Abstract
Mycoplasma infection is the most prevalent contamination in cell culture. Analysis of cell culture in laboratories from different countries shows that mycoplasma contamination ranges from 15% to 80% and, in some cases, even reaches 100% (Chernov et al., 2014). Whilst mycoplasma infection is not visible to the naked eye in cell culture, the consequences of mycoplasma contamination have been shown to induce a number of cellular changes, for example, increased resistance to chemotherapeutic drugs. Therefore, any results obtained from tissue culture studies, in the presence of mycoplasma contamination, potentially render the data invalid (Kim et al., 2015; Gedye et al., 2016). As such, mycoplasmas are not harmless bystanders and cannot be ignored in in vitro studies.
Keywords: Mycoplasma, Contamination, Transfection efficiency, HEK-293 cell, Cell culture
Mycoplasma infection is the most prevalent contamination in cell culture. Analysis of cell culture in laboratories from different countries shows that mycoplasma contamination ranges from 15% to 80% and, in some cases, even reaches 100% (Chernov et al., 2014). Whilst mycoplasma infection is not visible to the naked eye in cell culture, the consequences of mycoplasma contamination have been shown to induce a number of cellular changes, for example, increased resistance to chemotherapeutic drugs. Therefore, any results obtained from tissue culture studies, in the presence of mycoplasma contamination, potentially render the data invalid (Kim et al., 2015; Gedye et al., 2016). As such, mycoplasmas are not harmless bystanders and cannot be ignored in in vitro studies.
Human embryonic kidney 293 (HEK-293) cells are frequently used in the laboratory because of their reliable growth and propensity for transfection. They are utilized as an efficient factory to produce viral vectors such as recombinant adeno-associated virus (rAAV) and bioactive proteins following plasmid DNA transfection (Wang et al., 2014; Zhang et al., 2014; Lin et al., 2017; Strobel et al., 2019). Consequently, they are often cultured in biological research laboratories as well as biotechnology companies. However, the effect of mycoplasma contamination on plasmid DNA transfection efficiency has rarely been reported. Hence, the aim of this study is to identify whether mycoplasma contamination has any effect on plasmid DNA transfection efficiency in HEK-293 cells.
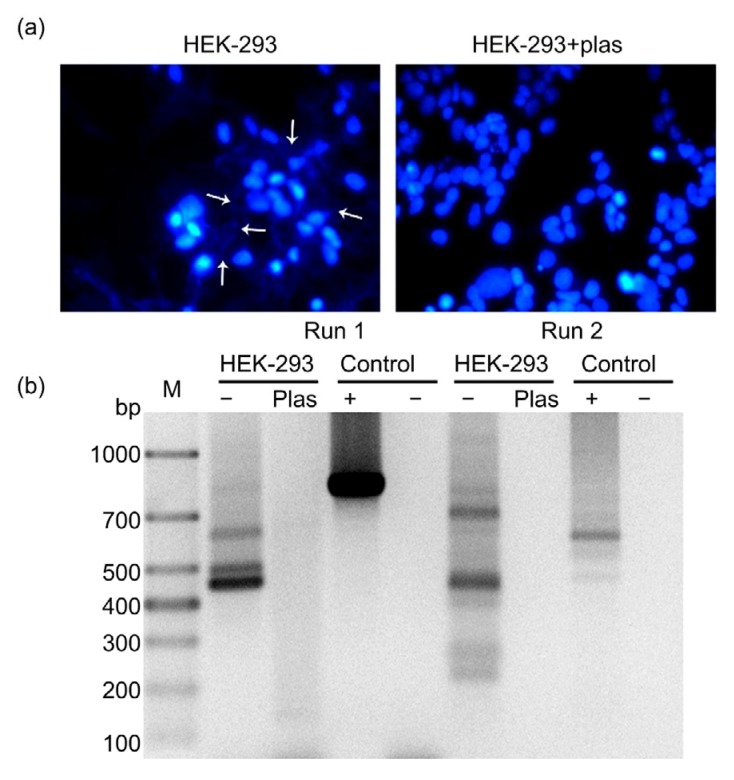
In order to detect mycoplasma contamination in HEK-293 cells, 4',6-diamidino-2-phenylindole (DAPI; 1 μg/mL; Sigma, D-8417, Germany) staining was used. As shown in Fig. 1a, the nucleus was stained blue, and abundant blue fluorescence was clearly observed out of nucleus in the cytoplasmic compartment, indicating mycoplasma contamination. Additionally, the presence of mycoplasma genome was assessed by polymerase chain reaction (PCR) using the Mycoplasma Detection Kit (TaKaRa, Japan) in the presence of appropriate controls to eliminate the possibility of false-negative and false-positive results. As illustrated in Fig. 1b, PCR products were present in the positive control lanes with a size of 810 bp in the first run and 590 bp in the second run. The absence of bands in the negative control (H2O) indicated the accuracy of this experiment. PCR analysis of mycoplasma-contaminated cells led to the generation of PCR products ranging in size from 400 to 700 bp in the first run and from 200 to 300 bp in the second run.
Fig. 1.
Detection of mycoplasma contamination and its eradication by Plasmocin™ in HEK-293 cells
The original HEK-293 cells, as well as HEK-293 cells treated with anti-mycoplasma treatment, Plasmocin™, for two weeks, were analyzed for mycoplasma contamination by DAPI staining (a) and PCR (b). Plas, Plasmocin™; M: mark
Several methods have been employed to eliminate mycoplasma including physical (e.g., autoclaving), chemical (e.g., treating with detergent), immunological (e.g., in vivo passage of cells in nude mice), and chemotherapeutical (e.g., antibiotic) procedures. In this study, antibiotic treatment was used, which is the simplest and the most effective approach (Uphoff and Drexler, 2014). Plasmocin™ has been frequently utilized by researchers due to its outstanding performance in eliminating mycoplasma (Uphoff et al., 2012; Boslett et al., 2014). Therefore, we applied Plasmocin™ to HEK-293 cells to eliminate the mycoplasma contamination. Following the administration of Plasmocin™ (25 μg/mL; InvivoGen, France) for two weeks, the extra-nuclear punctate pattern of blue fluorescence in HEK-293 could no longer be observed (Fig. 1a). Additionally, mycoplasma-specific PCR products were barely detectable in samples generated from Plasmocin™-treated cells (Fig. 1b), confirming the successful elimination of mycoplasma in these cells.
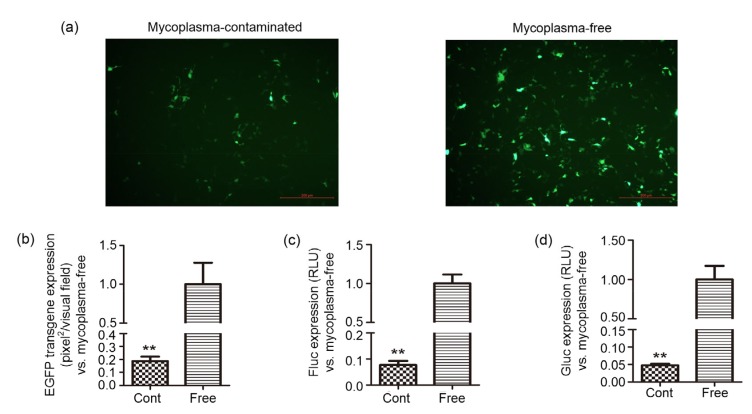
To determine the effect of mycoplasma contamination on plasmid DNA transfection efficiency, three plasmids (pdsAAV-CBAp-EGFP, pssAAV-CBAp-Fluc-EYFP, and pdsAAV-CBAp-Gluc) that are frequently used in packaging rAAV vectors were tested. These plasmids were added to mycoplasma-contaminated and Plasmocin™-treated (confirmed as mycoplasma-free) HEK-293 cells using the standard protocol for the common transfection reagent, polyethylenimine (PEI). Transfection efficiency was initially assessed by fluorescence microscopy. Fig. 2a showed that the number of visible green dots was lower, and the intensity of green fluorescence in mycoplasma-infected cells was apparently weaker than that in mycoplasma-eradicated cells. Further analysis indicated that the area of green fluorescence in mycoplasma-contaminated cells was approximately 20% of that observed in mycoplasma-free cells (Fig. 2b). Consistent with the findings with the enhanced green fluorescent protein (EGFP)-expressing plasmid, the levels of the two luciferases (firefly luciferase (Fluc) and Gaussia luciferase (Gluc)) were significantly decreased in mycoplasma-infected cells, with only 6% Fluc and 5% Gluc of those observed in mycoplasma-eradicated HEK-293 cells (Figs. 2c and 2d). Therefore, the protein expression post-transfection in mycoplasma-contaminated cells was all dramatically lower than that in mycoplasma-eradicated cells. These data suggest that the attenuated transfection efficiency of plasmid DNA can be attributed to mycoplasma infection in HEK-293 cells.
Fig. 2.
Effect of mycoplasma contamination on the transfection efficiency of plasmid DNA
Mycoplasma-contaminated and mycoplasma-free HEK-293 cells were seeded, followed by transfection with the plasmids pdsAAV-CBAp-EGFP, pssAAV-CBAp-Fluc-EYFP, and pdsAAV-CBAp-Gluc encoding different reporter genes. Twenty-four hours post-transfection the efficiency of expression of these genes was analyzed. For pdsAAV-CBAp-EGFP, cells were observed under the fluorescence microscope (scale bar=200 µm) (a), and the pixels of green fluorescence were calculated (b). The levels of Fluc (c) and Gluc (d) were measured by a spectral scanning reader after adding their specific substrates. Data are expressed as mean±standard deviation (n=3). **P<0.01, vs. mycoplasma-free cells. RLU, relative luciferase unit; Cont, contaminated
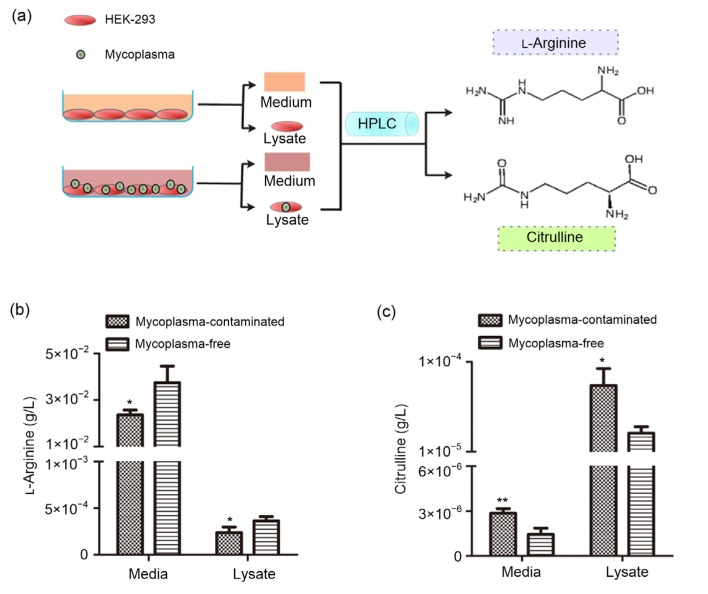
L-Arginine deprivation is a proven consequence of mycoplasma contamination in tissue culture cells. The microbial enzyme, arginine deiminase (ADI), is abundant in mycoplasma and has a high affinity for L-arginine and catalyzes L-arginine to citrulline (Riess et al., 2018). Because of this particular characteristic of mycoplasma, L-arginine consumption and citrulline accumulation has been confirmed as an indicator of mycoplasma infection in cultured cells in vitro (Capiaumont et al., 1995). Therefore, we collected the cell medium and lysate from mycoplasma-contaminated and mycoplasma-eradicated cells and measured the concentrations of L-arginine and citrulline by high-performance liquid chromatography (HPLC) (Fig. 3a). The results showed that in mycoplasma-infected HEK-293 cells, the L-arginine levels in cell medium and lysate fell significantly by approximately 30% (Fig. 3b). Conversely, the citrulline in cell medium and lysate was remarkably increased by 1-and 2-fold, respectively (Fig. 3c).
Fig. 3.
Identification of L-arginine consumption and citrulline accumulation in mycoplasma-contaminated HEK-293 cells
Schematic representation (a) of measuring the concentrations of L-arginine (b) and citrulline (c) in both cell media and lysates from mycoplasma-contaminated and mycoplasma-free HEK-293 cells. Data are expressed as mean±standard deviation (n=3). * P<0.05, ** P<0.01, vs. mycoplasma-free cells
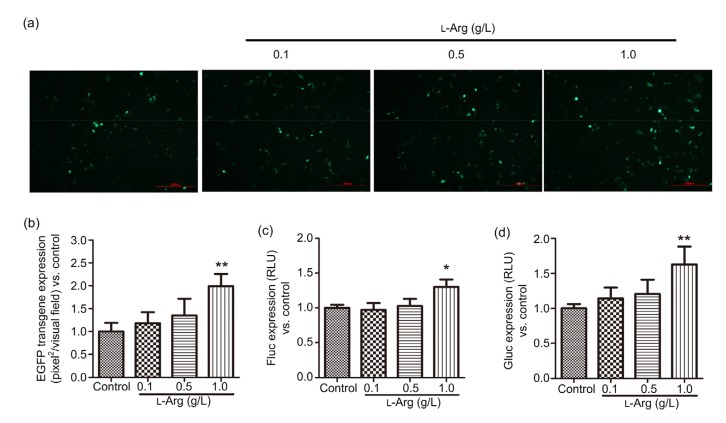
Next, we evaluated whether L-arginine supplementation in mycoplasma-contaminated HEK-293 cells could improve the transfection efficiency. To this end, we added L-arginine (24 h pre-and post-transfection) at concentrations ranging from 0.1 to 1.0 g/L, which showed no toxicity on cell viability (Fig. S1). As shown in Fig. 4a, when mycoplasma-contaminated cells were transfected with a plasmid driving the expression of EGFP, more green dots were observed in L-arginine-treated cells. Additionally, L-arginine (1.0 g/L) significantly increased the green fluorescence by almost one-fold (Fig. 4b). Consistent with the results obtained with plasmid pdsAAV-CBAp-EGFP, when mycoplasma-positive cells were transfected with plasmids driving the expression of luciferase, the Fluc (Fig. 4c) and Gluc (Fig. 4d) levels in the 1.0 g/L L-arginine-treated cells were slightly augmented by 30% and 60%, respectively. When mycoplasma-contaminated HEK-293 cells were treated with 1.0 g/L L-arginine, which is almost 10-fold higher than that in conventional Dulbecco’s modified Eagle medium (DMEM; 84 mg/L), the transgene expression was elevated by less than one-fold. These data, therefore, suggest that L-arginine supplementation was less effective than Plasmocin™ to enhance transfection efficiency in mycoplasma-infected cells.
Fig. 4.
Effect of L-arginine supplementation on plasmid DNA transfection efficiency in mycoplasma-contaminated HEK-293 cells
Mycoplasma-contaminated HEK-293 cells were transfected with plasmids pdsAAV-CBAp-EGFP, pssAAV-CBAp-Fluc-EYFP, and pdsAAV-CBAp-Gluc. Twenty-four hours pre-and post-transfection, cells were administrated with L-arginine (0.1, 0.5, and 1.0 g/L). The transfection efficiencies of these reporter genes were analyzed 24 h post-transfection by measuring the transgene expression. For pdsAAV-CBAp-EGFP, cells were observed under the fluorescence microscope (scale bar=200 µm) (a) and the pixels of green fluorescence were calculated (b). The levels of Fluc (c) and Gluc (d) were measured using a spectral scanning reader. Data are expressed as mean±standard deviation (n=3). * P<0.05, ** P<0.01, vs. control cells. L-Arg, L-arginine; RLU, relative luciferase unit
Collectively, our results highlight that mycoplasma, a notorious cell culture contaminant, can remarkably diminish plasmid DNA transfection efficiency in HEK-293 cells. Mycoplasma contamination, as well as the resulting poor plasmid DNA transfection efficiency, can be effectively reversed by Plasmocin™, a commercially available anti-mycoplasma reagent. This catastrophic effect of poor transfection efficiency is related to L-arginine deprivation in mycoplasma-contaminated cells, but L-arginine supplementation is not a valid strategy to boost transfection efficiency (when measured by transgene expression). Thus, for researchers who utilize HEK-293 cells to produce therapeutic proteins and viruses based on plasmid DNA transfection, we highly recommend the regular and necessary monitoring for the presence of mycoplasma, as well as the prompt and adequate elimination by anti-mycoplasma treatment if necessary, in order to sustain stable and efficient plasmid DNA transfection efficiency.
List of electronic supplementary materials
Cytotoxicity of L-arginine in HEK-293 cells
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81803929) and the Changhai Hospital Fund for Young Scholars (No. CH201810), China
Contributors: Zi-fei YIN, Ya-ni ZHANG, Juan DU, and Bin-bin CHENG designed this study. Zi-fei YIN and Ya-ni ZHANG performed the experiments. Shu-fang LIANG and Sha-sha ZHAO contributed to data analysis. Zi-fei YIN wrote the manuscript, while Juan DU and Bin-bin CHENG edited the manuscript. All authors read and approved the final manuscript. Therefore, all authors had full access to all the data in the study and take responsibility for the integrity and security of the data.
Electronic supplementary materials: The online version of this article (https://doi.org/10.1631/jzus.B1900380) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Zi-fei YIN, Ya-ni ZHANG, Shu-fang LIANG, Sha-sha ZHAO, Juan DU, and Bin-bin CHENG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Boslett B, Nag S, Resnick A. Detection and antibiotic treatment of Mycoplasma arginini contamination in a mouse epithelial cell line restore normal cell physiology. Biomed Res Int, 2014:532105. 2014 doi: 10.1155/2014/532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capiaumont J, Legrand C, Dousset B, et al. Arginine consumption as a monitor of mycoplasma infection of cultured cells. In Vitro Cell Dev Biol Anim. 1995;31(7):497–498. doi: 10.1007/bf02634026. [DOI] [PubMed] [Google Scholar]
- 3.Chernov VM, Chernova OA, Sanchez-Vega JT, et al. Mycoplasma contamination of cell cultures: vesicular traffic in bacteria and control over infectious agents. Acta Naturae. 2014;6(3):41–51. [PMC free article] [PubMed] [Google Scholar]
- 4.Gedye C, Cardwell T, Dimopoulos N, et al. Mycoplasma infection alters cancer stem cell properties in vitro. Stem Cell Rev. 2016;12(1):156–161. doi: 10.1007/s12015-015-9630-8. [DOI] [PubMed] [Google Scholar]
- 5.Kim BC, Kim SY, Kwon YD, et al. Mycoplasma detection and elimination are necessary for the application of stem cell from human dental apical papilla to tissue engineering and regenerative medicine. Biomater Res, 19:6. 2015 doi: 10.1186/s40824-015-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin B, Peng GQ, Feng HP, et al. Purification and characterization of a bioactive alpha-fetoprotein produced by HEK-293 cells. Protein Expr Purif. 2017;136:1–6. doi: 10.1016/j.pep.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Riess C, Shokraie F, Classen CF, et al. Arginine-depleting enzymes–an increasingly recognized treatment strategy for therapy-refractory malignancies. Cell Physiol Biochem. 2018;51(2):854–870. doi: 10.1159/000495382. [DOI] [PubMed] [Google Scholar]
- 8.Strobel B, Zuckschwerdt K, Zimmermann G, et al. Standardized, scalable, and timely flexible adeno-associated virus vector production using frozen high-density HEK-293 cell stocks and CELLdiscs. Hum Gene Ther Methods. 2019;30(1):23–33. doi: 10.1089/hgtb.2018.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uphoff CC, Drexler HG. Eradication of mycoplasma contaminations from cell cultures. Curr Protoc Mol Biol. 2014;106:28.5.1–28512. doi: 10.1002/0471142727.mb2805s106. [DOI] [PubMed] [Google Scholar]
- 10.Uphoff CC, Denkmann SA, Drexler HG. Treatment of mycoplasma contamination in cell cultures with Plasmocin. J Biomed Biotechnol, 2012:267678. 2012 doi: 10.1155/2012/267678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LN, Wang Y, Lu Y, et al. Pristimerin enhances recombinant adeno-associated virus vector-mediated transgene expression in human cell lines in vitro and murine hepatocytes in vivo. J Integr Med. 2014;12(1):20–34. doi: 10.1016/s2095-4964(14)60003-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YH, Wang Y, Yusufali AH, et al. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med. 2014;12(6):483–494. doi: 10.1016/s2095-4964(14)60057-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cytotoxicity of L-arginine in HEK-293 cells