was funded by the National Cancer Institute (NCI), with review and collaboration from the National Cancer Institute of Canada and the NCI Cancer Therapy Evaluation Program. The first two authors assume full responsibility for the accuracy and completeness of the data and vouch for the data analysis and for the fidelity of the trial to the protocol (available with the full text of this article at NEJM.org). All the drafts of the manuscript were prepared and approved by all the authors. The trial data were reviewed by a data and safety monitoring committee every 6 months.
AstraZeneca provided the trial medications at no cost to the enrolled patients. AstraZeneca provided comments on an early draft of the manuscript but contractually was not allowed to approve or disapprove of the submission of the manuscript for publication. AstraZeneca was not provided with the trial data and did not participate in the statistical analysis. The statistical analysis plan is available with the protocol.
Patients
The trial design and the characteristics of the patients at baseline have been published previously.1 The trial enrolled postmenopausal women with estrogen-receptor–positive or progesterone-receptor–positive metastatic breast cancer who had a Zubrod’s performance-status score of 0 to 2 (on a scale of 0 to 5, with higher scores indicating greater disability; a score of 0 indicates that the patient is fully active, 1 that the patient is restricted in strenuous activity but is ambulatory, and 2 that the patient is unable to work but is ambulatory and capable of self-care and up and about >50% of waking hours). No previous chemotherapy, hormonal therapy, or immunotherapy for metastatic disease was allowed. Previous treatment with adjuvant tamoxifen was allowed and was a stratification factor. Neoadjuvant or adjuvant chemotherapy or aromatase inhibitor therapy had to be completed more than 12 months before enrollment.
We randomly assigned patients in a 1:1 ratio to receive standard-dose anastrozole alone or anastrozole plus fulvestrant. Fulvestrant was administered at a loading dose of 500 mg on day 1, with 250 mg administered on days 14 and 28 and then 250 mg administered as maintenance therapy every 28 days. At the time of progression, in the absence of visceral crisis, crossover to fulvestrant was strongly encouraged. Near the end of the trial, a loading dose followed by an increased maintenance dose of fulvestrant of 500 mg per month was shown to be more effective than 250 mg per month, and patients were permitted this dose thereafter if they had disease progression.2 The enrollment goal was 690 eligible patients equally assigned to each of the two groups, with randomization stratified according to adjuvant tamoxifen use.
Statistical Analysis
The primary end point was progression-free survival, which was defined as the time from randomization to progression or death from any cause. Secondary end points included overall survival and safety. The primary statistical analysis was an intention-to-treat analysis that used stratified log-rank tests, followed by Cox regression to estimate the hazard ratio and 95% confidence interval.
The trial had 90% power at a two-sided alpha level of 0.05 to detect hazard ratios consistent with an expected median progression-free survival of 10 months in the monotherapy group, as compared with 13 months in the combination-therapy group, and with an expected median overall survival of 36 months and 48 months, respectively. Subgroup comparisons were conducted within the prespecified stratification factor (adjuvant tamoxifen therapy). Post hoc subgroup analyses were also conducted. There was no prespecified plan for adjustment for multiple comparisons. P values are reported for comparisons between the two intervention groups for the analyses of progression-free survival and overall survival. For the other analyses, point estimates and 95% confidence intervals are reported. The confidence intervals were not adjusted for multiple comparisons, and inferences drawn from them may not be reproducible.
Results
Patients
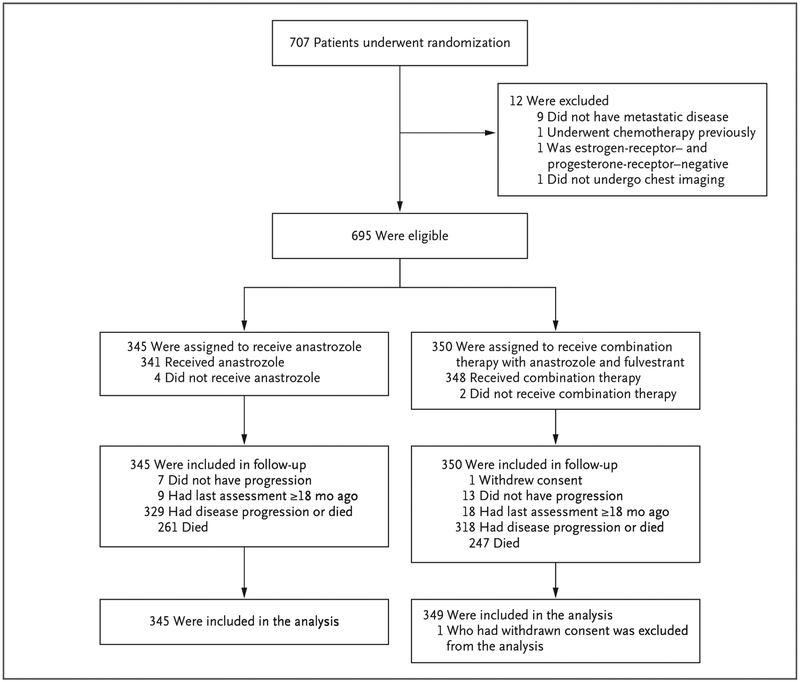
A total of 707 patients underwent randomization from June 2004 through June 2009 (Fig. 1). A total of 13 patients were excluded (12 ineligible patients and 1 who withdrew consent), leaving 694 patients who had data that could be analyzed.1 The median age of the patients was 65 years; 40% of the patients had received adjuvant tamoxifen previously, and 33% had received adjuvant chemotherapy previously. A total of 8% of the patients in the anastrozole-alone group and 10% of those in the combination-therapy group had cancer that was positive for human epidermal growth factor receptor 2 (HER2). Further information regarding the characteristics of the patients and their disease at baseline has been reported previously.1
Figure 1.
Enrollment, Randomization, and Follow-up of the Patients.
In the group that received anastrozole alone, 45% of the patients crossed over to receive fulvestrant (including at least 5 patients who received the 500-mg maintenance dose) at the time of progression. At least 9 of 349 patients in the combination-therapy group began receiving the 500-mg maintenance dose after progression (after February 2011, because of the approval of the higher-dose fulvestrant therapy by the Food and Drug Administration). (Because centers were not required to report switching from 250 mg to 500 mg, these numbers are underestimates.)
Updated Number of Events
In the additional 5 years of follow-up from the original report to the present report, the number of events of disease progression or death increased from 565 to 647, but the hazard ratios changed only slightly. The number of deaths increased from 330 to 508, but again the hazard ratios were little changed. Because of more deaths and thus improved power, the estimated hazard ratio became more certain, with a P value changing from 0.05 to 0.03, and allowed for the estimation of 5-year survival rates.
Progression-free Survival
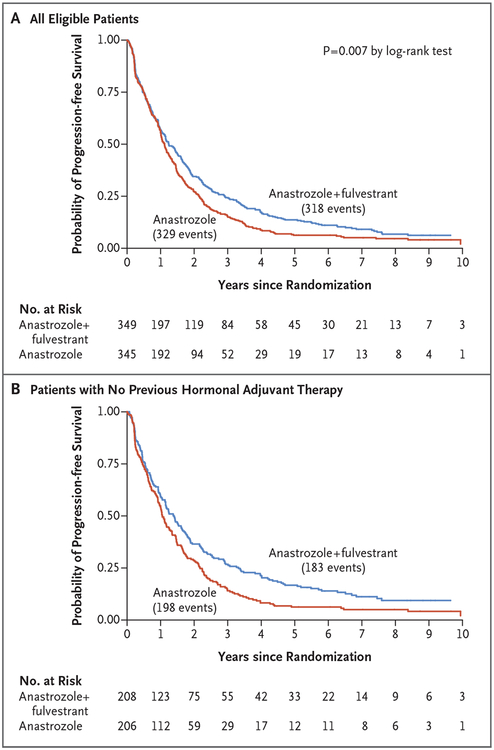
Updated outcomes regarding progression-free survival are presented in Figure 2. There were 647 events of disease progression or death (329 events in the anastrozole-alone group and 318 in the combination-therapy group) among 694 eligible patients (345 and 349 patients, respectively). The median follow-up among the patients who did not have disease progression was 7 years, with a maximum of 12 years. Overall, the median progression-free survival was 13.5 months in the anastrozole-alone group and 15.0 months in the combination-therapy group (hazard ratio for progression or death, 0.81; 95% CI, 0.69 to 0.94; stratified P = 0.007 by the log-rank test). In a subgroup analysis of the two strata, among women who had not received tamoxifen previously (414 [60%]), the median progression-free survival was 12.7 months in the anastrozole-alone group, as compared with 16.7 months in the combination-therapy group (hazard ratio, 0.73; 95% CI, 0.60 to 0.89); among women with previous exposure to adjuvant tamoxifen (280 [40%]), the median progression-free survival was similar in the two groups (13.9 months and 13.6 months, respectively; hazard ratio, 0.93; 95% CI, 0.73 to 1.19).
Figure 2. Kaplan–Meier Curves for Progression-free Survival, According to Trial Group.
Curves are shown for the overall trial population (Panel A) as well as for the subgroup of patients who had not received adjuvant endocrine therapy previously (Panel B).
Overall Survival
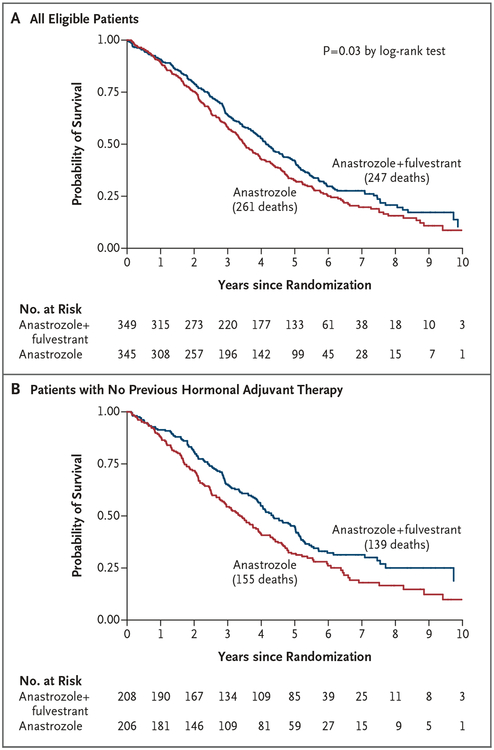
Table 1 and Figure 3 show the final outcomes regarding overall survival. Prolonged overall survival was seen in the group that received combination therapy: the median overall survival was 42.0 months in the anastrozole-alone group and 49.8 months in the combination-therapy group, on the basis of 261 and 247 deaths, respectively. The difference between the survival curves was significant (hazard ratio, 0.82; 95% CI, 0.69 to 0.98; P = 0.03 by the log-rank test).
Table 1.
Overall Survival with Anastrozole plus Fulvestrant, as Compared with Anastrozole Alone.*
| Variable | No. of Patients | No. of Deaths | Hazard Ratio for Death (95% CI) | P Value | Median Overall Survival | Patients Alive at 5 Yr (95% CI) |
|---|---|---|---|---|---|---|
| mo | % | |||||
| Total trial population | 694 | 0.82 (0.69–0.98) | 0.03 | |||
| Anastrozole | 345 | 261 | 42.0 | 33 (28–38) | ||
| Anastrozole plus fulvestrant | 349 | 247 | 49.8 | 42 (37–47) | ||
| Previous endocrine therapy† | ||||||
| Yes | 280 | 0.97 (0.74–1.27) | — | |||
| Anastrozole | 139 | 106 | 43.5 | 34 (26–42) | ||
| Anastrozole plus fulvestrant | 141 | 108 | 48.2 | 38 (30–46) | ||
| No | 414 | 0.73 (0.58–0.92) | — | |||
| Anastrozole | 206 | 155 | 40.3 | 32 (25–38) | ||
| Anastrozole plus fulvestrant | 208 | 139 | 52.2 | 45 (38–51) | ||
Shown are the stratified analyses of overall survival with combination therapy (anastrozole plus fulvestrant) as compared with anastrozole alone. The median overall survival, the hazard ratios for death with 95% confidence intervals, and the stratified P values (calculated by the log-rank test) are shown for the overall trial population and for the two indicated subgroups that were based on previous receipt or nonreceipt of endocrine therapy. The percentages of patients alive at 5 years, along with 95% confidence intervals, are shown for the overall trial population and for the two indicated subgroups.
P = 0.09 for interaction in this subgroup analysis.
Figure 3. Kaplan–Meier Curves for Overall Survival, According to Trial Group.
Curves are shown for the overall trial population (Panel A) as well as for the subgroup of patients who had not received adjuvant endocrine therapy previously (Panel B).
In a subgroup analysis involving women who had not received tamoxifen previously, the median overall survival was 40.3 months in the anastrozole-alone group, as compared with 52.2 months in the combination-therapy group (hazard ratio, 0.73; 95% CI, 0.58 to 0.92); among women with previous exposure to adjuvant tamoxifen, the median overall survival was 43.5 months and 48.2 months, respectively (hazard ratio, 0.97; 95% CI, 0.74 to 1.27) (P = 0.09 for interaction). Patients in the group that received anastrozole alone who crossed over had postprogression survival that was similar to that among patients who received combination therapy (results not significant; data not shown).
Post Hoc Subgroup Analyses
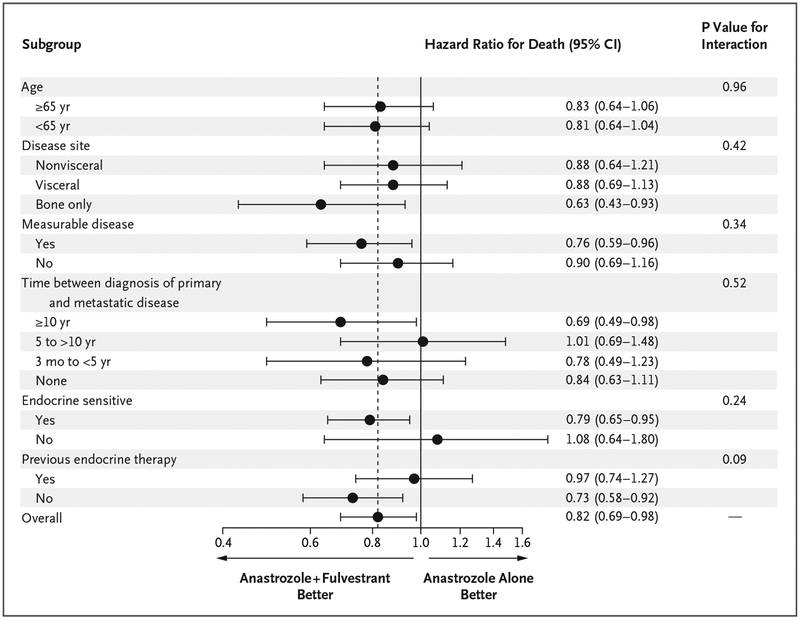
Additional post hoc subgroup analysis was performed to evaluate overall survival (Fig. 4). None of the P values for interaction were significant in any of the subgroup analyses. Patients who had been treated with tamoxifen were categorized according to whether there had been more than 6.5 years between the first diagnosis and trial enrollment or randomization or 6.5 years or less. The former group was combined with the population of patients who had not received endocrine therapy previously, and this population was designated as the endocrine-sensitive population; in contrast, the latter group was designated as the endocrine-refractory (acquired endocrine resistance) population. The cutoff point of 6.5 years was chosen to allow for 6 months of adjuvant chemotherapy, 5 years of adjuvant tamoxifen therapy, and a final 1-year tamoxifen-free period before relapse, given the standard definitions of endocrine-sensitive disease and endocrine-refractory disease.
Figure 4. Subgroup Analysis of Overall Survival.
Shown are the results of subgroup analyses of the treatment effect on overall survival. Hazard ratios for death in the group that received combination therapy with fulvestrant plus anastrozole, as compared with the group that received anastrozole alone, are shown along with 95% confidence intervals.
A total of 9% of the patients in the anastrozole-alone group and 12% in the combination- therapy group had disease that was resistant to endocrine therapy. In the endocrine-sensitive population, the median overall survival was 42.3 months (95% CI, 38.9 to 47.8) in the anastrozole-alone group and 50.7 months (95% CI, 46.6 to 58.3) in the combination-therapy group; in the endocrine-refractory population, the values were 39.2 months (95% CI, 30.2 to 50.0) and 35.1 months (95% CI, 26.8 to 50.1), respectively. In the endocrine-sensitive population, the hazard ratio for death was 0.79 (95% CI, 0.65 to 0.95), but it was 1.08 (95% CI, 0.65 to 1.80) in the endocrine-refractory population (P = 0.24 for interaction). In patients who had received the initial diagnosis more than 10 years before the first metastases, overall survival was 65.4 months with combination therapy and 49.7 months with anastrozole alone (hazard ratio, 0.69; 95% CI, 0.49 to 0.98). However, the P value for interaction was 0.52, indicating that a significant differential effect of the intervention according to the time from diagnosis to metastases was not shown. The hazard ratio for death in the analysis of overall survival generally favored combination therapy in all subgroups, including subgroups of patients with visceral metastases, those with nonvisceral metastases, and those with metastases only in bone.
Toxic Effects
Since the initial report, no additional toxic effects of grade 4 or 5 have been reported in the combination-therapy group. The previously reported toxic effects of grade 5 in this group included pulmonary emboli (in two patients) and a cerebrovascular ischemic event (in one patient). One patient in this group had grade 4 pulmonary emboli, and one had grade 4 neutropenia or lymphopenia. In the anastrozole-alone group, one additional patient had a grade 4 thromboembolism since the previous report. The previously reported grade 4 toxic effects in this group included thrombosis or embolism, arthralgia, thrombocytopenia, and dyspnea (in one patient each).
As of the data-cutoff date for the current report, toxic effects of grade 3 have occurred in 51 of 348 patients (15%) in the combination-therapy group and in 43 of 338 patients (13%) in the anastrozole-alone group (P = 0.47). These events included musculoskeletal pain, fatigue, hot flashes, mood alterations, and gastrointestinal symptoms, at frequencies of 1 to 4%. Few patients discon tinued treatment owing to adverse events or side effects (5 patients in the anastrozole-alone group and 12 in the combination-therapy group).
Discussion
In this trial, we found that combination therapy with anastrozole plus fulvestrant significantly prolonged, as compared with treatment with anastrozole alone, the primary and secondary end points of progression-free survival (P = 0.007) and long-term overall survival (P = 0.03) when used as first-line therapy for hormone-receptor–positive metastatic breast cancer in postmenopausal women. Furthermore, sequential therapy with anastrozole and fulvestrant (45% of patients crossed over to fulvestrant alone) did not negate the significance of the long-term overall survival benefit with the combination therapy as compared with anastrozole. Furthermore, this improvement was seen despite the use of a maintenance dose of fulvestrant (after the first-month loading dose) that was lower than the now-standard higher dose (i.e., 250 mg rather than 500 mg per month). The significant benefit with the combination therapy was observed despite longer progression-free survival and overall survival in the anastrozole-alone group than was projected at the start of the trial, with the results in the combination-therapy group even surpassing the projected survival in that group.
The absolute median prolongation in overall survival of 7.8 months was greater than the prolongation in progression-free survival of 1.5 months owing to late divergence of the progression-free survival curves after the median and early divergence of the overall survival curves before the median. However, the relative benefit with regard to overall survival is similar to the relative benefit in progression-free survival (hazard ratio for disease progression or death, 0.81; hazard ratio for death, 0.82). Postprogression survival was similar in the two groups, which reflects the finding that, despite crossover to fulvestrant in the anastrozole-alone group and the multiple lines of postprogression therapies typically administered in these patients, the progression-free survival benefit of up-front combination therapy resulted in prolonged overall survival. This additional benefit occurred in the absence of clinically significant between-group differences in the incidence of toxic effects of grade 3 to 5 or the discontinuation of treatment, even with long-term follow-up and despite a longer duration of combination therapy.
These results of our trial (S0226) are in contrast to the results of two similarly conducted prospective, randomized trials of single-agent aromatase inhibitors as compared with the combination of an aromatase inhibitor plus fulvestrant (the FACT [Fulvestrant and Anastrozole Combination at First Relapse Trial] and SoFEA [Study of Faslodex with or without Concomitant Arimidex vs. Exemestane Following Progression on Nonsteroidal Aromatase Inhibitors] trials).3,4 However, important differences distinguish the S0226 trial and these other trials. The FACT trial was smaller and included a more heterogeneous population that included both premenopausal and postmenopausal women as well as women with locally advanced and metastatic disease. Moreover, the requirement of first relapse for enrollment in the FACT trial excluded the untreated patients who had a first diagnosis of breast cancer with simultaneous metastasis and included a higher percentage of patients with previous exposure to antiestrogen therapy and thus a higher percentage of patients with recent exposure to antiestrogen therapy than were included in the current trial. Indeed, progression-free survival and overall survival in the FACT trial were shorter than in the S0226 trial. Moreover, because of chance alone, patients with liver metastasis who have a poor prognosis and patients with previous exposure to antiestrogen therapy were overrepresented in the combination-therapy group of the FACT trial. These factors — along with our observation that recent exposure to an antiestrogen, such as tamoxifen, predicts a lack of superiority of antiestrogen fulvestrant–containing therapy to therapy with an aromatase inhibitor alone — may explain the null results with combination therapy in the FACT trial. In support, the CONFIRM (Comparison of Faslodex in Recurrent or Metastatic Breast Cancer) trial showed that recent exposure to adjuvant tamoxifen therapy was associated with survival of just 2 years, even with high-dose fulvestrant.5
In the case of the SoFEA trial, in which the addition of fulvestrant to anastrozole therapy did not result in better outcomes than treatment with exemestane alone, only patients with acquired endocrine resistance (who had disease progression while they were receiving an aromatase inhibitor) were enrolled.4 The overall survival in the SoFEA trial was less than 2 years, which was as expected in the context of acquired endocrine resistance, and this survival level is much shorter than the overall survival in the S0226 trial (approximately 46 months). One would expect little benefit from any endocrine therapy in patients with acquired endocrine resistance.
In this regard, patients enrolled in the S0226 trial were more similar to those in two other trials, the FIRST (Fulvestrant First-Line Study Comparing Endocrine Treatments) and FALCON (Fulvestrant and Anastrozole Compared in Hormonal Therapy Naïve Advanced Breast Cancer) trials, in which patients with advanced breast cancer who had not had any previous exposure to endocrine therapy (77% of the patients in the FIRST trial and 100% of those in the FALCON trial) were randomly assigned to receive single-agent fulvestrant (at a dose of 500 mg per month) or anastrozole alone.6,7 Similar to the results of the S0226 trial, in the FIRST and FALCON trials, the benefit of fulvestrant therapy as compared with treatment with anastrozole alone was particularly compelling in the population of patients who had not received endocrine therapy previously. In addition, in the S0226 trial, patients who had more than 10 years between diagnosis and metastasis had the most benefit from the combination therapy regardless of previous tamoxifen use. Overall, the percentage of patients alive at 5 years was 42% with the combination therapy, as compared with 33% with anastrozole therapy in the trial that involved only patients with metastatic disease, and this result occurred despite the inclusion of some patients with a Zubrod’s performance-status score of 2, some with endocrine-refractory disease, and some with HER2-positive disease.
In the FALCON trial, which compared fulvestrant with anastrozole, patients with nonvisceral disease had a marked prolongation in progression-free survival, but similar findings were not observed with fulvestrant in patients with visceral disease.7 In contrast, in the S0226 trial, the two subgroups had a trend toward longer progression-free survival and overall survival with the combination therapy than with anastrozole alone, and the interaction test for differential benefit was not significant. The strategy of using anastrozole plus fulvestrant therapy may remain effective in the context of visceral metastasis, which is often an indication of a high-volume disease and probably multiple clones. Moreover, when we compare across trials, in the pure population of patients who had never received endo crine therapy, the hazard ratio in the group receiving fulvestrant-containing therapy, as compared with a common control group receiving anastrozole, was 0.73 (in both the analyses of progression-free and overall survival) in the S0226 trial, as compared with 0.80 (in the analysis of progression-free survival; overall survival not yet reported) in the FALCON trial.
It is also important to note from the aforementioned trials and the trials of molecularly targeted agents such as cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors and antiangiogenic agents such as bevacizumab in patients with hormone-receptor–positive breast cancer that populations of patients who had not received endocrine therapy previously or who had endocrine-sensitive disease represent a substantial portion of the populations in trials of first-line and subsequent endocrine therapy.6–16 Therefore, these are important considerations in cross-trial comparisons.6–16
In conclusion, at a maximum of 12 years of follow-up in patients without disease progression, the combination of the selective estrogen-receptor down-regulator fulvestrant and the aromatase inhibitor anastrozole, given as first-line endocrine therapy, resulted in superior long-term progression-free survival and overall survival, as compared with anastrozole alone, among postmenopausal women with hormone-receptor–positive metastatic breast cancer. The results suggest that the benefits were particularly notable in women who had not received endocrine therapy previously.
Acknowledgments
Supported by grants (CA180888, CA180819, CA180863, CA189808, CA180801, CA189952, CA189953, CA180858, CA46282, and CA13612) from the National Cancer Institute of the NIH and by AstraZeneca.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or AstraZeneca.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
References
- 1.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 2012; 367: 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 2010; 28: 4594–600. [DOI] [PubMed] [Google Scholar]
- 3.Bergh J, Jönsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 2012; 30: 1919–25. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 2013; 14: 989–98. [DOI] [PubMed] [Google Scholar]
- 5.Di Leo A, Jerusalem G, Torres R, et al. First-line vs second-line fulvestrant for hormone receptor-positive advanced breast cancer: a post-hoc analysis of the CONFIRM study. Breast 2018; 38: 144–9. [DOI] [PubMed] [Google Scholar]
- 6.Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol 2015; 33: 3781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016; 388: 2997–3005. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 9.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–48. [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–36. [DOI] [PubMed] [Google Scholar]
- 11.Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–46. [DOI] [PubMed] [Google Scholar]
- 12.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–15. [DOI] [PubMed] [Google Scholar]
- 13.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018; 36: 2465–72. [DOI] [PubMed] [Google Scholar]
- 14.Dickler MN, Barry WT, Cirrincione CT, et al. Phase III trial evaluating letrozole as first-line endocrine therapy with or without bevacizumab for the treatment of postmenopausal women with hormone receptor-positive advanced-stage breast cancer: CALGB 40503 (Alliance). J Clin Oncol 2016; 34: 2602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martín M, Loibl S, von Minckwitz G, et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the Letrozole/Fulvestrant and Avastin (LEA) study. J Clin Oncol 2015; 33: 1045–52. [DOI] [PubMed] [Google Scholar]
- 16.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018; 379: 1926–36. [DOI] [PubMed] [Google Scholar]