Abstract
Background
Metastatic or recurrent thyroid cancer often behaves aggressively, and approximately two‐thirds of patients present with radioiodine resistance. Effective therapies to suppress thyroid cancer metastasis are urgently needed. Nevirapine has been proved to suppress tumor growth and induce differentiation in several tumor cells, but has not previously been evaluated in metastasis of thyroid cancer. The present study aimed to investigate the effect of nevirapine on migration and invasion in dedifferentiated thyroid cancer cells.
Methods
Human dedifferentiated thyroid cancer cell line (WRO 82‐1) was subject to real‐time quantitative PCR, western blot and transwell migration/invasion assays. The liver metastasis in tumor xenografts of nude mice was subject to hematoxylin‐eosin (HE) staining.
Results
Nevirapine significantly repressed cell migration and invasion in WRO 82‐1 cells, and surprisingly significantly decreased liver metastatic tumor in the nude mouse model of dedifferentiated thyroid cancer compared with that of the control. Moreover, nevirapine significantly decreased the expression of IL‐6 mRNA and phosphorylation of JAK2 (Y1007+Y1008) and STAT3 (Tyr 705) in WRO 82‐1 cells compared with those in control cells.
Conclusion
Our findings suggest that nevirapine significantly repressed migration and invasion/metastasis in WRO 82‐1 cells and tumor xenografts, which may be related to inhibition of IL‐6/STAT3 signaling pathway. It promises great potential as a novel therapy for thyroid cancer, especially for those patients with metastasis.
Keywords: Dedifferentiated thyroid cancer, IL‐6/STAT3 signaling pathway, invasion, migration, nevirapine
Introduction
Thyroid cancer is one of the fastest growing cancers worldwide.1, 2 It has two outstanding features. The first is the high rate of metastasis to lymph nodes or other organs, which is as high as 30% to 59.1%.1, 3, 4, 5 The second is the resistance to chemotherapy and conventional external beam radiotherapy.6, 7 Metastatic thyroid cancer behaves aggressively and about two‐thirds of patients with metastatic disease ultimately present with radioiodine refractory disease.8, 9 Once it metastasizes to other organs it is hard to treat and therefore it is important to exploit new therapeutic agents which are effective in the treatment of metastatic thyroid cancer.
Nevirapine, the first non‐nucleoside reverse transcriptase inhibitor approved by the US Food and Drug Administration (FDA) in 1996, is an important component of highly active antiretroviral therapy widely prescribed for human immunodeficiency virus‐1 (HIV‐1) infection.10, 11, 12 Recently, accumulating findings have shown that nevirapine acts as a cytostatic and differentiating agent by modulating gene expression in progenitor cells and several human cancers, such as melanoma, prostate cancer, lung cancer, colon cancer, acute myeloid leukemia (AML) and so on.13, 14, 15, 16, 17 In our previous study, nevirapine promoted the mRNA expression of sodium iodide symporter (NIS) in anaplastic thyroid cancer. However, there has not previously been any study published on the effects of nevirapine on cell migration and invasion/metastasis in thyroid cancer.
Tumor migration and invasion/metastasis is a complicated process and is often correlated with extracellular matrix hydrolysis, a process mediated by several proteolytic enzymes, including MMPs which are proteinases involved in the migration and invasion of malignant cells.18 There are more than 20 MMPs involved, among these MMPs, MMP2 and MMP9 are pivotal for the invasion and metastasis of cancers.19, 20, 21
The role of the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) pathway is a well‐known mediator of tumorigenesis in different tumors, and is associated with migration and invasion of cancers by increasing expression of MMP2 and MMP9.22, 23, 24 Phosphorylation of STAT3 on Tyr705 is the major mechanism of STAT3 activation, which is mediated by JAK upon stimulation of the heterodimeric gp130/cytokine‐specific receptor complex by the IL‐6 family of cytokines, including IL‐6.25
The present study was undertaken to explore the effects of nevirapine on cell migration and invasion/metastasis and the underlying mechanism in WRO 82‐1 cells, which was derived from a metastatic lesion of a patient with follicular thyroid carcinoma26, 27, 28 and in athymic mouse xenografts.
Methods
Cell culture
The human dedifferentiated thyroid carcinoma cell WRO 82‐1 was purchased from Sigma‐Aldrich (Munich, Germany) and cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) at 37°C in a humidified atmosphere with 5% CO2. Nevirapine (purity, ≥99%) was purchased from the National Institute of Control of Pharmaceutical and Biological Products in China, dissolved in dimethyl sulfoxide (DMSO) to prepare a 250 mM stock solution and then diluted in medium immediately before use. The cells were treated with various concentrations of nevirapine (100 and 200 μM) prior to being subjected to various assays. In this study, negative control cells (0 μM) were treated with culture medium containing 0.1% DMSO. Incubation was carried out continuously for 72 hours.
Transwell migration assay
WRO 82‐1 cells were treated with 200 μM nevirapine for 72 hours. The migration assay was carried out according to the manufacturer's protocol. Briefly, migration chambers (Corning, USA) were placed in a 24‐well plate, then 200 μL of WRO 82‐1 cells (2.5 × 105/mL in serum‐free medium) were seeded on each transwell filter with 8 μM porosity and 750 μL culture media with 10% FBS added in the lower chamber. The cells were then incubated at 37°C in a humidified atmosphere with 5% CO2 for 24 hours, washed twice in PBS, fixed with 4% formaldehyde at room temperature for 15 minutes, permeabilized by 100% methanol at room temperature for 20 minutes, and then stained with crystal violet for 20 minutes at room temperature. The nonmigrated cells were scraped off with cotton swabs. Cells that had migrated through the pores were counted in five randomly selected fields under a light microscope at 20× objective magnification. The experiments were performed in triplicate.
Transwell invasion assay
Cell invasion was assessed using transwell cell culture chambers, according to the manufacturer's protocol. We used 24‐well cell culture inserts (Corning, USA) with a polyethylene terephthalate membrane (8 μM pores). The membranes of each upper chamber were coated with Matrigel (100 μg/cm2; BD) and then incubated at 37°C overnight for gelling. Before each assay, the WRO 82‐1 cells were treated with nevirapine at the indicated concentration for 72 hours. For cell invasion assay, 200 μL of WRO 82‐1 cells (5 × 105/mL in serum‐free medium) were seeded in the upper chamber in serum‐free medium, and 750 μL of media supplemented with 10% FBS was added to the lower chamber. The cells were then incubated at 37°C for 48 hours, washed twice with PBS, fixed with 3.7% formaldehyde at room temperature for two minutes, permeabilized by 100% methanol at room temperature for 20 minutes, and then stained with crystal violet for 15 minutes at room temperature. The cells that adhered to the upper surface of the chamber were carefully removed using cotton swabs, and those on the bottom surface of the membrane were imaged, after which cells in five randomly selected fields were counted under a light microscope (Leica, Germany) at 20× objective magnification. Experiments were performed in triplicate.
Real‐time quantitative PCR
Total RNA was extracted using TriZol reagent (TaKaRa, Japan), according to the manufacturer's instructions, and then subjected to reverse transcription to synthesize cDNA using the First Strand cDNA Synthesis Kit (TaKaRa, Japan). Quantitative real‐time PCR was performed with SYBR Green (GenStar, China) with an ABI PRISM 7500 Real‐time PCR System (Applied Biosystems, USA). Target gene expression levels were calculated using ΔΔCt and comparative methods after being normalized to GAPDH expression levels. The primer sequences were as follows: IL‐6 forward 5′‐ATG TAG CCG CCC CAC ACA GA‐3′, reverse 5′‐GCA TCC ATC TTT TTC AGC CAT C‐3′; VEGFA forward 5′‐AGG GCA GAA TCA TCA CGA AGT‐3′, reverse 5′‐AGG GTC TCG ATT GGA TGG CA‐3′; MMP‐2 forward 5′‐ CTG GGA GCA TGG CGA TGG ATA‐3′, reverse 5′‐ GGA AGC GGA ATG GAA ACT TG‐3′; MMP‐9 forward 5′‐ GCC ATG TCT GCT GTT TTC TAG AGG‐3′, reverse 5′‐ CAC ACT CCA GGC TCT GTC CTC TTT‐3′; and GAPDH forward 5′‐CAG AAC ATC ATC CCT GCC TCT AC‐3′, reverse 5′‐ TTG AAG TCA GAG GAG ACC ACC TG‐3′.
Western blot analysis
The cells were washed with PBS after being treated with nevirapine for 72 hours, and then lysed with RIPA buffer containing protease inhibitors cocktail 1× protease inhibitor cocktail (100:1). The lysate was centrifuged at 12 000 rpm for 20 minutes to remove debris. The supernatant was diluted with 5× loading buffer (4:1) and denatured in a boiling water bath (95°C) for 5 minutes, then preserved at −80°C. Protein concentrations were estimated by BCA assay. The 30 μg of protein extract was separated by SDS‐PAGE and then transferred to PVDF membranes, which were treated with 5% nonfat dry milk for one hour at room temperature. The membranes were later probed with the following primary antibodies overnight at 4°C, according to the manufacturer's protocol: rabbit polyclonal anti‐MMP2 (1:500, Affinity #AF0577, USA), rabbit monoclonal anti‐MMP9 (1:1000, Abcam #ab38898, UK), rabbit monoclonal anti‐JAK2 (1:2000, Abcam #ab108596), rabbit monoclonal anti‐pJAK2 (Y1007+Y1008) (1:1000, Abcam #ab32101), rabbit monoclonal anti‐pSTAT3 (Tyr705) (1:1000, Abcam #ab76315,UK), rabbit monoclonal anti‐STAT3 (1:2000, Abcam #ab68153, UK), VEGF (1:500, Affinity #AF5131, USA) and rabbit polyclonal anti‐GAPDH (1:5000, Proteintech #10494‐1‐AP, China). The membranes were then washed and incubated with HRP‐conjugated secondary anti‐rabbit IgG antibody (1:10000, Proteintech #SA00001‐2, China) for one hour at room temperature, after which the blots were detected by an enhanced ECL chemiluminescence system (Millipore, USA). The quantification of band intensity was performed using ImageJ software (Rawak Software, Germany).
Tumor xenografts and animal treatment
A total of 10 four‐week‐old BALB/c (nu/nu) male nude mice (Vital River, China) were randomly assigned to two groups, kept in accordance with Chinese community guidelines. Mice were inoculated subcutaneously with WRO 82‐1 cells (1 × 106/mouse) suspended in 300 μL PBS. Mice in the treated group were treated orally five days a week with nevirapine (150 mg/kg/day) in DMSO freshly diluted 1:4 with physiological solution, and the negative control mice were treated with vehicle (physiological solution with 20% DMSO). Treatment was started 10 days after tumor implant (tumor xenograft larger than 5 mm). Three weeks after tumor implant, animals were sacrificed and their tumors were dissected. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed and approved by the Ethics Committee of Qianfoshan Hospital, Shandong University.
Hematoxylin‐eosin (HE) staining
The tissues were immersion‐fixed in 4% paraformaldehyde for 24 hours, embedded in paraffin, and cut into 4 μM slices. After deparaffinization and rehydration, the sections were stained with HE staining to observe the morphology by microscope (Leica, Heidelberg, Germany).
Statistical analysis
All data were expressed as mean ± SD and analyzed with GraphPad Prism 5 (GraphPad Software, California, USA). Student's t‐test was used to evaluate the statistical difference between groups in SPSS 20.0 software (SPSS, Inc., Chicago, USA). A P‐value of <0.05 was considered statistically significant.
Results
Nevirapine significantly reduced metastasis in the liver of athymic mice
After inoculation of WRO 82‐1 cells in BALB/c nude mice, the animals received nevirapine treatment. Three weeks after treatment, we were surprised to see that the tumor metastasized to liver (Fig 1b) was reduced significantly compared with that of the control (Fig 1a), and the larger metastases were accompanied by necrosis in the liver of the control group. To further confirm the above observation, TTF‐1 and TG, two thyroid‐specific indicators were detected by immunohistochemistry, but the results were negative which may be related to the less expression of both proteins in dedifferentiated thyroid cancer. Subsequently, in order to further confirm whether metastases were derived from dedifferentiated thyroid cancer, HE staining, to observe cell morphology, was performed. The result in Figure 1c presented the spindle cells accompanied by a large number of pathological mitotic figures (arrow) and atypical giant cells (circle), which are the pathological features of dedifferentiated thyroid cancer. From the above we can see that dedifferentiated thyroid cancer metastasized to the liver and nevirapine inhibited the metastasis. In addition, it should be noted that although thyroid cancer is most likely to metastasize to the lungs, we did not find metastases by visual inspection and HE staining.
Figure 1.
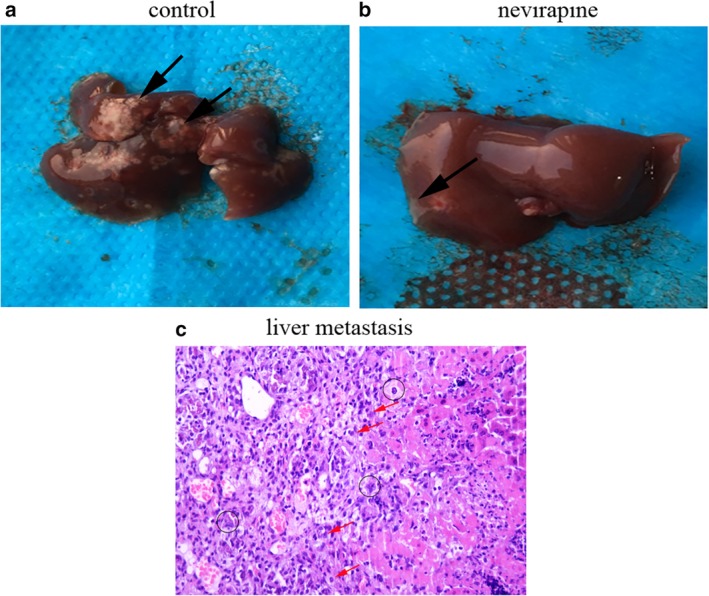
Nevirapine suppresses thyroid cancer cell metastasis in vivo. (a) The livers of control mice and (b) nevirapine‐treated mice were inspected for metastases. (c) The cell morphology was observed by HE staining. Thyroid cancer metastases are indicated by arrows and circles.
To further explore the mechanism, we conducted an in vitro study with the dedifferentiated thyroid cancer cell line WRO 82‐1.
Nevirapine inhibited the migration of WRO 82‐1 cells
To evaluate the effect of nevirapine on the migration of dedifferentiated thyroid cancer cells, we performed transwell migration assay. WRO 82‐1 cells were cultured and then incubated with different doses (0, 100 and 200 μM) of nevirapine for 72 hours. Treatment with various doses of nevirapine for 72 hours significantly decreased the migration rates of cells. As shown in Figure 2, migration rates of WRO 82‐1 cells receiving 200 μM nevirapine were significantly decreased to 34.38 ± 2.35% compared with the cells without nevirapine with statistical significance (P < 0.001). These data showed that nevirapine inhibited cell migration in the dedifferentiated cell line.
Figure 2.
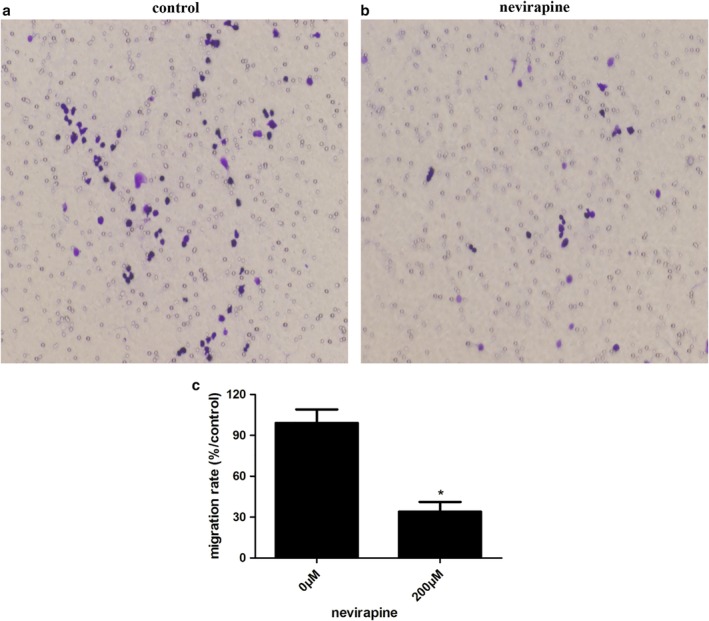
Nevirapine inhibited WRO 82‐1 cell migration. (a) Cell migration was detected by transwell migration assay. (b) The percentage of migration cells is shown as the mean ± SD of five replicate experiments. *P < 0.05.
Nevirapine inhibited the invasion of WRO 82‐1 cells
The capacity of cell invasion was performed with a Matrigel‐coated filter. As shown in Figure 3, the invasion rates were significantly decreased to 31.47 ± 2.76% in WRO 82‐1 cell treated with 200 μM nevirapine for 72 hours compared with those of untreated cells, respectively. The results indicated that the ability of traversing Matrigel‐coated layer was decreased significantly (P < 0.001) in nevirapine‐treated WRO 82‐1 cells compared with that of untreated cells, suggesting that nevirapine significantly inhibited the invasion of WRO 82‐1 cells.
Figure 3.
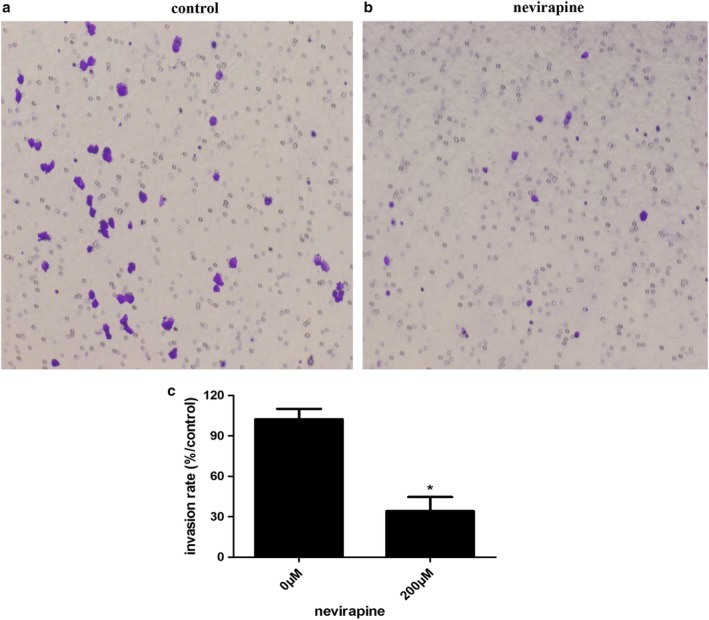
Nevirapine inhibited WRO 82‐1 cell invasion. (a) Cell invasion was detected by transwell invasion assay. (b) The percentage of invasion cells is shown as the mean ± SD of five replicate experiments. *P < 0.05.
Nevirapine downregulated expression of both mRNAs and proteins related to migration and invasion in WRO 82‐1 cells
Several proteins are closely related to the migration and invasion of cancers, such as VEGFA, MMP2, and MMP9. Therefore, we assessed the effects of nevirapine on the mRNA and protein expression of these proteins by quantitative real‐time PCR (Fig 4) and western blotting (Fig 5), respectively. WRO 82‐1 cells were treated with nevirapine of 0, 100 and 200 μM for 72 hours. The results demonstrated that treatment with 100 and 200 μM nevirapine significantly decreased VEGFA, MMP2 and MMP9 expression in a dose‐dependent manner in WRO 82‐1 cells compared with untreated controls. WRO 82‐1 cells treated with nevirapine (Fig 4) had a significantly lower mRNA expression of VEGFA, MMP2 and MMP9 compared with cells treated with 0 μM nevirapine (all P < 0.05). Nevirapine also significantly decreased the protein expression of VEGFA, MMP2 and MMP9 compared with cells treated with 0 μM nevirapine (except the protein expression of VEGFA in 100 μM nevirapine‐treated cells, all P < 0.05; Fig 5).
Figure 4.
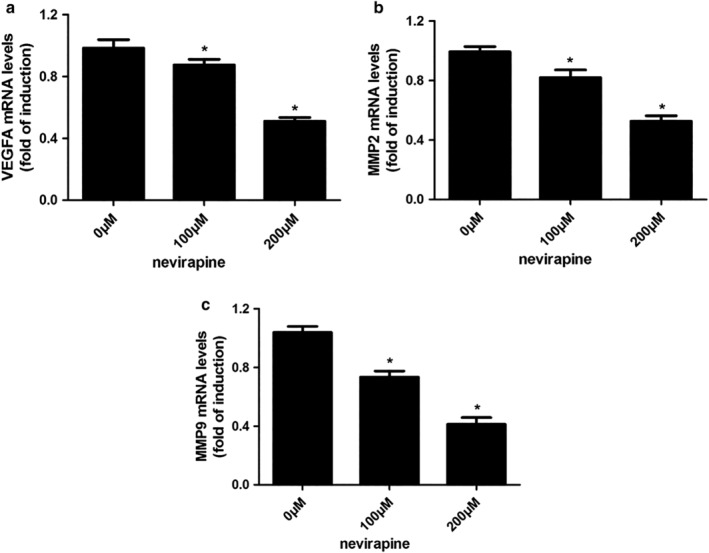
Nevirapine downregulated the expression of mRNAs related to migration and invasion in WRO 82‐1 cells. Changes of mRNA expression of (a) VEGFA, (b) MMP2 and (c) MMP9 were detected by quantitative real‐time PCR. n = 3, *P < 0.05.
Figure 5.
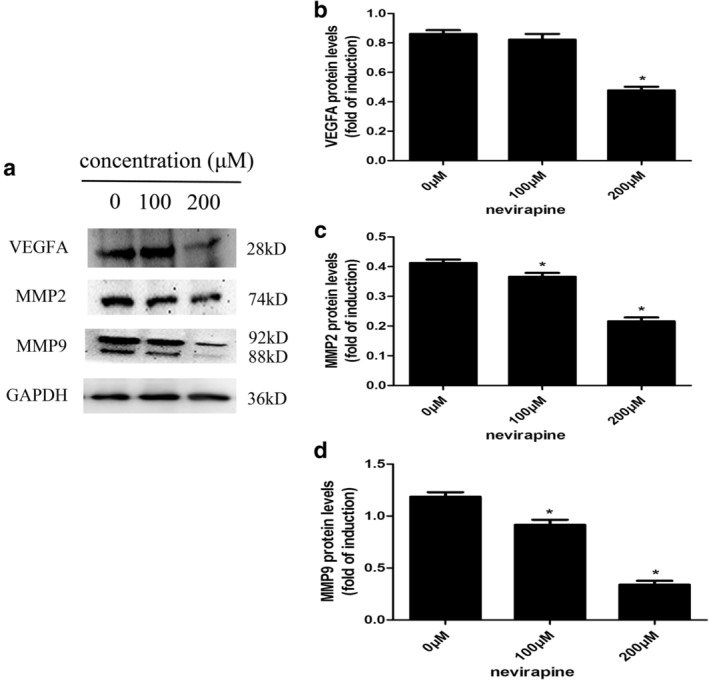
Nevirapine downregulated the expression of proteins related to migration and invasion in WRO 82‐1 cells. (a) Changes of protein expression of protein expression of VEGFA, MMP2 and MMP9 were determined by western blotting. The histograms show the results of the quantitative analysis of changes in (b) VEGFA, (c) MMP2 and (d) MMP9 protein expression. n = 3, *P < 0.05.
Possible mechanisms by which nevirapine inhibited the migration and invasion of thyroid cancer by inhibiting IL‐6/STAT3 signaling pathway
The effects of nevirapine on mRNA expression of IL‐6 were assessed by quantitative real‐time PCR (Fig 6a) and protein expression of pJAK2 (Y1007+Y1008)/JAK2 and pSTAT3 (Tyr705)/STAT3 by western blot (Fig 6b). The results showed that treatment with 100 and 200 μM nevirapine for 72 hours significantly decreased mRNA expression of IL‐6 and phosphorylation of JAK2 (Y1007+Y1008) and STAT3 (Tyr705) in WRO 82‐1 cells compared with untreated cells (all P < 0.05). Nevirapine with 200 μM reduced phosphorylation expression of JAK2 (Y1007+Y1008) and STAT3 (Tyr705) by 56.0% and 46.1%, respectively (all P < 0.05).
Figure 6.
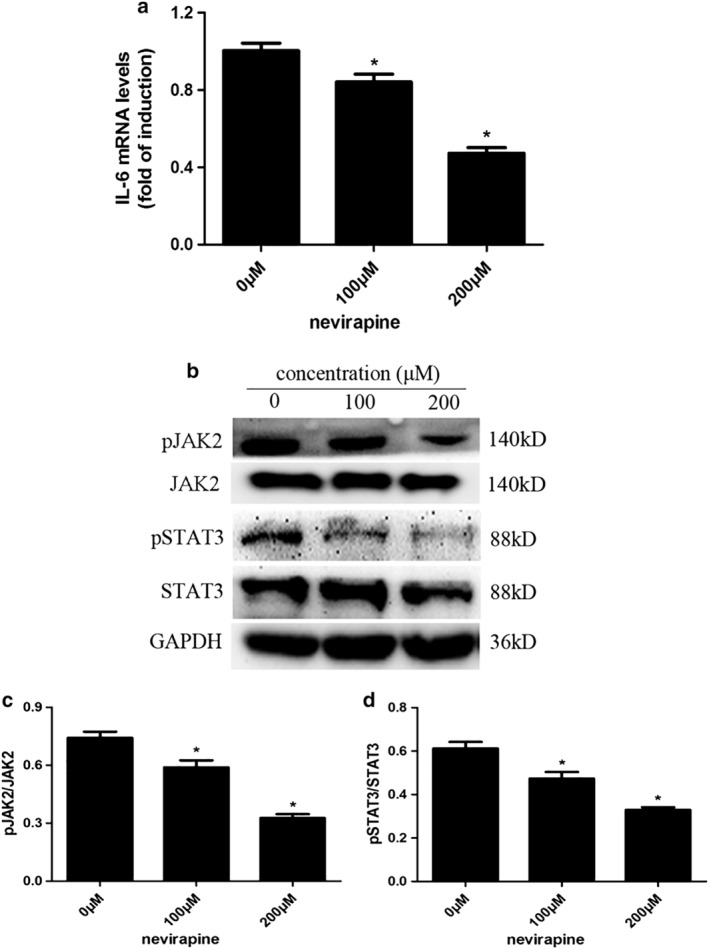
Nevirapine inhibited IL‐6/STAT3 signaling pathway. (a) The expression of IL‐6 mRNA was tested by quantitative real‐time PCR. (b) The phosphorylation of JAK2 (Y1007+Y1008) and STAT3 (Tyr705) was determined by western blot analysis and represented by the ratios of (c) pJAK2 (Y1007+Y1008)/JAK2 and pSTAT3 (Tyr705)/STAT3. n = 3, *P < 0.05.
Discussion
Nevirapine plays a vital role in differentiation of progenitor cells or redifferentiation of transformed cells, and in inhibiting genesis and progression of human tumors,13, 14, 15, 16, 17 but there has not previously been any published research on thyroid cancer. We hypothesized that nevirapine could inhibit the invasion and metastasis of thyroid cancer and, as expected, our results showed that nevirapine suppressed the migration and invasion in dedifferentiated thyroid cancer cell in a dose‐ and time‐dependent manner. Furthermore, nevirapine could significantly ameliorate tumor metastasis in vivo.
Nevirapine, as an immunomodulator, has been shown to inhibit the secretion of cytokine IL‐6 in HIV‐infected patients.29 Accumulating studies suggest that thyroid cancer is also an autoimmune disease.30, 31, 32 We found that nevirapine could inhibit IL‐6 mRNA expression in thyroid cancer by RT‐PCR. To elucidate the mechanism underlying the effects of IL‐6 in thyroid cancer, we assessed the expression of its downstream genes. STAT3, a transcription factor, binds to IL‐6 responsive elements located in the promoter region of various acute‐phase genes33 and is indicated to be phosphorylated on its Tyr705 in response to IL‐6.34 JAK2, an upstream activator of STAT3 and STAT5, has been identified as an oncogenic protein35, 36 and upregulated expression is associated with more malignant behavior of tumor cells in a large number of human cancers, such as cancers of the breast, ovary, and prostate.37, 38, 39, 40 To determine whether nevirapine could inhibit IL‐6 ‐induced expressions of pJAK2 and pSTAT3 in thyroid cancer, we examined expressions of pJAK2 (Y1007+Y1008) and pSTAT3 (Tyr705) by western blot analysis. Our data showed that nevirapine inhibited distant metastasis of tumor xenografts and phosphorylation of pJAK2 and pSTAT3 proteins in WRO 82‐1 cells, indicating that nevirapine exerts its anticancer effects possibly by inhibiting IL‐6/JAK2/STAT3 signaling pathway.
MMPs play a critical role in tumor invasion and metastasis by degrading the molecules constituting the extracellular matrix (ECM).19, 41, 42 Of the more than 20 known human MMPs, MMP2 and MMP9 seem to play crucial roles in tumor invasion and metastasis due to their ability to degrade the ECM, observed in a variety of cancer cell lines.43, 44 Here, we detected the effects of nevirapine on MMP‐2 and MMP‐9 expression in WRO 82‐1 cells and tumor xenografts. Western blot analysis showed that MMP2 and MMP9 expression was significantly attenuated in cells in a dose‐ and time‐dependent manner, immunohistochemistry also demonstrated that nevirapine decreased the expression of MMP‐2 and MMP‐9. These results indicated that nevirapine may exert its anti‐invasion and antimetastasis effects by regulating MMP2 and MMP9 expression.
Finally, we successfully constructed tumor xenografts in nude mice, and observed liver metastasis of thyroid carcinoma. Treatment with nevirapine resulted in smaller numbers and volumes in liver metastatic tumor. However, since liver metastases were unintentionally found during the experiment, they are less intuitive than those in nude mice inoculated subcutaneously with WRO 82‐1 cells labeled with luciferase. Improvements will be conducted in further research to explore these mechanisms in depth. Nevertheless, our findings suggest that nevirapine plays a crucial role in attenuating metastasis in thyroid cancer.
There is a limitation for this study. The detailed mechanism of inhibiting migration and invasion by nevirapine in dedifferentiated thyroid cancer cells was not performed in our study, but the next part of our work will be to explore in depth how nevirapine works to inhibit migration and invasion by IL‐3/STAT3 pathway.
In summary, these data suggest that nevirapine has inhibitory effects in human dedifferentiated thyroid cancer by targeting the IL‐6/STAT3 signaling pathway, and our findings demonstrate that nevirapine may be useful as a therapy against human thyroid cancer.
Disclosure
The authors declare that they have no conflict of interest.
Acknowledgment
This study was funded by National Natural Science Foundation of China (81070637, 81570742).
Contributor Information
Jianjun Dong, Email: cwc_ll@sdu.edu.cn.
Lin Liao, Email: liaolin@sdu.edu.cn.
References
- 1. Huang LL, Wang Z, Cao CJ et al AEG‐1 associates with metastasis in papillary thyroid cancer through upregulation of MMP2/9. Int J Oncol 2017; 51: 812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yapa S, Mulla O, Green V, England J, Greenman J. The role of chemokines in thyroid carcinoma. Thyroid 2017; 27: 1347–59. [DOI] [PubMed] [Google Scholar]
- 3. Ren L, Xu Y, Liu C, Wang S, Qin G. IL‐17RB enhances thyroid cancer cell invasion and metastasis via ERK1/2 pathway‐mediated MMP‐9 expression. Mol Immunol 2017; 90: 126–35. [DOI] [PubMed] [Google Scholar]
- 4. Ieni A, Vita R, Magliolo E et al One‐third of an archivial series of papillary thyroid cancer (years 2007‐2015) has coexistent chronic lymphocytic thyroiditis, which is associated with a more favorable tumor‐node‐metastasis staging. Front Endocrinol 2017; 8: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai WY. Controversies on the extent of thyroidectomy for differentiated thyroid cancer. J Surg Concepts Pract 2003; 8: 289–92. (in Chinese). [Google Scholar]
- 6. Hong CM, Ahn BC. Redifferentiation of radioiodine refractory differentiated thyroid cancer for reapplication of I‐131 therapy. Front Endocrinol 2017; 8: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antonelli A, La Motta C. Novel therapeutic clues in thyroid carcinomas: The role of targeting cancer stem cells. Med Res Rev 2017; 37: 1299–317. [DOI] [PubMed] [Google Scholar]
- 8. Zhang CY, Zhang L, Yu HX, Bao JD, Sun Z, Lu RR. Curcumin inhibits invasion and metastasis in K1 papillary thyroid cancer cells. Food Chem 2013; 139: 1021–8. [DOI] [PubMed] [Google Scholar]
- 9. Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine‐refractory differentiated thyroid cancer: The sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol 2014; 2: 830–42. [DOI] [PubMed] [Google Scholar]
- 10. Inzaule SC, Weidle PJ, Yang C et al Prevalence and dynamics of the K65R drug resistance mutation in HIV‐1‐infected infants exposed to maternal therapy with lamivudine, zidovudine and either nevirapine or nelfinavir in breast milk. J Antimicrob Chemother 2016; 71: 1619–26. [DOI] [PubMed] [Google Scholar]
- 11. Famiglini V, Silvestri R. Focus on chirality of HIV‐1 non‐nucleoside reverse transcriptase inhibitors. Molecules 2016; 21: E221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhatti L, Gladstein J. Once‐daily nevirapine XR: A brief overview of the safety and efficacy of a new formulation. J Int Assoc Physicians AIDS Care 2012; 11: 369–73. [DOI] [PubMed] [Google Scholar]
- 13. Mangiacasale R, Pittoggi C, Sciamanna I et al Exposure of normal and transformed cells to nevirapine, a reverse transcriptase inhibitor, reduces cell growth and promotes differentiation. Oncogene 2003; 22: 2750–61. [DOI] [PubMed] [Google Scholar]
- 14. Sciamanna I, Landriscina M, Pittoggi C et al Inhibition of endogenous reverse transcriptase antagonizes human tumor growth. Oncogene 2005; 24: 3923–31. [DOI] [PubMed] [Google Scholar]
- 15. Sbardella G, Mai A, Bartolini S et al Modulation of cell differentiation, proliferation, and tumor growth by dihydrobenzyloxopyrimidine non‐nucleoside reverse transcriptase inhibitors. J Med Chem 2011; 54: 5927–36. [DOI] [PubMed] [Google Scholar]
- 16. Carlini F, Ridolfi B, Molinari A et al The reverse transcription inhibitor abacavir shows anticancer activity in prostate cancer cell lines. PLOS One 2010; 5: e14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinibaldi‐Vallebona P, Lavia P, Garaci E, Spadafora C. A role for endogenous reverse transcriptase in tumorigenesis and as a target in differentiating cancer therapy. Genes Chromosomes Cancer 2006; 45: 1–10. [DOI] [PubMed] [Google Scholar]
- 18. Loffek S, Schilling O, Franzke CW. Series "matrix metalloproteinases in lung health and disease": Biological role of matrix metalloproteinases: A critical balance. Eur Respir J 2011; 38: 191–208. [DOI] [PubMed] [Google Scholar]
- 19. Lan H, Hong W, Fan P, Qian D, Zhu J, Bai B. Quercetin inhibits cell migration and invasion in human osteosarcoma cells. Cell Physiol Biochem 2017; 43: 553–67. [DOI] [PubMed] [Google Scholar]
- 20. Cheng G, Gao F, Sun X, Bi H, Zhu Y. Paris saponin VII suppresses osteosarcoma cell migration and invasion by inhibiting MMP2/9 production via the p38 MAPK signaling pathway. Mol Med Rep 2016; 14: 3199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manoj GS, Kumar TR, Varghese S, Murugan K. Effect of methanolic and water extract of Leucobryum bowringii Mitt. on growth, migration and invasion of MCF 7 human breast cancer cells in vitro. Indian J Exp Biol 2012; 50: 602–11. [PubMed] [Google Scholar]
- 22. Patyra K, Jaeschke H, Lof C et al Partial thyrocyte‐specific Gαs deficiency leads to rapid‐onset hypothyroidism, hyperplasia, and papillary thyroid carcinoma‐like lesions in mice. FASEB J 2018; 32: 6239–51. [DOI] [PubMed] [Google Scholar]
- 23. Liu H, Ren G, Wang T et al Aberrantly expressed Fra‐1 by IL‐6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial‐mesenchymal transition. Carcinogenesis 2015; 36: 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michaud‐Levesque J, Bousquet‐Gagnon N, Beliveau R. Quercetin abrogates IL‐6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res 2012; 318: 925–35. [DOI] [PubMed] [Google Scholar]
- 25. Sosonkina N, Starenki D, Park JI. The role of STAT3 in thyroid cancer. Cancer 2014; 6: 526–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Estour B, Van Herle AJ, Juillard GJF et al Characterization of a human follicular thyroid carcinoma cell line (UCLA RO 82 W‐l). Virchows Arch B Cell Pathol Incl Mol Pathol 1989; 57: 167–74. [DOI] [PubMed] [Google Scholar]
- 27. Van Herle AJ, Agatep ML, Padua DN 3rd et al Effects of 13 cis‐retinoic acid on growth and differentiation of human follicular carcinoma cells (UCLA R0 82 W‐1) in vitro. J Clin Endocrinol Metab 1990; 71: 755–63. [DOI] [PubMed] [Google Scholar]
- 28. Svejgaard B, Wehland M, Ma X et al Common effects on cancer cells exerted by a random positioning machine and a 2D clinostat. PLOS One 2015; 10: e0135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diaz‐Delfin J, del Mar Gutierrez M, Gallego‐Escuredo JM et al Effects of nevirapine and efavirenz on human adipocyte differentiation, gene expression, and release of adipokines and cytokines. Antiviral Res 2011; 91: 112–9. [DOI] [PubMed] [Google Scholar]
- 30. Tesselaar MH, Smit JW, Nagarajah J, Netea‐Maier RT, Plantinga TS. Pathological processes and therapeutic advances in radioiodide refractory thyroid cancer. J Mol Endocrinol 2017; 59: R141–R14. [DOI] [PubMed] [Google Scholar]
- 31. Mould RC, van Vloten JP, AuYeung AWK, Karimi K, Bridle BW. Immune responses in the thyroid cancer microenvironment: Making immunotherapy a possible mission. Endocr Relat Cancer 2017; 24: T311–29. [DOI] [PubMed] [Google Scholar]
- 32. French JD, Haugen BR, Thyroid‐specific T. cells in patients with differentiated thyroid cancer: Implications for immune‐based therapies? J Clin Endocrinol Metab 2017; 102: 2131–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akira S, Nishio Y, Inoue M et al Molecular cloning of APRF, a novel IFN‐stimulated gene factor 3 p91‐related transcription factor involved in the gp130‐mediated signaling pathway. Cell 1994; 77: 63–71. [DOI] [PubMed] [Google Scholar]
- 34. Zhong Z, Wen Z, Darnell JE Jr. Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin‐6. Science 1994; 264: 95–8. [DOI] [PubMed] [Google Scholar]
- 35. Bromberg JF, Wrzeszczynska MH, Devgan G et al Stat3 as an oncogene. Cell 1999; 98: 295–303. [DOI] [PubMed] [Google Scholar]
- 36. Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene 2000; 19: 2474–88. [DOI] [PubMed] [Google Scholar]
- 37. Ward Y, Lake R, Martin PL et al CD97 amplifies LPA receptor signaling and promotes thyroid cancer progression in a mouse model. Oncogene 2013; 32: 2726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Min H, Wei‐hong Z. Constitutive activation of signal transducer and activator of transcription 3 in epithelial ovarian carcinoma. J Obstet Gynaecol Res 2009; 35: 918–25. [DOI] [PubMed] [Google Scholar]
- 39. Liu X, He Z, Li CH, Huang G, Ding C, Liu H. Correlation analysis of JAK‐STAT pathway components on prognosis of patients with prostate cancer. Pathol Oncol Res 2012; 18: 17–23. [DOI] [PubMed] [Google Scholar]
- 40. Ai T, Wang Z, Zhang M et al Expression and prognostic relevance of STAT3 and cyclin D1 in non‐small cell lung cancer. Int J Biol Markers 2012; 27: e132–8. [DOI] [PubMed] [Google Scholar]
- 41. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006; 25: 9–34. [DOI] [PubMed] [Google Scholar]
- 42. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2: 161–74. [DOI] [PubMed] [Google Scholar]
- 43. Pei S, Yang X, Wang H et al Plantamajoside, a potential anti‐tumor herbal medicine inhibits breast cancer growth and pulmonary metastasis by decreasing the activity of matrix metalloproteinase‐9 and ‐2. BMC Cancer 2015; 15: 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salem N, Kamal I, Al‐Maghrabi J et al High expression of matrix metalloproteinases: MMP‐2 and MMP‐9 predicts poor survival outcome in colorectal carcinoma. Future Oncol 2016; 12: 323–31. [DOI] [PubMed] [Google Scholar]


