Abstract
Crohn’s disease (CD) is an inflammatory bowel disease, which can involve any region of the gastrointestinal tract. First described in 1932 as terminal ileitis or regional enteritis, it predominately involves the ileum with or without colonic involvement. Isolated colonic CD was first described in 1960 and since then the phenotypic classification of CD has evolved to stratify patients into isolated ileal, ileocolonic, or isolated colonic involvement. In the current review we evaluate the published literature regarding differences in epidemiology, natural history, pathogenesis, response to therapy, and disease monitoring, when stratified by disease location. Based on the available evidence consideration could be given to a new classification for CD, which splits it into ileum dominant (isolated ileal and ileocolonic) and isolated colonic disease. This may allow for a more optimized approach to clinical care and scientific research for CD.
Keywords: Crohn’s disease, classification, ileal, colonic
INTRODUCTION
Crohn’s disease (CD) is characterized by the transmural inflammation anywhere along the gastrointestinal tract. Morgagni recognized the condition as early as 1761, but it wasn’t until 1932 that Burrill Bernard Crohn first submitted the condition to the American Medical Association for consideration as a unique disease under the entity of terminal ileitis.(1) Later that year he changed the classification to regional ileitis,(2) and eventually the disease was re-named Crohn’s disease. The first case series of CD were actually published in 1913 by Thomas Kennedy Dalziel where he reported on 9 cases, some of which had involvement of the colon. In fact, it is suspected that one of the original reports of ulcerative colitis in 1859 by Wilks may have actually been colonic CD.(1) In 1960 Hugh Evelyn Lockhard-Mummery published a series of cases distinguishing colonic CD from ulcerative colitis,(3) and from there the disease was grouped into a common disease of Crohn’s disease and subsequently clinically stratified by location: terminal ileum (L1), colon (L2), ileocolonic (L3), and upper GI location (L4). This classification evolved in an effort to stratify patients at higher risk for disease progression, and to identify those who would most benefit from early combination therapy with immunosuppressants and biologics. Although disease location remains stable over time after diagnosis;(4) distinct differences exist in the presentation and risk for progression or complications based on location, which may influence management decisions. In this review we will highlight those differences and how disease location, namely the presence or absence of ileal involvement, impacts response to therapy and outcomes, and some pathophysiological considerations for possibly transitioning towards a classification of ileum-dominant versus isolated colonic CD.
EPIDEMIOLOGY AND NATURAL HISTORY
Prevalence and demographics
Isolated colonic involvement occurs in up to one-third of CD patients, with recent trends suggesting a significant decline in prevalence (p=0.02).(5) Recent analyses from the IBD Genetic Consortium observed that among late onset CD patients (> 55 years of age), the prevalence of colonic CD was greater than ileal CD.(6) A notable observation is that there is a higher prevalence of smokers with ileal CD as compared with colonic CD (50% vs. 38%, p<0.01).(5, 7) Smoking quantity and behavior has been associated with risk for development of CD, and among those with established CD it is associated with an increased risk for disease-related complications,(7–9) which are seen more often in ileal CD versus colonic CD as discussed below. The prevalence and negative effects of smoking of smoking in CD is higher and more pronounced in female CD patients.(7, 10, 11) Therefore it would be expected that ileal CD might have a female predominance but in fact it is isolated colonic CD which has a female predominance.(5, 12–14) Furthermore, oral contraceptive use by females has been associated with the development of CD, but this association is strongest for colonic CD.(5, 15)
Clinical presentation
The Swiss IBD Cohort Study Group found that ileal disease location was associated with a significantly increased risk for > 24 month delay in diagnosis (OR 1.69, p=0.025).(16) This delay in diagnosis for ileal CD may be related to the fact that there is a stronger correlation between symptoms and disease activity in colonic CD, and therefore ileal CD patients do not present until symptoms of complications have developed due to differences in bowel diameter.(17)
Ileal CD is more often associated with the development of perianal disease and isolated colonic CD is more often associated with extra-intestinal manifestations.(5, 18) The increased prevalence of arthritis/arthralgias and ocular manifestations with colonic CD is thought to occur as a result of a shared and unique peptide in the human colon, eye, and joint.(19) It should be noted, however, that spondylitis is more often associated with ileal inflammation including sub-clinical ileal inflammation.(5, 18) Erythema nodosum and pyoderma gangrenosum are frequently associated with eye and joint involvement, along with isolated colonic involvement.(19, 20) The development of extra-intestinal manifestations is also more often seen in smokers and female IBD patients.(21, 22) The development of primary sclerosing cholangitis (PSC) specifically in CD is strongly associated with a colonic disease location, and these patients more often have a non-stricturing and non-penetrating disease phenotype (a phenotype more closely resembling ulcerative colitis).(23–25) Notably, a negative association between smoking and PSC has been observed, however, it is unclear if this simply a function of varying prevalence for smoking between ileal and colonic CD patients.(10)
Clinical course
A population-based cohort study (1970–2004) observed that relative to isolated colonic involvement, isolated ileal involvement was associated with a 7-fold (HR 7.76) increased risk for change in disease behavior and 9-fold (HR 9.25) increased risk for developing an intestinal complication.(26) CD patients with ileocolonic involvement were observed to have a risk for change in disease behavior (HR 5.63) and intestinal complication (HR 5.74) closer to that of patients with isolated ileal involvement.(26) A recent prediction tool observed that, after accounting for the NOD2 mutation, perianal disease, and CBir1 positivity, small bowel CD was associated with a 2-fold increased risk for a complication (HR 2.12), and left sided colonic CD was associated with approximately 30% reduction in risk for a complication (HR 0.73).(27) A post-hoc analysis of the REACT adult CD trial similarly observed that ileocolonic and isolated ileal disease location carried a 2 fold increased risk (vs. colonic disease) for disease-related complications or surgery over a 24 month period.(28)
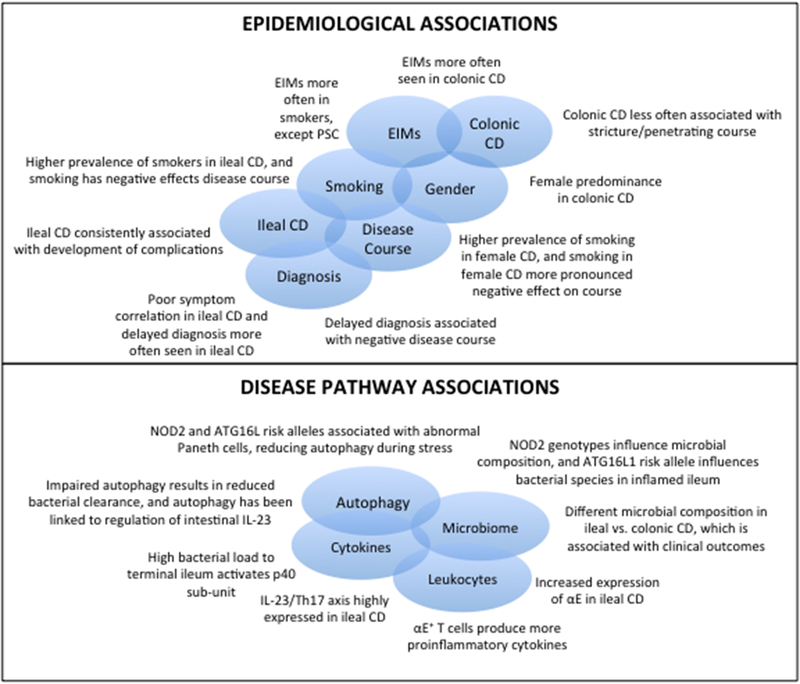
Taken together, there appear to be consistent epidemiological associations across studies that begin to shape the viewpoint that ileal and colonic CD have different natural history, and that potentially, considering them as separate diseases may enhance future research in understanding the pathogenesis of CD. (Figure 1) However, several important epidemiological questions remain which require further investigation. (Table 1)
Figure 1:
Epidemiological and Pathogenic Associations Supporting Splitting of Crohn’s disease into Ileal-Dominant and Isolated Colonic Crohn’s disease
Table 1:
Notable Observations and Further Scientific Questions Remaining
| Observation | Questions Remaining |
|---|---|
| Female CD are more often smokers, smoking is associated with increased risks for disease complications, and the negative effects of smoking on disease course are more pronounced in female CD patients. There is a higher prevalence of smokers in ileal CD and ileal CD itself is also associated with an increased risk for disease complications. However, isolated colonic CD is female predominant and isolated colonic CD more often has a benign disease course. | Are female isolated colonic CD patients more often non-smokers and how does smoking influence the disease course in colonic CD and ileal CD separately? Do female ileal CD patients who smoke represent the highest risk sub-group for complications? |
| EIMs are more often seen in colonic CD, female CD, and in smokers, however, there is a lower prevalence of smokers among colonic CD patients. Furthermore, PSC specifically is strongly associated with colonic CD yet smoking appears to have a negative association with the development of PSC. | How does smoking and gender influence the development of EIMs in colonic CD and ileal CD separately and is the negative association between smoking and PSC true or a function of varying prevalence for smoking in ileal and colonic CD? |
| Ileal CD is associated with increased risk for delayed diagnosis, and delayed diagnosis is associated with risk for disease progression and complications at presentation. | Are delays in diagnosis for ileal CD related to variability in clinical manifestations and poor correlations between symptoms and disease activity in ileal CD relative to colonic CD? What are early clinical markers of ileal CD that could be used to avoid delays in diagnosis and allow for early therapeutic interventions to offset the natural history and risk for complications? |
| NOD2 and ATG16L risk alleles are associated with abnormal Paneth cells and reductions in autophagy during stress. When combined with abnormal unfolded protein response through Xbp1 deletion, this has been shown to result in spontaneous ileitis in animal models, supporting its role in the pathogenesis of ileal CD. Furthermore, targeting autophagy through mTOR inhibition has been observed to improve CD activity. | Does targeting mTOR inhibition have relatively greater effectiveness in improving symptoms in ileal vs. colonic CD, and what impact does it have on long-term risks of disease-related complications given the association between NOD2 and disease-related complications? |
| Impaired autophagy results in reduced bacterial clearance, and this predominately impacts Paneth cells and small intestine. The high bacterial load to the small intestine has been shown to activate the p40 sub-unit of the IL-23 pathway, and the IL-23/Th17 axis has been observed to be highly expressed in ileal CD. | What is the differential impact of therapies targeting IL-23 in ileal and colonic CD, and how does concomitant mTOR inhibition impact treatment effectiveness and reductions in disease-related complications? |
| αE+ T cells produce more proinflammatory cytokines and αE integrin expression is increased in ileal CD. αEβ7 expressing T-lymphocytes are present in highest numbers in the ileum and α4β1-VCAM-1 axis is essential for T effector cell homing to the ileum in CD. | What is the association between integrin expression and future risk of disease complications across disease location sub-types? |
| Differential microbial composition in ileal and colonic CD, with specific species being associated with clinical outcomes. NOD2 genotype influences microbial composition and specific gene expression events involved in acute inflammatory responses to microbes are associated with a risk for penetrating disease complications. | How do shifts in microbial composition influence longitudinal risk for disease-related complications in ileal and colonic CD and can microbiome manipulation impact this natural history? |
PATHOGENESIS
Colonic CD lays genetically midway between ileal CD and UC, with specific genes being predominantly associated with ileal vs. colonic disease.(5, 29, 30) Several distinct differences in key disease pathways have been observed between ileal and colonic CD.
Autophagy
Autophagy is important for clearance of intracellular microorganisms, and is interrelated with endoplasmic reticulum stress. GWAS studies identified ATG16L1 as the first autophagy gene to be associated with CD.(31) The risk-associated SNP in ATG16L1 is a missense mutation that leads to reduced amounts of ATG16L1 protein within cells, thereby reducing autophagy during periods of stress (inflammation), resulting in reduced clearance of intracellular pathogens and increased cytokine production.(32) The disease-associated reduction in ATG16L1 expression impacts Paneth cells (located in the small intestine), a key component of mucosal defense. Mice with reduced ATG16L1 expression have been observed to have morphologically-abnormal Paneth cells with reductions in the number and size of secretory granules,(33) and when the unfolded protein response is compromised through deletion of Xbp1 simultaneously with impaired autophagy in Paneth cells, a CD-like spontaneous ileitis develops.(34) A study of 119 CD patients observed that abnormalities in Paneth cells were associated with the number of CD-associated NOD2 risk alleles, and the cumulative number of NOD2 and ATG16L1 risk alleles had additive effects on the proportion of abnormal Paneth cells. Patients with >20% of Paneth cells being abnormal had shorter times to disease recurrence and progression, particularly after surgery.(35) In support of targeting Paneth cell abnormalities and potentially autophagy is a small case series using sirolimus, a mTOR inhibitor that upregulates autophagy, to alleviate CD activity.(36) A prior randomized controlled trial also demonstrated that everolimus, another selective mTOR inhibitor, was comparable to azathioprine for maintaining steroid-free remission in moderate-severe CD (although unfortunately the authors did not report subgroup analyses based on disease location).(37)
Th17 Pathway
The importance of the IL-23/IL-17-Th17 axis in CD has been well established.(38) Serum IL-22 levels are increased in CD, associated with disease activity and severity, independent of NOD2 status, and modulated by IL23R polymorphisms.(39) Thus, serum IL-22 levels serve as a potential marker of Th17 cell activity in CD patients. Early studies observing a correlation between IL-22 and disease activity noted that the highest levels of IL-22 mRNA in intestinal epithelial cells were observed in CD patients with ileal involvement.(40) CD patients also exhibit increased mucosal IL-12p35 in the non-inflamed ileum.(41) IL-17A+ IFN-γ+ and IL-22+ IFN-γ+ T cell subsets have been shown to accumulate specifically in the inflamed terminal ileum of CD patients,(42) and the correlation between IL-17A and IFN-γ with IL-23p19 have been shown to be specific to the ileal mucosa.(41) Animal studies have observed that a high bacterial load in the terminal ileum activates p40 gene transcription in lamina propria dendritic cells through NF-kappaB, suggesting a predisposition of the terminal ileum to develop chronic inflammatory responses through IL-23.(43) Furthermore, sequential longitudinal data have shown that a transition from histologic to endoscopic disease activity in the neo-terminal ileum is marked by an abundance of Th1 cytokines, an increase in IL-17A, and the induction of IL-6 and IL-23. By contrast, a mixed Th1/Th17 response was seen with no tumor necrosis factor (TNF)-α induction in samples with established lesions.(44) Subclinical intestinal inflammation seen in ankylosing spondylitis and CD is also marked by a strong and significant up-regulation of IL-23p19 transcripts in the terminal ileum.(45) In these patients it was also observed that Paneth cells were a major source of IL-23, however, only in CD was the IL-23 associated with up-regulation of IL-17 as well as IL-17-inducing cytokines such as IL-6 and IL-1beta along with overexpression of IL-12p35 and IFN-γ observed.(45) A recent clinical trial for risankizumab (anti-IL-23) demonstrated that IL-22 gene expression was significantly reduced in ileal biopsies from treated patients and there was a reduction in serum IL-22 levels, which correlated with the clinical response.(46)
Leukocyte Trafficking
The integrin α4β7 binds to mucosal addressin cell adhesion molecule-1 (MAdCAM-1), whereas integrin α4β1 binds to vascular cell adhesion molecule-1 (VCAM-1), and fibronectin within the gut. Integrin α4β7 is the proposed major gut homing receptor. αEβ7 binds to E-cadherin and is thought to impact T cell retention within the gut. αE+ T-cells have been shown to produce more proinflammatory cytokines,(47) αE integrin expression is increased in the ileum relative to the colon in CD, and this association is unaffected by disease activity or concomitant medication use.(48) αEβ7 expressing T-lymphocytes are present in highest numbers in the ileum, with a descending gradient along the intestine and colon. α4β7 expressing T-lymphocytes, in contrast, are fewest in the ileum and more abundant in the descending colon.(49) The α4β1-VCAM-1 axis is essential for T effector cell homing to the ileum in CD, and accordingly α4 and α4β1 blockade reduce ileal homing; by contrast α4β7 blockade has less impact on homing.(50) This might lead to the hypothesis that anti-integrins targeting α4 or β7 individually would be more effective for treating both ileal and colonic CD, whereas those selectively targeting α4β7 might be less effective for ileal involvement.
The anti-integrin biologics approved or currently under investigation target the α4 (natalizumab, anti-α4; α4β1 and α4β7), α4β7 (vedolizumab and abrilumab), and β7 (etrolizumab, anti-β7; α4β7 and αEβ7) integrin sub-units. Natalizumab could have been a potentially highly effective therapy for ileal CD given its targeting of the α4 sub-unit, but its use is limited by the development of life-threatening progressive multi-focal leukoencephalopathy (PML) and is now only available through special access programs. In ulcerative colitis, an association between αE expression and response to etrolizumab has been observed,(51) and it can be anticipated that a similar observation may be made in ileal CD given the abundance of αEβ7 expressing T-lymphocytes in the ileum, however, proof of concept for this stratified approach within ileal CD is needed.
Microbiome
Microbial community profiles have been shown to be significantly different between ileal and colonic CD.(52–54) This distinct separation is independent of biopsy site, inflammatory state, patient condition (remission or relapse), or genotype.(55, 56) Ileal CD patients have been observed to have a lower abundance of Faecalibacterium prausnitzii, Roseburia, and subsets of Clostridiales, and a higher abundance of Enterobacteriaceae, Ruminococcus gnavus, and Escherichia coli as compared to colonic CD or healthy co-twins.(53, 55–58) The relative abundance of Fusobacterium is also strongly correlated with disease activity in patients with ileal, but not colonic CD.(55) A low frequency of F. prausnitzii is associated with an increased risk of relapse and post-op recurrence in CD.(59–61) The abundance and number of E. coli correlate with ileal disease severity,(57, 62) and it has been observed that adherent/invasive E. coli is more abundant in the ileum of CD patients (as compared to healthy controls or colonic CD) and restricted to the inflamed ileal mucosa.(57, 63) The F. prausnitzii-E. coli index (F-E index) has also been shown to discriminate between healthy controls, ulcerative colitis patients, and between disease phenotypes within CD such as ileal vs. ileocolonic vs. colonic only CD.(62)
NOD2 genotypes influence microbial composition in humans and temporal development and composition of the host microbiota.(64) Patients homozygous for the ATG16L1 risk allele have increased numbers of Fusobacteriaceae in inflamed ileal tissue, whereas those with the protective allele have decreased Enterobacteriaceae and Bacteroidaceae, and increased Lachnospiraceae. The impact of the disease-associated ATG16L1 allele does not appear to affect bacterial composition in the non-inflamed ileum, however. Monocytes homozygous for the ATG16L1 risk allele display impaired killing of adherent/invasive E. coli under inflammatory conditions compared with those homozygous for the ATG16L1 protective allele.(65) Studies in the RISK cohort, a pediatric inception cohort, observed that isolated ileal disease or NOD2 status in themselves were not predictive of a complication but rather, specific gene expression events involved in acute inflammatory responses to microbes were more closely associated with a risk for penetrating disease complications.(58) Ruminococcus was most prominently implicated in stricturing complications, and Veillonella was implicated in the risk for penetrating complications with the frequency being increased specifically in the ileum in this cohort.(58) Prior studies have observed that anti-TNFα therapy shifts the relative abundance of E. coli but not F. prausnitzii,(66) and steroids and smoking are associated with shifts in F. prausnitzii specifically.(60) The RISK cohort observed that early anti-TNFα use was associated with a reduction in risk for penetrating complications but not stricturing complications. Whether this is due to a differential effect on the microbiome will require further exploration, but the evidence would suggest that if a differential effectiveness in ileal vs. colonic CD is present for biologics, it may be driven by variations in microbial composition and/or shifts in microbial composition upon the initiation of therapy.
RESPONSE TO THERAPY
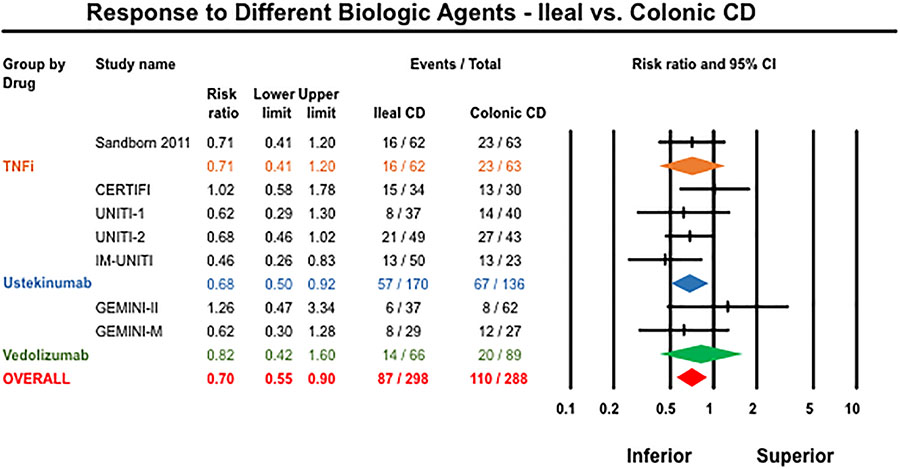
Isolated ileal disease location has been observed to be a negative predictor of responses to anti-TNFα therapy in several cohort studies.(5) Post-hoc analysis of certolizumab pegol RCT demonstrated that colonic CD (vs. placebo: OR 2.39) and ileocolonic CD (vs. placebo: OR 2.07) patients were more likely to achieve remission at week 6 compared to those with isolated ileal disease (vs. placebo: OR 0.42).(67) Post-hoc analyses of vedolizumab and ustekinumab phase 3 trial programs did not formally assess the comparison between ileal and colonic CD. We therefore performed an updated meta-analysis including the certolizumab pegol trial in combination with the GEMINI trial program and the CERTIFI and UNITI trial programs for CD (these were the only trials to report disease location-specific outcomes).(68) We observed that patients with isolated ileal CD (vs. colonic CD) were significantly less likely to achieve a response or remission with the biologic intervention (29% vs. 38%; Relative Risk [RR] 0.70, 95% CI 0.56–0.87, I2=0%) (Figure 2). It should be noted that for ustekinumab, clinical response was reported as the primary outcome (100-point reduction in CDAI), whereas other trials reported clinical remission (CDAI<150). Thus, heterogeneity in trial design limits the direct applicability of these results, and differential impacts on regional mucosal inflammation versus symptoms in patients with strictures and fistula may confound these data. However, they do appear to suggest a trend towards lower response rates in ileal CD patients that will require further evaluation using individual participant data that also includes endoscopic outcomes, which are more likely to capture true differences in response rates.
Figure 2:
Meta-analysis of response to biologic agents stratified by disease location
FECAL BIOMARKERS AND DISEASE MONITORING
There remains a frequent inability to accurately assess disease activity and response to therapy in ileal CD as compared to colonic CD due to the presence of impassable ileal strictures, a lack of well-validated and widely accepted radiographic end-points, and variability in reported accuracy for non-invasive biomarkers. Furthermore, current endoscopic indices do not have validated differential cut-offs for defining moderate-severe ileitis versus ileocolitis. Finally, approximately 5–10% of patients will have isolated small bowel disease beyond the reach of conventional ileocolonoscopy, further adding to this burden. Ileal CD patients are therefore likely under-represented in clinical trials and they represent an at-risk population where treat-to-target disease monitoring and therapy adjustment cannot be easily applied.
It has been observed that ileal CD patients may not have markedly elevated fecal calprotectin levels despite having large or very large ulcers.(69) Pooled analyses utilizing capsule endoscopy as a gold standard have suggested that FC (50 ug/g) has a modest sensitivity (83%) for ruling out active ileal CD.(70) More recent studies suggested that a cut-off of 50 ug/g correlated poorly with ileal disease location assessed using capsule endoscopy.(71) This discrepancy across cohorts when using capsule endoscopy as a gold standard may be a result of variation in correlation between FC and different small bowel capsule endoscopic disease activity indices.(72) When using cross sectional imaging as a gold standard for disease activity, some studies have suggested an optimal cut-off around 150 ug/g for defining active ileal CD,(73) whereas others have suggested a cut-off of around 200 ug/g when using balloon assisted enteroscopy with or without cross sectional imaging as gold standards.(74, 75) Thus, FC may correlate with ileal CD activity, but the optimal cut-off is yet to be determined and is significantly influenced by the diagnostic tool and scoring index used as the gold standard.
Ileocolonoscopy scoring of disease activity in CD can be accomplished using the Crohn’s Disease Endoscopic Index of Severity (CDEIS) or the Simple Endoscopic Score for Crohn’s Disease (SES-CD). Although both indices allow for a segment-based assessment of disease activity, the score is created by combining findings in all segments. For example, an ileal CD patient with very large (> 2cm) ileal ulcers, a narrowing that cannot be passed, >75% of ileal surface area involved with inflammation, and >30% of ileal surface area involved with ulceration, would have an SES-CD score of 12 that would be classified as moderate endoscopic activity. In contrast, a colonic CD patient with small apthous (< 0.5mm) ulcers throughout the colon, no narrowings, < 50% of colonic surface area involved with inflammation, and < 10% of colonic surface area involved with ulceration would also have an SES-CD score of 12 and be classified as having moderate endoscopic activity. These patients are, however, very different in their risks for complication, probability of response to therapy, and likely need for surgery, and this example highlights the need for separate endoscopic indices for ileal- and colonic-dominant CD.
SEROLOGIC BIOMARKERS
Antibodies to Saccharomyces cerevisiae (ASCA) have been observed primarily in CD patients, with ASCA+ CD patients more often having ileal or ileocolonic disease location, and more often requiring ileocecal resection compared to ASCA- negative CD patients.(76–78) Notably in one cohort study, ASCA was again associated with ileal disease location and all ASCA+ CD patients who also had CARD15 mutations were observed to have ileal disease location.(79) Deoxyribonuclease (DNase)-sensitive perinuclear antineutrophil cytoplasmic antibodies (DNase-sensitive pANCA) are more often observed in UC patients than CD patients (70% vs. 18%), however, when pANCA is observed in CD patients it is predominately a CD colitis phenotype.(76, 79, 80)
CONCLUSION
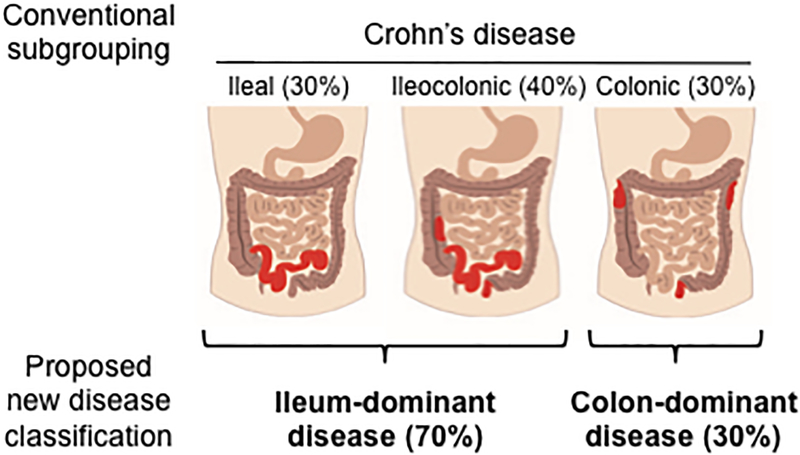
The data presented here suggest that ileal and colonic CD could potentially be separate diseases and consideration should be given to a distinct classification system for ileal-dominant (isolated ileal and ileocolonic) and isolated colonic CD. (Figure 3) Although the prior literature does not provide clear concrete justification for this split, we have highlighted observations that help to support this concept and highlight key questions remaining that will need to be studied in a prospective manner. Furthermore, adoption of a revised nomenclature across clinical and academic platforms will require a broader consensus discussion in the field, but we hope to start that discussion with this review.
Figure 3:
Prior anatomic location of Crohn’s disease and proposed new classification
Grant support:
SS is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK117058, the American College of Gastroenterology Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award. NVC is supported by the American Gastroenterology Association Research Scholar Award. BSB is supported by the the Crohn’s and Colitis Foundation Career Development Award and UCSD KL2 1KL2TR001444. JRN is supported by RO1 DK108670. PBE is supported by the Wayne and Gladys Valley Foundation, the Chiba University-UCSD Immunology Initiative and NIH AI079145.
Footnotes
Conflicts of interest: PSD: Consulting Takeda, Janssen, Prometheus; Research support Takeda, Janssen, Pfizer, Abbvie, Buhlmann, Polymedco; SS: Research support Abbvie, Pfizer; Consulting for Pfizer, Abbvie, Takeda, AMAG Pharmaceuticals; NVC: Consulting Boehringer Ingelheim, Takeda, Janssen, Pfizer, Progenity, Prometheus; Research support: R-Biopharm, Takeda, UCB. JTC: Grant support Takeda. JRN: Grant support Takeda. PBE: None. LE: Consultant for Orphagen Pharmaceuticals, lab service agreement with Takeda California. KEB: None. WJS: research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos; consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Prizer, Precision IBD, Progenity, Prometheus Laboratories, Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Precision IBD, Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech - consultant, stock options; Progenity - consultant, stock; Oppilan Pharma - employee, stock options; Escalier Biosciences - employee, stock options; Precision IBD - employee, stock options; Ventyx Biosciences – employee, stock options; Vimalan Biosciences – employee, stock options.
References
- 1.Mulder DJ, Noble AJ, Justinich CJ, Duffin JM. A tale of two diseases: the history of inflammatory bowel disease. J Crohns Colitis. 2014;8(5):341–8. [DOI] [PubMed] [Google Scholar]
- 2.Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. 1932. Mt Sinai J Med. 2000;67(3):263–8. [PubMed] [Google Scholar]
- 3.Lockhart-Mummery HE, Morson BC. Crohn’s disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut. 1960;1:87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Loftus EV Jr., Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105(2):289–97. [DOI] [PubMed] [Google Scholar]
- 5.Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: a systematic review of isolated colonic Crohn’s disease: the third IBD? Gut. 2017;66(2):362–81. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Haritunians T, Landers C, Potdar AA, Yang S, Huang H, et al. Late-Onset Crohn’s Disease Is A Subgroup Distinct in Genetic and Behavioral Risk Factors With UC-Like Characteristics. Inflamm Bowel Dis. 2018;24(11):2413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. J Crohns Colitis. 2014;8(8):717–25. [DOI] [PubMed] [Google Scholar]
- 8.To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther. 2016;43(5):549–61. [DOI] [PubMed] [Google Scholar]
- 9.Lang BM, Biedermann L, van Haaften WT, de Valliere C, Schuurmans M, Begre S, et al. Genetic polymorphisms associated with smoking behaviour predict the risk of surgery in patients with Crohn’s disease. Aliment Pharmacol Ther. 2018;47(1):55–66. [DOI] [PubMed] [Google Scholar]
- 10.Biedermann L, Fournier N, Misselwitz B, Frei P, Zeitz J, Manser CN, et al. High Rates of Smoking Especially in Female Crohn’s Disease Patients and Low Use of Supportive Measures to Achieve Smoking Cessation--Data from the Swiss IBD Cohort Study. J Crohns Colitis. 2015;9(10):819–29. [DOI] [PubMed] [Google Scholar]
- 11.Cosnes J, Carbonnel F, Beaugerie L, Le Quintrec Y, Gendre JP. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology. 1996;110(2):424–31. [DOI] [PubMed] [Google Scholar]
- 12.Cleynen I, Gonzalez JR, Figueroa C, Franke A, McGovern D, Bortlik M, et al. Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013;62(11):1556–65. [DOI] [PubMed] [Google Scholar]
- 13.Cleynen I, Boucher G, Jostins L, Schumm LP, Zeissig S, Ahmad T, et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387(10014):156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ananthakrishnan AN, Shi HY, Tang W, Law CC, Sung JJ, Chan FK, et al. Systematic Review and Meta-analysis: Phenotype and Clinical Outcomes of Older-onset Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(10):1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornish JA, Tan E, Simillis C, Clark SK, Teare J, Tekkis PP. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103(9):2394–400. [DOI] [PubMed] [Google Scholar]
- 16.Vavricka SR, Spigaglia SM, Rogler G, Pittet V, Michetti P, Felley C, et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(3):496–505. [DOI] [PubMed] [Google Scholar]
- 17.Bamba S, Tsujikawa T, Ban H, Imaeda H, Inatomi O, Nishida A, et al. Predicting Mucosal Healing in Crohn’s Disease Using Practical Clinical Indices with Regard to the Location of Active Disease. Hepatogastroenterology. 2014;61(131):689–96. [PubMed] [Google Scholar]
- 18.Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21(8):1982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhagat S, Das KM. A shared and unique peptide in the human colon, eye, and joint detected by a monoclonal antibody. Gastroenterology. 1994;107(1):103–8. [DOI] [PubMed] [Google Scholar]
- 20.Farhi D, Cosnes J, Zizi N, Chosidow O, Seksik P, Beaugerie L, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87(5):281–93. [DOI] [PubMed] [Google Scholar]
- 21.Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(3):239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severs M, van Erp SJ, van der Valk ME, Mangen MJ, Fidder HH, van der Have M, et al. Smoking is Associated With Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(4):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iny O, Yanai H, Matalon S, Santo E, Shibolet O, Dotan I, et al. Crohn’s Disease Behavior and Location is Altered when Associated with Primary Sclerosing Cholangitis. Isr Med Assoc J. 2018;1(20):25–9. [PubMed] [Google Scholar]
- 24.O’Toole A, Alakkari A, Keegan D, Doherty G, Mulcahy H, O’Donoghue D. Primary sclerosing cholangitis and disease distribution in inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(4):439–41. [DOI] [PubMed] [Google Scholar]
- 25.Boonstra K, van Erpecum KJ, van Nieuwkerk KM, Drenth JP, Poen AC, Witteman BJ, et al. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2270–6. [DOI] [PubMed] [Google Scholar]
- 26.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr.. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139(4):1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel CA, Horton H, Siegel LS, Thompson KD, Mackenzie T, Stewart SK, et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Alimentary pharmacology & therapeutics. 2016;43(2):262–71. [DOI] [PubMed] [Google Scholar]
- 28.Guizzetti L, Zou G, Khanna R, Dulai PS, Sandborn WJ, Jairath V, et al. Development of Clinical Prediction Models for Surgery and Complications in Crohn’s Disease. J Crohns Colitis. 2018;12(2):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590–605. [DOI] [PubMed] [Google Scholar]
- 30.Verstockt B, Smith KG, Lee JC. Genome-wide association studies in Crohn’s disease: Past, present and future. Clin Transl Immunology. 2018;7(1):e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39(2):207–11. [DOI] [PubMed] [Google Scholar]
- 32.Murthy A, Li Y, Peng I, Reichelt M, Katakam AK, Noubade R, et al. A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014;506(7489):456–62. [DOI] [PubMed] [Google Scholar]
- 33.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Bock J, Martinez-Naves E, et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503(7475):272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanDussen KL, Liu TC, Li D, Towfic F, Modiano N, Winter R, et al. Genetic variants synthesize to produce paneth cell phenotypes that define subtypes of Crohn’s disease. Gastroenterology. 2014;146(1):200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massey DC, Bredin F, Parkes M. Use of sirolimus (rapamycin) to treat refractory Crohn’s disease. Gut. 2008;57(9):1294–6. [DOI] [PubMed] [Google Scholar]
- 37.Reinisch W, Panes J, Lemann M, Schreiber S, Feagan B, Schmidt S, et al. A multicenter, randomized, double-blind trial of everolimus versus azathioprine and placebo to maintain steroid-induced remission in patients with moderate-to-severe active Crohn’s disease. Am J Gastroenterol. 2008;103(9):2284–92. [DOI] [PubMed] [Google Scholar]
- 38.Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152(2):374–88 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, Paschos E, et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14(2):204–12. [DOI] [PubMed] [Google Scholar]
- 40.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G827–38. [DOI] [PubMed] [Google Scholar]
- 41.Verdier J, Begue B, Cerf-Bensussan N, Ruemmele FM. Compartmentalized expression of Th1 and Th17 cytokines in pediatric inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18(7):1260–6. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Doty AL, Tang Y, Berrie D, Iqbal A, Tan SA, et al. Enrichment of IL-17A(+) IFN-gamma(+) and IL-22(+) IFN-gamma(+) T cell subsets is associated with reduction of NKp44(+) ILC3s in the terminal ileum of Crohn’s disease patients. Clin Exp Immunol. 2017;190(1):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112(5):693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zorzi F, Monteleone I, Sarra M, Calabrese E, Marafini I, Cretella M, et al. Distinct profiles of effector cytokines mark the different phases of Crohn’s disease. PLoS One. 2013;8(1):e54562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciccia F, Bombardieri M, Principato A, Giardina A, Tripodo C, Porcasi R, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60(4):955–65. [DOI] [PubMed] [Google Scholar]
- 46.Feagan BG, Sandborn WJ, D’Haens G, Panes J, Kaser A, Ferrante M, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389(10080):1699–709. [DOI] [PubMed] [Google Scholar]
- 47.Yamada D, Kadono T, Masui Y, Yanaba K, Sato S. β7 Integrin Controls Mast Cell Recruitment, whereas αE Integrin Modulates the Number and Function of CD8+ T Cells in Immune Complex–Mediated Tissue Injury. The Journal of Immunology. 2014;192(9):4112–21. [DOI] [PubMed] [Google Scholar]
- 48.Ichikawa R, Lamb CA, Eastham-Anderson J, Scherl A, Raffals L, Faubion WA, et al. AlphaE Integrin Expression Is Increased in the Ileum Relative to the Colon and Unaffected by Inflammation. J Crohns Colitis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smids C, Horjus Talabur Horje CS, van Wijk F, van Lochem EG. The Complexity of alpha E beta 7 Blockade in Inflammatory Bowel Diseases. J Crohns Colitis. 2017;11(4):500–8. [DOI] [PubMed] [Google Scholar]
- 50.Zundler S, Fischer A, Schillinger D, Binder MT, Atreya R, Rath T, et al. The alpha4beta1 Homing Pathway Is Essential for Ileal Homing of Crohn’s Disease Effector T Cells In Vivo. Inflamm Bowel Dis. 2017;23(3):379–91. [DOI] [PubMed] [Google Scholar]
- 51.Tew GW, Hackney JA, Gibbons D, Lamb CA, Luca D, Egen JG, et al. Association Between Response to Etrolizumab and Expression of Integrin alphaE and Granzyme A in Colon Biopsies of Patients With Ulcerative Colitis. Gastroenterology. 2016;150(2):477–87 e9. [DOI] [PubMed] [Google Scholar]
- 52.Dicksved J, Halfvarson J, Rosenquist M, Jarnerot G, Tysk C, Apajalahti J, et al. Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J. 2008;2(7):716–27. [DOI] [PubMed] [Google Scholar]
- 53.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139(6):1844–54 e1. [DOI] [PubMed] [Google Scholar]
- 54.Tyler AD, Kirsch R, Milgrom R, Stempak JM, Kabakchiev B, Silverberg MS. Microbiome Heterogeneity Characterizing Intestinal Tissue and Inflammatory Bowel Disease Phenotype. Inflamm Bowel Dis. 2016;22(4):807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naftali T, Reshef L, Kovacs A, Porat R, Amir I, Konikoff FM, et al. Distinct Microbiotas are Associated with Ileum-Restricted and Colon-Involving Crohn’s Disease. Inflamm Bowel Dis. 2016;22(2):293–302. [DOI] [PubMed] [Google Scholar]
- 56.Willing B, Halfvarson J, Dicksved J, Rosenquist M, Jarnerot G, Engstrand L, et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis. 2009;15(5):653–60. [DOI] [PubMed] [Google Scholar]
- 57.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. 2007;1(5):403–18. [DOI] [PubMed] [Google Scholar]
- 58.Kugathasan S, Denson LA, Walters TD, Kim MO, Marigorta UM, Schirmer M, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet. 2017;389(10080):1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rajca S, Grondin V, Louis E, Vernier-Massouille G, Grimaud JC, Bouhnik Y, et al. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm Bowel Dis. 2014;20(6):978–86. [DOI] [PubMed] [Google Scholar]
- 60.Li E, Hamm CM, Gulati AS, Sartor RB, Chen H, Wu X, et al. Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS One. 2012;7(6):e26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105(43):16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez-Siles M, Martinez-Medina M, Busquets D, Sabat-Mir M, Duncan SH, Flint HJ, et al. Mucosa-associated Faecalibacterium prausnitzii and Escherichia coli co-abundance can distinguish Irritable Bowel Syndrome and Inflammatory Bowel Disease phenotypes. Int J Med Microbiol. 2014;304(3–4):464–75. [DOI] [PubMed] [Google Scholar]
- 63.Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127(2):412–21. [DOI] [PubMed] [Google Scholar]
- 64.Rehman A, Sina C, Gavrilova O, Hasler R, Ott S, Baines JF, et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60(10):1354–62. [DOI] [PubMed] [Google Scholar]
- 65.Sadaghian Sadabad M, Regeling A, de Goffau MC, Blokzijl T, Weersma RK, Penders J, et al. The ATG16L1-T300A allele impairs clearance of pathosymbionts in the inflamed ileal mucosa of Crohn’s disease patients. Gut. 2015;64(10):1546–52. [DOI] [PubMed] [Google Scholar]
- 66.Busquets D, Mas-de-Xaxars T, Lopez-Siles M, Martinez-Medina M, Bahi A, Sabat M, et al. Anti-tumour Necrosis Factor Treatment with Adalimumab Induces Changes in the Microbiota of Crohn’s Disease. J Crohns Colitis. 2015;9(10):899–906. [DOI] [PubMed] [Google Scholar]
- 67.Sandborn WJ, Schreiber S, Feagan BG, Rutgeerts P, Younes ZH, Bloomfield R, et al. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(8):670–8 e3. [DOI] [PubMed] [Google Scholar]
- 68.Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther. 2018. [DOI] [PubMed] [Google Scholar]
- 69.Gecse KB, Brandse JF, van Wilpe S, Lowenberg M, Ponsioen C, van den Brink G, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol. 2015;50(7):841–7. [DOI] [PubMed] [Google Scholar]
- 70.Kopylov U, Yung DE, Engel T, Avni T, Battat R, Ben-Horin S, et al. Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28(10):1137–44. [DOI] [PubMed] [Google Scholar]
- 71.Yousuf H, Aleem U, Egan R, Maheshwari P, Mohamad J, McNamara D. Elevated Faecal Calprotectin Levels are a Reliable Non-Invasive Screening Tool for Small Bowel Crohn’s Disease in Patients Undergoing Capsule Endoscopy. Dig Dis. 2018;36(3):202–8. [DOI] [PubMed] [Google Scholar]
- 72.Koulaouzidis A, Douglas S, Plevris JN. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn’s Disease Activity Index. Dig Dis Sci. 2012;57(4):987–93. [DOI] [PubMed] [Google Scholar]
- 73.Cerrillo E, Beltran B, Pous S, Echarri A, Gallego JC, Iborra M, et al. Fecal Calprotectin in Ileal Crohn’s Disease: Relationship with Magnetic Resonance Enterography and a Pathology Score. Inflamm Bowel Dis. 2015;21(7):1572–9. [DOI] [PubMed] [Google Scholar]
- 74.Arai T, Takeuchi K, Miyamura M, Ishikawa R, Yamada A, Katsumata M, et al. Level of Fecal Calprotectin Correlates With Severity of Small Bowel Crohn’s Disease, Measured by Balloon-assisted Enteroscopy and Computed Tomography Enterography. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2017;15(1):56–62. [DOI] [PubMed] [Google Scholar]
- 75.Kawashima K, Ishihara S, Yuki T, Fukuba N, Sonoyama H, Kazumori H, et al. Fecal Calprotectin More Accurately Predicts Endoscopic Remission of Crohn’s Disease than Serological Biomarkers Evaluated Using Balloon-assisted Enteroscopy. Inflamm Bowel Dis. 2017;23(11):2027–34. [DOI] [PubMed] [Google Scholar]
- 76.Zholudev A, Zurakowski D, Young W, Leichtner A, Bousvaros A. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn’s disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. 2004;99(11):2235–41. [DOI] [PubMed] [Google Scholar]
- 77.Quinton JF, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42(6):788–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koutroubakis IE, Petinaki E, Mouzas IA, Vlachonikolis IG, Anagnostopoulou E, Castanas E, et al. Anti-Saccharomyces cerevisiae mannan antibodies and antineutrophil cytoplasmic autoantibodies in Greek patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96(2):449–54. [DOI] [PubMed] [Google Scholar]
- 79.Linskens RK, Mallant-Hent RC, Murillo LS, von Blomberg BM, Alizadeh BZ, Pena AS. Genetic and serological markers to identify phenotypic subgroups in a Dutch Crohn’ s disease population. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2004;36(1):29–34. [DOI] [PubMed] [Google Scholar]
- 80.Linskens RK, Mallant-Hent RC, Groothuismink ZM, Bakker-Jonges LE, van de Merwe JP, Hooijkaas H, et al. Evaluation of serological markers to differentiate between ulcerative colitis and Crohn’s disease: pANCA, ASCA and agglutinating antibodies to anaerobic coccoid rods. Eur J Gastroenterol Hepatol. 2002;14(9):1013–8. [DOI] [PubMed] [Google Scholar]