Abstract
Oligometastatic non-small cell lung cancer (NSCLC) describes an intermediate stage of NSCLC between localized and widely-disseminated disease. This stage of NSCLC is characterized by a limited number of metastases and a more indolent tumor biology. Currently, the management of oligometastatic NSCLC involves radical treatment (radiotherapy or surgery) that targets the metastatic lesions and the primary tumor to achieve disease control. This approach offers the potential to achieve prolonged survival in patients who, in the past, would have only received palliative measures. The optimal therapeutic strategies for the different scenarios of oligometastatic disease (intracranial vs extracranial disease, synchronous vs metachronous) remain undefined. Given the lack of head-to-head studies comparing radiotherapy to surgery in these patients, the decision to apply surgery or radiotherapy (with or without systemic treatment) must be based on prognostic factors that allow us to classify patients. This classification will allow us to select the most appropriate therapeutic strategy on an individualized basis. In the future, the molecular or microRNA profiles will likely improve the treatment selection process. The objective of the present article is to review the most relevant scientific evidence on the management of patients with oligometastatic NSCLC, focusing on the role of radiotherapy and surgery. We also discuss areas of controversy and future directions.
Keywords: Non-small cell lung cancer, Metastasectomy, Oligometastases, Stereotactic ablative radiotherapy, Stereotactic body radiation therapy, Radiosurgery
Core tip: In recent years, numerous studies, including two randomized phase II trials, have demonstrated that local treatment, either radiotherapy or surgery, of the primary tumor and metastases improves progression-free survival and overall survival in patients who present with oligometastatic non-small cell lung cancer at diagnosis and in those who respond to the initial systemic therapy. As we await the results of ongoing randomized phase III trials, the main international clinical guidelines recommend a multimodal strategy to manage this subgroup of oligometastatic patients. Current guidelines recommend systemic therapy combined with local treatment of the metastases and, if applicable, the primary tumor.
INTRODUCTION
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer-related death worldwide. Approximately 80% of newly-diagnosed lung cancers in Europe are non-small cell lung cancer (NSCLC), and 60%-70% of these patients have advanced disease at diagnosis[1]. Traditionally, these patients have been managed exclusively with chemotherapy and palliative treatments aimed at relieving symptoms and improving quality of life. Previously, patients with advanced NSCLC were not considered candidates for curative-intent treatment due to their poor prognosis, with a median survival of 8-11 mo and a 5-year overall survival (OS) rate of only 4%-6%[2]. Today, however, the situation has changed significantly. In recent years, advances in diagnostic imaging techniques, including 18F-FDG-positron-emission tomography (PET)/computed tomography (CT) and brain magnetic resonance imaging (MRI), have substantially increased the tumor detection rate. These advances, together with the emergence of targeted therapies such as anti-epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK), have increased survival in patients with metastatic disease, leading to a growing number of patients characterized by a limited number of metastases at only a few sites. This newly-defined patient subgroup represents an intermediate stage of NSCLC between locally-advanced and widely-disseminated disease. Patients with oligometastatic disease have a better long-term prognosis than those with more advanced NSCLC and may benefit from systemic therapy combined with local treatment (mainly radiotherapy and/or surgery) directed at the metastatic lesions[2].
The 8th edition of the Tumor, Node, Metastasis published by the International Association for the Study of Lung Cancer includes, for the first time, oligometastatic disease. These patients are divided into three distinct categories according to their prognosis: Stage M1a: Involvement of the lung alone; M1b: Single extrathoracic metastasis; and M1c: Multiple extrathoracic metastases in one or more organs[3]. In this context, the aim of this article is to review the most relevant scientific evidence for the management of patients with oligometastatic NSCLC, with a focus on the role of surgery and radiotherapy. We also discuss current controversies and future directions.
DEFINITION OF THE OLIGOMETASTATIC STATE
The oligometastatic state was first described by Hellman and Weichselbaum[4] in an editorial published in 1995, in which the authors proposed their “step-wise” and “seed and soil” hypotheses. Oligometastasis refers to a clinical stage in which patients present a limited number of metastases to a few sites (a single organ or a few organs). These lesions may be more indolent than those typically observed in patients with multiple metastases, and thus these oligometastatic patients have a better prognosis. The incidence of oligometastatic NSCLC is estimated to range from 20%-50%[5] of patients with NSCLC, depending on the specific definition used to define oligometastatic disease (i.e. number of metastases: Three vs five and time of presentation: Synchronous vs metachronous). The most common metastatic site in NSCLC is the brain (60%), followed by multiorgan metastases to the contralateral lung, lymph nodes, liver (23%), and adrenal glands (10%)[6].
Data from observational studies suggest that the recurrence pattern in up to 60% of patients diagnosed with oligometastatic NSCLC treated with systemic therapy is mainly local, involving the same sites affected at the time of diagnosis[7]. This pattern of progression supports the application of local treatment to target the individual metastases and the primary tumor in order to improve local control and progression-free survival (PFS). Conceivably, an increase in PFS could improve OS by preventing the uncontrolled growth of the oligometastatic lesions, thus reducing or preventing the progressive dysfunction of the involved organ(s). In recent years, numerous studies, mostly retrospective, have evaluated the treatment of oligometastatic NSCLC. Most of those studies found that administering aggressive local treatment to all of the metastatic lesions improved both PFS and OS.
Oligometastatic disease can be subclassified according to the initial diagnosis and the systemic therapy. The term synchronous or de novo oligometastases refers to the presence at diagnosis of a limited number of metastases. By contrast, oligo-recurrence refers to the development of new, metachronous metastases after definitive treatment of the locoregional thoracic disease[8]. Two other terms, oligoprogression and oligo-resistance, are used to describe patients with disseminated disease at diagnosis that partially respond to systemic treatment (disease stabilization), except for the development of a limited number of metastases that progress (oligoprogression) or persist (oligo-resistance) after systemic chemotherapy. Oligoprogression and oligo-resistance are common in patients treated with targeted therapies (anti-EGFR/anti-ALK) due to acquired resistance after treatment with tyrosine kinase inhibitors (TKI).
To identify accurately oligometastatic patients, the use of modern imaging techniques, such as PET and brain MRI, is essential to detect the presence of occult metastases that are not visible with conventional imaging techniques. MRI is superior to CT in the detection of brain metastases, often detecting lesions that are not visible on CT. The use of PET/CT imaging is associated with better survival outcomes because metastases detected with PET/CT, which are not visible on standard CT, result in stage migration. Approximately 15% of patients with NSCLC[9,10] who are initially classified as stage I-III by CT will be upstaged to stage IV if PET/CT imaging is performed. These more advanced imaging tests can detect occult metastases in patients initially considered oligometastatic, thus obviating the application of aggressive local therapy in patients unlikely to benefit from treatment. In this regard, Tönnies et al[9] sought to determine whether the use of staging PET/CT in patients with NSCLC with a single surgically-resected metastasis had any influence on survival. Those authors compared patients who underwent surgery after preoperative staging with PET/CT (n = 66) vs conventional CT (n = 115). Five-year survival in the PET/CT group was 58% vs 33% in the conventional CT group (P = 0.01). It is important to note that the vast majority of published reports about oligometastatic NSCLC were conducted prior to the PET/CT era.
METASTASIS-DIRECTED LOCAL THERAPIES
Surgery has traditionally been the main treatment option in oligometastatic patients, with approximately 55% of patients undergoing surgical resection[11]. However, the use of less invasive ablative techniques, principally stereotactic radiosurgery (SRS), has increased significantly in recent years. SRS, which was first used to treat brain metastases[5], involves the administration of ablative doses of radiation (delivered in one or more sessions) to the intracranial lesion. SRS is a highly precise technique that minimizes the dose to the surrounding healthy tissues. The application of SRS outside the brain is known as stereotactic ablative radiotherapy (SABR) or stereotactic body radiation therapy (SBRT). No studies have yet directly compared surgery to radiotherapy in oligometastatic disease. Moreover, many of the available studies have included patients who received both surgery and radiotherapy[5]. Although other ablative techniques, including radiofrequency ablation and cryoablation, have been used in oligometastatic patients, their role for this indication is limited and thus not discussed in the present review.
Treatment selection (i.e. surgery or radiotherapy) for oligometastatic patients will depend on several variables: Age; performance status; comorbidities; time of appearance of the metastases relative to the primary tumor (metachronous metastases have a better prognosis); number of lesions (prognosis is better in patients with a single metastasis); localization of the metastases (prognosis is better for metastases located in the brain, lung, and adrenal glands); extension of the primary tumor; and mediastinal lymph node involvement (better prognosis in stage N0 disease)[5]. It is important to interpret cautiously the results of the studies that have compared metastasis-directed treatments given the potential for selection bias due to the better prognosis of these patients (good performance status, technically operable, etc).
Surgery
Traditionally, surgical metastasectomy is the most common approach to oligometastatic NSCLC. The indication for surgery depends on various metastasis-related factors (size, number, and location of the metastases) and patient-specific factors (age, performance status, comorbidities, and prognosis). The strongest evidence supporting the benefits of surgical metastasectomy comes from studies involving patients with brain metastases. Patchell et al[12] randomized 48 patients (77% with NSCLC) with a single brain metastasis to receive either whole brain radiotherapy (WBRT) or surgical metastasectomy followed by WBRT. The results showed an increase in local control and OS in the surgically-treated group. Nevertheless, few studies have specifically evaluated the surgical resection of extracranial metastases in NSCLC. Of the studies that are available, most are retrospective and heterogenous, including patients treated a range of different radical intent approaches (surgery, SBRT, radiosurgery, or other ablative therapies)[11,13]. A study conducted in Germany[14] examined the role of surgery in NSCLC patients with synchronous, solitary metastatic lesions. At 5 years, OS was 38%; median survival was longer in patients without mediastinal lymph node involvement (50 mo vs 19 mo, P = 0.015). OS rates were better in patients with localized lung metastases compared to those with extrathoracic lesions (5-year OS: 48.5% vs 23.6%). Johnson et al[15] reported a 5-year OS rate of 58% in 22 patients with synchronous oligometastatic NSCLC without mediastinal node involvement who underwent surgery or radiotherapy to the primary tumor and metastases.
Radiotherapy
Several local ablative treatments, including radiotherapy, can be used to treat oligometastatic NSCLC. Technological advances of the last decade have made it possible to administer high ablative doses with great precision to various sites. Brain metastases are treated with SRS while SBRT is used for extracranial lesions. An important advantage of these high-dose treatments is that they require fewer fractions, and thus the treatment duration is shorter than with conventional fractionation schedules. SRS and SBRT are considered safe, with minimal treatment-related toxicity and only requiring a brief interruption of systemic therapy. In addition, ablative radiotherapy can be administered to all tumor sites throughout the body. Historically, surgical resection was the preferred local therapy for oligometastases in patients with NSCLC[16]. However, in many cases, these metastatic lesions are unresectable, or the patient is considered inoperable, or the long postoperative recovery period may require an unacceptable delay in the initiation of systemic therapy. As a result, less invasive treatments such as radiotherapy are often necessary. A recent survey (published in 2017) of more than 1000 radiation oncologists from 43 countries[17] found that the use of SBRT to treat oligometastatic NSCLC is growing. The most common pattern of recurrence in patients with oligometastatic NSCLC who undergo first-line systemic therapies is local recurrence alone[18]. This pattern of progression supports the application of aggressive local treatments to the primary tumor and the metastatic sites to increase PFS[19] and, ultimately, OS. SBRT has proven beneficial in patients with a single metastasis as well as in those with multiple metastases[20], with local control rates ranging from 70%-90% and grade (G) ≥ 3 toxicity rates less than 10%[2]. Hasselle et al[21] investigated the role of SBRT in 25 patients with oligometastatic NSCLC (1-5 metastases). At a median follow-up of 14 mo, the median PFS and OS rates in that series were 7.6 and 22.7 mo, respectively. Several variables were associated with worse PFS: > two sites treated with SBRT; non-adenocarcinoma histology; prior systemic therapy; and progression after systemic therapy.
PRINCIPAL STUDIES OF OLIGOMETASTATIC NSCLC
Retrospective studies
One of the most important retrospective studies was carried out by López Guerra et al[22]. From January 2000 through June 2011, 78 consecutive patients with oligometastatic NSCLC (< 5 metastases) at diagnosis underwent definitive radiochemotherapy (≥ 45 Gy) to the primary tumor. Most of these patients (61/78; 78.2%) had only a single metastatic lesion, most (42%) located in the brain. The oligometastases were treated with local therapy in 44 patients (56%). The 2-year OS in the full cohort was 32%. Several variables were associated with improved OS: Tumor volume (gross tumor volume ≤ 124 cm3), performance status, administration of ≥ 63 Gy to the primary tumor, and definitive local treatment of the oligometastases. Severe (grade ≥ 3) pulmonary and esophageal toxicity were observed in 6.4% and 19.4% of patients, respectively.
Another important retrospective study of oligometastatic NSCLC was performed by Mordant et al[23]. That study included 94 patients with NSCLC who had a solitary, extrathoracic synchronous metastasis. The primary tumor and metastases were treated surgically in all patients (69 of 94 metastases resected). The most common metastatic site was the brain (n = 57), followed by bone (n = 14), adrenal glands (n = 12), liver (n = 5), and skin (n = 5). Mediastinal node status was distributed as follows: N0 (n = 46), N1 (n = 17), and N2 (n = 32). The 5-year OS was 16% (median survival, 13 mo). Induction therapy, adenocarcinoma, N0 staging, and lobectomy were all associated with better prognosis, but surgical metastasectomy was not. These findings raise doubts about the superiority of surgical resection versus radiotherapy and/or chemotherapy as the treatment of choice for solitary synchronous M1b metastases.
During the 2006-2016 time period, several small (20 to 60 patients) retrospective studies[21-25] of oligometastatic NSCLC were published. In those studies, both the primary tumor and the metastases received radical treatment (either surgery or radiotherapy). The median survival in those studies ranged from 13-22 mo, and the incidence of grade ≥ 3 toxicity was low.
Merino Lara et al[26] recently reported results of a Canadian study involving 108 patients with metastatic NSCLC treated with extracranial SBRT for oligometastasis (n = 66), oligoprogression (n = 20), and local control of dominant tumors (n = 22). In the full cohort, median OS and PFS were, respectively, 27.3 and 4.4 mo. Patients treated with SBRT for oligometastasis presented longer OS and PFS (39.3 and 7.6 mo, respectively) than those treated for oligoprogression (21.1 and 3.3 mo, respectively) with better local control of dominant tumors (11.8 and 2.2 mo, respectively). The application of SBRT also delayed the time to starting/changing systemic therapy (SCST): Cumulative 1-year incidence = 21.5%. Predictors of time to SCST were: Tumor size ≤ 4 cm (1-year SCST: 11.3% vs 36.4% in tumors > 4 cm) and EGFR/ALK mutational status (1-year SCST in EGFR/ALK positive disease: 45.5% vs 16% in negative EGFR/ALK). The local failure rate at 1 year was 15.6%. Predictors of local failure at 1 year were tumor size (local failure: 7.7% in tumors ≤ 4 cm vs 26.6% in tumors > 4 cm) and treatment with previous systemic therapy (local failure without systemic therapy, 13.2% vs 17.7% with previous systemic therapy). Two patients developed grade ≥ 3 toxicity (respiratory events) and three patients developed radiation-induced bone fractures.
The Shanghai Pulmonary Hospital group[27] evaluated 145 patients with stage IV EGFR-mutant NSCLC and ≤ 5 metastases at diagnosis. Of these patients, 35.2% received consolidative local ablative therapy (LAT) to all metastases (all-LAT group), 37.9% received LAT to the primary tumor or to the oligometastases (part-LAT group), and 26.9% did not receive LAT (non-LAT group). All patients received first-line anti-EGFR therapy (TKI). Median OS in the all-LAT, part-LAT, and non-LAT groups was, respectively, 40.9, 34.1, and 30.0 mo (P < 0.001). Median PFS was 20.6, 15.6, and 13.9 mo (P < 0.001), respectively. There was a significant difference in OS between the all-LAT group and the other groups, but no difference between the part-LAT and the non-LAT groups. Adverse effects (grade ≥ 3) included pneumonitis (7.7%) and esophagitis (16.9%).
Another recent study conducted in Asia[28] evaluated 231 patients with oligometastatic, EGFR-mutant lung adenocarcinoma treated with TKI alone (n = 88) or TKI plus local treatment of the metastases (n = 143). PFS (15 mo vs 10 mo) and OS (34 mo vs 21 mo) were both significantly better in the local metastasis-directed treatment.
Clinical trials
Several clinical trials, including two randomized phase II trials, have been conducted to evaluate the efficacy and safety of local ablative therapies (surgery or SBRT) in patients with oligometastatic NSCLC (Table 1).
Table 1.
Prospective trials of local treatment in oligometastatic disease
| Author | n | Primary site, n | Treatment groups | Type of treatment | Endpoints | Toxicity |
| Rusthoven et al[7], 2009 | 47 | CRC: 15; Lung: 10; breast: 4; other: 10 | SBRT | Phase I: 36-60 Gy in 3 fx; Phase II: 60 Gy in 3 fx. | LC 2 yr: 92%; OS 2 yr: 30% | G3: 2%; No RILD |
| Salama et al[29], 2011 | 61 | NSCLC: 11; SCLC: 5; H&N: 5. Other: 40 | SBRT | 3 fx: 8 Gy 3 fx: 20 Gy | MFu: 20.9 mo; PFS 2 yr: 22%; OS 2 yr: 56.7% | G3 (n = 2) |
| Iyengar et al[88], 2014 | 24 | NSCLC | SBRT: 1 fx: 19-24 Gy; 3 fx: 27-33 Gy; 5 fx: 35-40 Gy | SBRT + erlotinib | MFU: 11.6 mo; PFS: 14.7 mo;; OS: 20.4 mo | G3 (n = 2); G4 (n = 1); G5 (n = 1) (after SBRT to three sites) |
| Collen et al[104], 2014 | 26 | NSCLC | SBRT after primary treatment | 50 Gy in 10 fx | MFu: 16.4 mo; PFS 1 yr: 45%, median 11.2 mo; OS 1 yr: 67% | G2: 15% G3: 8% (n = 2) |
| Gomez et al[33], 2018 | 49 | NSCLC | LCT (CRT/Surgery of all lesions) vs maintenance therapy/obs. | SBRT n = 5; Surgery n = 3; pemetrexed n = 16, erlotinib n = 2; afatinib n = 1, bevacizumab | Median PFS: 14.2 mo/4.4 mo (P < 0.05) Median OS: 41.2 mo/17.0 mo (P < 0.05) | Similar, G3 |
| Iyengar et al[31], 2018 | 29 | NSCLC | Maintenance Ct alone vs SBRT followed by maintenance Ct | Ct: carboplatin + pemetrexed; SBRT: 1, 3 and 5 fractions with 21-27 Gy, 26.5-33 GY and 30-37.5 Gy respectively | MFu: 9.6 mo Median PFS: -Ct: 3.5 mo -SBRT: 9.7 mo (P < 0.05); Median OS: - no difference | G3 n: 4 vs 2; G4 n: 1 (ct only); G5 n: 3 vs 6. No grade 3 or higher toxicity attributable to SBRT |
| De Ruysscher et al[30], 2018 | 40 | NSCLC | Primary: RT or CRT, LCT (surgery, SBRT) | SBRT: 54 Gy of bone metastases. CNS: 1 fx: 8-20 Gy single or 24 Gy × 3 fx. PORT: 60 Gy | Median OS: 13.5 mo; 2-6 yr OS: 23.3%-10.3% Median PFS: 12.1 mo; 2-6 yr PFS: 51.3%-2.5% | G3 esophagitis: 15%; > G4: 0% |
CRC: Colorectal cancer; NSCLC: Non-small cell lung cancer; SCLC: Small cell lung cancer; CNS: Central nervous system; ECOG: Eastern Cooperative Oncology Group; PORT: Postoperative radiotherapy; PFS: Progression-free survival; MFU: Median follow up; OS: Overall survival; LCT: Local consolidative therapy; Ct: Chemotherapy; RT: Radiotherapy; fx: Fraction; CRT: Chemoradiotherapy; SBRT: Stereotactic body radiotherapy; LC: Local control; RILD: Radiation-induced liver disease; H&N: Head and neck.
A phase I/II trial[7] assessed the efficacy and tolerability of SBRT (36 Gy in phase I and 60 Gy in phase II, delivered in 3 fractions) in 47 cancer patients (21.3% with lung cancer) who presented 1-3 liver metastases (63 lesions in total). Most (69%) of the patients had received at least one (range, 0 to 5) previous systemic treatments for metastatic disease, and 45% had extrahepatic disease at study inclusion. Median follow-up was 16 mo. Only one patient (2%) developed toxicity ≥ grade 3. One- and 2-year local control rates after SBRT were 95% and 92%, respectively. The 2-year local control rate was 100% in patients with lesions ≤ 3 cm in diameter. Median OS was 20.5 mo.
In 2012, Salama et al[29] reported the results of a dose escalation (SBRT) trial involving 61 patients with one to five metastases (total: 113 metastatic lesions). Only 11 patients in that study had NSCLC. Median follow-up was 20.9 mo. Treatment tolerance was good. At 2 years, PFS and OS rates were, respectively, 22% and 56.7%. In the patients whose tumors progressed, most (72%) were oligoprogression (one to three metastases). Two-year OS was 60.3% in patients with metastases versus 21.9% in patients with four to five metastases.
A prospective, single-arm, phase II trial[13,30] evaluated the role of SBRT in 39 patients with oligometastatic NSCLC (≤ metastases). Most (74%) of the patients had local stage III disease, and 87% had a single metastasis, most commonly in the brain (44%). In 95% of the patients, the primary treatment included chemotherapy. Median OS and PFS were, respectively, 13.5 and 12.1 mo. At 3, 5, and 6 years, OS rates were 10.3%, 7.7%, and 5.1%, respectively; the corresponding values for PFS were 7.7%, 7.7%, and 2.5%. Only three patients (7.7%) developed local recurrence. The treatment was well-tolerated, with no cases of grade ≥ 3 toxicity
Iyengar et al[31] conducted a phase II randomized clinical trial involving 29 patients with oligometastatic NSCLC, without EGFR or ALK mutations, who achieved partial response or stable disease after chemotherapy. Patients were randomized to maintenance chemotherapy alone (n = 15) or SBRT followed by maintenance chemotherapy (n = 14). The trial was closed prematurely after the interim analysis revealed a significant increase in PFS in the SBRT group (9.7 mo vs 3.5 mo) versus the chemotherapy alone group (P = 0.01). Toxicity was similar in both arms. There were fewer recurrences overall in the SBRT group.
In 2016, Gomez et al[32] reported the results of a prospective, multicenter, randomized phase 2 study involving 74 patients with oligometastatic NSCLC (≤ 3 metastases). All patients received standard first-line systemic therapy consisting of chemotherapy and EGFR or ALK inhibitors. Patients with ≤ 3 metastases after systemic therapy were randomized to receive either local consolidation therapy (LCT) with surgery +/- radiotherapy to the primary tumor and metastases, with or without maintenance treatment or maintenance treatment (including observation, at the clinician’s discretion) alone. The patient groups were comparable with regards to lymph node involvement, EGFR/ALK status, response to first-line systemic therapy, brain metastases, and number of metastases. The study was closed after the first interim analysis (49 patients recruited) due to the treatment efficacy observed in the experimental arm. At a median follow-up of 12.39 mo, the study achieved the main objective: A statistically significant increase in PFS in the 25 patients who received LCT (11.9 mo) versus the 24 patients in the non-LCT group (3.9 mo, P = 0.0054). In addition, time to the appearance of a new lesion was longer in the LCT arm (11.9 mo vs 5.7 mo, P = 0.0497), suggesting that LCT may have altered the natural course of the disease, either by limiting the potential for subsequent dissemination or by altering systemic anticancer immune responses to facilitate longer control of subclinical disease. Adverse events were similar in the two groups, with no treatment-related grade 4 adverse events or deaths. The lead author of that study presented updated results at the American Society for Radiation Oncology congress in 2018[33]. At a median follow-up of 38.8 mo, the PFS benefit was durable, with a median PFS of 14.2 mo in the LCT arm versus 4.4 mo in the maintenance therapy/observation arm (P = 0.014). The extended follow up also demonstrated that LCT improved OS, with a median OS of 41.2 mo in the treatment arm versus 17.0 mo in the control arm (P = 0.017). No additional grade 3 or higher toxicities were observed in either arm. This was the first randomized clinical trial to demonstrate an increase in OS in patients with oligometastatic NSCLC. The main limitations of the study were the limited sample size, molecular heterogeneity (including EGFR and ALK patients), and the definition of oligometastatic disease (which was defined after systemic therapy).
In the American Society for Radiation Oncology 2018 congress, Palma et al[34] presented results of the international (Australia, Canada, Scotland, and Netherlands) SABR-COMET trial that included 99 patients with oligometastatic cancer treated from 2012 to 2016. All of the patients had good performance status (Eastern Cooperative Oncology Group (ECOG) 0-1). The most common primary tumor types were breast (n = 18), lung (n = 18), colorectal (n = 18), and prostate (n = 16) cancer. The inclusion criteria allowed for up to 5 metastatic lesions, although 93% had only 1-3 lesions. Median age was 68 years, and 59% of the patients were men. Patients were randomized 2:1 to SABR plus standard palliative care (treatment group) or palliative care alone (control arm). The baseline characteristics of the two groups were comparable. Some of the patients who developed new metastatic lesions during the trial were successfully treated with additional ablation therapy. At a median follow-up of 27 mo, median PFS was 12 mo in the treatment group versus 6 mo in the control arm (P = 0.001). At 5 years, 46% of patients in the SABR arm remained alive versus only 24% in the control arm. The median OS was numerically better in the SABR group: 41 mo [95% confidence interval (CI), 26 mo to “not reached”] versus 28 mo (95%CI: 19-33 mo) in the control group (P = 0.09). Adverse events (AEs) ≥ grade 2 were more common in the SABR group (30% vs 9%). The most common AEs were fatigue, dyspnea, and pain (muscle, joint, bone, or other types). Three AE-related deaths occurred in the investigational arm. No differences in quality of life were observed. At 6 mo post-treatment, overall scores on the Functional Assessment of Cancer Therapy General questionnaire were high in both the SABR and control arms: 82.5 versus 82.6, respectively (P = 0.992).No significant between-group differences were observed on the functional, emotional, physical, and social subscales of the Functional Assessment of Cancer Therapy General. A follow-up phase III study (SABR-COMET-3) will evaluate this treatment approach in patients with up to three metastatic lesions. The phase III SABR-COMET-10 will evaluate the same treatment approach but with lower doses of radiation in patients with up to 10 lesions.
A recent multicentric phase 2 trial[35] evaluated 147 patients with oligometastatic cancer (≤ 5 metastases), 21.8% of whom had lung cancer. All patients received SBRT. At a median follow-up of 41.3 mo [interquartile range: 14.6-59], median OS was 42.3 mo and 5-year OS was 43%. Local and metastatic PFS rates were 74% and 17%, respectively. Late toxicity ≥ 3 was 1.4%. There was a significant improvement in patient-reported quality of life at months 6 and 12.
Petty et al[36] conducted a phase II trial involving 27 patients with oligometastatic NSCLC (≤ 5 metastases) who had responded to three to six cycles of platinum-based chemotherapy. All patients received radiochemotherapy to the primary tumor and the metastases. Although the study closed prematurely due to poor recruitment, the study endpoint (PFS > 6 mo) was met (P < 0.0001). Median PFS and OS were, respectively, 11.2 and 28.4 mo. OS and PFS in patients with brain metastases or lymph node involvement did not differ significantly from the overall results.
PROGNOSTIC FACTORS FOR OLIGOMETASTATIC NSCLC
One of the keys to treatment selection in oligometastatic NSCLC is to identify patients likely to benefit from aggressive metastasis-directed therapies. However, only a relatively small percentage (15%-25%) of oligometastatic patients will have an extended disease-free interval (DFI) following ablative treatment of the metastatic lesions. The prognostic factors associated with survival include the following: Number of metastases; mediastinal node involvement; time until onset of metastases (DFI > 6 mo); histology; age; performance status; diagnosis-specific graded prognostic assessment (DS-GPA classification)[5] in patients with brain metastasis; pathological T-staging; the metastatic location (brain or adrenal glands versus other locations); treatment of the primary tumor (the type of surgical resection, intrathoracic small radiotherapy treatment volumes); and the molecular profile.
Although nearly all of the studies to date have included patients with up to five metastases, most of those patients had three or fewer metastases. Moreover, half of the patients in the meta-analysis by Ashworth et al[6] had only a single metastasis. In general, prognosis is better in patients with few metastases versus those with multiple metastases, regardless of whether metastasis-directed therapy is administered or not[37]. In this regard, the study by Albain et al[38] is worth highlighting. They evaluated 2531 patients with disseminated NSCLC, finding better OS in patients with a single metastasis (8.7 mo) versus those with multiple metastases in a single organ (6.2 mo) versus patients with multiple metastases in multiple organs (5.1 mo). Similar results were obtained by the International Association for the Study of Lung Cancer [3] in a validation study conducted in the context of preparing the 8th edition of the American Joint Committee on Cancer staging manual. In that study, OS was longer (median, 17.8 mo) in patients with a single extrathoracic metastasis than in those with multiple metastases (13.6 mo, P < 0.001).
Mediastinal node involvement is an important adverse prognostic factor for OS in patients with oligometastatic NSCLC. In this regard, a study of 39731 patients with M1a NSCLC included in the Surveillance, Epidemiology, and End Results database demonstrated that patients without lymph node involvement had the best cancer-specific survival, followed by patients with N1 disease; there were no differences in cancer-specific survival between patients with N2 or N3 disease[39].
The timing of the appearance of metastases is another important prognostic factor evaluated in the meta-analysis by Ashworth et al[6], which included a total of 757 patients with oligometastatic (one to five metastases) NSCLC. The metastases were synchronous in 75.5% of patients and metachronous in the other 24.5%. Treatment consisted of ablative therapy (mainly surgery) plus curative-intent treatment of the primary tumor. The median patient age was 61.1 years. Most patients had good performance status. The majority of patients (96.5%) had only one or two metastases. Median OS was 26 mo. The following factors were associated with worse OS: Synchronous metastasis, N-stage, and a non-adenocarcinoma histology. Patients were stratified into three prognostic groups based on an analysis of the study data: Low-risk: Metachronous metastases (5-year OS, 47.8%); intermediate risk: Synchronous metastases and N0 disease (5-year OS, 36.2%); and high risk: Synchronous metastases and N1/N2 disease (5-year OS, 13.8%).
In patients with brain metastases, predictors of survival include performance status, extent of extracranial disease, patient age, and number of brain metastases. This is known as the diagnosis-specific graded prognostic assessment (DS-GPA). Median survival according to DS-GPA values are as follows: 0-1: 3 mo; 1.5-2: 5.5 mo; 2.5-3: 9.4 mo; and 3.5-4: 14 mo[40].
In a retrospective study of 168 patients with oligometastatic NSCLC (≤ 5 lesions), Parikh et al[37] assessed the role of performance status and histology on outcomes, finding that N2-3 nodal status was an unfavorable prognostic factor. In that study, ECOG performance status ≥ 2, squamous cell histology, and metastases to multiple organs were all associated with a greater risk of death. By contrast, definitive local therapy to the primary tumor was associated with prolonged OS.
The role of pathologic T-stage on treatment outcomes was evaluated in a retrospective study of 29 patients with synchronous, single-organ metastatic NSCLC treated by lung resection and metastasis-directed therapy. Median survival was 20.5 mo, with a 5-year OS of 36%. Pathologic T stage was a predictor of survival. Median survival was significantly longer in patients with pT1-2 disease (26 mo) versus those with pT3-4 disease (8 mo, P = 0.007)[41].
The site of the metastatic lesion is another important prognostic factor. Lung, brain, and adrenal gland metastases appear to be associated with better survival than bone or liver metastases[42]. Griffoen et al[25] retrospectively evaluated 61 patients with one to three synchronous metastases treated with radical intent. Those authors found that the factors associated with improved survival after first progression were: Surgical excision of the primary lung tumor, presence of brain metastases (13.0 mo vs 3.3 mo, P = 0.002), and absence of bone metastases (11.9 mo vs 3.3 mo, P = 0.004). The study conducted to validate externally the prognostic value of the newly-proposed M staging classification system included a total of 1024 patients with stage IV NSCLC, showing that patients with lung metastases (M1a) had a better prognosis than those with extrathoracic metastases (M1a: 22.5 mo vs M1b: 17.8 mo vs M1c: 13.6 mo, P < 0.001).
Franceschini et al[43] recently evaluated patient-, treatment-, and disease-related variables that could potentially predict response to SBRT and survival. That study included 358 oligometastatic patients (≤ 5 lesions to three or fewer sites) from different primary tumors (23.7% NSCLC). At a median follow-up of 31.8 mo, 6- and 24-mo local control rates were, respectively, 94.6% and 78.9%; the corresponding PFS rates were 66.1% and 18.4%. Median OS was 34.7 mo (95%CI: 29.6-43.8), and 6- and 24-mo OS rates were 96% and 63.5%, respectively. On the multivariate analysis, pulmonary and nodal metastases were both associated with longer OS. Local response was also associated with OS. By contrast, primary lung cancer, older age, and metastases in locations other than those irradiated were all independent predictors of shorter OS.
Frost et al[44] conducted a retrospective propensity score analysis of 180 patients with synchronous oligometastatic (≤ 4 metastases) lung cancer (including NSCLC and neuroendocrine lung cancer) who received local treatment (surgery or SBRT) to all metastatic sites (experimental group) or standard chemotherapy combined, if needed, palliative-intent local treatment (control group). The experimental group had significantly better PFS (25.1 mo vs 8.2 mo, hazard ratio (HR) = 0.30, 95%CI: 0.21-0.43, P < 0.001) and OS (60.4 mo vs 22.5 mo, HR = 0.42, 95%CI: 0.28-0.62, P < 0.001). The most important prognostic factor was local treatment of the metastases. Several variables were associated with better PFS: adenocarcinoma histology, stage T1a, lymph node stage (N0-2 vs 3), and single versus multiple metastases. Performance status (ECOG 0-1 vs 2) was associated with better OS.
The meta-analysis conducted by Li et al[45] examined 24 studies (clinical trials and comparative retrospective studies) to identify prognostic factors in oligometastatic NSCLC. A total of 1935 patients were included. On the univariate analysis, neither age, nor smoking status, nor type of metastasis had any influence on OS. By contrast, several variables were significant prognostic factors for OS: Female sex (HR = 1.21, 95%CI: 1.02-1.45, P = 0.03), stage pN0 (HR = 1.82, 95%CI: 1.40-2.36, P < 0.00001), and adenocarcinoma (HR = 1.44, 95%CI: 1.10-1.88, P = 0.008). On the multivariate analysis, stage pN0 disease was associated with significantly better OS compared to stage pN1 disease (HR = 1.63, 95%CI: 1.27-2.10, P = 0.001) but not when compared to stage pN2 (HR = 2.01, 95%CI: 0.80-5.03, P = 0.14). In the subgroup analyses, none of the following variables had a significant impact on OS: thoracic stage, primary tumor, tumor histology, or number of oligometastases. However, patients who received aggressive/radical local treatment of the primary tumor had better OS (HR = 0.56, 95%CI: 0.37-0.83, P = 0.001), as did patients who received aggressive/radical local treatment of the oligometastases (HR = 0.54, 95%CI: 0.36-0.82, P < 0.00001).
The molecular profile of the patients also appears to be a prognostic factor associated with survival. Lussier et al[46] identified a specific microRNA expression that identified the patients most likely to remain oligometastatic after metastasis-directed treatment and therefore associated with a better prognosis.
SPECIFIC SITUATIONS
Brain oligometastases
Lung cancer is the main source of brain metastases, and 25%-30% of lung cancer patients present brain metastases at diagnosis. In certain cases, radical management of these metastases is associated with better outcomes: Limited number of metastases, good performance status, and favorable histology[47,48]. The main treatment approaches for this indication are surgery, WBRT, and SRS (Figure 1).
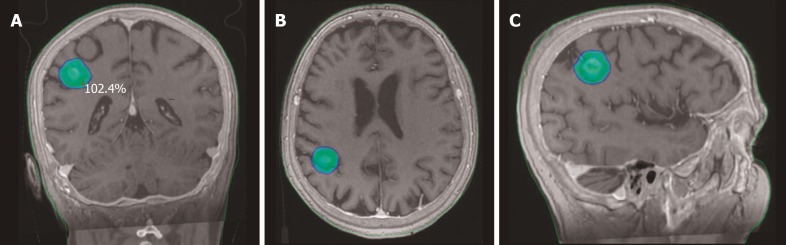
Figure 1.
Stereotactic body radiation therapy dose distribution for a single brain metastasis from non-small cell lung cancer. Planning computed tomography fused with magnetic resonance images. A: Coronal view; B: Axial view; C: Sagittal view.
Data from retrospective case series show that patients with NSCLC with a single synchronous brain metastasis who undergo radical treatment of the primary tumor and the brain metastasis have better OS (7%-24% at 5 years)[49,50]. One of the first and most important studies was conducted by Patchell et al[12], who carried out a prospective randomized trial involving patients (77% with NSCLC) with a single brain metastasis treated by surgery or surgery-WBRT. The surgery-WBRT arm had a better OS with a lower rate of recurrence (18% vs 70%).
SRS is another treatment option in this postoperative scenario. In 2017, Lamba et al[51] carried out a meta-analysis of retrospective studies, finding that SRS of the surgical cavity appears to yield survival outcomes and local and distant control rates that are comparable to WBRT. In a recent phase III trial, Mahajan et al[52] randomized patients (n = 128) who had undergone complete resection of one to three brain metastases to either observation or SRS. Those authors observed no local recurrences in 72% of the SRS group versus 43% of the observation group. This finding supports the use of SRS as an alternative to postoperative WBRT in this patient profile. In non-surgical patients, the RTOG 9508 phase III clinical trial evaluated 331 patients with one to three brain metastases from different primary tumors (64% lung cancer). The patients were randomized to receive either WBRT or WBRT plus an SRS boost to all lesions. On the univariate analysis, there was a survival benefit for the SRS boost only in the patients with a single brain metastasis (mean survival, 6.5 mo vs 4.9 mo, P = 0.0393)[53].
An important consideration in the treatment of brain oligometastases is the need to avoid the neurocognitive morbidity associated with WBRT. A randomized trial of 132 patients with up to four brain metastases (maximum diameter, 3 cm), compared SRS alone to SRS-WBRT and reported similar survival outcomes (8 mo vs 7.5 mo), indicating that patients with brain oligometastases could be treated with SRS alone[54]. Bhatnagar et al[55] evaluated the results of SRS alone in 205 patients (42% with NSCLC) with four to fifteen brain metastases. The 1-year local control rate was 71%, with a mean OS of 8 mo. The total treatment volume was a better predictor of survival than the number of metastases, a finding that raised the possibility that the concept of brain oligometastasis may be more closely associated with tumor volume than with the number of lesions. The recent meta-analysis by Tsao et al[56] compared WBRT to WBRT-SRS and SRS to WBRT-SRS, finding that combined treatment offers no OS benefit compared to SRS alone. While WBRT improved local control, toxicity with SRS was much lower[56].
There are no randomized trials comparing surgery-WBRT to SRS-WBRT, although one phase III trial compared surgery-WBRT to SRS for the treatment of single brain metastasis (≤ 3 cm in diameter). Although that study was closed early due to low recruitment, there were no significant survival differences between the two groups (64 patients)[57].
There is some controversy surrounding the optimal management of NSCLC patients with brain metastases who present an actionable oncogenic driver mutation (e.g., EGFR or ALK). Different phase III trials with third-generation anti-EGFR/ALK TKI, with ability to cross the blood-brain barrier, have shown CNS (central nervous system) response rates of up to 80% and an increase in OS[58,59]. The greater efficacy to treat CNS diseases raises the option of treating these patients with TKI alone, thus allowing for a delay in radiotherapy until progression is detected. However, there are still no randomized studies comparing these strategies directly and prospectively. On the other hand, Magnuson et al[61] reported results of a multi-institutional analysis of 351 patients treated with SRS followed by EGFR-TKI or WBRT followed by EGFR-TKI, or EGFR-TKI followed by SRS or WBRT upon intracranial progression. There were significant differences among the three groups in median OS: 46, 30, and 25 mo, respectively (P < 0.001), showing better results with the early local treatment.
Adrenal oligometastasis
The lung is the main site of origin for most adrenal gland metastases[5]. The incidence of these lesions has increased in recent years due to the increasing use of PET-CT and MRI. Unilateral involvement is more common when no other metastases are present (50% of cases); however, if metastatic involvement is present in other sites, unilateral adrenal involvement is only observed in 25% of cases[62]. Traditionally, adrenalectomy has been the treatment of choice in selected patients[63]. The results of laparoscopic surgery are equivalent to open surgery, with less morbidity[64].
Recently, SBRT has become an excellent alternative to surgery for the treatment of adrenal oligometastasis[65] (Figure 2). Published case series, most with > 12 mo of follow-up, suggest that toxicity ≥ G2 is practically nonexistent. Moreover, the excellent local control rates (> 90%) are highly promising. Probably the most notable case series published to date is the 34 patient series reported by Scorsetti et al[66]. This study has the longest median follow-up to date (41 mo), with excellent local control rates (93%).
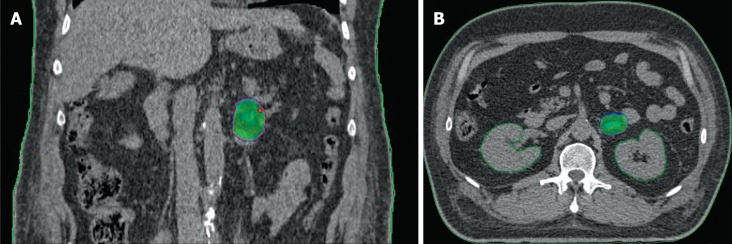
Figure 2.
Example of stereotactic body radiation therapy plan for adrenal gland metastases from non-small cell lung cancer with 95% isodose highlighted in dose heatmap. A: Coronal view; B: Axial view.
Holy et al[67] reported a median OS of 23 mo, comparable to surgical series, in patients with NSCLC with a single adrenal metastasis treated with SBRT (40 Gy in five fractions). Casamassima et al[68] reported results from a series of patients with adrenal metastases from different primary tumors (including NSCLC). Those patients received SBRT (36 Gy in three fractions), obtaining a 2-year local control rate of 90%. To date, a range of different fractionation schedules and doses have been used to treat adrenal gland metastases. Some authors, such as Li et al[69], have proposed a biologically-effective dose (BED) 10 > 100 Gy (as in other metastatic sites), suggesting that this BED provides greater local control.
The ideal candidate for radical intent local treatment to the adrenal metastasis remains to be defined. Similarly, we still need to define the optimal treatment, either surgery or SBRT. Given the lack of comparative studies, current evidence suggests that SBRT yields comparable local control rates to surgery, with less morbidity and lower mortality rates. Moreover, SBRT is easier to combine with systemic treatment.
Liver oligometastases
While liver metastasis is less common than in other locations in patients with NSCLC, the presence of such lesions is an unfavorable prognostic factor[5]. Although surgery remains the treatment of choice for these lesions, estimates suggest that only 10%-20% of patients are eligible for surgery[70]. Several different ablative techniques have been investigated, with reported local control rates as high as 90%[71,72]. However, these ablative treatments are limited by the patient’s reserve of healthy liver tissue, the risks of bleeding, lesion size, and the presence of nearby vascular structures[73]. SBRT has become a non-surgical option in these patients (Figure 3).
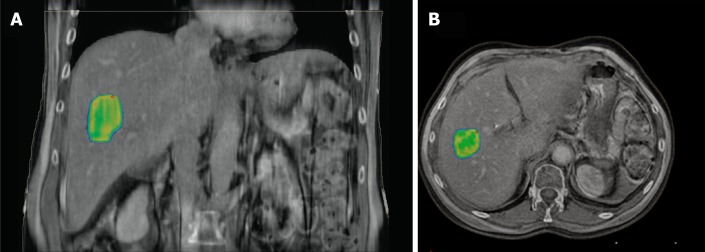
Figure 3.
Image capture from stereotactic body radiation therapy planning session showing concentration of radiation dose to the liver metastases. Planning computed tomography fused with magnetic resonance images. A: Coronal view; B: Axial view.
Numerous retrospective and prospective studies have evaluated SBRT for liver oligometastases, all of which have included patients with liver metastases from different primary tumors (Table 2). Of these, the recent study carried out by Mahadevan et al[70] stands out. They evaluated 427 patients with a total of 568 liver metastases (12% secondary to lung cancer) treated with SBRT. OS was longer (25 mo vs 15 mo) in small volume metastatic lesions (< 40 cm3) and in lesions that received a BED 10 ≥ 100 Gy (27 mo vs 15 mo), which also yielded better local control (77.2% vs 59.6%). Tumor histology had no impact on local control rates. While we await the results of ongoing prospective studies, the available evidence indicates that the high local control rates obtained with SBRT appear to be similar to those achieved with other local treatments, with a good toxicity profile.
Table 2.
Publishes results for stereotactic body radiation therapy-treated liver metastases
| Study | Number of lesions | Patients, n | Primary tumor | Dose/fractions | Toxicity | Mean follow-up, mo | Control, local | Survival |
| Blomgren et al[74] | Variable | 31 | Various | 8-66 Gy/1-4 | 2 Hemorrhagic gastritis | 1.5-3.8 | 80% | Not reported |
| Hoyer et al[75] | 1-6 (< 6 cm) | 44 | Various | 45 Gy/3 | 1 liver failure 2 severe GI toxicity | 52 | 2-yr: 86% | 1-yr: 67% 2-yr: 38% |
| Mendez Romero et al[76] | 1-3 (< 7 cm) | 25 | Various | 37.5 Gy/3 | 4 acute grade ≥ 3 1 late grade 3 | 12.9 | 2-yr: 86% | 1-yr: 85% 2-yr: 62% |
| Rusthoven et al[7] | 1-3 (< 6 cm) | 47 | Various | 60 Gy/3 | < 2% late | 16 | 2-yr: 92% < 3 cm: 100% | mean, 17.6 mo |
| Lee et al[77] | Variable | 68 | Various | 28-60 Gy/3 | Acute: 8 GIII, 1 GIV | 10.8 | 1-yr: 71% | 18 m: 47% |
| Ambrosino et al[78] | 1-3 (< 6 cm) | 27 | Various | 25-60 Gy/3 | Not reported | 13 | 74% | Not reported |
| Goodman et al[79] | 1-5 (< 5 cm) | 26 | Various | 18-30 Gy/1 | Late: 4 GII | 17.3 | 1-yr: 77% | 1-yr: 62% 2-yr: 49% |
| Rule et al[80] | 1-5 | 27 | Various | 30 Gy/3 50-60 Gy/5 | No ≥ G2 | 20 | 30 Gy: 56% 50 Gy: 89%60 Gy: 100% | 30 Gy: 56% at 2-yr 50 Gy: 67% at 2-yr 60 Gy: 50% at 2-yr |
| Scorsetti et al[81] | 1-3 (< 6 cm) | 61 | Various | 52.5-75/3 | No ≥ G3 | 24 | 91% | 1-yr: 80%2-yr: 70% |
| Mahadevan et al[70] | Variable | 427 | Various | 45 (12-60) / 3 (1-5) | Not reported | 14 (1-91) | Median: 52 mo 1-yr: 84%2-yr: 72% | Mean: 22 mo 1-yr: 74% 2-yr: 49% |
GI: Gastrointestinal.
Lung oligometastases
Surgical resection of lung metastases has a long history. The influential “Analysis of the International Registry of Pulmonary Metastases” was published in 1997[82]. In that study, 5206 cases of pulmonary metastases (of various different histologies) were evaluated, showing 5- and 15-year OS rates of 36% and 22%, respectively. The following were independent prognostic factors: Number of metastases, complete resection, and long DFI. Pulmonary metastases secondary to lung cancer are one of the most common metastatic locations. However, only a minority of patients are candidates for surgery. Local control rates range from 85%-95% after lobectomy and from 50%-70% after wedge resections[83,84].
A meta-analysis of 757 patients with oligometastatic NSCLC found that, among patients who underwent radical-intent local treatment, survival was better in those who had metachronous versus synchronous disease (P < 0.001)[6]. Various series have shown that survival rates achieved with lobectomy or pneumonectomy are comparable to sublobar resection but with greater morbidity[85,86].
In recent years, SBRT has become an excellent alternative to surgery for this indication (Figure 4). Rusthoven et al[7] carried out a phase I/II trial to evaluate the efficacy and tolerability of SBRT in patients with one to three lung metastases (≤ 7 cm in diameter). Radiotherapy was delivered in three fractions, and the dose was safely escalated from 48 to 60 Gy in phase I. The study included 38 patients (13.2% with primary NSCLC) and 63 metastatic lesions. Most (71%) of the patients received systemic treatment. The incidence of G3 toxicity was 8%, with no cases of G4 toxicity. Local control rates at 1 and 2 years were 100% and 96%, respectively. In the Mayo Clinic study carried out by Milano et al[87], 121 patients with ≤ 5 metastases of differing histologies (including NSCLC) received SBRT to all metastatic lesions. At 2 years, local control was 74% in patients with non-breast cancer tumor histologies, and mean survival was 18 mo. OS was longer in patients with a clinical response or stable disease after systemic treatment administered prior to SBRT. Iyengar et al[88] performed a randomized trial to assess SBRT in patients with oligometastatic NSCLC. SBRT was administered in one (19-24 Gy), three (27-33 Gy), or five sessions (35-40 Gy). All metastases (up to six extracranial lesions) were irradiated and patients received concurrent erlotinib after progression following first-line chemotherapy. PFS and OS were, respectively, 14.7 and 20.4 mo, which was higher than that from previous reports in patients who developed progression to first-line treatment without SBRT. A retrospective analysis at the University of Chicago included 25 patients (62 lesions) diagnosed with oligometastatic NSCLC (one to five lesions). At a mean follow-up of 14 mo, DFS and OS were, respectively, 7.6 and 22.7 mo[21]. The aforementioned study by Gomez et al[32] and Gómez et al[33] are among the most important conducted to date in oligometastatic NSCLC. Of the 25 patients who received local consolidative therapy after systemic treatment, 23 cases involved the lung, which is therefore the most important site in this study.
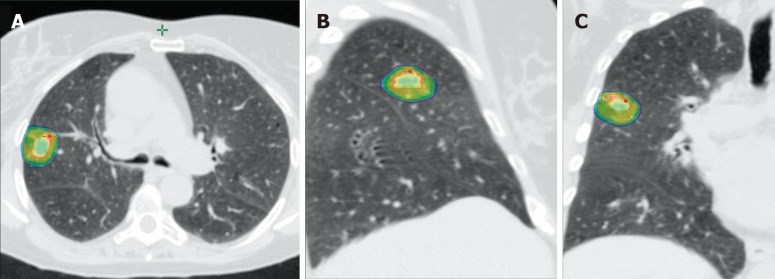
Figure 4.
Example of stereotactic body radiation therapy planning using 4D-computed tomography for a patient with right lung metastases from non-small cell lung cancer. A: Isodose distribution in the axial plane; B: Isodose distribution in the sagittal plane; C: Isodose distribution in the coronal plane.
To date, no randomized trials have directly compared SBRT to surgery in the treatment of lung metastases. However, based on the currently available results of both treatments, SBRT seems to be the better option due to greater patient tolerability and because it is easier to combine with systemic treatment. Pending comparative results, the two treatments appear to provide similar local control.
Oligometastases in other localizations
The use of local treatment is growing as is the number of oligometastatic possible sites[5]. Local therapy is now used to treat oligometastatic lesions in the bones, the spinal cord, and lymph nodes. Surgery has long been the treatment of choice for bone metastases in selected patients. However, due to the significant morbidity and mortality associated with surgery, the indications for this approach in bone metastases are limited[89]. The evidence base to support spinal cord or lymph node surgery in oligometastatic NSCLC is limited, which is why surgery is only indicated in cases in which a consensus decision by a multidisciplinary team has been reached after evaluating other therapeutic options.
The growing and increasingly effective use of local treatment of oligometastatic lesions is primarily due to the emergence of SBRT. Several recent reviews have evaluated the available body of evidence, mainly case series, for SBRT in these three metastatic sites (2, 3, and 4)[90-92]. Most of the studies published to date share several characteristics. For example, they all include patients with metastases to various sites, with differing histologies, treatment schemes, and a good tolerance profile (with exceptionally low rates of G3-4 toxicity). Moreover, in most published series, local control rates generally range from 80%-85% (or higher). Pending the publication of prospective studies, the decision to administer local treatment to these sites must be based on a consensus agreement among specialists after a thorough evaluation of the patient and careful consideration of the optimal local treatment.
MANAGMENT OF THE PRIMARY TUMOR IN OLIGOMETASTASIC NSCLC
Management of the primary tumor in patients with oligometastatic NSCLC should be evaluated individually and based on the available evidence. Currently, as we can see in previous sections, the most robust evidence is for patients diagnosed with synchronous intracranial metastases (one to three lesions). There is some evidence suggesting that treatment of the primary thoracic tumor and mediastinal lymph nodes in oligometastatic patients is associated with better survival, as shown in the studies discussed previously[6,37,38,41]. Li et al[93] performed a meta-analysis of seven retrospective, observational cohort studies involving 668 synchronous oligometastatic NSCLC patients. Of those 668 patients, 227 (34.0%) received aggressive thoracic therapy (surgery and/or radiotherapy, total dose > 40 Gy). Aggressive thoracic therapy was associated with a significant improvement in OS in the full cohort (HR = 0.48, P < 0.00001) and in three subgroups: Patients with metastases to a single organ (HR = 0.42), those with a solitary brain metastasis (HR = 0.49), and patients with stage I-II thoracic disease (HR = 0.38). In addition, 4-year OS rates were significantly higher in patients who received aggressive thoracic therapy (12.6% vs 2.0%). Several studies have shown that definitive treatment of the primary tumor is a favorable prognostic factor for survival in NSCLC, as reflected in the results of the meta-analysis conducted by Ashworth and colleagues[6]. The main treatments for the primary tumor are surgery, primarily lobectomy, and radiotherapy, with or without chemotherapy (depending on the disease stage). Radiotherapy can be normofractionated or hypofractionated (SBRT) in patients with negative node disease, which is a favorable prognostic factor.
In their case series, Lopez Guerra et al[22] evaluated prognostic factors in oligometastatic lung cancer, finding that small tumor volumes, radiotherapy doses > 63 Gy, and good functional status were all associated with better survival. Similarly, Griffoen et al[25] retrospectively analyzed 61 patients, finding that small radiation volumes and surgical treatment of the primary tumor were both prognostic factors for better survival.
Recently, two interesting studies have been published to evaluate the role of irradiating the primary tumor in oligometastatic NSCLC. In the first one, Petrelli et al[94] conducted a systematic review and meta-analysis of all published prospective trials and retrospective studies (up to July 2018) that compared radiotherapy versus no radiotherapy to the primary lung tumor in patients with synchronous oligometastatic NSCLC. A total of 924 patients from 21 studies were included. Patients who received thoracic irradiation of the primary tumor had significantly better OS (HR = 0.44, 95%CI: 0.32-0.6, P < 0.001) and PFS (HR = 0.42, 95%CI: 0.33-0.55, P < 0.001). The second study conducted by Arrieta et al[95] is a phase II prospective trial to evaluate patients (n = 37) diagnosed with stage IV NSCLC with ≤ 5 synchronous metastases at any location, including the CNS. All patients received four cycles of systemic treatment. The metastases and primary tumor were both irradiated in patients who responded (stable disease or partial response) to systemic therapy. Nineteen patients (51.4%) showed a complete response (CR) to radiotherapy on PET-CT imaging. The PFS for the full cohort was 23.5 mo (95%CI: 13.6-33.3). OS was better in the patients with CR on PET-CT, leading the authors to conclude that CR is an important prognostic factor.
LOCAL TREATMENT COMBINED WITH SYSTEMIC THERAPY
Although nowadays treatment of oligometastatic NSCLC with a radical intention is a routine practice, the optimal treatment sequence remains controversial because the data to support this recommendation come mostly from retrospective or small prospective studies. Therefore an individualized assessment of every patient is necessary to develop a radical-intent treatment plan and should always be determined by a multidisciplinary team. Several factors must be considered, including: Local extension of primary tumor, the tumor type (presence or absence of target mutations); the number, size and location of the metastases; the time of diagnosis (i.e. synchronous vs metachronous), and other patient-related factors such as the presence or absence of symptoms secondary to the disease, performance status, and comorbidities. Importantly, there are no clear criteria available to guide patient selection nor is there an standard strategy to combine ablative local treatment with systemic therapy in this patient profile.
In patients who present with synchronous metastases at diagnosis, an accepted strategy is to initiate systemic treatment and then re-stage the patient to confirm disease response (i.e. absence of progression) before administering radical treatment to the primary tumor and metastases. This was the strategy used in the two phase II randomized trials, both of which found better PFS and OS in patients who received radical local therapy[31,33].
A second strategy could be radical treatment of all metastatic lesions followed by systemic consolidation treatment. This approach was used by De Ruysscher et al[30] in a phase II prospective trial with 39 patients. The results were poor, with a 5-year PFS of only 8%, probably because this strategy does not include an option to select patients who respond to systemic therapy.
The use of more effective and less toxic treatments such as immunotherapy and targeted therapies has brought about a change in the natural history of NSCLC. There is a new subset of patients who present with oligoprogression during systemic treatment. In those patients, the local therapy of the progressive metastases is also associated with a longer PFS and OS while allowing continuation with the same systemic treatment[96,97].
With regard to the treatment strategy in patients with metastatic involvement limited to the CNS, there is a stronger consensus in favor of initiating treatment with SRS or surgical resection. In the subgroup of patients with activating mutations (EGFR or ALK) with asymptomatic lesions, it is considered reasonable to initiate targeted therapy before radiotherapy[98-100]. However, SRS follow ed by EGFR-TKI, an approach used in the study by Magnuson et al[61], is an alternative option to consider in these patients.
RECOMENDATIONS IN CLINICAL GUIDELINES
The oligometastatic state is based on the concept of long-term disease control, which is achieved through the aggressive local treatment of metastases. For this reason, clinical guidelines have already begun to incorporate local treatment techniques such as SBRT.
Spanish Society of Medical Oncology
The Spanish Society of Medical Oncology guidelines recommend local treatment (SBRT or surgery) in oligometastatic patients with up to five metastases, provided that all of lesions are susceptible to radical treatment[98].
The guidelines define four different clinical scenarios eligible for local treatment: (1) Metastases limited in number and location at the time of diagnosis. These metastases and the primary tumor must be susceptible to local treatment; (2) Limited number of metastases after primary treatment. All lesions must be susceptible to radical treatment; (3) Limited number of metastases that progress after systemic treatment; primary tumor and the other metastases remained controlled; and (4) Oligo-recurrence in patients who received radical-intent treatment, with one to five metachronous metastases susceptible to radical treatment.
The guidelines recommend that patients with oligometastatic disease at diagnosis receive radical treatment with systemic therapy plus local treatment to the primary tumor and metastases. This recommendation is based on two phase II studies showing that local treatment after systemic treatment improves PFS.
These guidelines also note that NSCLC patients who receive targeted therapy and develop progression can be treated with SBRT in order to continue receiving the targeted treatment. In patients with asymptomatic brain metastases susceptible to treatment with TKIs, the guidelines recommend starting with TKI therapy and postponing local treatment (surgery or radiosurgery).
National Comprehensive Cancer Network
The National Comprehensive Cancer Network guidelines include local treatment (SBRT or surgery) of metastases in well-selected patients with good ECOG performance status who have received radical treatment to the intrathoracic disease[101].
In patients with limited brain metastases, the guidelines recommend local treatment of these lesions (by surgery or radiosurgery). In patients with asymptomatic brain metastases with mutations, it is acceptable to continue with TKI, only administering local treatment if the lesions become symptomatic.
SBRT or surgery to the oligometastatic lesions (one to five metastases) is recommended provided that all sites can be safely treated. In patients who develop disease progression in a limited number of sites following systemic treatment, the use of SBRT may extend the duration of the benefit from that line of systemic treatment. These recommendations also apply to patients with driver mutations that progress to targeted therapy in some locations. In these cases, SBRT can be applied to allow the patient to continue with the same treatment. If SBRT is not feasible at the oligoprogression sites, the guidelines recommend using other hypofractionated treatments.
European Society for Medical Oncology
The European Society for Medical Oncology guidelines[99] recommend local treatment with SBRT or surgery in the following situations: (1) Patients with a limited number of brain metastases who fulfil criteria for class I-II recursive partitioning analysis (RPA). WBRT can be obviated if the patient is closely monitored using brain MRI after local treatment; (2) In patients with mutations (EGFR, ALK, etc) and asymptomatic brain metastases, TKI can be started and radiosurgery delayed; (3) Stage IV patients with extracranial oligometastases and one to three synchronous metastases. In this case, DFS may improve if systemic treatment is combined with local treatment (SBRT). The guidelines recommend presenting these cases to a multidisciplinary tumor board due to the current lack of evidence; (4) In patients with mutations who develop oligoprogression. SBRT or surgery should be considered to prolong DFS and to continue with systemic therapy; and (5) In single contralateral pulmonary lesions that would be considered as a second synchronous tumor and would be treated with curative intent if possible.
FUTURE DIRECTIONS AND CLINICAL TRIALS CURRENTLY UNDERWAY
Based on the current evidence, local treatment-either surgery or SBRT-of patients with oligometastatic NSCLC is associated with good survival rates with acceptable toxicity[33,34,102]. Numerous prospective studies are currently underway to corroborate these results. The following table summarizes the relevant trials that are currently registered in ClinicalTrials.gov (Table 3)[103].
Table 3.
Clinical trials in patients with oligometastatic non-small cell lung cancer
| Clinical trial identifier | Title | Objective |
| NCT01185639 | Stereotactic Body Radiation Therapy (SBRT) in Metastatic Non-Small Cell Lung Cancer | To evaluate the efficacy of SBRT in oligometastatic NSCLC patients who achieve partial response or stable disease after 4 cycles of ChT. The hypothesis is that SBRT after 4 cycles of ChT is feasible, safe, with good local control, and improves time to progression |
| NCT 02314364 | A Trial of Integrating SBRT With Targeted Therapy in Stage IV Oncogene-driven NSCLC | To evaluate SBRT in patients with stage 4 NSCLC and EGFR, ALK, or ROS1 mutations who have been treated with erlotinib, gefitinib, or other targeted drugs |
| NCT03275597 | Phase Ib Study of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Non-small Lung Cancer (NSCLC) With Dual Immune Checkpoint Inhibition | To evaluate the safety and tolerability of SBRT combined with durvalumab and tremelimumab for oligometastatic NSCLC |
| NCT02975609 | Phase II Trial of SBRT Compared With Conventional Radiotherapy for Oligometastatic Non-Small Cell Lung Cancer | To compare the efficacy and safety of SBRT vs conventional radiotherapy after response to 4-6 cycles of chemotherapy. Outcome measures for both groups include PFS, OS, and adverse effects |
| NCT03808337 | Investigating the Effectiveness of Stereotactic Body Radiotherapy (SBRT) in Addition to Standard of Care Treatment for Cancer That Has Spread Beyond the Original Site of Disease | To determine if SBRT to all oligometastatic lesions can extend time to progression |
| NCT02417662 | Stereotactic Ablative Radiotherapy for Oligometastatic Non-small Cell Lung Cancer (SARON) | Phase III study of efficacy and safety in oligometastatic NSCLC patients comparing conventional ChT alone to ChT plus conventional radiotherapy to the primary tumor and SBRT to the metastases |
SBRT: Stereotactic body radiation therapy; NSCLC: Non-small cell lung cancer; ChT: Chemotherapy; PFS: Progression-free survival; OS: Overall survival.
CONCLUSION
Oligometastatic NSCLC is a heterogeneous disease. These patients may present brain and body metastases, which can be synchronous or metachronous. Most studies performed to date have limited the number of metastases to no more than five lesions, thus limiting our ability to extrapolate results from those studies to patients with more widely disseminated disease. Nevertheless, due to the technological advances and improvements in the therapeutic strategy in recent years, this an exciting time for specialists who manage oligometastatic NSCLC. In recent years, numerous studies, including two randomized phase II trials, have demonstrated that local treatment, either radiotherapy or surgery, of the primary tumor and metastases improves PFS and OS in patients who present with oligometastatic NSCLC at diagnosis and in those who respond to the initial systemic therapy.
As we await the results of ongoing randomized phase III trials, the main international clinical guidelines recommend a multimodal strategy to manage this subgroup of oligometastatic patients. Current guidelines recommend systemic therapy combined with local treatment of the metastases and, if applicable, the primary tumor. However, the optimal therapeutic approach for the specific type of oligometastatic disease (intracranial vs extracranial, synchronous vs metachronous) remains to be defined. Given that no studies have been performed to compare radiotherapy to surgery in this setting, treatment selection (surgery vs radiotherapy, with or without systemic treatment) must be based on individualized prognostic factors, which allow us to classify patients in order to select the most appropriate therapeutic strategy for that particular patient. In the future, the availability of molecular or microRNA profiles is expected to improve patient selection.
Footnotes
Conflict-of-interest statement: The authors declare having no conflicts of interests related to this article.
Manuscript source: Invited manuscript
Peer-review started: March 19, 2019
First decision: August 2, 2019
Article in press: September 13, 2019
Specialty type: Oncology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Deng B, Mogulkoc R S-Editor: Ma RY L-Editor: Filipodia E-Editor: Liu MY
Contributor Information
Felipe Couñago, Department of Radiation Oncology, Hospital Universitario Quirónsalud Madrid, Pozuelo de Alarcón, Madrid 28223, Spain; Department of Radiation Oncology, Hospital La Luz, Madrid 28003, Spain; Clinical Department, Faculty of Biomedicine, Universidad Europea, Madrid 28223, Spain.
Javier Luna, Department of Radiation Oncology, Hospital Fundación Jiménez Díaz, Madrid 28040, Spain.
Luis Leonardo Guerrero, Department of Radiation Oncology, Hospital La Luz, Madrid 28003, Spain.
Blanca Vaquero, Department of Radiation Oncology, Hospital La Luz, Madrid 28003, Spain.
María Cecilia Guillén-Sacoto, Department of Medical Oncology, Hospital La Luz, Madrid 28003, Spain.
Teresa González-Merino, Department of Medical Oncology, Hospital La Luz, Madrid 28003, Spain.
Begoña Taboada, Department of Radiation Oncology, Complexo Hospitalario Universitario Santiago de Compostela, Santiago de Compostela 15706, Spain.
Verónica Díaz, Department of Radiation Oncology, Hospital Universitario Puerta del Mar, Cádiz 11009, Spain. fcounago@gmail.com.
Belén Rubio-Viqueira, Department of Medical Oncology, Hospital Universitario Quirónsalud Madrid, Pozuelo de Alarcón, Madrid 28223, Spain.
Ana Aurora Díaz-Gavela, Department of Radiation Oncology, Hospital Universitario Quirónsalud Madrid, Pozuelo de Alarcón, Madrid 28223, Spain; Department of Radiation Oncology, Hospital La Luz, Madrid 28003, Spain; Clinical Department, Faculty of Biomedicine, Universidad Europea, Madrid 28223, Spain.
Francisco José Marcos, Department of Radiation Oncology, Hospital Universitario Quirónsalud Madrid, Pozuelo de Alarcón, Madrid 28223, Spain; Department of Radiation Oncology, Hospital La Luz, Madrid 28003, Spain; Clinical Department, Faculty of Biomedicine, Universidad Europea, Madrid 28223, Spain.
Elia del Cerro, Department of Radiation Oncology, Hospital Universitario Quirónsalud Madrid, Pozuelo de Alarcón, Madrid 28223, Spain; Department of Radiation Oncology, Hospital La Luz, Madrid 28003, Spain; Clinical Department, Faculty of Biomedicine, Universidad Europea, Madrid 28223, Spain.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Juan O, Popat S. Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer. Clin Lung Cancer. 2017;18:595–606. doi: 10.1016/j.cllc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A, 3rd, Goldstraw P, Rami-Porta R International Association for Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2015;10:1515–1522. doi: 10.1097/JTO.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 4.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Bergsma DP, Salama JK, Singh DP, Chmura SJ, Milano MT. Radiotherapy for Oligometastatic Lung Cancer. Front Oncol. 2017;7:210. doi: 10.3389/fonc.2017.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, Congedo MT, Gomez DR, Wright GM, Melloni G, Milano MT, Sole CV, De Pas TM, Carter DL, Warner AJ, Rodrigues GB. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2014;15:346–355. doi: 10.1016/j.cllc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M, Gaspar LE, Schefter TE. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 8.Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–111. doi: 10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tönnies S, Tönnies M, Kollmeier J, Bauer TT, Förster GJ, Kaiser D, Wernecke KD, Pfannschmidt J. Impact of preoperative 18F-FDG PET/CT on survival of resected mono-metastatic non-small cell lung cancer. Lung Cancer. 2016;93:28–34. doi: 10.1016/j.lungcan.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Dinan MA, Curtis LH, Carpenter WR, Biddle AK, Abernethy AP, Patz EF, Jr, Schulman KA, Weinberger M. Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non-small-cell lung cancer, 1998-2003. J Clin Oncol. 2012;30:2725–2730. doi: 10.1200/JCO.2011.40.4392. [DOI] [PubMed] [Google Scholar]
- 11.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 13.De Ruysscher D, Wanders R, van Baardwijk A, Dingemans AM, Reymen B, Houben R, Bootsma G, Pitz C, van Eijsden L, Geraedts W, Baumert BG, Lambin P. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450) J Thorac Oncol. 2012;7:1547–1555. doi: 10.1097/JTO.0b013e318262caf6. [DOI] [PubMed] [Google Scholar]
- 14.Tönnies M, Pfannschmidt J, Bauer TT, Kollmeier J, Tönnies S, Kaiser D. Metastasectomy for synchronous solitary non-small cell lung cancer metastases. Ann Thorac Surg. 2014;98:249–256. doi: 10.1016/j.athoracsur.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KK, Rosen JE, Salazar MC, Boffa DJ. Outcomes of a Highly Selective Surgical Approach to Oligometastatic Lung Cancer. Ann Thorac Surg. 2016;102:1166–1171. doi: 10.1016/j.athoracsur.2016.04.086. [DOI] [PubMed] [Google Scholar]
- 16.Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer. 2010;69:251–258. doi: 10.1016/j.lungcan.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Lewis SL, Porceddu S, Nakamura N, Palma DA, Lo SS, Hoskin P, Moghanaki D, Chmura SJ, Salama JK. Definitive Stereotactic Body Radiotherapy (SBRT) for Extracranial Oligometastases: An International Survey of >1000 Radiation Oncologists. Am J Clin Oncol. 2017;40:418–422. doi: 10.1097/COC.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 18.Mehta N, Mauer AM, Hellman S, Haraf DJ, Cohen EE, Vokes EE, Weichselbaum RR. Analysis of further disease progression in metastatic non-small cell lung cancer: implications for locoregional treatment. Int J Oncol. 2004;25:1677–1683. [PubMed] [Google Scholar]
- 19.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, Salm M, Horswell S, Escudero M, Matthews N, Rowan A, Chambers T, Moore DA, Turajlic S, Xu H, Lee SM, Forster MD, Ahmad T, Hiley CT, Abbosh C, Falzon M, Borg E, Marafioti T, Lawrence D, Hayward M, Kolvekar S, Panagiotopoulos N, Janes SM, Thakrar R, Ahmed A, Blackhall F, Summers Y, Shah R, Joseph L, Quinn AM, Crosbie PA, Naidu B, Middleton G, Langman G, Trotter S, Nicolson M, Remmen H, Kerr K, Chetty M, Gomersall L, Fennell DA, Nakas A, Rathinam S, Anand G, Khan S, Russell P, Ezhil V, Ismail B, Irvin-Sellers M, Prakash V, Lester JF, Kornaszewska M, Attanoos R, Adams H, Davies H, Dentro S, Taniere P, O'Sullivan B, Lowe HL, Hartley JA, Iles N, Bell H, Ngai Y, Shaw JA, Herrero J, Szallasi Z, Schwarz RF, Stewart A, Quezada SA, Le Quesne J, Van Loo P, Dive C, Hackshaw A, Swanton C TRACERx Consortium. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 20.Jones CM, Brunelli A, Callister ME, Franks KN. Multimodality Treatment of Advanced Non-small Cell Lung Cancer: Where are we with the Evidence? Curr Surg Rep. 2018;6:5. doi: 10.1007/s40137-018-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasselle MD, Haraf DJ, Rusthoven KE, Golden DW, Salgia R, Villaflor VM, Shah N, Hoffman PC, Chmura SJ, Connell PP, Vokes EE, Weichselbaum RR, Salama JK. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol. 2012;7:376–381. doi: 10.1097/JTO.0b013e31824166a5. [DOI] [PubMed] [Google Scholar]
- 22.Lopez Guerra JL, Gomez D, Zhuang Y, Hong DS, Heymach JV, Swisher SG, Lin SH, Komaki R, Cox JD, Liao Z. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int J Radiat Oncol Biol Phys. 2012;84:e61–e67. doi: 10.1016/j.ijrobp.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mordant P, Arame A, De Dominicis F, Pricopi C, Foucault C, Dujon A, Le Pimpec-Barthes F, Riquet M. Which metastasis management allows long-term survival of synchronous solitary M1b non-small cell lung cancer? Eur J Cardiothorac Surg. 2012;41:617–622. doi: 10.1093/ejcts/ezr042. [DOI] [PubMed] [Google Scholar]
- 24.Khan AJ, Mehta PS, Zusag TW, Bonomi PD, Penfield Faber L, Shott S, Abrams RA. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC) Radiother Oncol. 2006;81:163–167. doi: 10.1016/j.radonc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Griffioen GH, Toguri D, Dahele M, Warner A, de Haan PF, Rodrigues GB, Slotman BJ, Yaremko BP, Senan S, Palma DA. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer. 2013;82:95–102. doi: 10.1016/j.lungcan.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Merino Lara T, Helou J, Poon I, Sahgal A, Chung HT, Chu W, Soliman H, Ung Y, Verma S, Cheema P, Cheng S, Khanna S, Erler D, Zhang L, Cheung P. Multisite stereotactic body radiotherapy for metastatic non-small-cell lung cancer: Delaying the need to start or change systemic therapy? Lung Cancer. 2018;124:219–226. doi: 10.1016/j.lungcan.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, Zhou C. Consolidative Local Ablative Therapy Improves the Survival of Patients With Synchronous Oligometastatic NSCLC Harboring EGFR Activating Mutation Treated With First-Line EGFR-TKIs. J Thorac Oncol. 2018;13:1383–1392. doi: 10.1016/j.jtho.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Hu F, Xu J, Zhang B, Li C, Nie W, Gu P, Hu P, Wang H, Zhang Y, Shen Y, Wang S, Zhang X. Efficacy of Local Consolidative Therapy for Oligometastatic Lung Adenocarcinoma Patients Harboring Epidermal Growth Factor Receptor Mutations. Clin Lung Cancer. 2019;20:e81–e90. doi: 10.1016/j.cllc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, Villaflor VM, Stadler WM, Hoffman PC, Cohen EE, Connell PP, Haraf DJ, Vokes EE, Hellman S, Weichselbaum RR. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 30.De Ruysscher D, Wanders R, Hendriks LE, van Baardwijk A, Reymen B, Houben R, Bootsma G, Pitz C, van Eijsden L, Dingemans AC. Progression-Free Survival and Overall Survival Beyond 5 Years of NSCLC Patients With Synchronous Oligometastases Treated in a Prospective Phase II Trial ( NCT 01282450) J Thorac Oncol. 2018;13:1958–1961. doi: 10.1016/j.jtho.2018.07.098. [DOI] [PubMed] [Google Scholar]
- 31.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, Pulipparacharuvil S, Choy H, Timmerman RD. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018;4:e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez DR, Blumenschein GR, Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE, Gibbons DL, Karam JA, Kavanagh BD, Tang C, Komaki R, Louie AV, Palma DA, Tsao AS, Sepesi B, William WN, Zhang J, Shi Q, Wang XS, Swisher SG, Heymach JV. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez DR, Tang C, Zhang J, Blumenschein GR, Hernandez M, Lee JJ, Ye R, Camidge DR, Skoulidis F, Doebele R, Gaspar LE, Gibbons DL, Karam J, Kavanagh BD, Palma DA, Louie AV, Tsao A, Sepesi B, Swisher SG, Heymach J. Local Consolidative Therapy (LCT) Improves Overall Survival (OS) Compared to Maintenance Therapy/Observation in Oligometastatic Non-Small Cell Lung Cancer (NSCLC): Final Results of a Multicenter, Randomized, Controlled Phase 2 Trial. Int J Radiat Oncol. 2018;102:1604. [Google Scholar]
- 34.Palma DA, Olson RA, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy LA, Lock MI, Rodrigues G, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Liu MC, Moore K, Currie S, Bauman GS, Warner A, Senan S. Stereotactic Ablative Radiation Therapy for the Comprehensive Treatment of Oligometastatic Tumors (SABR-COMET): Results of a Randomized Trial. Int J Radiat Oncol. 2018;102:S3–4. [Google Scholar]
- 35.Sutera P, Kalash R, Clump DA, D'Ambrosio D, Mihai A, Burton SA, Heron DE. Stereotactic Ablative Radiation Therapy for Unresectable Colorectal Oligometastases. Adv Radiat Oncol. 2018;4:57–62. doi: 10.1016/j.adro.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petty WJ, Urbanic JJ, Ahmed T, Hughes R, Levine B, Rusthoven K, Papagikos M, Ruiz JR, Lally BE, Chan M, Clark H, D'Agostino RB, Jr, Blackstock AW. Long-Term Outcomes of a Phase 2 Trial of Chemotherapy With Consolidative Radiation Therapy for Oligometastatic Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2018;102:527–535. doi: 10.1016/j.ijrobp.2018.06.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh RB, Cronin AM, Kozono DE, Oxnard GR, Mak RH, Jackman DM, Lo PC, Baldini EH, Johnson BE, Chen AB. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;89:880–887. doi: 10.1016/j.ijrobp.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–1626. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 39.Dai C, Ren Y, Xie D, Zheng H, She Y, Fei K, Jiang G, Chen C. Does Lymph Node Metastasis Have a Negative Prognostic Impact in Patients with NSCLC and M1a Disease? J Thorac Oncol. 2016;11:1745–1754. doi: 10.1016/j.jtho.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 40.Milano MT, Vokes EE, West HJ, Salama JK. Oligometastatic non-small cell lung cancer - UpToDate [Internet] Available from: https://www.uptodate.com/contents/oligometastatic-non-small-cell-lung-cancer?search=oligometastatico&source=search_result&selectedTitle=1~69&usage_type=default&display_rank=1.
- 41.Collaud S, Stahel R, Inci I, Hillinger S, Schneiter D, Kestenholz P, Weder W. Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer. 2012;78:234–238. doi: 10.1016/j.lungcan.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Divisi D, Barone M, Zaccagna G, Gabriele F, Crisci R. Surgical approach in the oligometastatic patient. Ann Transl Med. 2018;6:94. doi: 10.21037/atm.2018.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franceschini D, De Rose F, Franzese C, Comito T, Di Brina L, Radicioni G, Evangelista A, D'Agostino GR, Navarria P, Scorsetti M. Predictive Factors for Response and Survival in a Cohort of Oligometastatic Patients Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2019;104:111–121. doi: 10.1016/j.ijrobp.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 44.Frost N, Tessmer A, Schmittel A, van Laak V, Raspe M, Ruwwe-Glösenkamp C, Brunn M, Senger C, Böhmer D, Ochsenreither S, Temmesfeld-Wollbrück B, Furth C, Schmidt B, Neudecker J, Rückert JC, Suttorp N, Witzenrath M, Grohé C. Local ablative treatment for synchronous single organ oligometastatic lung cancer-A propensity score analysis of 180 patients. Lung Cancer. 2018;125:164–173. doi: 10.1016/j.lungcan.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Zhu R, Li D, Li N, Zhu X. Prognostic factors of oligometastatic non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2018;10:3701–3713. doi: 10.21037/jtd.2018.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lussier YA, Xing HR, Salama JK, Khodarev NN, Huang Y, Zhang Q, Khan SA, Yang X, Hasselle MD, Darga TE, Malik R, Fan H, Perakis S, Filippo M, Corbin K, Lee Y, Posner MC, Chmura SJ, Hellman S, Weichselbaum RR. MicroRNA expression characterizes oligometastasis(es) PLoS One. 2011;6:e28650. doi: 10.1371/journal.pone.0028650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 48.Ostrom QT, Wright CH, Barnholtz-Sloan JS. Brain metastases: epidemiology. Handb Clin Neurol. 2018;149:27–42. doi: 10.1016/B978-0-12-811161-1.00002-5. [DOI] [PubMed] [Google Scholar]
- 49.Villaruz LC, Kubicek GJ, Socinski MA. Management of non-small cell lung cancer with oligometastasis. Curr Oncol Rep. 2012;14:333–341. doi: 10.1007/s11912-012-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu C, Chang EL, Hassenbusch SJ, 3rd, Allen PK, Woo SY, Mahajan A, Komaki R, Liao Z. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006;106:1998–2004. doi: 10.1002/cncr.21818. [DOI] [PubMed] [Google Scholar]
- 51.Lamba N, Muskens IS, DiRisio AC, Meijer L, Briceno V, Edrees H, Aslam B, Minhas S, Verhoeff JJC, Kleynen CE, Smith TR, Mekary RA, Broekman ML. Stereotactic radiosurgery versus whole-brain radiotherapy after intracranial metastasis resection: a systematic review and meta-analysis. Radiat Oncol. 2017;12:106. doi: 10.1186/s13014-017-0840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, Settle S, Prabhu SS, Lang FF, Levine N, McGovern S, Sulman E, McCutcheon IE, Azeem S, Cahill D, Tatsui C, Heimberger AB, Ferguson S, Ghia A, Demonte F, Raza S, Guha-Thakurta N, Yang J, Sawaya R, Hess KR, Rao G. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ., Jr Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 54.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 55.Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 56.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 57.Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299–307. doi: 10.1007/s11060-007-9510-4. [DOI] [PubMed] [Google Scholar]
- 58.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T ALEX Trial Investigators. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 59.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 60.Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, Geater SL, Orlov S, Cortinovis D, Yu CJ, Hochmair M, Cortot AB, Tsai CM, Moro-Sibilot D, Campelo RG, McCulloch T, Sen P, Dugan M, Pantano S, Branle F, Massacesi C, de Castro G., Jr First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 61.Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, Beal K, Amini A, Patil T, Kavanagh BD, Camidge DR, Braunstein SE, Boreta LC, Balasubramanian SK, Ahluwalia MS, Rana NG, Attia A, Gettinger SN, Contessa JN, Yu JB, Chiang VL. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol. 2017;35:1070–1077. doi: 10.1200/JCO.2016.69.7144. [DOI] [PubMed] [Google Scholar]
- 62.Bazhenova L, Newton P, Mason J, Bethel K, Nieva J, Kuhn P. Adrenal metastases in lung cancer: clinical implications of a mathematical model. J Thorac Oncol. 2014;9:442–446. doi: 10.1097/JTO.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradley CT, Strong VE. Surgical management of adrenal metastases. J Surg Oncol. 2014;109:31–35. doi: 10.1002/jso.23461. [DOI] [PubMed] [Google Scholar]
- 64.Strong VE, D'Angelica M, Tang L, Prete F, Gönen M, Coit D, Touijer KA, Fong Y, Brennan MF. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol. 2007;14:3392–3400. doi: 10.1245/s10434-007-9520-7. [DOI] [PubMed] [Google Scholar]
- 65.Plichta K, Camden N, Furqan M, Hejleh TA, Clamon GH, Zhang J, Flynn RT, Bhatia SK, Smith MC, Buatti JM, Allen BG. SBRT to adrenal metastases provides high local control with minimal toxicity. Adv Radiat Oncol. 2017;2:581–587. doi: 10.1016/j.adro.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scorsetti M, Alongi F, Filippi AR, Pentimalli S, Navarria P, Clerici E, Castiglioni S, Tozzi A, Reggiori G, Mancosu P, Ricardi U. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: a retrospective analysis of 34 patients. Acta Oncol. 2012;51:618–623. doi: 10.3109/0284186X.2011.652738. [DOI] [PubMed] [Google Scholar]
- 67.Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187:245–251. doi: 10.1007/s00066-011-2192-z. [DOI] [PubMed] [Google Scholar]
- 68.Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, Bonucci I, Agresti B, Simontacchi G, Doro R. Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys. 2012;82:919–923. doi: 10.1016/j.ijrobp.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Shi Z, Wang Z, Liu Z, Wu X, Shen Z, Li B, Song Y, Zhu X. Treating adrenal tumors in 26 patients with CyberKnife: a mono-institutional experience. PLoS One. 2013;8:e80654. doi: 10.1371/journal.pone.0080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahadevan A, Blanck O, Lanciano R, Peddada A, Sundararaman S, D'Ambrosio D, Sharma S, Perry D, Kolker J, Davis J. Stereotactic Body Radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch® Patient Registry. Radiat Oncol. 2018;13:26. doi: 10.1186/s13014-018-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kennedy A. Radioembolization of hepatic tumors. J Gastrointest Oncol. 2014;5:178–189. doi: 10.3978/j.issn.2078-6891.2014.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahnken AH, Pereira PL, de Baère T. Interventional oncologic approaches to liver metastases. Radiology. 2013;266:407–430. doi: 10.1148/radiol.12112544. [DOI] [PubMed] [Google Scholar]
- 73.Rhim H, Goldberg SN, Dodd GD, 3rd, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21 Spec No:S17–35; discussion S36-9. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. [DOI] [PubMed] [Google Scholar]
- 74.Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 75.Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, Kiil Berthelsen A, Grau C, Aage Engelholm S, Von der Maase H. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 76.Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, Nuyttens JJ, Brandwijk RP, Verhoef C, Ijzermans JN, Levendag PC. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 77.Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, Cummings B, Ringash J, Tse RV, Knox JJ, Dawson LA. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 78.Ambrosino G, Polistina F, Costantin G, Francescon P, Guglielmi R, Zanco P, Casamassima F, Febbraro A, Gerunda G, Lumachi F. Image-guided robotic stereotactic radiosurgery for unresectable liver metastases: preliminary results. Anticancer Res. 2009;29:3381–3384. [PubMed] [Google Scholar]
- 79.Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, Gibbs IC, Fisher GA, Koong AC. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 80.Rule W, Timmerman R, Tong L, Abdulrahman R, Meyer J, Boike T, Schwarz RE, Weatherall P, Chinsoo Cho L. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081–1087. doi: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 81.Scorsetti M, Arcangeli S, Tozzi A, Comito T, Alongi F, Navarria P, Mancosu P, Reggiori G, Fogliata A, Torzilli G, Tomatis S, Cozzi L. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys. 2013;86:336–342. doi: 10.1016/j.ijrobp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 82.Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, Johnston M, McCormack P, Pass H, Putnam JB, Jr International Registry of Lung Metastases. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 83.Nakamura H, Kazuyuki S, Kawasaki N, Taguchi M, Kato H. History of limited resection for non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 2005;11:356–362. [PubMed] [Google Scholar]
- 84.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22; discussion 622-3. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 85.De Leyn P, Moons J, Vansteenkiste J, Verbeken E, Van Raemdonck D, Nafteux P, Decaluwe H, Lerut T. Survival after resection of synchronous bilateral lung cancer. Eur J Cardiothorac Surg. 2008;34:1215–1222. doi: 10.1016/j.ejcts.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 86.Voltolini L, Rapicetta C, Luzzi L, Ghiribelli C, Paladini P, Granato F, Gallazzi M, Gotti G. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. Eur J Cardiothorac Surg. 2010;37:1198–1204. doi: 10.1016/j.ejcts.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 87.Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 88.Iyengar P, Kavanagh BD, Wardak Z, Smith I, Ahn C, Gerber DE, Dowell J, Hughes R, Abdulrahman R, Camidge DR, Gaspar LE, Doebele RC, Bunn PA, Choy H, Timmerman R. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol. 2014;32:3824–3830. doi: 10.1200/JCO.2014.56.7412. [DOI] [PubMed] [Google Scholar]
- 89.Wood TJ, Racano A, Yeung H, Farrokhyar F, Ghert M, Deheshi BM. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081–4089. doi: 10.1245/s10434-014-4002-1. [DOI] [PubMed] [Google Scholar]
- 90.Bedard G, McDonald R, Poon I, Erler D, Soliman H, Cheung P, Chung H, Chu W, Loblaw A, Chow E, Sahgal A. Stereotactic body radiation therapy for non-spine bone metastases--a review of the literature. Ann Palliat Med. 2016;5:58–66. doi: 10.3978/j.issn.2224-5820.2015.07.01. [DOI] [PubMed] [Google Scholar]
- 91.Huo M, Sahgal A, Pryor D, Redmond K, Lo S, Foote M. Stereotactic spine radiosurgery: Review of safety and efficacy with respect to dose and fractionation. Surg Neurol Int. 2017;8:30. doi: 10.4103/2152-7806.200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jereczek-Fossa BA, Ronchi S, Orecchia R. Is Stereotactic Body Radiotherapy (SBRT) in lymph node oligometastatic patients feasible and effective? Rep Pract Oncol Radiother. 2015;20:472–483. doi: 10.1016/j.rpor.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li D, Zhu X, Wang H, Qiu M, Li N. Should aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysis. J Thorac Dis. 2017;9:310–317. doi: 10.21037/jtd.2017.02.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrelli F, Ghidini A, Cabiddu M, Tomasello G, De Stefani A, Bruschieri L, Vitali E, Ghilardi M, Borgonovo K, Barni S, Trevisan F. Addition of radiotherapy to the primary tumour in oligometastatic NSCLC: A systematic review and meta-analysis. Lung Cancer. 2018;126:194–200. doi: 10.1016/j.lungcan.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 95.Arrieta O, Barrón F, Maldonado F, Cabrera L, Corona-Cruz JF, Blake M, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, García O, Arén O, De la Garza J. Radical consolidative treatment provides a clinical benefit and long-term survival in patients with synchronous oligometastatic non-small cell lung cancer: A phase II study. Lung Cancer. 2019;130:67–75. doi: 10.1016/j.lungcan.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 96.Ou SH, Jänne PA, Bartlett CH, Tang Y, Kim DW, Otterson GA, Crinò L, Selaru P, Cohen DP, Clark JW, Riely GJ. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25:415–422. doi: 10.1093/annonc/mdt572. [DOI] [PubMed] [Google Scholar]
- 97.Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA, Jr, Aisner DL, Gaspar LE, Kavanagh BD, Doebele RC, Camidge DR. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Majem M, Juan O, Insa A, Reguart N, Trigo JM, Carcereny E, García-Campelo R, García Y, Guirado M, Provencio M. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018) Clin Transl Oncol. 2019;21:3–17. doi: 10.1007/s12094-018-1978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:863–870. doi: 10.1093/annonc/mdy474. [DOI] [PubMed] [Google Scholar]
- 100.Churilla TM, Weiss SE. Emerging Trends in the Management of Brain Metastases from Non-small Cell Lung Cancer. Curr Oncol Rep. 2018;20:54. doi: 10.1007/s11912-018-0695-9. [DOI] [PubMed] [Google Scholar]
- 101.National Comprehensive Cancer Network Clinical Practice Guidelines. Non-Small Cell Lung Cancer (Version 3. 2019) Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 102.Fleckenstein J, Petroff A, Schäfers HJ, Wehler T, Schöpe J, Rübe C. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer. 2016;16:348. doi: 10.1186/s12885-016-2379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.ClinicalTrials. gov. [Accessed on 03.09.19] Available from: https://clinicaltrials.gov.
- 104.Collen C, Christian N, Schallier D, Meysman M, Duchateau M, Storme G, De Ridder M. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol. 2014;25:1954–1959. doi: 10.1093/annonc/mdu370. [DOI] [PubMed] [Google Scholar]