Abstract
Purpose
To report the normal live birth and birth defect rates pre- and post- preimplantation genetic testing for chromosomal structural rearrangements (PGT-SR) in reciprocal translocation carriers who have experienced two or more unfavorable pregnancy histories.
Methods
We conducted a retrospective cohort study of 194 couples who underwent 265 PGT-SR cycles between January 2013 and August 2016. The rates of miscarriage, normal live birth, and birth defect pre- and post- PGT-SR treatment were recorded. The types of birth defect were also categorized.
Results
Before PGT-SR treatment, the 194 couples with reciprocal translocation had a previous reproductive history consisting of 592 pregnancies in total: 496 (83.8%) were miscarriages; 29 (4.9%) ended by induced abortion due to unintended pregnancy; 36 (6.1%) had birth defects; and 17 (2.9%) were normal live births. After PGT-SR treatment, there were 118 clinical pregnancies. Of these pregnancies, 13 (11.0%) were miscarriages, 101 (85.6%) were normal live births, and 4 (3.4%) had birth defects. In total, 14 different disorders were noted in the prenatal and postnatal examinations. Before the PGT-SR treatment, multiple birth defects, central nervous system abnormalities, and congenital heart defects were the three most common congenital malformations. Excluding for methylmalonic acidemia, there were only single and mild birth defects after the PGT-SR treatment.
Conclusions
After the PGT-SR treatment, the reciprocal translocation carriers who had previously experienced two or more unfavorable pregnancy outcomes had a low risk of miscarriages and birth defects. The rate of normal live births per pregnancy was higher after PGT-SR treatment.
Keywords: Preimplantation genetic testing, Balanced translocation, Birth defect, Miscarriage, Live birth
Introduction
For couples with reciprocal translocation, the outcomes of pregnancies without interventions have a high risk of recurrent miscarriage (RM) and malformed offspring with an unbalanced genome [1–3]. Since it was first applied in 1989 [4], the evolving method of preimplantation genetic testing (PGT) has been available to precisely detect chromosome abnormalities, and it has become a predominant treatment for embryo karyotype diagnosis. After PGT for chromosomal structural rearrangement (PGT-SR) treatment, pregnancy outcomes of reciprocal carriers with recurrent miscarriage have been reported to improve, with a decrease in miscarriage rates and an increase in the ongoing pregnancy rates [5, 6]. How PGT-SR treatment affects the rates of birth defects and normal live births patients with reciprocal translocation remain understudied.
Normal live birth and birth defect rates are informative parameters in genetic counseling, but these have not been used in previous work [7–9]. Patients with an unfavorable reproductive history such as a birth defect can be anxious about whether PGT-SR treatment can minimize suffering and help them have a normal baby. Information about normal live births and birth defects is necessary to provide satisfactory genetic counseling.
To evaluate the effectiveness of PGT-SR treatment in reciprocal couples with unfavorable pregnancy outcomes and to provide additional data for genetic counseling, we investigated the rates of normal live births and birth defects of them before and after treatment.
Materials and methods
Study design
We conducted a retrospective cohort study of reciprocal translocation carriers who received PGT-SR treatment between January 2013 and August 2016. The inclusion criteria were as follows: (1) either the male or female of the couple was a reciprocal translocation carrier; (2) the couple had experienced two or more unfavorable pregnancy outcomes as miscarriage, stillbirth, or birth defect; and (3) no family genetic disease. In total, 194 couples were eligible.
All the patients accepted genetic counseling before decision. Reviews of genetic and pregnancy histories were taken first. The explanation described the advantages and limitations of PGT-SR procedures. All of the patients who received PGT-SR underwent cryopreserved cycles and the premise of biopsy was at least one good-quality day 5/6 embryos. All the normal/balanced embryo transfers were comprised of singletons. Written consent was signed by couples.
Information about the pregnancy outcomes pre-PGT-SR was extracted from the medical records, and follow-up information of pregnancy outcomes post-PGT-SR was collected via telephone interviews or medical records.
Array-CGH procedure
The array-comparative genomic hybridization (CGH) procedure was performed based on the manufacturer’s protocol. Trophectoderm cells of the blastocysts were biopsied and collected into sterile tubes preparing for polymerase chain reaction (PCR). The extracted DNA from the washed samples and reference DNA (male genomic) was processed using whole genome amplification (WGA). The labeled and amplified samples and the reference DNAs with cy3 and cy5 fluorophores were competitively hybridized and applied to specific arrays for translocations in the array-CGH format (Illumina, San Diego, USA). After washing, microarray slides were scanned with a laser scanner (InnoScan 900). The scanned images were analyzed using a software package BlueFuse Multi Software (Illumina, San Diego, USA). The euploid embryos were defined as having chromosome ratios within a ± 0.3 log2 ratio [10].
Clinical data and definition
The data on maternal age, PGT-SR cycles, number of biopsy embryos, array-CGH on blastocysts, balanced embryos, transferred embryos, and clinical pregnancy outcomes after embryo transfer were calculated. The recorded pregnancy outcomes consisted of ectopic pregnancy, abortion (e.g., miscarriage, induced abortion due to an unexpected pregnancy), normal live birth, and birth defect. The types of birth defects were recorded in detail and classified mainly by structural or anatomical system.
A clinical pregnancy was defined as an ultrasound-detected gestational sac at 5–6 weeks after embryo transfer. Miscarriage was defined as the natural death of a pregnancy before the fetus could survive outside the uterus (from the time of conception until 20 weeks’ gestation). Induced abortion was defined as abortion caused on purpose, or for therapeutic reasons (elective abortion or therapeutic abortion). A normal live birth was defined as the birth of a phenotypically normal child. A birth defect was defined as structural or functional changes that affected almost all or parts of the body. Birth defects could be detected either prenatally or after birth and included terminations for defects at any gestational period and also cerebral palsy [11].
Statistical analysis
In this study, the categorical data of pregnancy outcomes are presented as proportions and continuous data as age are presented as means and standard deviations.
Results
We studied194 couples with reciprocal translocation who were referred for PGT-SR treatment after two or more unfavorable pregnancies between January 2013 and August 2016. The average maternal age was 29.61 ± 4.26 years (range 22–43). Of 194 couples, 165(85.1%) were maternal age < 35 years and 29 (14.9%) were maternal age ≥ 35 years. Of the194 couples with reciprocal translocation, 111 (57.2%) had female carriers and 83 (42.8%) had male carriers.
Pregnancy history of the reciprocal carrier couples
Of total 194 couples, 55.2% (107/194) had experienced two or more times unfavorable pregnancy outcomes, 27.3% (53/194) had experienced three unfavorable outcomes, 8.2% (16/194) had experienced four times, and 9.3% (18/194) had experienced five or more times. Among those couples, 32 (16.5%) couples suffered from offspring malformations and 162 (84.5%) couples had high risk of miscarriage, only 17 (8.8%) couples had experienced normal live birth.
The 194 couples with reciprocal translocation had a reproductive history of 592 pregnancies in total. Of 592 pregnancies, 496 (83.8%) had ended as miscarriage; 29 (4.9%) had terminated by elective abortion due to an unexpected pregnancy; and 36 (6.1%) pregnancies had experienced birth defect. Only 17 (2.9%) had been normal live birth (Table 1).
Table 1.
Pregnancy outcomes of 194 reciprocal carriers pre- and post-PGT-SR
| Pre-PGT n (%) |
Post-PGT n (%) |
|
|---|---|---|
| Achieved clinic pregnancy | ||
| No | / | 80 (41.2) |
| Yes | 194 | 114 (58.8) |
| Numbers of unfavorable pregnancy | ||
| 0 | / | 97 (85.1) |
| 1 | / | 17 (14.9) |
| 2 | 107 (55.2) | |
| 3 | 53 (27.3) | |
| 4 | 16 (8.2) | |
| ≥ 5 | 18 (9.3) | |
| Mean (per couple) | 2.8 | 0.09 |
| Birth defect | ||
| Yes | 32 (16.5) | 4 (3.5) |
| No | 162 (83.5) | 110 (96.5) |
| Normal live birth | ||
| Yes | 17 (8.8) | 101 (88.6) |
| No | 177 (91.2) | 13 (11.4) |
| Total number of pregnancies | 592 | 118 |
| Types of pregnancy outcome | ||
| Ectopic pregnancy | 10 (1.7) | / |
| Miscarriage | 496 (83.8) | 13 (11.0) |
| Induced abortion | ||
| Elective abortion | 29 (4.9) | / |
| Therapeutic abortion | 2 (0.3) | / |
| Stillbirth | 1 (0.2) | / |
| Birth defect | 36 (6.1) | 4 (3.4) |
| Normal live birth | 17 (2.9) | 101 (85.6) |
Embryo result and pregnancy outcome of the PGT-SR cycles in reciprocal carriers
In total, 265 cycles of PGT-SR were carried out for the 194 couples with reciprocal translocation, and 968 embryos were tested via array-CGH. The average start cycles were 1.32 per couple. Eighteen (6.7%) cycles were canceled due to poor ovary response or the unavailability of a good quality embryo, and 87 (31.7%) cycles were canceled because of unavailability of a normal/balanced embryo. Among all the good-quality embryos, 98.9% (968/979) were successfully tested, and 26.7% (258/968) of them were normal/balanced.
Post-PGT-SR treatment, 114 (58.8%, 114/194) couples achieved clinical pregnancy. Among the couples with clinical pregnancies, 101 (88.6%, 101/114) had normal offspring. Only 4 (3.5%, 4/114) couples had malformed offspring compared with 32 (16.5%, 32/194) couples pre-PGT-SR. No patients underwent an induced abortion in the post-PGT-SR group, but four birth defects existed in this group. They were polydactyly, heart defect, congenital inguinal hernia, and methylmalonic acidemia.
After the 179 normal/balanced embryos were transferred, 65.9% (118/179) reached clinical pregnancy. Of the clinical pregnancies, 11.0% (13/118) terminated as miscarriages, which was significantly decreased compared with miscarriage rates (83.8%) before PGT-SR treatment. There were 85.6% (101/118) normal live birth, and 3.4% (4/118) of these had birth defect. The normal live birth rates were 56.4% (101/179) per embryo transfer (ET) and 38.1% (101/265) per started cycles (Table 1).
Types of birth defects in reciprocal carriers pre- and post-PGT-SR treatment
There were 36 pregnancies from 32 carriers with birth defects before PGT-SR treatment. After the PGT-SR treatment in the same 32 couples, there were 50.0% (16/32) pregnancies free of birth defects and had a normal live birth. Some of the couples (31.3%, 10/32) were incapable of having normal/balanced embryo. Four couples who did not experience any birth defects before treatment had a baby with birth defects post-PGT-SR. It is noteworthy that one of them was single gene disorders (methylmalonic acidemia) and the couples denied any family history of genetic disorder. The information about carrier couples with birth defect pregnancies extracted from follow-up records is listed in Table 2.
Table 2.
Types of birth defects among 40 reciprocal carriers pre- and post-PGT-SR
| Karyotypes | Maternal age | N | Birth defect pre-PGT | N | Birth defect post-PGT | Pregnancy outcome after PGT treatment |
|---|---|---|---|---|---|---|
| 46, XX,t(1;11)(q41;q24) | 28 | 2 |
(1) Holoprosencephaly (2) Hydrocephalus |
Normal live birth | ||
| 46, XX,t(1;3)(q25;p21) | 33 | 2 |
(1) Heart defects (2) Cerebral palsy |
Normal live birth | ||
| 46, XY,t(3;7)(q21;q32) | 32 | 2 |
(1) Heart defect and Pulmonary hypoplasia (2) Fetal nuchal cystic hygroma |
Implantation failure | ||
| 46, XX,t(9;16)(p10;p10) | 30 | 2 |
(1) Severe multiple defect (2) 21 trisomy |
Normal live birth | ||
| 46, XX,t(15;16)(q26;p11.2) | 29 | 1 | Renal and heart defects | Normal live birth | ||
| 46, XX,t(6;13)(q22;q33) | 36 | 1 | Fetal nuchal cystic hygroma and Orofacial clefts | Without normal embryo | ||
| 46, XY,t(3;22)(p24;q13) | 40 | 1 | Hydronephrosis and heart defect | Without normal embryo | ||
| 46, XY,t(10;20)(p11.2;q13.1) | 25 | 1 | Coronary artery fistula and single umbilical artery | Normal live birth | ||
| 46, XX,t(3;10)(q29;q21) | 30 | 1 | Heart defect and eye defect | Miscarriage | ||
| 46, XY,t(7;9)(p13;p21) | 25 | 1 | Fetal nuchal cystic hygroma and acromphalus | Normal live birth | ||
| 46, XX,t(1;3)(q42;q21) | 30 | 1 | Unbalanced karyotype and multiple defect | Implantation failure | ||
| 46, XY,t(6;14)(p23;q24) | 36 | 1 | Hydrocephalus | Normal live birth | ||
| 46, XY,t(6;8)(q15;p23) | 29 | 1 | Hydrocephalus | Implantation failure | ||
| 46, XX,t(8;13)(q22;q22) | 26 | 1 | Brain defect | Without normal embryo | ||
| 46, XX,t(7;21)(q22;q22) | 29 | 1 | Brain defect | Normal live birth | ||
| 46, XX,t(9;11)(q34;q23) | 30 | 1 | Heart defect | Without normal embryo | ||
| 46, XY,t(1;10)(q42;q22) | 29 | 1 | Heart defect | Normal live birth | ||
| 46, XY,t(4;10)(p11;p15),1qh+ | 31 | 1 | Heart defect | Normal live birth | ||
| 46, XX,t(13;15)(q13;q25) | 31 | 1 | Heart defect | Normal live birth | ||
| 46, XY,t(4;10)(p10;p10) | 27 | 1 | Fetal nuchal cystic hygroma | Without normal embryo | ||
| 46, XX,t(2; 11)(q35;q22) | 27 | 1 | Fetal nuchal cystic hygroma | Normal live birth | ||
| 46, XX,t(17;20)(p10;p13) | 31 | 1 | Fetal nuchal cystic hygroma | Implantation failure | ||
| 46, XY,t(3;4)(p23;q43) | 29 | 1 | Unbalanced karyotype | Without normal embryo | ||
| 46,XY,t(1;16)(q32;p13.3) | 29 | 1 | Unbalanced karyotype | Without normal embryo | ||
| 46, XX,t(11;22)(q25;q12) | 27 | 1 | 22 trisomy | Without normal embryo | ||
| 46, XX,t(1;11)(q32;p24) | 26 | 1 | Orofacial clefts | Normal live birth | ||
| 46, XX,t(3;4)(p13;q35) | 28 | 1 | Orofacial clefts | Normal live birth | ||
| 46, XX,t(6;7)(q21;p13) | 39 | 1 | Cerebral palsy | Without normal embryo | ||
| 46, XY,t(2q;15q) | 31 | 1 | Pleural effusion | Miscarriage | ||
| 46, XY,t(5;18)(q?23;q?21) | 35 | 1 | Congenital hip dislocation | Without normal embryo | ||
| 46, XX,t(7;9)(p15;q13) | 33 | 1 | Limbs defect | Normal live birth | ||
| 46, XX,t(5;18)(p11;q11.1) | 26 | 1 | Acromphalus | Normal live birth | ||
| 46, XY,t(3;8)(p25;q22) | 28 | 1 | Polydactyly | Birth defect | ||
| 46, XY,t(3:21)(p13;q22) | 23 | 1 | Heart defect | Birth defect | ||
| 46, XY,t(3;7)(q27;p15) | 24 | 1 | Congenital inguinal hernia | Birth defect | ||
| 46, XX,t(6;15)(q21;q24) | 24 | 1 | Methylmalonic acidemia | Birth defect | ||
| Total | 36 | 4 |
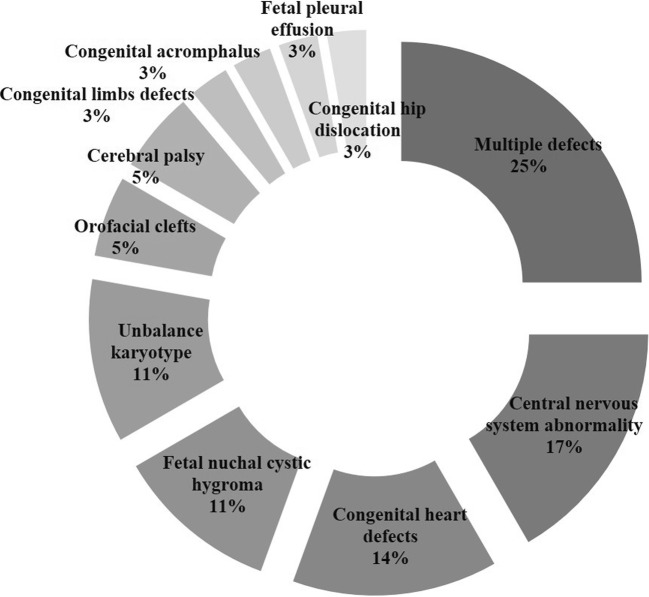
In total, 14 different disorders were found upon prenatal and postnatal examination. The types of birth defects fell into two categories: multiple defects and single defect. Multiple defects accounted for 25% (9/36) in birth defect category pre-PGT-SR, and the remaining defects were single defect. Among the single defects, central nervous system abnormalities and congenital heart defects were the most common. Figures 1 and 2 show the categories of birth defects pre- and post-PGT-SR treatment.
Fig. 1.
Birth defects category pre-PGT-SR (n = 36)
Fig. 2.
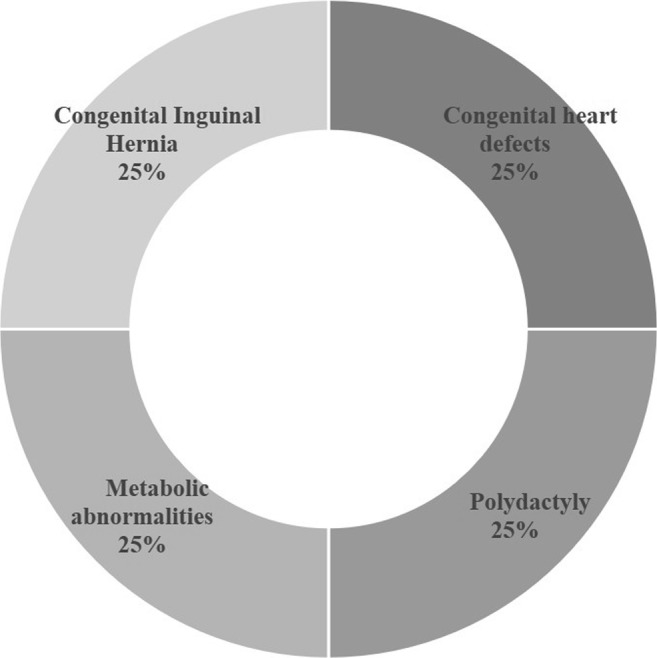
Birth defects category post-PGT-SR (n = 4)
Discussion
We evaluated the effectiveness of PGT-SR treatment for reciprocal translocation carriers who had two or more unfavorable pregnancies. The PGT-SR treatment lowered the incidence of miscarriage and birth defect rates and increased normal live birth rates.
The incidence of miscarriage in natural pregnancies (83.8%) of reciprocal translocation carriers in our study is consistent with previous studies (83.1–84.6%) [3, 12], and the average number of miscarriages (2.8) in our study also agree with the range reported in previous work (2.9–4.3) [13]. The incidence of birth defects pre-PGT-SR per couple (16.5%) is consistent with other study (16.3%) [3], and the two most common types of birth defects were multiple defects and heart defect. The rate of normal live birth (2.9%) was obviously lower than in previous studies (range 44–47.2%) [14, 15]. These rates may have been cofounded by China’s one child policy, because couples with reciprocal translocation who have a healthy baby generally do not come to genetic consulting and tried to have another baby.
Similar to other studies on PGT-SR for reciprocal translocation, over half of the embryos transfer were canceled because the unavailability of normal/balanced embryos [7]. Once the normal/balanced embryos were transferred, the rates of live birth per ET (58.7%, 105/179) were similar with live birth rates per ET of other 24-chromosome screening studies (50–52%) [9, 16]. The live birth rates per couple(54.1%, 105/194) in our study were higher than live birth rates of fluorescence in situ hybridization (FISH)-PGT-SR in reciprocal carriers who had two or more miscarriage (31–32%) [13, 17]. To date, no studies reported birth defect information of reciprocal translocation carriers before and after 24-chromosome screening PGT-SR., The birth defect rates per pregnancy(3.4%, 4/118) found in this study are at the low end of the reported birth defect rates from studies focusing on safety of FISH-PGT-SR (range 2.6–16.7%) [18–20].
Compared with naturally conceived pregnancy outcomes in the reciprocal translocation carriers, the live birth rates per couple (54.1%, 105/194) in our study agree with subsequent live birth rates per couple in previous work (47.2–63%) [15, 21], but lower than a long-term (5.8 years) cumulative live birth rates per carrier couple (83%) [14]. That means a woman who can get consecutive series of pregnancies naturally has 83% chance of having a normal live birth in 5 years. The ideal cumulative live birth rate is based on a certain number of pregnancies. During the process of having a normal baby, woman with reciprocal translocation would experience times of miscarriage (54%) or terminated pregnancy due to the fetal abnormality (2.4%) [14]. When PGT-SR treatment was applied, the miscarriage rate was lower (11.0%) and no induced abortion was reported, and normal live birth rates per pregnancy (85.6% 101/118) were higher than other studies reporting live birth rates per pregnancy (55.3–63%) [12, 14]. Few studies have reported birth defect rates when following-up naturally pregnancy. One study has noted four birth defects that all came from reciprocal carriers (2.5%, 4/157) [14]. Those reported birth defect were two children with an unbalanced karyotype, a severely handicapped child with Potter’s syndrome, and one child with esophageal atresia. The rates of birth defect per reciprocal couple in our study were 2.1% (4/194). The types of birth defects were polydactyly, heart defect, congenital inguinal hernia, and methylmalonic acidemia. Among those disorders, methylmalonic acidemia was single gene disorders and no unbalanced karyotypes. We therefore suggest that the mechanism of lower birth defects is related to the chromosomal screening. Chromosomal abnormality was considered to be a risk factor for many birth defects, such as congenital heart defect and fetal nuchal cystic hygroma [22]. The PGT-SR treatment could avoid unbalanced chromosomal abnormality as well as lowering the risk of some birth defects.
We found that the normal live birth rates per carrier couple following 24-chromosome screening PGT-SR treatment were higher than reported studies with FISH-PGT-SR (52.1% vs 31–32%) [13, 17]. The observed live birth rates were comparable with subsequent natural pregnancy [15, 21]. After a clinical pregnancy, the normal live birth reached 85.6% per pregnancy after the PGT-SR treatment. This finding suggests that 24-chromosome screening is a good choice for reciprocal carriers to avoid high risk of miscarriage and to achieve acceptable normal live birth rates.
Currently, the focus of genetic counseling is on unbalanced karyotype offspring in reciprocal carriers. However, when genetic counseling for reciprocal translocation couples suffered from birth defect, the question about their risk of having a child with phenotypes of birth defect is unanswerable. We found that the unbalanced karyotype was not the dominant phenotypes of birth defects in the offspring of reciprocal carriers. Severe multiple defects dominated. After the PGT-SR treatment, the birth defect phenotypes were mild and no unbalanced karyotypes were found during the prenatal examination (Table 2). This trend in birth defect rate suggests that reciprocal translocation carriers with severe multiple birth defects might benefit from PGT-SR treatment.
This study considered the effect of PGT-SR treatment on the birth defects and normal live births and categorized birth defects in reciprocal translocation carriers before and after PGT-SR treatment. Until now, no study had reported information on birth defect and normal live birth information of reciprocal translocation carriers before and after 24-chromosome screening PGT-SR treatment.
The information about pregnancies pre-PGT-SR is limited, and most of the couples did not realize the importance of chromosome testing in their birth defect offspring. After post-PGT-SR treatment, there were 105 carriers who had a live birth (including normal live birth and live birth with defect). Only 14 (13.3%) of these patients opted for an amniocentesis and 28.5% (4/14) of their offspring were found to be reciprocal carriers. In addition, some embryos were not been transferred before analysis. Therefore, we cannot rule out the possibility of differences between the final pregnancy rates and the present data in this study. As this was a retrospective study, the sample size post-PGT-SR remained small. Further prospective, large-size cohort studies are necessary for solid conclusion.
In conclusion, the reciprocal translocation carriers who had experienced two or more unfavorable pregnancy outcomes had a low risk of miscarriage and severe birth defect after PGT-SR treatment. Their chance of having a normal live birth treatment was higher than has been reported after FISH-PGT-SR and was comparable with subsequent naturally pregnancies. Reciprocal translocation carriers who have had offspring with severe multiple birth defects might benefit from PGT-SR treatment.
Acknowledgments
We sincerely thank Mei Li’s group for helping us with follow-up investigation and data collection. We are grateful to PGT-SR staff and all patients of Center for Reproductive Medicine, Shandong Provincial Hospital Affiliated to Shandong University who made this study possible.
Funding information
This study was financially supported by grant from the National Key Research and Development Program of China (2016YFC1000202) and General Program of National Natural Science Foundation of China (81671522).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neri G, Serra A, Campana M, Tedeschi B. Reproductive risks for translocation carriers: cytogenetic study and analysis of pregnancy outcome in 58 families. Am J Med Genet. 1983;16:535–561. doi: 10.1002/ajmg.1320160412. [DOI] [PubMed] [Google Scholar]
- 2.Kozlowska K, Panasiuk B, Stasiewicz-Jarocka B, Lurie IW, Chrzanowska K, Lenkiewicz M, et al. Probability rate of unbalanced offspring at birth and risk of unfavorable pregnancy outcomes in families of carriers of chromosomal reciprocal translocations involving chromosome 7. Ginekol Pol. 2013;84:992–1004. doi: 10.17772/gp/1671. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Xu J, Yin M, Chen M, Ren D. Pregnancy outcomes of 194 couples with balanced translocations. Zhonghua Fu Chan Ke Za Zhi. 2006;41:592–596. [PubMed] [Google Scholar]
- 4.Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344:768–770. doi: 10.1038/344768a0. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Aoyama N, Kawasaki N, Hayashi H, Xiaohui T, Abe T, Kuroda T. Reproductive outcomes following preimplantation genetic diagnosis using fluorescence in situ hybridization for 52 translocation carrier couples with a history of recurrent pregnancy loss. J Hum Genet. 2016;61:687–692. doi: 10.1038/jhg.2016.39. [DOI] [PubMed] [Google Scholar]
- 6.Fischer J, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2010;94:283–289. doi: 10.1016/j.fertnstert.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 7.Keymolen K, Staessen C, Verpoest W, Liebaers I, Bonduelle M. Preimplantation genetic diagnosis in female and male carriers of reciprocal translocations: clinical outcome until delivery of 312 cycles. Eur J Hum Genet. 2012;20:376–380. doi: 10.1038/ejhg.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan YQ, Tan K, Zhang SP, Gong F, Cheng DH, Xiong B, Lu CF, Tang XC, Luo KL, Lin G, Lu GX. Single-nucleotide polymorphism microarray-based preimplantation genetic diagnosis is likely to improve the clinical outcome for translocation carriers. Hum Reprod. 2013;28:2581–2592. doi: 10.1093/humrep/det271. [DOI] [PubMed] [Google Scholar]
- 9.Idowu D, Merrion K, Wemmer N, Mash JG, Pettersen B, Kijacic D, Lathi RB. Pregnancy outcomes following 24-chromosome preimplantation genetic diagnosis in couples with balanced reciprocal or Robertsonian translocations. Fertil Steril. 2015;103:1037–1042. doi: 10.1016/j.fertnstert.2014.12.118. [DOI] [PubMed] [Google Scholar]
- 10.Colls P, Escudero T, Fischer J, Cekleniak NA, Ben-Ozer S, Meyer B, Damien M, Grifo JA, Hershlag A, Munné S. Validation of array comparative genome hybridization for diagnosis of translocations in preimplantation human embryos. Reprod BioMed Online. 2012;24:621–629. doi: 10.1016/j.rbmo.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson MD, Sierra S. Reproductive outcomes in recurrent pregnancy loss associated with a parental carrier of a structural chromosome rearrangement. Hum Reprod. 2006;21:1076–1082. doi: 10.1093/humrep/dei417. [DOI] [PubMed] [Google Scholar]
- 13.Franssen MTM, Musters AM, van der Veen F, Repping S, Leschot NJ, Bossuyt PMM, Goddijn M, Korevaar JC. Reproductive outcome after PGD in couples with recurrent miscarriage carrying a structural chromosome abnormality: a systematic review. Hum Reprod Update. 2011;17:467–475. doi: 10.1093/humupd/dmr011. [DOI] [PubMed] [Google Scholar]
- 14.Franssen MTM, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PMM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]-control study. BMJ. 2006;332:759–763. doi: 10.1136/bmj.38735.459144.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozawa N, Maruyama T, Nagashima T, Ono M, Arase T, Ishimoto H, Yoshimura Y. Pregnancy outcomes of reciprocal translocation carriers who have a history of repeated pregnancy loss. Fertil Steril. 2008;90:1301–1304. doi: 10.1016/j.fertnstert.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 16.Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, Ubaldi FM, Iammarrone E, Gordon A, Pantos K. PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod. 2011;26:1925–1935. doi: 10.1093/humrep/der082. [DOI] [PubMed] [Google Scholar]
- 17.Pundir J, Magdalani L, El-Toukhy T. Outcome of preimplantation genetic diagnosis using FISH analysis for recurrent miscarriage in low-risk reciprocal translocation carriers. Eur J Obstet Gynecol Reprod Biol. 2016;203:214–219. doi: 10.1016/j.ejogrb.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 18.Bay B, Ingerslev HJ, Lemmen JG, Degn B, Rasmussen IA, Kesmodel US. Preimplantation genetic diagnosis: a national multicenter obstetric and neonatal follow-up study. Fertil Steril. 2016;106:1363–1369.e1. doi: 10.1016/j.fertnstert.2016.07.1092. [DOI] [PubMed] [Google Scholar]
- 19.Sharpe AN, Choudhary M. Reproductive outcome following pre-implantation genetic diagnosis (PGD) - an analysis of UK national database over two decades. Fertil Steril. 2015;104:e281. doi: 10.1016/j.fertnstert.2015.07.882. [DOI] [Google Scholar]
- 20.Desmyttere S, De Rycke M, Staessen C, Liebaers I, De Schrijver F, Verpoest W, et al. Neonatal follow-up of 995 consecutively born children after embryo biopsy for PGD. Hum Reprod. 2012;27:288–293. doi: 10.1093/humrep/der360. [DOI] [PubMed] [Google Scholar]
- 21.Sugiura-Ogasawara M, Aoki K, Fujii T, Fujita T, Kawaguchi R, Maruyama T, et al. Subsequent pregnancy outcomes in recurrent miscarriage patients with a paternal or maternal carrier of a structural chromosome rearrangement. J Hum Genet. 2008;53:622. doi: 10.1007/s10038-008-0290-2. [DOI] [PubMed] [Google Scholar]
- 22.Van Der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJM. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. 2011. [DOI] [PubMed]