Abstract
Objective
The study aimed to investigate the impact of the peak E2 level during controlled ovarian hyperstimulation (COS) on the cumulative live birth rate (cLBR) in non-PCOS women with normal ovarian reserve.
Materials and methods
Women between 20 and 39 years were included. Donor cycles and patients who never experienced embryo transfer were excluded. Multivariable regression and smooth curve fitting were applied for statistical analysis.
Results
A total of 1141 patients were included. The mean age, basal AFC, peak E2 level, and number of retrieved oocyte were 30.0 ± 3.7 years old, 16.8 ± 6.7, 3911.0 ± 1302.9 pg/ml, and 13.6 ± 5.5, respectively. In the overall population of the cohort, cLBR, miscarriage rate, and preterm birth rate were 66.9%, 7.4%, and 13.7%, respectively. The results of multivariable regression analysis failed to show the impact of peak E2 on the cLBR [OR (95%CI) 0.995 (0.982, 1.009), P = 0.486]. However, the result of smooth curve fitting indicated that when the peak E2 was lower than 2185 pg/ml, the cLBR increased about 12% with 100 pg/ml increasing of the peak E2. When the peak E2 was higher than 6136 pg/ml, the cLBR decreased about 10% with 100 pg/ml increasing of the peak E2.
Conclusion
We concluded that the peak E2 level on hCG trigger day is associated with the cLBR in a segmental pattern. There should be an appropriate range of the peak E2 level during COS to achieve a relative best cLBR in non-PCOS patients using stimulating protocol mainly based on GnRH agonist; however, the cutoff value must vary in different centers.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01568-w) contains supplementary material, which is available to authorized users.
Keywords: In vitro fertilization, Cumulative live birth rate, Peak estradiol, Retrospective cohort study
Introduction
Along with the worsened living environment and the advanced maternal age, the incidence of infertility has immensely increased in recent years. Since the first IVF baby Louis Brown was born in Britain 40 years ago, the technology of in vitro fertilization and embryo transfer (IVF-ET) has become the most effective treatment for infertility and has been widely used worldwide [1]. It is acknowledged that the success rate of IVF has been greatly improved during the last four decades. However, the outcomes of many patients remain unsatisfying probably because of some intrinsic limitations of this technology [2].
IVF is a multiple step process starting from ovarian stimulation with gonadotropins, followed by oocyte retrieval under sedation with subsequent fertilization in the laboratory, and culture of embryos prior to embryo transfer into the uterus. The controlled ovarian hyperstimulation (COH) is the first step aiming to induce maturation of multiple oocytes, and hence maximizing the chance of achieving a successful pregnancy. An obvious side effect of COH is that it puts patients, oocytes, and even embryos under a supraphysiologic estradiol (E2) environment.
Previous studies investigating the impact of the supraphysiologic E2 on the clinical outcomes mostly reported the clinical pregnancy rate rather than live birth rate as the main outcome measurement. Imudia et al. [3] retrospectively compared clinical outcomes, including clinical pregnancy rate, miscarriage rate, ectopic pregnancy rate, and so on, between patients with serum peak E2 below 90th percentile and those with peak E2 above 90th percentile. Nevertheless, the difference between the two groups was not statistically significant. Statistical significance was neither observed using multivariable logistic regression analysis. Other previous investigators also failed to draw a consistent conclusion regarding to the relationship between supraphysiologic E2 during COS and clinical outcomes of IVF. Early studies with a small sample size showed a tendency that high peak E2 level was associated with higher number of oocytes, higher number of transferrable embryos, adequate endometrial thickness, and finally patients’ clinical outcomes [4–7]. Kara et al. retrospectively analyzed the relationship between the peak E2 and the clinical outcomes in 203 patients undergoing IVF/ICSI treatment. The result suggested that the number of oocytes and the clinical pregnancy rate in patients with peak E2 higher than 4000 pg/ml were higher than in patients with peak E2 lower than 4000 pg/ml [4]. However, recent large studies showed the unfavorable impact of extremely high peak E2 on the clinical pregnancy rate. For example, Bu et al. [8] enrolled 7112 cases in a retrospective study to investigate the association between the ratio of the peak E2 to the number of oocytes (E2/O) and the clinical pregnancy rate, and the results suggested that the lowest clinical pregnancy rate was found in the highest E2/O ratio group. They further compared the peak E2 level in the highest 10% E2/O ratio group with that in the rest of the population and found that the peak E2 level was significantly higher, while the clinical pregnancy rate significantly lower in the highest 10% E2/O ratio group than that in the control group (6711.85 pg/ml vs 4670.89 pg/ml, P = 0.000; 39.27% vs 45.67%, P = 0.001).
Standing in patients’ shoes, clinical pregnancy is not the ultimate goal for patients who seek for infertility treatment. They care more about the chance of taking a live baby home. Moreover, in consideration of economic burden, patient compliance, OHSS, and oocyte retrieval operation-related complications, the purpose of COS is to obtain multiple mature oocytes to maximize the opportunity of achieving a successful pregnancy in one stimulation cycle. Therefore, we prefer to choose the cumulative live birth rate (cLBR) as the better outcome measure with more practical significance. Unfortunately, few studies set the cLBR as the primary outcome when investigating the relationship between the peak E2 level and the clinical outcomes of IVF. Only one small study with 71 high ovarian responders reported by Steward et al. [9] suggested that the live birth rate was significantly reduced in the group with the peak E2 level higher than 5000 pg/ml than in the group with the peak E2 level lower than 5000 pg/ml (OR = 0.095 with 95% CI 0.01–0.90, P = 0.041). Due to the very small sample size of a carefully selected population, the power of the statistical analysis and the extrapolation was severely weakened.
The objective of the present study was to investigate the impact of the peak E2 level during COH on the cLBR, as well as the miscarriage rate and the preterm delivery rate in non-PCOS population with normal ovarian reserve. Some related perinatal complications were also simply described in this study.
Materials and methods
Patients coming to our center for infertility treatment from Jan 1, 2016, to Dec 31, 2016, were screened for the inclusion and exclusion. Women aged 20–39 years old were with normal ovarian reserve [defined as the basal antral follicular count (AFC) > 7] [10] and without diagnosis of polycystic ovary syndrome (PCOS) according to Rotterdam diagnostic criteria for PCOS [11]. Donor cycles and patients who had previous IVF treatment(s) and who did not receive embryo transfer were excluded from the cohort.
Baseline demographic parameters included female age (years), body mass index (BMI) (kg/m2), duration of infertility, and infertility diagnosis. Baseline IVF characteristics recorded were basal follicular plasma follicle-stimulating hormone (FSH) level (IU/L), basal E2 level (pg/ml), and AFC, all of which were measured on menstrual cycle day 2 or day 3. Ovarian stimulation parameters documented for all patients included ovarian stimulation regimens, duration of ovarian stimulation (days), total dose of gonadotropins (IU), follicular count on hCG day (≥ 14 mm), peak E2 level (pg/ml) on the day of hCG trigger, and mean number of oocytes retrieved. Embryo variables included fertilization method, development stage of transferred embryos, and mean number of embryos transferred. The main outcome measurement was cLBR. The secondary outcome measurements were miscarriage rate and preterm birth weight. Data on low birth weight (LBW), macrosomia, congenital malformation, perinatal death, ectopic pregnancy, heterotopic pregnancy, moderate to severe ovarian hyperstimulation syndrome (OHSS), and gestational hypertension were also briefly described. All parameters of baseline characteristics and ovarian stimulation, as well and as all embryos transferred in FET cycles, were from the first stimulation cycle.
For the GnRH-agonist (GnRHa) protocol, GnRHa 0.1 mg by subcutaneous injection was administered daily in the mid-luteal phase of the menstrual cycle, lasting for 10–14 days until the pituitary down-regulation was confirmed, then the ovarian stimulation with gonadotropin (Gn) commenced. For the GnRH-antagonist protocol, ovarian stimulation began on the second day of the cycle and the antagonist was administered as soon as the leading follicle reached a diameter of ≥ 14 mm on average, and the administration continued until the day of hCG administration. For the prolonged protocol, 3.75 mg long-acting GnRHa was administered on day 2 of the menstrual cycle, 28 days later, the second 3.75 mg long-acting GnRHa was administered. Trans-vaginal ultrasound was performed and serum basal FSH, LH, and E2 tested on the 14–20th day after the second long-acting GnRHa injection for down-regulation confirmation. Ovarian stimulation with Gn commenced when the down-regulation was confirmed. Vitrification was used for the embryo cryopreservation in this cohort.
Embryo assessment, including cleavage-stage embryo assessment and blastocyst assessment, was performed based on the recommendation of Istanbul consensus workshop on embryo assessment [12].
Categorical variables were expressed as number of cases (n) with percentage (%), and continuous variables were expressed as mean ± SD. The Shapiro–Wilk normality test was used to check for normality in addition to visual inspection of the distributions. For the purpose of the study, E2 levels on the day of hCG trigger were taken into account. Univariable linear regression analysis was used to assess the association between the outcomes and any of the following variables: female age, BMI, basal AFC, basal FSH, basal E2, duration of infertility, infertility diagnosis, ovarian stimulation protocol, total Gn dose, peak E2 level on hCG trigger day, follicular count on hCG trigger day (≥ 14 mm), mean number of oocytes retrieved, method of fertilization, development stage of transferred embryos, and fresh or frozen embryo transfer. Stratification analysis was performed and each stratification was adjusted for all abovementioned factors except the stratification factor itself. Interactions between the stratification factors and the exposure factors were also tested. All parameters in the univariable regression analysis, except the peak E2 level on hCG trigger day, were adjusted in the adjusted multivariable model I, and the general additive model was applied as the adjusted multivariable model II, in which all categorical variables were adjusted and the continuous variables in Model I were adjusted by curve fitting. P for trend was used to demonstrate whether the trend of cLBR, miscarriage rate, and preterm delivery rate with E2 was statistically significant. Unadjusted and adjusted odds ratios (ORs) with 95% confident intervals (CIs) were calculated from the corresponding models. Smooth curve of cLBR to the peak E2 was fitted, and the cutoff values of peak E2 for its effect on cLBR were calculated. To increase statistical power, the OR value represented the change of the rate for each main outcome measurement per 100 pg/ml increase of the peak E2 level in the present study. Statistical significance was reached at P < 0.05, and all statistical analyses were performed using statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowrStats software (http://www.mpowerstats.com, X&Y Solution, Inc., Boston, MA).
The institutional review board at the Fourth Military Medical University, Tangdu Hospital, reviewed and approved this study protocol.
Results
Characteristics of the studied population
A total of 5963 patients underwent the ovarian stimulation and oocyte retrieval during the study period. Of these, 4882 (81.9%) patients were excluded due to any of the following reasons: older than 39 years old (723 patients, 12.1%), not the first stimulation cycle (891 patients, 14.9%), no embryo transferred by the time of data collection (2488 patients, 47.1%), PCOS patients (584 patients, 9.8%), impaired ovarian reserve (171 patients, 2.9%), and incomplete records (25 patients, 0.4%). The remaining 1141 patients met the eligibility criteria. Overall demographics including baseline infertility characteristics, ovarian stimulation parameters, embryo parameters, and perinatal outcomes of the studied cohort are displayed in Table 1. Briefly, the mean female age was 30.0 ± 3.7 years, and the mean BMI was 22.1 ± 2.8 kg/m2. These patients with a mean basal AFC of 16.8 ± 6.7 underwent ovarian stimulation with GnRH-agonist-based protocols in 93.9% (1071/1141) of cycles, with a mean stimulation duration of 11.3 ± 2.1 days, and required a mean Gn dosage of 1953.4 ± 898.9 IU. The mean E2 level on hCG trigger day was 3911.0 ± 1302.9 pg/ml. The mean number of oocytes retrieved was 13.6 ± 5.5. Among all embryos, 72.8% were from conventional IVF, 21.1% from ICSI, and the remaining 6.1% from IVF + ICSI (half-half and IVF plus rescued ICSI). Nine hundred and ninety-one (991, 88.2%) patients received cleavage stage embryo transfer; 124 (11.0%) patients received single blastocyst transfer and the remaining 9 (0.8%) patients received a cleavage-stage embryo plus a blastocyst transfer. In 16 cases, the information of the development stage of the transferred embryos was not available. The cLBR of this cohort was 66.9%, and the miscarriage rate and the preterm delivery rate were 7.4% and 13.7%, respectively. Other perinatal complications are listed in Table 1. The patients were equally divided into three groups based on the peak E2 level from low to high, and the demographic parameters within each group were also calculated and compared. Notably, the incidence of macrosomia in the highest peak E2 level group was significantly lower than in the lowest peak E2 level group (4.2% vs 9.1%, P = 0.036). The result was consistent with our previous study findings, showing that the peak E2 level was negatively associated with the birth weight.
Table 1.
Demographic characteristics of the study population
| Characteristics | Patients were equally divided into three groups according to the peak E2 level from low to high | P low versus middle | P high versus middle | |||
|---|---|---|---|---|---|---|
| n = 1141 | Low (n = 380) | Intermediate (n = 380) | High (n = 381) | |||
| Peak E2 level on hCG trigger day (100 pg/ml) | 39.1 ± 13.0 | 24.6 ± 5.9 | 39.4 ± 3.3 | 53.3 ± 7.0 | < 0.001 | < 0.001 |
| Female age (years) | 30.0 ± 3.7 | 30.5 ± 3.8 | 30.0 ± 3.7 | 29.5 ± 3.5 | 0.042 | 0.056 |
| BMI (kg/m2) | 22.1 ± 2.8 | 22.5 ± 3.0 | 22.1 ± 2.8 | 21.6 ± 2.5 | 0.030 | 0.020 |
| Infertility duration (years) | 3.8 ± 2.7 | 3.8 ± 2.8 | 3.6 ± 2.4 | 3.9 ± 2.8 | 0.244 | 0.119 |
| Basal AFC | 16.8 ± 6.7 | 14.5 ± 6.0 | 17.3 ± 7.0 | 18.5 ± 6.4 | < 0.001 | 0.020 |
| Basal FSH (IU/L) | 7.3 ± 3.3 | 7.4 ± 2.7 | 7.1 ± 2.8 | 7.2 ± 3.1 | 0.076 | 0.680 |
| Basal E2 (pg/ml) | 53.8 ± 35.5 | 53.7 ± 40.2 | 55.0 ± 33.0 | 52.6 ± 33.0 | 0.639 | 0.325 |
| Infertility etiology (data missing, n = 2) | 0.398 | 0.808 | ||||
| Tubal factor (n [%]) | 653 (57.2) | 232 (61.1) | 205 (54.0) | 216 (56.7) | ||
| Male factor (n [%]) | 126 (11.0) | 38 (10.0) | 43 (11.3) | 45 (11.8) | ||
| Mixed factor (n [%]) | 291 (25.5) | 86 (22.6) | 105 (27.6) | 100 (26.3) | ||
| Endometriosis (n [%]) | 5 (0.4) | 2 (0.5) | 2 (0.5) | 1 (0.3) | ||
| Unexplained (n [%]) | 66 (5.8) | 22 (5.8) | 25 (6.6) | 19 (5.0) | ||
| Stimulation protocol | < 0.001 | 0.543 | ||||
| GnRH-agonist protocol (n [%]) | 1071 (93.9) | 329 (86.6) | 371 (97.6) | 371 (97.4) | ||
| GnRH-antagonist protocol (n [%]) | 69 (6.1) | 51 (13.4) | 8 (2.1) | 10 (2.6) | ||
| Other (n [%]) | 1 (0.1) | 0 (0.0) | 1 (0.3) | 0 (0.0) | ||
| Duration of stimulation (days) | 11.3 ± 2.1 | 11.3 ± 2.0 | 11.6 ± 2.4 | 11.0 ± 2.0 | 0.104 | < 0.001 |
| Follicular count on hCG day (≥ 14 mm)Follicular count on hCG day (≥ 14 mm)Follicular count on hCG day (≥ 14 mm) | 11.2 ± 3.6 | 8.70 ± 3.2 | 11.9 ± 3.2 | 13.0 ± 2.9 | < 0.001 | < 0.001 |
| Total dose of Gn administered (IU) | 1953.4 ± 898.9 | 2349.3 ± 963.0 | 1906.2 ± 868.3 | 1604.5 ± 682.2 | < 0.001 | < 0.001 |
| Number of oocytes retrieved | 13.6 ± 5.5 | 9.8 ± 3.9 | 14.3 ± 4.9 | 16.7 ± 5.0 | < 0.001 | < 0.001 |
| Method of fertilization | 0.462 | 0.957 | ||||
| IVF (n [%]) | 831 (72.8) | 288 (75.8) | 273 (71.8) | 270 (70.9) | ||
| ICSI (n [%]) | 241 (21.1) | 72 (19.0) | 83 (21.8) | 86 (22.6) | ||
| IVF + ICSI (n [%]) | 69 (6.1) | 20 (5.3) | 24 (6.3) | 25 (6.6) | ||
| Mean number of embryos transferred | 1.9 ± 0.4 | 1.9 ± 0.3 | 1.9 ± 0.3 | 1.8 ± 0.4 | 0.266 | 0.062 |
| Development of transferred embryos (data missing, n = 16) | 0.018 | 0.216 | ||||
| 1 or 2 Grade 1 D3 embryos (n [%]) | 231 (20.5) | 72 (19.2) | 80 (21.2) | 79 (21.2) | ||
| 1 or 2 Grade 2 D3 embryos (n [%]) | 487 (43.3) | 181 (48.1) | 153 (40.6) | 153 (41.1) | ||
| 1 Grade 1 and 1 Grade 2 D3 embryos (n [%]) | 274 (24.4) | 101 (26.9) | 97 (25.7) | 76 (20.4) | ||
| Single blastocyst (n [%]) | 124 (11.0) | 20 (5.3) | 43 (11.4) | 61 (16.4) | ||
| Embryo + blastocyst (n [%]) | 9 (0.8) | 2 (0.5) | 4 (1.1) | 3 (0.8) | ||
| Fresh or frozen embryo transfer | < 0.001 | < 0.001 | ||||
| Fresh embryo transfer (n [%]) | 377 (33.0) | 200 (52.6) | 121 (31.8) | 56 (14.7) | ||
| FET (n [%]) | 764 (67.0) | 180 (47.4) | 259 (68.2) | 325 (85.3) | ||
| Methods of endometrium preparation in FET cycles | < 0.001 | < 0.001 | ||||
| HRT (n [%]) | 321 (28.1) | 65 (17.1) | 106 (27.9) | 150 (39.4) | ||
| Natural ovulation cycle (n [%]) | 415 (36.4) | 107 (28.2) | 139 (36.6) | 169 (44.4) | ||
| Down-regulation cycle (n [%]) | 366 (32.1) | 198 (52.1) | 116 (30.5) | 52 (13.7) | ||
| Mild stimulation cycle (n [%]) | 39 (3.4) | 10 (2.6) | 19 (5.0) | 10 (2.6) | ||
| cLBR (n [%]) | 763 (66.9) | 251 (66.1) | 257 (67.6) | 255 (66.9) | 0.644 | 0.836 |
| Miscarriage rate (n [%]) | 84 (7.4) | 28 (7.4) | 24 (6.3) | 32 (8.4) | 0.565 | 0.271 |
| Preterm birth (n [%]) | 156 (13.7) | 45 (11.8) | 52 (13.7) | 59 (15.5) | 0.447 | 0.481 |
| Macrosomia (n [%]) | 66 (7.2) | 27 (9.1) | 26 (8.4) | 13 (4.2) | 0.758 | 0.028 |
| LBW (n [%]) | 151 (16.5) | 40 (13.5) | 52 (16.8) | 59 (18.9) | 0.256 | 0.510 |
| Congenital malformation (n [%]) | 1 (0.1) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0.311 | 0.654 |
| Perinatal death (n [%]) | 3 (0.3) | 2 (0.6) | 1 (0.3) | 0 (0.0) | 0.549 | 0.491 |
| Ectopic pregnancy (n [%]) | 2 (0.2) | 1 (0.3) | 0 (0.0) | 1 (0.3) | 0.311 | 0.325 |
| Heterotopic pregnancy (n [%]) | 2 (0.2) | 0 (0.0) | 1 (0.3) | 1 (0.3) | 0.323 | 0.980 |
| Moderate to severe OHSS (n [%]) | 5 (0.5) | 0 (0.0) | 4 (1.2) | 1 (0.3) | 0.047 | 0.166 |
| Gestational hypertension (n [%]) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0.755 | 0.325 |
| Live births | ||||||
| Singletons (n [%]) | 654 (71.3%) | 219 (74.0%) | 230 (74.7%) | 205 (65.5%) | 0.846 | 0.013 |
| Twins (n [%]) | 263 (28.7%) | 77 (26.0%) | 78 (25.3%) | 108 (34.5%) | ||
AFC antral follicular count, BMI body mass index, cLBR cumulative live birth rate, E2 estradiol, FET frozen embryo transfer, FSH follicular stimulating hormone, hCG human chorionic gonadotropin, HRT hormone replacement treatment, IVF in vitro fertilization, ICSI intracytoplasmic single sperm injection, LBW low birth weight
Outcomes of univariable analysis
The outcome of univariable analysis did not show any significant impact of peak E2 level on the cLBR [OR (95%CI) 1.00 (0.99, 1.01), P = 0.822] or on the miscarriage rate or the preterm delivery rate. The results suggested the cLBR decreased with the increasing BMI [OR (95%CI) 1.58 (1.02, 2.43), P = 0.039]. Patients with male infertility factor only had better cLBR when compared with patients with tubal factor [OR (95%CI) 1.53 (1.11, 2.11), P = 0.009]. ICSI significantly increased the cLBR as compared with the conventional IVF [OR (95%CI) 0.55(0.33, 0.89), P = 0.015]. Embryo quality of transferred embryos had a positive impact on the cLBR, and interestingly, single blastocyst transfer resulted in significantly lower cLBR [OR (95%CI) 0.55(0.33, 0.89), P = 0.015] but twice higher miscarriage rate in the univariable analysis [OR (95%CI) 2.32 (1.04, 5.19), P = 0.040] (Table 2).
Table 2.
Univariable analysis for the potential factors influencing clinical outcomes of IVF/ICSI
| Exposure | Statistics | cLBR | Miscarriage rate | Preterm birth | |||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | ||
| Female age (years) | Mean ± SD | ||||||
| 30.0 ± 3.7 | 0.975 (0.943, 1.008) | 0.139 | 1.056 (0.996, 1.120) | 0.069 | 0.970 (0.926, 1.016) | 0.064 | |
| BMI (kg/m2) | 22.1 ± 2.8 | 0.953 (0.912, 0.995) 0.953 (0.912, 0.995) 0.953 (0.912, 0.995) | 0.030 | 1.009 (0.933, 1.092) | 0.821 | 1.036 (0.977, 1.098) | 0.241 |
| Infertility duration (years) | 3.8 ± 2.7 | 1.016 (0.970, 1.065) | 0.507 | 1.020 (0.941, 1.105) | 0.635 | 0.940 (0.877, 1.008) | 0.084 |
| Basal AFC | 16.8 ± 6.7 | 0.983 (0.966, 1.001) | 0.068 | 1.025 (0.994, 1.057) | 0.117 | 1.023 (0.998, 1.047) | 0.067 |
| Basal FSH (IU/L) | 7.3 ± 3.3 | 0.990 (0.950, 1.032) | 0.639 | 0.983 (0.902, 1.071) | 0.692 | 1.010 (0.956, 1.067) | 0.726 |
| Basal E2 (pg/ml) | 53.8 ± 35.5 | 1.001 (0.997, 1.005) | 0.573 | 0.993 (0.984, 1.002) | 0.14 | 0.998 (0.993, 1.004) | 0.54 |
| Peak E2 on hCG trigger day (100 pg/ml) | 39.1 ± 13.2 | 1.001 (0.992, 1.012) | 0.822 | 1.003 (0.986, 1.021) | 0.708 | 1.012 (0.999, 1.025) | 0.076 |
| Antral follicular count on hCG day (≥ 14 mm) | 11.2 ± 3.6 | 1.017 (0.983, 1.053) | 0.341 | 1.035 (0.973, 1.101) | 0.278 | 1.015 (0.968, 1.064) | 0.537 |
| Total dose of Gn administered (IU) | 1953.4 ± 898.9 | 1.000 (0.999, 1.000) | 0.878 | 1.000 (0.999, 1.000) | 0.857 | 0.999 (0.999, 1.000) | 0.033 |
| Number of oocytes retrieved | 3.8 ± 2.7 | 1.004 (0.981, 1.027) | 0.736 | 1.010 (0.970, 1.051) | 0.646 | 1.020 (0.989, 1.051) | 0.215 |
| Infertility etiology (data missing, n = 2) | n [%] | ||||||
| Tubal factor (n [%]) | 653 (55.3) | 1 | 1 | 1 | |||
| Male factor (n [%]) | 126 (10.7) | 1.582 (1.023, 2.426) | 0.039 | 0.709 (0.314, 1.603) | 0.409 | 0.868 (0.484, 1.556) | 0.634 |
| Mixed factor (n [%]) | 291 (24.7) | 1.142 (0.851, 1.533) | 0.376 | 1.183 (0.721, 1.942) | 0.506 | 0.964 (0.641, 1.451) | 0.862 |
| Unexplained (n [%]) | 66 (5.6) | 1.003 (0.590, 1.706) | 0.991 | 0.186 (0.025, 1.365) | 0.098 | 1.729 (0.919, 3.250) | 0.089 |
| Stimulation protocol (data missing, n = 4) | |||||||
| GnRH-agonist protocol (n [%]) | 1071 (93.9) | 1 | 1 | 1 | |||
| GnRH-antagonist protocol (n [%]) | 69 (6.1) | 1.225 (0.717, 2.092) | 0.458 | 0.981 (0.384, 2.508) | 0.968 | 0.372 (0.134, 1.036) | 0.059 |
| Method of fertilization | |||||||
| IVF (n [%]) | 831 (72.8) | 1 | 1 | 1 | |||
| ICSI (n [%]) | 241 (21.1) | 1.532 (1.109, 2.113) | 0.009 | 0.782 (0.438, 1.398) | 0.407 | 0.803 (0.520, 1.238) | 0.32 |
| IVF + ICSI (n [%]) | 69 (6.1) | 1.568 (0.899, 2.733) | 0.113 | 0.725 (0.256, 2.054) | 0.545 | 0.559 (0.237, 1.319) | 0.184 |
| Development stage and embryo quality of transferred embryos (data missing, n = 16) | |||||||
| 1 or 2 Grade 1 D3 embryos (n [%]) | 231 (20.5) | 1 | 1 | 1 | |||
| 1 or 2 Grade 2 D3 embryos (n [%]) | 487 (43.3) | 0.425 (0.295, 0.611) | < 0.0001 | 1.501 (0.767, 2.935) | 0.236 | 1.228 (0.774, 1.949) | 0.383 |
| 1 Grade 2 and 1 Grade 1 D3 embryos (n [%]) | 274 (24.4) | 0.524 (0.350, 0.784) | 0.002 | 1.437 (0.687, 3.006) | 0.336 | 1.297 (0.781, 2.154) | 0.316 |
| Single blastocyst (n [%]) | 124 (11.0) | 0.551(0.331, 0.892) | 0.015 | 2.322 (1.043, 5.192) | 0.040 | 0.480 (0.213, 1.086) | 0.078 |
| Fresh or frozen embryo transfer | |||||||
| Fresh embryo transfer (n [%]) | 377 (33.0) | 1 | 1 | 1 | |||
| FET (n [%]) | 764 (67.0) | 0.899 (0.691, 1.171) | 0.431 | 1.632 (0.972, 2.740) | 0.064 | 0.985 (0.688, 1.410) | 0.934 |
Factors with less than 10 cases were not included in the univariable analysis: endometriosis (n = 5) in infertility factors, other stimulation strategy (n = 1) in stimulation strategies and blastocyst + embryo transfer (n = 9) in development of transferred embryos
Outcomes of multivariable analysis
Taking all confounding factors into account [female age, BMI, infertility duration infertility factors, basal FSH, basal E2, follicular count on hCG day (≥ 14 mm), total Gn dose, stimulation regimen, method of fertilization, basal AFC, development of transferred embryos, and fresh or frozen embryo transfer], the results of multivariable regression analysis failed to show any impact of peak E2 level on the cLBR [OR (95%CI) 0.995 (0.982, 1.009), P = 0.486], miscarriage rate [OR (95%CI) 0.996 (0.973, 1.020), P = 0.740], or preterm delivery rate [OR (95%CI) 1.014 (0.997, 1.033), P = 0.114] in the general population of the studied cohort (Table 3).
Table 3.
Outcomes of multivariable regression analysis of the impact of peak E2 level on hCG trigger day (100 pg/ml) on cLBR, miscarriage rate, and preterm birth rate
| Outcomes | Non-adjusted | P | Adjusted I | P |
|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | |||
| cLBR | 1.001 (0.992, 1.011) | 0.822 | 0.995 (0.982, 1.009) | 0.288 |
| Miscarriage rate | 1.003 (0.986, 1.021) | 0.708 | 0.996 (0.973, 1.020) | 0.87 |
| Preterm birth | 1.012 (0.999, 1.025) | 0.076 | 1.014 (0.997, 1.033) | 0.153 |
The following factors were adjusted in model I: female age, BMI, infertility duration, infertility etiology, basal FSH, basal E2, follicular count on hCG day (≥ 14 mm), total dose of Gn administered, stimulation protocol, method of fertilization, basal AFC, development of transferred embryos, and fresh or frozen embryo transfer. The general additive model was applied as the adjusted model II, in which all categorical variables were adjusted and the continuous variables in Model I were adjusted by curve fitting
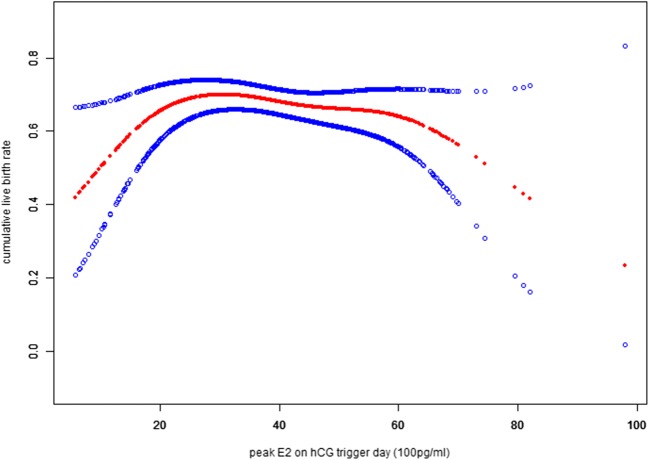
However, the smooth curve fitting results demonstrated a clear positive association between cLBR and the peak E2 level in the first segment of the curve, followed by a plateau, and a negative trend afterwards (Fig. 1). The quantitative analysis suggested that when peak E2 level was lower than 2185 pg/ml, the cLBR increased by about 12% with every 100 pg/ml increase of the peak E2 level, and the trend was statistically significant. When the peak E2 level was between 2185 and 6136 pg/ml, the cLBR only slightly decreased (0.4%). However, with the peak E2 level that was higher than 6136 pg/ml, the cLBR decreased more remarkably (10%), but the effect value was not statistically significant, probably because of the relative small sample size in the extremely high peak E2 level group.
Fig. 1.
The smooth curve fitting results demonstrated a clear positive association between cLBR and the peak E2 level in the first segment of the curve, followed by a plateau, and a negative trend afterwards
Outcomes of stratification and interaction analyses
The impact of peak E2 level on the outcomes of IVF/ICSI was further analyzed with multivariable regression model in several subgroups. The results suggested that the peak E2 level did not impact the cLBR, the miscarriage rate, or the preterm delivery rate in most subgroups (Table 4). Interactions between the stratification factors and the peak E2 level were tested, and results showed that majority of the stratification factors failed to have the interaction effect with the peak E2 level on outcomes of cLBR, the miscarriage rate, and the preterm delivery rate. Interestingly, the result implicated that the effect of peak E2 level on the cLBR was different between subgroups of different infertility diagnosis, as well as between subgroups of different follicular count on hCG trigger day. In the subgroup of unexplained infertility diagnosis, the cLBR was negatively associated with the peak E2 level; specifically, the cLBR decreased by nearly 15% with every 100 pg/ml increase of the peak E2 level [OR (95%CI) 0.866 (0.774, 0.968), P = 0.011]. In patients with high follicular count on hCG trigger day (the mean number of follicular count on trigger day was 14.93 ± 2.0), the cLBR was also negatively associated with the peak E2 level. Although the effect value was much smaller, the P value showed significance [OR (95%CI) 0.966 (0.944, 0.989), P = 0.004]. The effect of peak E2 level on the preterm delivery rate was also different in subgroups of different embryo transfer strategies based on the quality of embryos. The preterm delivery rate in the subgroup receiving transfer of mixed quality embryos significantly increased with the increasing of the peak E2 level, but the effect value was also small [OR (95%CI) 1.044 (1.010, 1.080), P = 0.012].
Table 4.
Outcomes of multivariable regression analysis of the impact of peak E2 level on hCG trigger day (100 pg/ml) on cLBR, miscarriage rate, and preterm birth rate in subgroup patients
| Stratification characteristics | N = 1141 | cLBR | P for interaction | Miscarriage rate | P for interaction | Preterm birth | P for interaction | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95%CI) | P | Adjusted OR (95%CI) | P | Adjusted OR (95%CI) | P | |||||
| Infertility etiology (data missing, n = 2) | 0.020 | 0.470 | 0.465 | |||||||
| Tubal factor (n [%]) | 653 (55.34) | 0.984 (0.967, 1.002) | 0.08 | 1.010 (0.979, 1.041) | 0.538 | 1.015 (0.991, 1.040) | 0.219 | |||
| Male factor (n [%]) | 126 (10.68) | 1.015 (0.965, 1.069) | 0.557 | 1.040 (0.906, 1.195) | 0.573 | 1.015 (0.947, 1.088) | 0.677 | |||
| Mixed factor (n [%]) | 291 (24.66) | 1.010 (0.983, 1.037) | 0.464 | 0.968 (0.924, 1.014) | 0.173 | 1.016 (0.982, 1.052) | 0.354 | |||
| Unexplained (n [%]) | 66 (5.59) | 0.866 (0.774, 0.968) | 0.011 | 1.031 (0.859, 1.238) | 0.743 | 1.075 (0.971, 1.190) | 0.165 | |||
| Stimulation strategy | 0.760 | 0.278 | 0.118 | |||||||
| GnRH-agonist protocol (n [%]) | 1071 (93.87) | 1.002 (0.992, 1.012) | 0.757 | 1.003 (0.985, 1.021) | 0.777 | 1.009 (0.995, 1.022) | 0.215 | |||
| GnRH-antagonist protocol (n [%]) | 69 (6.05) | 1.009 (0.970, 1.050) | 0.658 | 1.013 (0.950, 1.081) | 0.694 | 1.027 (0.959, 1.100) | 0.445 | |||
| Follicular count on hCG day (≥ 14 mm)Follicular count on hCG day (≥ 14 mm) | 0.016 | 0.748 | 0.507 | |||||||
| Low (n [%]) (Mean ± SD 7.1 ± 1.8) | 355 (31.2) | 1.016 (0.987, 1.046) | 0.281 | 0.967 (0.916, 1.022) | 0.239 | 1.011 (0.970, 1.052) | 0.614 | |||
| Intermediate (n [%]) (Mean ± SD 11.0 ± 0.8) | 370 (32.5) | 0.992 (0.967, 1.017) | 0.527 | 1.003 (0.955, 1.054) | 0.902 | 1.006 (0.971, 1.041) | 0.751 | |||
| High (n [%]) (Mean ± SD 14.93 ± 2.0) | 414 (36.4) | 0.966 (0.944, 0.989) | 0.004 | 1.010 (0.974, 1.048) | 0.58 | 1.026 (0.997, 1.056) | 0.078 | |||
| Method of fertilization | 0.562 | 0.169 | 0.890 | |||||||
| IVF (n [%]) | 831 (72.8) | 0.998 (0.987, 1.009) | 0.72 | 1.009 (0.990, 1.028) | 0.375 | 1.011 (0.996, 1.026) | 0.147 | |||
| ICSI (n [%]) | 241 (21.1) | 1.007 (0.985, 1.030) | 0.548 | 0.972 (0.933, 1.013) | 0.183 | 1.015 (0.984, 1.047) | 0.339 | |||
| IVF + ICSI (n [%]) | 69 (6.1) | 1.017 (0.976, 1.060) | 0.429 | 1.035 (0.955, 1.120) | 0.404 | 1.026 (0.961, 1.095) | 0.442 | |||
| Development stage of transferred embryos (data missing, n = 16) | 0.628 | 0.078 | 0.000 | |||||||
| 1 or 2 Grade 1 D3 embryos (n [%]) | 231 (20.5) | 0.986 (0.953, 1.021) | 0.433 | 1.004 (0.938, 1.074) | 0.912 | 1.012 (0.968, 1.058) | 0.607 | |||
| 1 or 2 Grade 2 D3 embryos (n [%]) | 487 (43.3) | 0.997 (0.977, 1.017) | 0.746 | 0.995 (0.958, 1.034) | 0.806 | 1.010 (0.983, 1.038) | 0.472 | |||
| 1 Grade 2 and 1 Grade 1 D3 embryos (n [%]) | 274 (24.4) | 0.981 (0.956, 1.007) | 0.154 | 1.008 (0.962, 1.056) | 0.728 | 1.044 (1.010, 1.080) | 0.012 | |||
| Single blastocyst (n [%]) | 124 (11.0) | 1.013 (0.963, 1.065) | 0.623 | 0.989 (0.907, 1.079) | 0.808 | 0.806 (0.618, 1.052) | 0.112 | |||
| Fresh or frozen embryo transfer | 0.216 | 0.655 | 0.272 | |||||||
| Fresh embryo transfer (n [%]) | 377 (33.0) | 0.990 (0.964, 1.017) | 0.468 | 1.032 (0.978, 1.089) | 0.247 | 1.014 (0.979, 1.049) | 0.437 | |||
| FET (n [%]) | 764 (67.0) | 0.993 (0.978, 1.009) | 0.402 | 0.989 (0.963, 1.016) | 0.429 | 1.019 (0.998, 1.040) | 0.082 | |||
Factors with less than 10 cases were not included in the stratification analysis: endometriosis (n = 5) in infertility factors, other stimulation strategy (n = 1) in stimulation strategies and blastocyst + embryo transfer (n = 9) in development of transferred embryos. The following factors, expect the stratification factor itself, were adjusted in the multivariable analysis: female age, BMI, infertility duration, infertility etiology, basal FSH, basal E2, follicular count on hCG day (≥ 14 mm), total dose of Gn administered, stimulation protocol, method of fertilization, basal AFC, development of transferred embryos, and fresh or frozen embryo transfer
Discussion
This retrospective cohort study analyzed 1141 non-PCOS infertile women who underwent the first stimulation cycle. In the present cohort, the mean level of peak E2 and the mean cLBR were 3911.02 ± 1302.93 pg/ml and 66.9%, respectively. When taking into account the confounding factors including female age, BMI, infertility duration, infertility factors, basal FSH, basal E2, follicular count on hCG trigger day (≥ 14 mm), total dose of Gn, stimulation regimen, method of fertilization, basal AFC, development stage of transferred embryos, and fresh or frozen embryo transfer, the results of multivariable analysis and the smooth curve fitting indicated that when the peak E2 level was lower than 2185 pg/ml, the cLBR increased by about 12% with every 100 pg/ml increase of the peak E2 level. When the peak E2 was extremely high, the cLBR was negatively associated with the level of peak E2 level. And, the peak E2 level had a negative impact on the cLBR in patients with unexplained infertility diagnosis, but not on the miscarriage rate or the preterm delivery rate in general or subgroup populations.
The vast majority of the studies investigating the impact of the peak E2 level on the clinical outcomes used the clinical pregnancy rate as the main outcome measurement, which was considered the second best one compared with the live birth rate (LBR) as the efficacy indicator of IVF/ICSI. However, there was only a small study of 71 hyperresponders suggesting the negative association between the peak E2 level and the LBR [9]. The result of our study also suggested a negative relationship between the peak E2 level and the cLBR in overall population, although it failed to reach statistical significance [OR (95%CI) 0.995 (0.982, 1.009), P = 0.486]. The sample size of this study was much bigger, and interestingly, the curve fitting suggested an obvious segmental rather than a linear pattern of the peak E2 level’s effect on the cLBR. Specifically, when the peak E2 level was relatively low (< 2185 pg/ml), their association was positive, but the trend of the effect changed when the peak E2 level increased to above 6361 pg/ml.
The positive association between the peak E2 level and the cLBR in the first segment of the curve suggested the importance of the number of mature oocytes for the success of IVF/ICSI. In women of childbearing age, E2 is primarily secreted by the follicular granulosa cells in ovaries in a menstrual cycle-dependent pattern [13]. In physiologic condition, it increases with the process of follicular maturation and reaches surge before ovulation. Therefore, serum peak E2 level was closely associated with the oocyte yield in COH. Wei et al. performed a retrospective study in 129 patients undergoing IVF/ICSI who were divided into low peak E2 level group (< 1005.89 pmol/L) and high peak E2 level group (> 1005.89 pmol/L), and then found higher clinical pregnancy rate in the high peak E2 level group [6]. Siddhartha et al. also reported that the favorable clinical pregnancy rate was obtained when the peak E2 level was high [7]. Eight-nine (69) cases were included in their analysis and were divided into five groups according to the peak E2 level from low to high (group I < 1000 pg/mL, group II 1000–2000 pg/ml, group III 2000–3000 pg/ml, group IV 3000–4000 pg/ml, and group V > 5000 pg/ml). The result showed that the highest oocyte yield and highest clinical pregnancy rate were found in group V. Another similar study divided 128 patients into 3 groups based on the peak E2 level (group I < 1500 pg/mL, group II 1500–3000 pg/ml, and group III > 3500 pg/ml) and found that the clinical pregnancy rate significantly increased along with the increasing of the peak E2 level [14].
A plenty of studies reached the consistent conclusion with ours, that there was a proper range of peak E2 level, within which the optimal and stable clinical outcomes would be achieved (2185–6361 pg/ml in the present study). For example, Joo et al. concluded that in younger patients (< 38 years), the optimal clinical pregnancy rate was achieved when the peak E2 level was between 3000 and 4000 pg/mL, while in older patients (> 38 years), the range of peak E2 level with favorable clinical pregnancy rate decreased to 2000–3000 pg/mL [15]. Mittal et al. analyzed the ratio of the peak E2 level to the number of AFC (E/fol) on hCG trigger day, and the result also found the existence of a proper range of E/fol ratio (200–299.99 pg/ml per follicle) with the best clinical pregnancy rate.
Extremely high peak E2 level was detrimental to the outcomes of IVF/ICSI in the general population in this study. Although the P value was not significant, the effect value was remarkable (the cLBR decreased by nearly 10% with every 100 pg/ml increase of the peak E2), which was previously supported by Bu et al., who found the negative effect of too high peak E2 level (the highest 10 percentile) on the clinical pregnancy rate in population [8]. This negative association was also supported by the result of the stratification analysis based on the follicular count on hCG trigger day (Table 4). The cLBR significantly decreased with the increasing of the peak E2 level in population with the highest one third follicular count on hCG day, and the impact of the peak E2 level on the cLBR in this population was intrinsically different than in populations with fewer follicles on hCG day (P for interaction = 0.016). Given that E2 is secreted by the follicular granulosa cells, the follicular count on hCG trigger day was closely associated with the level of peak E2 level. But, the negative impact of high E2 level was only significant when a patient’s ovaries over responded. Periera et al. reported 2.3 times higher odds of term LBW when the E2 level was > 3069.2 pg/ml in a study of 2939 live singleton births conceived with fresh IVF-ET [16]. They further confirmed the predictive value of peak E2 level on hCG trigger day for LBW in singletons born after fresh embryo transfer in a retrospective cohort study involving 4071 normal responders [17]. A retrospective study conducted by Imudia et al. has shown that E2 levels of > 3450 pg/ml increase the odds of LBW [18]. When comparing perinatal outcomes between frozen embryo transfer cycles and fresh embryo cycles in randomized controlled trials [19–22], the singleton pregnancies were observed to have a higher mean birth weight and a lower incidence of SGA in FET cycles; meanwhile, the lower E2 levels were observed in the peri-implantation period of FET cycles, demonstrating the negative correlation between maternal serum E2 level and birth weight.
However, the explanation for the negative trend within the last segment of the cLBR-E2 curve varies by subgroups of fresh embryo transfer cycles versus FET cycles. In fresh embryo transfer cycles, it has been proved that the high E2 level during COH impaired the endometrial receptivity, through mechanisms such as reduced endometrial blood flow [23, 24], the advanced transition [25], and the histopathologic change [26, 27] of the endometrium. Endometrial gene expression was also altered by COH. A literature review summarized the impact of the COH on the secretome of the endometrium and suggested that the COH could induce the significant change of the endometrial secretome [28]. Ullah et al. [29] found that the proteome of the endometrial cancer cell line, Ishikawa cell, was altered when the cells were treated with 10−7 M E2. Among the proteins, C13, plasminogen and kininase-1, which are usually upregulated in the endometrial epitheliums within the window of implantation (WOI) were changed as confirmed by western blot. The findings had indicated the impact of E2 on the endometrial receptivity.
Few studies explored the impact of superphysiologic E2 on the clinical outcomes of IVF/ICSI in FET cycles. The negative relationship between the peak E2 level and the cLBR in FET cycles did not differ from that in the fresh embryo transfer cycle (P for interaction was 0.216). Notably, when dividing patients into three groups according to the peak E2 level from low to high, the percentage of FET cycles was significantly higher than that of fresh embryo transfer cycles in the highest peak E2 level group (Table 1). The difference was speculated that in clinical practice, the freeze-all strategy was preferred when the peak serum E2 was very high in order to avoid the OHSS. High serum E2 is known to be an important, though not an independent, predictive factor of OHSS [30]. Coincidently, in this study, the trend of the latter segment of the overall curve matched the curve fitted to only include FET cycles (supplementary Fig. 1), suggesting that the negative relationship in the latter segment of the overall curve was mostly contributed by the FET cycles.
It was hypothesized that the unfavorable impact of the peak E2 on the clinical outcomes in FET cycles was primarily because of the impact of the supraphysiologic E2 on the oocytes during COH, and the oocytes were the only subjects ever exposed to the supraphysiologic E2 milieu in the process of the COH. Our previous study has revealed the negative impact of the peak E2 level on birth weight of singletons born after FET [31], suggesting that the influence of the peak E2 level on the oocytes might be through epigenetic mechanisms so that the influence could subsequently be passed on to the embryos and their later implantation and development. Unfortunately, few studies have explored the molecular changes in oocytes generated by COH with the supraphysiologic E2 level. Tarumi et al. found that culture with E2 delayed or inhibited oocyte meiotic maturation, such as chromosome alignment on the metaphase plate and extrusion of the first polar body, suggesting that E2 induced abnormalities of follicular development [32]. Imudia et al. found that serum E2 above the 90th percentile on the hCG trigger day was associated with a significantly lower rate of normal fertilization (68.6 ± 20% vs. 71.6 ± 21%, P = 0.02) when compared with patients with a lower serum E2 threshold [3], indicating the detrimental influence of high E2 on the fertilization potential of the oocytes.
The stratification multivariable analysis also suggested the significant negative impact of peak E2 level on the cLBR in population with unexplained infertility. The diagnosis of unexplained infertility was made after excluding common causes of infertility through semen analysis, assessment of ovulation, endometriosis, and tubal patency test [33]. Unexplained infertility includes several heterogeneous conditions, one being age-related infertility [34]. Age is the best predictor for ovarian reserve and oocyte quality, as they decline steadily from before birth until the menopause [35]. The mean age of the 66 unexplained infertile patients in this cohort was significantly higher than that of the remaining patients with other infertility factors (31.26 ± 3.73 years vs 29.88 ± 3.68 years, P = 0.003). Moreover, the basal serum FSH (measured on the menstrual day 3), which was historically screened for ovarian reserve evaluation and which rises in reaction to decreased responsiveness of ovary [36], was significantly higher in the unexplained infertile women in this cohort (8.24 ± 5.40 IU/L vs 7.13 ± 2.65 IU/L). Therefore, we postulated that the ovarian reserve in these unexplained infertile patients decreased so that the oocytes with compromised quality might be more sensitive to the detrimental effect of the elevated E2, which could explain the significant negative relationship between cLBR and the peak E2 level in this subgroup. However, more robust data are needed to confirm the conclusion.
Additionally, it was noticed that the supraphysiologic E2 was significantly associated with the elevated preterm birth rate [OR (95%CI) 1.044 (1.010, 1.080), P = 0.012] in patients one class 1 and one class 2 D3 embryos transferred (n = 274). Further analysis showed significantly more twin births (34.8%) in this subgroup when compared with patients transferred only grade 1 D3 embryos, only grade 2 D3 embryos, and single blastocyst (34.1%, 26.4%, and 6.6%, P < 0.001). While the macrosomia was not significantly different between subgroups transferred embryos of different quality. Literatures also suggested that the multiple pregnancy contributed most to the high preterm delivery rate in IVF/ICSI [37, 38]. Hence, the positive relationship between the preterm delivery and the peak E2 level in subgroup patients transferred with mixed quality embryos might be with considerable bias induced by the high percentage of multiple pregnancy.
Further clinical studies investigating the impact of the peak E2 level during COH on the clinical outcomes of IVF/ICSI specifically in poor or high responders, as well as in fresh or frozen embryo transfer cycles, are warranted to facilitate the tailored treatment strategy for individual patients with different subfertile background.
Conclusion
This retrospective cohort study concluded that the peak E2 level on hCG trigger day was associated with the cLBR in a segmental pattern. The peak E2 level had negative impact on the cLBR in patients with unexplained infertility diagnosis, probably attributing to the impaired ovarian reserve and the oocyte quality. The peak E2 level does not impact the miscarriage rate in general or subgroup populations with normal ovarian reserve. The positive association between peak E2 level and the preterm delivery in patients being transferred with mixed quality embryos should be interpreted with caution because of the high multiple pregnancy percentage in this subgroup.
Electronic supplementary material
(PNG 323 kb)
(TIFF 408 kb)
Funding information
This work was funded by grants from the National Natural Science Foundation of China (NSFC, Grant/Award No. 81671463) and the Key Project and Development Plan-fund of Shaanxi province (Grant/Award No. 2017ZDCXL-SF-02-03).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wanlin Zhang and Ying Tian contributed equally to this work.
References
- 1.Crawford GE, Ledger WL. In vitro fertilisation/intracytoplasmic sperm injection beyond 2020. BJOG. 2019;126(2):237–243. doi: 10.1111/1471-0528.15526. [DOI] [PubMed] [Google Scholar]
- 2.De Neubourg D, Bogaerts K, Blockeel C, Coetsier T, Delvigne A, Devreker F, et al. How do cumulative live birth rates and cumulative multiple live birth rates over complete courses of assisted reproductive technology treatment per woman compare among registries? Hum Reprod (Oxford, England). 2016;31(1):93–99. doi: 10.1093/humrep/dev270. [DOI] [PubMed] [Google Scholar]
- 3.Imudia AN, Goldman RH, Awonuga AO, Wright DL, Styer AK, Toth TL. The impact of supraphysiologic serum estradiol levels on peri-implantation embryo development and early pregnancy outcome following in vitro fertilization cycles. J Assist Reprod Genet. 2014;31(1):65–71. doi: 10.1007/s10815-013-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kara M, Kutlu T, Sofuoglu K, Devranoglu B, Cetinkaya T. Association between serum estradiol level on the hCG administration day and IVF-ICSI outcome. Iran J Reprod Med. 2012;10(1):53–58. [PMC free article] [PubMed] [Google Scholar]
- 5.Rehman R, Jawaid S, Gul H, Khan R. Impact of peak estradiol levels on reproductive outcome of intracytoplasmic sperm injection. Pak J Med Sci. 2014;30(5):986–991. doi: 10.12669/pjms.305.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei M, Zhang XM, Gu FL, Lv F, Ji YR, Liu KF, et al. The impact of LH, E2, and P level of HCG administration day on outcomes of in vitro fertilization in controlled ovarian hyperstimulation. Clin Exp Obstet Gynecol. 2015;42(3):361–366. [PubMed] [Google Scholar]
- 7.Siddhartha N, Reddy NS, Pandurangi M, Tamizharasi M, Radha V, Kanimozhi K. Correlation of serum estradiol level on the day of ovulation trigger with the reproductive outcome of intracytoplasmic sperm injection. J Hum Reprod Sci. 2016;9(1):23–27. doi: 10.4103/0974-1208.178631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bu Z, Wang K, Guo Y, Su Y, Zhai J, Sun Y. Impact of estrogen-to-oocyte ratio on live birth rate in women undergoing in vitro fertilization and embryo transfer. Int J Clin Exp Med. 2015;8(7):11327–11331. [PMC free article] [PubMed] [Google Scholar]
- 9.Committee Opinion No. 690: Carrier Screening in the Age of Genomic Medicine. Obstet Gynecol. 2017;129(3):e35–40. 10.1097/aog.0000000000001951. [DOI] [PubMed]
- 10.Christianson MS, Shoham G, Tobler KJ, Zhao Y, Cordeiro CN, Leong M, et al. Measurement of antral follicle count in patients undergoing in vitro fertilization treatment: results of a worldwide web-based survey. J Assist Reprod Genet. 2015;32(10):1435–1440. doi: 10.1007/s10815-015-0555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group EASPCW Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Hum Reprod. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Alpha Scientists in Reproductive Medicine, ESHRE Special Interest Group Embryology Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reproductive BioMedicine Online. 2011;22(6):632–646. doi: 10.1016/j.rbmo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Vrtacnik P, Ostanek B, Mencej-Bedrac S, Marc J. The many faces of estrogen signaling. Biochem Med (Zagreb) 2014;24(3):329–342. doi: 10.11613/bm.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foroozanfard F, Moraveji SA, Taghavi SA, Karimi F. Association between serum estradiol level on the day of hCG administration and IVF-ICSI outcome. J Obstet Gynaecol India. 2016;66(3):170–173. doi: 10.1007/s13224-015-0687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93(2):442–446. doi: 10.1016/j.fertnstert.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 16.Pereira N, Reichman DE, Goldschlag DE, Lekovich JP, Rosenwaks Z. Impact of elevated peak serum estradiol levels during controlled ovarian hyperstimulation on the birth weight of term singletons from fresh IVF-ET cycles. J Assist Reprod Genet. 2015;32(4):527–532. doi: 10.1007/s10815-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira N, Elias RT, Christos PJ, Petrini AC, Hancock K, Lekovich JP, et al. Supraphysiologic estradiol is an independent predictor of low birth weight in full-term singletons born after fresh embryo transfer. Hum Reprod. 2017;32(7):1410–1417. doi: 10.1093/humrep/dex095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97(6):1374–1379. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–136. doi: 10.1056/NEJMoa1705334. [DOI] [PubMed] [Google Scholar]
- 21.Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, et al. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med. 2018;378(2):137–147. doi: 10.1056/NEJMoa1703768. [DOI] [PubMed] [Google Scholar]
- 22.Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet (London, England) 2019;393(10178):1310–1318. doi: 10.1016/s0140-6736(18)32843-5. [DOI] [PubMed] [Google Scholar]
- 23.Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. Comparison of endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound between stimulated and natural cycles in the same patients. Hum Reprod (Oxford, England). 2004;19(10):2385–2390. doi: 10.1093/humrep/deh384. [DOI] [PubMed] [Google Scholar]
- 24.Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. Factors affecting endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound during IVF treatment. Hum Reprod (Oxford, England) 2006;21(4):1062–1069. doi: 10.1093/humrep/dei442. [DOI] [PubMed] [Google Scholar]
- 25.Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78(5):1025–1029. doi: 10.1016/S0015-0282(02)03323-X. [DOI] [PubMed] [Google Scholar]
- 26.Lee YL, Liu Y, Ng PY, Lee KF, Au CL, Ng EH, et al. Aberrant expression of angiopoietins-1 and -2 and vascular endothelial growth factor-a in peri-implantation endometrium after gonadotrophin stimulation. Hum Reprod (Oxford, England). 2008;23(4):894–903. doi: 10.1093/humrep/den004. [DOI] [PubMed] [Google Scholar]
- 27.Zapantis G, Szmyga MJ, Rybak EA, Meier UT. Premature formation of nucleolar channel systems indicates advanced endometrial maturation following controlled ovarian hyperstimulation. Hum Reprod (Oxford, England). 2013;28(12):3292–3300. doi: 10.1093/humrep/det358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MQ, Jin LP. Ovarian stimulation for in vitro fertilization alters the protein profile expression in endometrial secretion. Int J Clin Exp Pathol. 2013;6(10):1964–1971. [PMC free article] [PubMed] [Google Scholar]
- 29.Ullah K, Rahman TU, Pan HT, Guo MX, Dong XY, Liu J, et al. Serum estradiol levels in controlled ovarian stimulation directly affect the endometrium. J Mol Endocrinol. 2017;59(2):105–119. doi: 10.1530/jme-17-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namavar Jahromi BMD, Parsanezhad MEMD, Shomali ZMD, Bakhshai PMD, Alborzi MMD, Moin Vaziri NMDP, et al. Ovarian Hyperstimulation syndrome: a narrative review of its pathophysiology, risk factors, prevention, classification, and management. Iranian J Med Sci. 2018;43(3):248–260. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Wanlin, Ma Yefei, Xiong Yujing, Xiao Xifeng, Chen Shuqiang, Wang Xiaohong. Supraphysiological serum oestradiol negatively affects birthweight in cryopreserved embryo transfers: a retrospective cohort study. Reproductive BioMedicine Online. 2019;39(2):312–320. doi: 10.1016/j.rbmo.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Tarumi W, Itoh MT, Suzuki N. Effects of 5α-dihydrotestosterone and 17β-estradiol on the mouse ovarian follicle development and oocyte maturation. PLoS One. 2014;9(6):e99423-e. doi: 10.1371/journal.pone.0099423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. 2014;69(2):109–115. doi: 10.1097/ogx.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 34.Somigliana E, Paffoni A, Busnelli A, Filippi F, Pagliardini L, Vigano P, et al. Age-related infertility and unexplained infertility: an intricate clinical dilemma. Hum Reprod (Oxford, England). 2016;31(7):1390–1396. doi: 10.1093/humrep/dew066. [DOI] [PubMed] [Google Scholar]
- 35.Ge ZJ, Schatten H, Zhang CL, Sun QY. Oocyte ageing and epigenetics. Reproduction (Cambridge, England) 2015;149(3):R103–R114. doi: 10.1530/rep-14-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 37.Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol. 2017;217(3):270–281. doi: 10.1016/j.ajog.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Adverse pregnancy, birth, and infant outcomes in twins: effects of maternal fertility status and infant gender combinations; the Massachusetts outcomes study of assisted reproductive technology. Am J Obstet Gynecol. 2017;217(3):330.e1–330e15. doi: 10.1016/j.ajog.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 323 kb)
(TIFF 408 kb)