ABSTRACT
Background
Naturally occurring carbon and nitrogen stable isotope ratios [13C/12C (CIR) and 15N/14N (NIR)] are promising dietary biomarkers. As these candidate biomarkers have long tissue residence times, long-term feeding studies are needed for their evaluation.
Objective
Our aim was to evaluate plasma, RBCs, and hair CIR and NIR as biomarkers of fish, meat, and sugar-sweetened beverage (SSB) intake in a 12-wk dietary intervention.
Methods
Thirty-two men (aged 46.2 ± 10.5 y; BMI: 27.2 ± 4.0 kg/m2) underwent a 12-wk inpatient dietary intervention at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in Phoenix, Arizona. The effects of fish, meat, and SSB intake on CIR and NIR were evaluated using a balanced factorial design, with each intake factor at 2 levels (present/absent) in a common, background diet (50% carbohydrate, 30% fat, 20% protein). Fasting blood samples were taken biweekly from baseline, and hair samples were collected at baseline and postintervention. Data were analyzed using multivariable regression.
Results
The postintervention CIR of plasma was elevated when diets included meat (β = 0.89, 95% CI: 0.73,1.05) and SSBs (β = 0.48, 95% CI: 0.32, 0.64). The postintervention NIR of plasma was elevated when diets included fish (β = 0.85, 95% CI: 0.64, 1.05) and meat (β = 0.61, 95% CI: 0.42, 0.8). Results were similar for RBCs and hair. Postintervention RBC CIR and NIR had strong associations with baseline, suggesting that turnover to the intervention diets was incomplete after 12 wk. Estimates of isotopic turnover rate further confirmed incomplete turnover of RBCs.
Conclusions
CIR was associated with meat and SSBs, and more strongly with meat. NIR was associated with fish and meat, and more strongly with fish. Overall, CIR and NIR discriminated between dietary fish and meat, and to a lesser extent SSBs, indicating their potential utility as biomarkers of intake in US diets. Approaches to make these biomarkers more specific are needed. This trial was registered at clinicaltrials.gov as NCT01237093.
Keywords: stable isotopes, biomarkers, food intake, carbon isotope, nitrogen isotope
Introduction
There is growing evidence that intakes of sugar-sweetened beverages (SSBs) and added sugars (ASs) (1–4) as well as meat (5–9) may have some detrimental effects on health outcomes. In most studies, however, diet assessment measures are based on self-report and are subject to error and selective biases, and can be burdensome to administer (10–12). Better methods of assessing free-living nutrient intake are needed to address the role of diet in chronic disease.
Biomarkers are unbiased, biochemical indicators of diet that can resolve many of the problems inherent in dietary self-reporting (13–15). Naturally occurring variations in carbon and nitrogen stable isotope ratios (13C/12C and 15N/14N, expressed as δ13C and δ15N values) have been proposed as candidate biomarkers for the intake of AS/SSBs (16–21), animal protein/meat (22–25), and fish (22, 26, 27), as they vary predictably among these foods and are captured and preserved in tissues (14). Nitrogen isotope ratios (NIRs) are generally elevated in animals relative to plants and are particularly high in fish (28–30). Carbon isotope ratios (CIRs) are naturally elevated in corn and sugar cane relative to other plant-based foods (14, 31, 32), conferring an elevated CIR to corn- and cane-derived sugars as well as meat from animals fed primarily on corn (beef cattle, pigs, poultry) (28, 30, 32, 33). Controlled studies are needed to evaluate the strength of these dietary influences on CIR and NIR in the United States, where the majority of sweeteners derive from corn or sugar cane (34). A recent 2-wk feeding study of postmenopausal women found associations with fish, meat, and animal protein intake, but not AS (25). Studies of longer duration and with more controlled intake levels are needed to complement and extend these findings.
The purpose of this study was to evaluate the response of CIR and NIR in plasma, RBCs, and hair to simultaneous variation in dietary intake of fish, meat, and SSBs in a highly controlled, 12-wk inpatient feeding study, as a first step toward determining how well these candidate dietary biomarkers reflect differences in these intakes. As blood biomarker data were collected biweekly, we also evaluated the changes from baseline in plasma and RBC CIR and NIR over time, following the shift to the intervention diet. We hypothesize that CIR will be elevated in diets containing meat and SSBs, whereas NIR will be elevated in diets containing fish and meat. In addition, we hypothesize that SSB intake will have no effect on NIR. Finally, we hypothesize that plasma CIR and NIR will show more complete turnover to the intervention diets than RBC CIR and NIR, due to differences in their protein turnover rates.
Methods
Subjects and study design
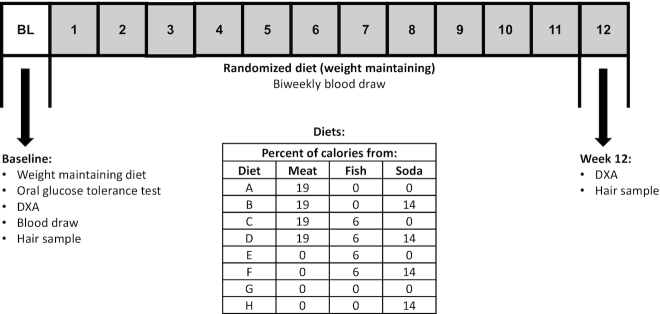
The Developing Biomarkers of Diet (DBD) Study was designed to examine the effects of meat, fish, and SSB intake on whole tissue (“bulk”) carbon and nitrogen stable isotope ratios (CIR and NIR) in a highly controlled, 12-wk dietary intervention. Participants were randomly assigned to 1 of 8 diets characterized by the presence or absence of SSB, meat, and fish, in a full factorial design, for a 12-wk inpatient dietary intervention study. In order to ensure a balanced design, no additional participants were assigned to a dietary treatment once 4 participants had completed it past week 8. Figure 1 provides the contents of the study diets: A: meat/no fish/no SSBs; B: meat/no fish/SSBs; C: meat/fish/no SSBs; D: meat/fish/SSBs; E: no meat/fish/no SSBs; F: no meat/fish/SSBs; G: no meat/no fish/no SSBs (vegetarian); and H: no meat/no fish/SSBs (vegetarian + SSBs). Investigators and study participants were blinded to the study arm until after randomization. All laboratory assays were performed blinded to treatment assignment.
FIGURE 1.
Study procedures, diets, and timeline. Diet A: meat/no fish/no SSBs; B: meat/no fish/SSBs; C: meat/fish/no SSBs; D: meat/fish/SSBs; E: no meat/fish/no SSBs; F: no meat/fish/SSBs; G: no meat/no fish/no SSBs (vegetarian); and H: no meat/no fish/SSBs (vegetarian + SSB). SSB, sugar-sweetened beverage.
Healthy, nondiabetic male volunteers were recruited at the Obesity and Diabetes Clinical Research Section of the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) in Phoenix, Arizona (clinicaltrials.gov identifier: NCT01237093). Female volunteers were excluded from the study due to the potential for higher concentrations of mercury in the fish diets, as the fish intake was higher than that recommended for premenopausal women contemplating pregnancy. Volunteers underwent an extensive screening process prior to study admission and were deemed free of health problems aside from increased adiposity and impaired glucose tolerance. Type 2 diabetes was excluded based on a 75 g oral-glucose-tolerance test (OGTT) administered on day 4 (35). The protocol was approved by the NIDDK Institutional Review Board (#11-DK-N018). All volunteers were informed of the risks of the study and gave informed consent prior to participation.
A schematic of the study protocol is provided in Figure 1. After 3 d of a standard weight-maintaining diet, volunteers underwent a 75 g, 3-h OGTT (glucose samples were run on an Analox GM9 glucose analyzer, Analox Technologies) and a DXA scan for body fat. Fasting weight was measured daily upon awakening to the nearest 0.1 kg on a calibrated scale. Height was measured to the nearest centimeter using a stadiometer. Body composition was measured at the start and end of the study by DXA (DPX-L, Lunar Corp, and Prodigy). Details of the procedure and calculations of fat mass and fat-free mass have been previously described (36). Two different DXA machines were used over the course of the study; a correction factor based on cross calibration was applied to those scans on the newer machine (37).
All volunteers continued the same weight-maintaining diet for a total of 1 wk before starting the dietary intervention (see below). Prior to starting the study diet, a scalp hair sample of 20–30 hairs (∼1/4 the thickness of a pencil) was collected by cutting close to the scalp, below the postoccipital bump, and taped at the noncut end for storage until measurement (38). This procedure was repeated at the end of the dietary intervention and CIR and NIR were measured in hair closest to the scalp.
Serum and plasma were collected at the start of the diet and biweekly from then on for assessment of CIR and NIR.
Experimental diets
All 8 experimental diets were designed to maintain body weight using Food Processor (Version 11.0.2, ESHA Research). The macronutrient content of all diets was held constant (20% protein, 30% fat, and 50% carbohydrate), but diets differed in the amount of meat, fish, and SSBs provided. Fish, meat, and SSBs were provided in the diet at 6, 19, and 14% of total calories/d, respectively (Figure 1). Only nonbreaded fish (canned tuna and canned salmon) were used. Diets containing meat included hamburgers, hot dogs, chicken, turkey, ham, roast beef, meatloaf, bacon, and sausage. Diets without meat included vegetarian alternatives, such as chik'n patties, chik'n enchiladas, chik'in nuggets, veggie hot dogs, veggie bacon, garden veggie patty, and veggie sausage (Morningstar Farms). For the diets that included SSBs, volunteers were given caffeinated soda (Coca-Cola or Signature Select Cola) during all meals except for their evening snack, when they were given a noncaffeinated drink (Sprite or Signature Select Lemon Lime). The background (nonmeat/fish/SSBs) components were similar between all diets to assure that background stable isotope ratios were similar across all diets.
Outcome measures: measurement of CIR and NIR in plasma, RBCs, and hair
The CIR and NIR of plasma, RBCs, and hair were analyzed at the Alaska Stable Isotope Facility at the University of Alaska Fairbanks. Aliquots of plasma (4.8 μL) and RBCs (1.9 μL) were pipetted into preweighed tin capsules (Elemental Microanalysis, IsoMass Scientific, Inc.), autoclaved, dried, and weighed to the nearest 0.001 mg using a microbalance. The 0.3 cm of hair most proximal to the scalp was cut and cleaned by two 30-min washes with sonication in 3:1 methanol: chloroform, followed by a 30 min wash with sonication in deionized water. Hair samples were then dried and ∼0.2–0.4 mg was weighed into tin capsules using a microbalance. Tin capsules containing plasma, RBCs, and hair were crushed and introduced into a Costech Elemental Analyzer (ECS 4010; Costech Analytical Technologies) using an autosampler. The elemental analyzer was interfaced to a Delta V Plus isotope ratio mass spectrometer via the Conflo IV interface (Thermo Scientific, Inc). Isotope ratios are presented in permil (‰) abundance of heavy isotope relative to reference values as follows: δX = (R sample – Rreference)/(Rreference) × 1000 (‰), where X is the heavy isotope, R is the ratio of heavy to light isotope (13C/12C or 15N/14N), and the reference values are internationally recognized standards calibrated to Vienna Pee Dee Belemnite (13C/12C = 0.01124) and atmospheric nitrogen (15N/14Natm-N = 0.003677). Analytical precision was assessed as the SD of laboratory working standards calibrated to the above reference materials that were measured after every 10th sample; these were typically within 0.2‰ for δ 13C and δ15N.
Statistical analyses
Our primary comparisons were of NIR and CIR concentrations for fish compared with no fish, meat compared with no meat, and SSBs compared with no SSBs. The primary outcome of the effect of each dietary factor on the 12-wk study value of NIR or CIR was assessed using a multivariable linear regression model. For plasma and RBCs, the model was further adjusted for baseline NIR or CIR; hair models were not adjusted for baseline values as a preintervention hair sample was not obtained for all participants. Regression models with only one dietary factor were also considered in a supportive analysis.
To further explore the degree to which CIR and NIR captured differences in the diets, a logistic regression prediction model was built for each dietary factor as a binary outcome (presence/absence), with plasma CIR and NIR as the predictors. The predictive accuracy of each model was summarized with the area under the receiver operator characteristic curve. The AUC was adjusted for optimism (inflated accuracy due to fitting and evaluating the model with the same data) using Harrell's bootstrapped AUC (39). Similar models were built using RBC CIR and NIR.
The correlation of the baseline CIR and NIR concentration with each follow-up measure was assessed with the Pearson correlation coefficient for RBCs and plasma. The kinetics were further described by fitting LOESS (LOcally WEighted Scatter-plot Smoother) curves, fitted separately to the longitudinal stable isotope ratio data for each of the combined study groups thought to be most similar in their effects on the stable isotope ratios. Namely, for each of the 4 groups defined by the 2 factors, meat (yes/no) and SSBs (yes/no), LOESS curves were calculated to describe the trend in CIR over time for plasma and RBCs. Similarly, LOESS curves were also fitted to the longitudinal NIR data separately for the 4 groups defined by fish (yes/no) and meat (yes/no). Prepost values of CIR and NIR were compared for the hair samples. To describe individual fractional incorporation rates by compartment and isotope ratio, exponential models were fitted for each individual as δX(t) = δX∞ − (δX∞ − δX0) e−λ time, with X representing 13C or 15N in plasma or RBCs. Each model sought to estimate the fractional incorporation rate (λ), the baseline δ13C or δ15N value (δX0), and the δ13C or δ15N value at equilibrium (δX∞) (40). Summary statistics are presented for the fractional incorporation rate, the half-life (t0.5), and the time to 90% incorporation (t0.9), which were calculated as −ln (1 − % incorporation/100)/λ.
Significance tests were 2-sided and performed at the α = 0.05 level. All analyses were done using R software version 3.4.2 (R Foundation for Statistical Computing).
Sample size
Our original planned sample size was to study 5 individuals/diet; however, we elected to analyze the data after completing 4 individuals/diet due to logistical issues around recruitment and length of stay. This reduced sample size maintained the original power of nearly 100% to detect differences in CIR and NIR for the main effects of fish compared with no fish, meat compared with no meat, and SSBs compared with no SSBs. Power was based on an expected within-group SD of 0.5‰, an expected dietary effect size of ≥1‰, and a 0.05 α level. With 16 per group, we maintained ∼80% power for a 0.5‰ change.
Results
Study volunteers
A total of 55 volunteers were screened for the study. Of these, 41 were admitted to the inpatient study, and of those admitted, 37 were randomized, and 32 completed the intervention through to week 8, with 4 participants assigned to each of the 8 intervention diets. Five randomized participants, who dropped out prior to 4 weeks due to lack of compliance, illness, or behavioral problems, were excluded from the analysis cohort. Details of recruitment and retention are provided in Supplemental Figure 1. Of the 32 “completed” subjects, 1 withdrew after week 8 of the intervention and 1 withdrew after week 10; the other 30 participants completed the full 12 wk. For the missing plasma and RBC CIR and NIR data, 8- or 10-wk measures were used to impute the 12-wk values. In addition, 1 participant was missing the postintervention hair sample. For the hair analysis, complete case data analyses are presented; this analysis assumes the data are missing completely at random.
The baseline characteristics of the volunteers are shown in Table 1. Subjects were similar across the 8 dietary treatments in age, body weight, BMI, % body fat, fasting or 2-h plasma glucose, and baseline CIR and NIR (Table 1). Weight stability (day of discharge − first full day as inpatient) over the course of the study averaged 0.5 ± 2.6 kg (0.7 ± 3.2%) among all participants combined. The average within-person change in % body fat from the first to the last DXA measurement was 1.1% (0.4, 1.9) (mean, 95% CI). The baseline CIR of hair was ∼1.5‰ higher than that of RBCs or plasma, whereas the baseline NIR of hair and plasma was ∼1.5‰ higher than that of RBCs, similar to previous findings (41, 42).
TABLE 1.
Baseline characteristics of study subjects (n = 32)1
| Characteristic | Total | Diet A M2 | Diet B M/S2 | Diet C M/F2 | Diet D M/F/S | Diet E F | Diet F F/S | Diet G no M/F/S | Diet H S |
|---|---|---|---|---|---|---|---|---|---|
| Male sex, n (%) | 32 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) |
| Race/ethnicity, n (%) | |||||||||
| White | 19 (59.4) | 2 (50) | 1 (25) | 3 (75) | 3 (75) | 1 (25) | 1 (25) | 4 (100) | 4 (100) |
| Native American | 10 (31.3) | 2 (50) | 3 (75) | 0 (0) | 1 (25) | 2 (50) | 2 (50) | 0 (0) | 0 (0) |
| Hispanic | 2 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) |
| African American | 1 (3.1) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Age, y | 46.3 (10.5) | 49.7 (8.9) | 45.0 (13.9) | 46.5 (12.9) | 45.0 (13.2) | 44.2 (7.6) | 45.3 (6.6) | 54.1 (11.5) | 40.1 (11.8) |
| BMI, kg/m2 | 27.2 (4.0) | 28.6 (5.5) | 23.7 (3.3) | 27.2 (2.8) | 28.3 (3.8) | 29.7 (4.2) | 24.5 (1.7) | 28.6 (5.2) | 27.0 (3.8) |
| Weight, kg | 83.9 (13.6) | 81.2 (10.4) | 73.0 (9.6) | 83.5 (12.6) | 94.3 (8.8) | 91.7 (18.0) | 74.0 (9.7) | 95.5 (15.3) | 77.8 (8.5) |
| % Body fat | 24.3 (6.0) | 22.0 (7.9) | 21.8 (4.1) | 25.5 (5.9) | 25.5 (5.1) | 27.3 (6.3) | 22.1 (6.8) | 24.9 (7.9) | 25.5 (7.0) |
| Glucose, g/dL | 92.7 (8.1) | 93.8 (2.1) | 90.8 (5.2) | 95.2 (7.9) | 93.2 (17.0) | 91.2 (10.2) | 95.0 (6.8) | 89.5 (9.9) | 92.8 (3.5) |
| CIR plasma, ‰ | −19.9 (0.7) | −20.3 (0.5) | −20.0 (1.2) | −19.9 (0.5) | −19.9 (0.4) | −19.7 (0.4) | −19.4 (0.7) | −20.2 (0.5) | −19.6 (0.8) |
| CIR RBC, ‰ | −19.6 (0.7) | −19.8 (0.6) | −19.7 (1.1) | −19.6 (0.1) | −19.6 (0.5) | −19.4 (0.4) | −19.1 (1.0) | −19.8 (0.5) | −19.4 (1.1) |
| CIR hair, ‰ | −17.2 (0.8) | −17.7 (0.7) | −16.9 (0.9) | −17.1 (0.2) | −17.2 (0.4) | −17.0 (0.6) | −16.8 (1.1) | −17.9 (0.8) | −16.9 (1.0) |
| NIR plasma, ‰ | 8.6 (0.3) | 8.4 (0.3) | 8.5 (0.3) | 8.6 (0.2) | 8.8 (0.3) | 8.6 (0.4) | 8.6 (0.2) | 8.5 (0.3) | 8.7 (0.1) |
| NIR RBC, ‰ | 7.0 (0.4) | 7.0 (0.6) | 6.9 (0.3) | 7.0 (0.4) | 7.1 (0.4) | 7.0 (0.5) | 7.1 (0.2) | 6.8 (0.3) | 7.1 (0.4) |
| NIR hair, ‰ | 8.3 (0.4) | 8.1 (0.6) | 8.2 (0.2) | 8.3 (0.4) | 8.5 (0.6) | 8.3 (0.1) | 8.3 (0.4) | 8.4 (0.4) | 8.2 (0.2) |
Values are mean (SD) unless otherwise noted.
M, F, and S denote the presence of meat, fish, and SSBs, respectively, in each diet.
Effects of fish, meat, and SSB intake
Analyses of our primary comparisons, the effects of meat, fish, and SSB intake on the plasma, RBC, and hair CIR and NIR, are presented in Table 2. The CIR of plasma at the end of the feeding period was significantly higher following intake of diets providing meat and SSBs (both P <0.001). Meat had a larger effect size than SSBs (β meat = 0.89, 95% CI: 0.73, 1.05, compared with βSSB = 0.48, 95% CI: 0.32, 0.64), despite meat and SSBs providing similar % of energy (19% compared with 14%). Fish intake had no effect on plasma CIR. The NIR of plasma at the end of the feeding period was significantly higher following the intake of diets providing fish and meat (both P <0.001). Fish had a larger effect size than meat (βfish = 0.85, 95% CI: 0.64, 1.05, compared with βmeat = 0.61, 95% CI: 0.42, 0.81), despite comprising a lower % of intake (6% compared with 19% of energy). As expected, SSB intake had no effect on plasma NIR. The CIR and NIR of RBCs and hair followed similar patterns (all P <0.001). Generally, effect sizes were similar between plasma and hair, and lower in RBCs. Plasma CIR at the end of the feeding period was significantly associated with baseline, whereas plasma NIR was not. In contrast, RBC CIR and NIR at the end of the feeding period were both strongly associated with baseline values. Supplemental Table 1 provides results for the analogous regression models including only 1 dietary factor at a time. Results were similar, as expected by the independence of diet and baseline factors provided by the randomization. The distribution of the final measurement of stable isotopes is compared by each dietary factor and compartment in Figure 2.
TABLE 2.
Regression coefficients (β) for CIR and NIR in plasma, RBC, and hair compartments for multivariate (all dietary factors) regression models
| CIR (as δ13C values) | NIR (as δ15N values) | |||||
|---|---|---|---|---|---|---|
| Diet | β | 95% CI | P value1 | β | 95% CI | P value1 |
| Plasma2 | ||||||
| Fish | −0.04 | (−0.20, 0.12) | 0.602 | 0.85 | (0.64, 1.05) | <0.001 |
| Meat | 0.89 | (0.73, 1.05) | <0.001 | 0.61 | (0.42, 0.81) | <0.001 |
| SSBs | 0.48 | (0.32, 0.64) | <0.001 | 0.13 | (−0.08, 0.34) | 0.206 |
| Baseline | 0.27 | (0.14, 0.40) | <0.001 | 0.25 | (−0.17, 0.66) | 0.236 |
| RBC2 | ||||||
| Fish | 0.08 | (−0.07, 0.22) | 0.307 | 0.46 | (0.29, 0.62) | <0.001 |
| Meat | 0.57 | (0.42, 0.72) | <0.001 | 0.43 | (0.26, 0.59) | <0.001 |
| SSBs | 0.33 | (0.18, 0.47) | <0.001 | −0.08 | (−0.24, 0.09) | 0.340 |
| Baseline | 0.53 | (0.42, 0.65) | <0.001 | 0.62 | (0.40, 0.85) | <0.001 |
| Hair3 | ||||||
| Fish | −0.03 | (−0.39, 0.32) | 0.841 | 0.66 | (0.40, 0.93) | <0.001 |
| Meat | 0.75 | (0.41, 1.10) | <0.001 | 0.80 | (0.53, 1.06) | <0.001 |
| SSBs | 0.53 | (0.17, 0.90) | 0.006 | 0.01 | (−0.25, 0.27) | 0.931 |
| Baseline | 0.25 | (0.02, 0.49) | 0.038 | 0.41 | (0.03, 0.78) | 0.034 |
P values calculated using a Wald test for 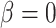 .
.
n = 32 for plasma and RBC models.
n= 29 for hair models. Analyses used the 0.3 cm of hair most proximal to the scalp.
FIGURE 2.
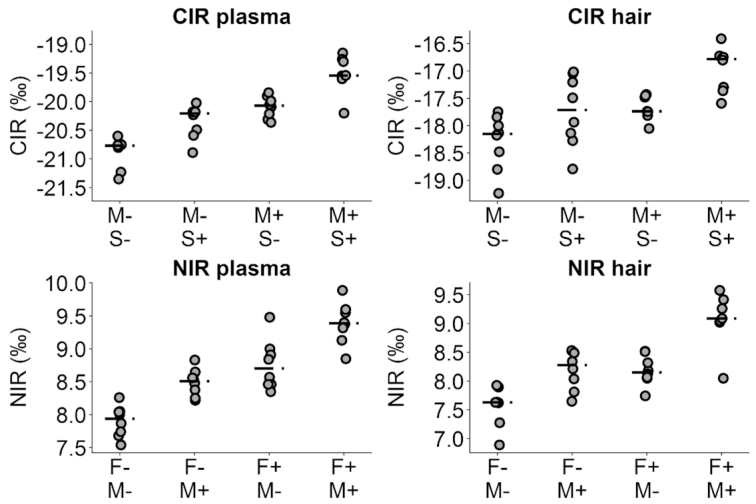
Scatterplots of final CIR and NIR in plasma and hair, by combinations of significant dietary effects. M, F, and S denote meat, fish, and SSBs, respectively, in each diet, and − and + denote absence and presence of the diet components at 19% (meat), 6% (fish), or 16% (SSBs) of energy. The CIR and NIR plasma plots display 32 subjects, with 8 subjects in each diet combination. The CIR hair plot displays 31 subjects, with 7 subjects in diet group M+, S−, and 8 in all others. The NIR hair plot displays 31 subjects, with 7 subjects in diet group F+, M+, and 8 in all others.
Plasma CIR and NIR, when included as predictors in a logistic regression model for the presence of a dietary factor, accurately predicted fish and meat consumption, with optimism-corrected AUC = 0.97 and 0.92, respectively. SSB intake was predicted with a more modest level of accuracy, with corrected AUC = 0.78 (Supplemental Figure 2). RBC CIR and NIR produced similar results, although with lower discrimination (data not shown).
Changes in stable isotope profiles over time
Due to the availability of biweekly blood draws, changes in plasma and RBC isotope ratios were assessed over time. Table 3 shows the correlation between the baseline measurements for each isotope and all subsequent follow-up measurements. The correlation for all variables decreased with increasing time, as tissues came closer to equilibrium with the intervention diets. In RBCs, the end of study value was still significantly correlated with the baseline value for both CIR and NIR, suggesting 12 wk was not long enough to completely turn over the effect of the prestudy diet. In plasma, the end of study values were not significantly associated with baseline, suggesting near-complete turnover; however, with 32 subjects this study had relatively low power to detect modest associations (e.g., there was only 28% power to detect a correlation of 0.25). Table 4 presents median (IQR) for the fractional incorporation rates (λ) and weeks to 50% and 90% incorporation for individual turnover models by isotope and compartment. These models did not converge for all individuals, likely because of the small number of data points and the magnitude of isotopic change, which varied randomly among individuals based on the baseline isotope ratio and the treatment assigned. Among the models that did converge, the median time to 90% incorporation was estimated to be 8 wk for plasma CIR, 12 wk for plasma NIR, 19 wk for RBC CIR, and 15 wk for RBC NIR. Figure 3 shows the trends over time of plasma CIR for the 4 study groups defined by their meat and SSB consumption; Figure 4 shows the trends over time of plasma NIR for the 4 study groups defined by their fish and meat consumption. Supplemental Figures 3–6 show the trends for CIR and NIR for RBCs and hair for the similarly defined study groups.
TABLE 3.
Correlation (P value) between baseline measurement and all subsequent follow-up measurements for CIR and NIR in plasma and RBCs (n = 32)1
| Week on intervention | ||||||
|---|---|---|---|---|---|---|
| Biomarker | 2 | 4 | 6 | 8 | 10 | 12 |
| CIR plasma | 0.72 (<0.001) | 0.53 (0.001) | 0.40 (0.023) | 0.27 (0.133) | 0.31 (0.083) | 0.21 (0.256) |
| NIR plasma | 0.62 (<0.001) | 0.49 (0.004) | 0.45 (0.009) | 0.38 (0.031) | 0.26 (0.149) | 0.31 (0.090) |
| CIR RBC | 0.94 (<0.001) | 0.89 (<0.001) | 0.83 (<0.001) | 0.79 (<0.001) | 0.70 (<0.001) | 0.66 (<0.001) |
| NIR RBC | 0.88 (<0.001) | 0.88 (<0.001) | 0.77 (<0.001) | 0.78 (<0.001) | 0.65 (<0.001) | 0.58 (<0.001) |
P values from the Fisher z transformation test.
TABLE 4.
Median, 25th percentile, and 75th percentile of the fractional incorporation rates (λ) of the subjects for whom the model converged1
| Biomarker | Number converged | Median λ2 (25th, 75th) | Median t0.5 (wk) | Median t0.9(wk) |
|---|---|---|---|---|
| CIR plasma | 16 | 0.28 (0.21, 0.33) | 2.5 | 8.2 |
| NIR plasma | 15 | 0.19 (0.13, 0.36) | 3.7 | 12.1 |
| CIR RBC | 13 | 0.12 (0.09, 0.21) | 5.9 | 19.1 |
| NIR RBC | 8 | 0.15 (0.10, 0.20) | 4.6 | 15.4 |
The median weeks until 50% turnover (half-life) and the median weeks to 90% turnover are also presented. For each biomarker, the number converged is out of 32 subjects. 2Units of λ are week−1.
Units of λ are week−1.
FIGURE 3.
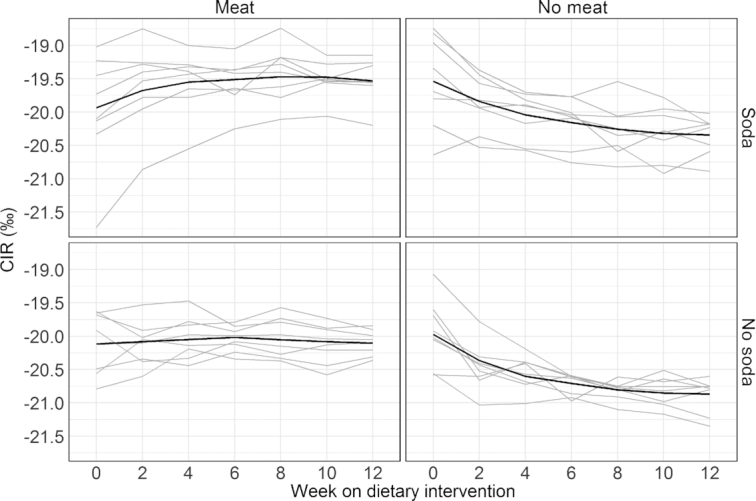
Plasma CIR measurements across all weeks for meat and SSB diet combinations. The gray lines are the individual trajectories within each diet group. The black line is a smoothed average curve within each diet group calculated using the LOESS. Each of the 4 plots displays 8 subjects in each diet combination.
FIGURE 4.
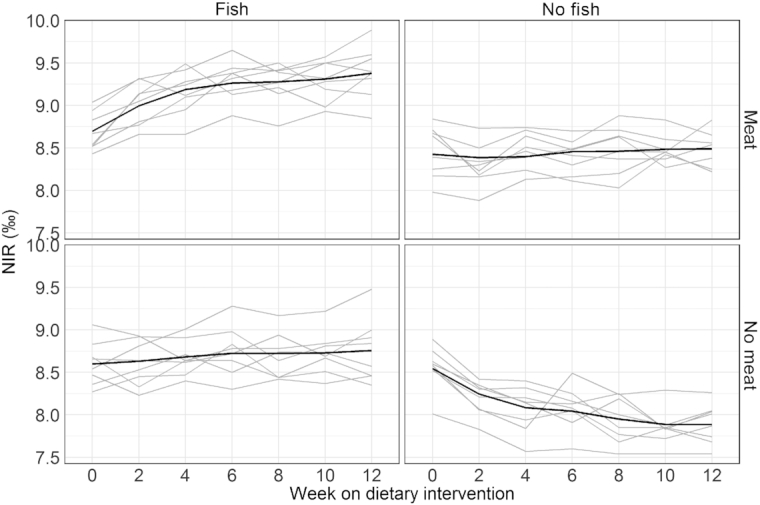
Plasma NIR measurements across all weeks for meat and fish diet combinations. The gray lines are the individual trajectories within each diet group. The black line is a smoothed average curve within each diet group calculated using the LOESS. Each of the 4 plots displays 8 subjects in each diet combination.
Discussion
In this highly controlled, 12-wk inpatient dietary intervention we showed that natural abundance CIR was significantly elevated by dietary meat and SSB intake, whereas natural abundance NIR was significantly elevated by dietary fish and meat intake. In combination, CIR and NIR were able to distinguish consumers of meat and fish very accurately, whereas there was more modest discriminatory power for SSB consumers. These results were consistent across sample type (plasma, RBCs, and hair). Our study also demonstrated the highly time-integrative nature of dietary biomarkers based on stable isotope ratios. Preliminary models of isotopic incorporation suggested that plasma biomarkers required 8–12 wk to achieve isotopic equilibration, whereas RBC biomarkers required 15–19 wk, observations that were further supported by the significant influence of baseline on postintervention isotope ratios, particularly in RBCs. This property means that CIR and NIR will reflect an integrated dietary signal over a period of 2 to 5 mo, depending on the blood fraction sampled. The similar dietary effects on hair CIR and NIR make these a good alternative for noninvasive sampling. These findings support the potential for stable isotope ratios to serve as biomarkers of the long-term intake of fish, meat, and/or SSBs in future studies of diet and health in US populations.
Both meat and SSB intake (at 14% and 19% of energy, respectively) increased the CIR of plasma, RBCs, and hair, whereas fish intake (at 6% of energy) did not. In the United States, all 3 food types have elevated CIR relative to other foods, SSBs due to the use of corn or sugar cane as a sweetener and primary ingredient (∼ −10 to −12‰), meat due to the use of corn as animal feed (∼ −13 to −19‰), and fish due to the modestly elevated carbon isotope ratios of phytoplankton and other aquatic plants (∼ −19 to −22‰) (28, 30, 32, 43, 44). Meat had a larger effect on the CIR of plasma and RBCs than did SSBs. This finding is consistent with a growing number of published studies that have evaluated associations with SSBs (or ASs) and meat (or animal protein) concurrently (21, 25, 45, 46), but is inconsistent with a study of a Virginia cohort (20). In hair, meat and SSBs had similar effect sizes. Naturally occurring CIR is of particular interest in the determination of SSB and AS intake in US populations. Several studies have used a cross-sectional design to correlate SSB and/or AS intake with blood CIR measures (19, 20, 32, 46). These studies should be interpreted cautiously in light of the potential confounding of CIR by meat and/or animal protein intake. Because blood and hair are composed primarily of protein their CIR is expected to reflect dietary protein sources to a large degree (47–49). Approaches to improve the specificity of the CIR for SSBs/ASs are needed, potentially by identifying analytes for which a greater proportion of carbon derives from sugars (50, 51).
In plasma, RBCs, and hair, NIR was significantly associated with intakes of both fish and meat. In plasma, the association with fish intake was greater despite the dietary “dose” (6% of energy) being approximately a third of that of meat intake (19% of energy). In RBCs and hair, the associations for fish and meat were similar. Fish has a higher NIR (∼10 to 20‰) than meat and other animal products (∼4 to 7‰), although both are elevated relative to the average of plant-based foods (∼1 to 6‰) (28–30, 43, 44). The strong association of NIR with fish intake is consistent with findings from both cohort and controlled feeding studies in the United States and Europe (22, 23, 25, 26, 30). The weaker association of NIR with meat intake is consistent with some studies examining associations with meat (22) or animal protein intake more generally (23, 24, 45) but is inconsistent with 2 US studies finding no association (20, 25). A recent analysis of the NIR of combined meals based on reported diets found that meal NIR was primarily influenced by fish content; beef and lamb content had a positive but only modest effect (52). These findings provide support for NIR as a biomarker of fish intake; however, caution is required given the potential for an association with meat or animal protein intake generally, especially in populations with low fish intake [e.g., (45)].
Our study is the first to assess the change in stable isotope biomarkers across a long-term (12 wk) dietary intervention with the level of dietary control afforded by an inpatient setting. Plasma and RBCs differed in the time course of their response to the intervention, as evidenced by their changing associations with baseline values over time and by models of isotopic incorporation from a subset of study participants. After 8 wk of dietary intervention, baseline values explained <15% of the variation in plasma CIR and NIR, and models of isotopic incorporation suggested that plasma was nearing complete isotopic turnover by the end of the 12-wk dietary intervention. In contrast, baseline values still explained >60% of the variation in RBC CIR and NIR after 8 wk of dietary intervention, and models of isotopic incorporation suggested that turnover was incomplete after 12 wk. The slow apparent turnover of RBCs is likely the result of both the replacement rate of RBCs (53) and the contribution of carbon and nitrogen deriving from large body reservoirs to RBC synthesis. Others have altered the diets of free-living volunteers to include more meat (54) or C4 plants, which have a higher CIR than C3 plants (55), over a 4-wk period. In the study in which NIR and CIR responded to the dietary intervention (55) they did not appear to stabilize during that period. The fact that CIR or NIR integrate diet over a long time period, particularly when measured in RBCs, suggests that they could provide useful time-integrated biomarkers for a usual diet.
A unique strength of this study was its balanced factorial design, which allowed us to test the effects of 3 dietary factors efficiently and under common experimental conditions. Other strengths include the high level of dietary control afforded by the use of an inpatient facility, and the duration of dietary control, which allowed us to evaluate stable isotope biomarkers over a time period appropriate to their long residence times. One limitation of the study was that it was only practical to test 2 levels of intake for each dietary factor due to the balanced factorial design. Thus, dose-response studies with multiple levels of intake are a natural next step for this research. Another limitation was that due to long residence times of stable isotope biomarkers and the length of the dietary intervention in this study, we were unable to equilibrate all participants to a common diet prior to shifting them to an experimental diet. Due to this, biomarker measurements at baseline were variable. This only allowed us to model isotopic incorporation rates in a subset of study subjects, thus, these findings should be viewed as preliminary.
In summary, we tested the effect of consuming fish, meat, and SSBs on the CIR and NIR of plasma, RBCs, and hair in a highly controlled 12-wk US dietary intervention. In all 3 sample types CIR was strongly associated with both meat and SSB intake, whereas NIR was strongly associated with both fish and meat intake. In plasma, the effect of meat on CIR was greater than the effect of SSBs, and the effect of fish on NIR was greater than the effect of meat. Approaches to improve biomarker specificity are needed for their application to USA populations. In combination, the 2 plasma isotope ratios could accurately discriminate between the sustained diets with fish compared with no fish and meat compared with no meat; whereas there was only a modest amount of discriminatory power for the SSBs compared with no SSBs. Plasma isotope ratios exhibited nearly complete turnover by the end of the 12-wk dietary intervention, whereas RBC isotope ratios did not. These results suggests that plasma biomarkers would be more appropriate for evaluating shorter-term dietary changes, whereas RBC biomarkers are more appropriate for assessing a usual diet over several months.
Supplementary Material
Acknowledgments
We thank Garrett Savory, Jynene Black, Pat Rivera, and Timothy Howe for assistance with laboratory analyses. We also thank the nursing and kitchen staff of the Obesity and Diabetes Clinical Research Section of the NIDDK in Phoenix, AZ, for their assistance with the clinical study and the preparation of the study diets.
The authors’ responsibilities were as follows—SBV: design and oversight of study; PAS: oversight of statistical analyses; EJO: statistical analysis; CAV: development of study diets; SB: study oversight on clinical research unit; JK: design and oversight of study; DMO: design of study and oversight of stable isotope measurement; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to this study.
Notes
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH through grant number 1R01DK109946 and intramural NIDDK funding. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIDDK or the NIH.
Supplemental Figures 1–6 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AS, added sugar; CIR, carbon isotope ratio; LOESS, Locally Weighted Scatter-plot Smoother; NIDDK, National Institutes of Diabetes and Digestive and Kidney Diseases; NIR, nitrogen isotope ratio; OGTT, oral-glucose-tolerance test; SSB, sugar-sweetened beverage.
References
- 1. Bundrick S, Thearle M, Venti C, Krakoff J, Votruba S. Soda consumption during ad libitum food intake predicts weight change. J Acad Nutr Diet. 2014;114(3):444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stinson E, Piaggi P, Ibrahim M, Venti C, Krakoff J, Votruba S. High fat and sugar consumption during ad libitum intake predicts weight gain. Obesity. 2018;26(4):689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma J, McKeown N, Hwang S, Hoffman U, Jacques P, Fox C. Sugar-sweetened beverage consumption is associated with change of visceral adipose tissue over 6 years of follow-up. Circulation. 2016;133:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scharf R, DeBoer M.. Sugar-sweetened beverages and children's health. Annual Review of Public Health. 2016;37(1):273–93. [DOI] [PubMed] [Google Scholar]
- 5. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi A, Knuppel S, Igbal K, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–73. [DOI] [PubMed] [Google Scholar]
- 6. Isanejad M, LaCroix AZ, Thomson CA, Tinker L, Larson JC, Qi Q, Qi L, Cooper-DeHoff RM, Phillips LS, Prentice RL et al.. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women's Health Initiative. Br J Nutr. 2017;117(11):1523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klurfeld D. Research gaps in evaluating the relationship of meat and health. Meat Sci. 2015;109:86–96. [DOI] [PubMed] [Google Scholar]
- 8. Sinha R, Cross A, Graubard B, Leitzmann M, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169(6):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wyness L. The role of red meat in the diet: nutrition and health benefits. Proc Nutr Soc. 2015;75(3):227–32. [DOI] [PubMed] [Google Scholar]
- 10. Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, Bingham S, Schoeller DA, Schatzkin A, Carroll RJ. Structure of dietary measurement error: results of the OPEN Biomarker Study. Am J Epidemiol. 2003;158(1):14–21. [DOI] [PubMed] [Google Scholar]
- 11. Stubbs J, O'Reilly L, Whybrow S, Fuller Z, Johnstone A, Livingstone M, Ritz P, Horgan GW. Measuring the difference between actual and reported food intakes in the context of energy balance under laboratory conditions. Br J Nutr. 2014;111:2032–43. [DOI] [PubMed] [Google Scholar]
- 12. Tarasuk V, Beaton GH. The nature and individuality of within-subject variation in energy intake. Am J Clin Nutr. 1991;54(3):464–70. [DOI] [PubMed] [Google Scholar]
- 13. Jenab M, Slimani N, Bictash M, Ferrari P, Bingham S. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125(5–6):507–25. [DOI] [PubMed] [Google Scholar]
- 14. O'Brien D. Stable isotope ratios as biomarkers of diet for health research. Ann Rev Nutr. 2015;35:565–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dragsted LO, Gao Q, Scalbert A, Vergères G, Kolehmainen M, Manach C, Brennan L, Afman LA, Wishart DS, Andres Lacueva C et al.. Validation of biomarkers of food intake–critical assessment of candidate biomarkers. Genes Nutr. 2018;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davy BM, Jahren AH, Hedrick VE, Comber DL. Association of δ(13)C in fingerstick blood with added sugars and sugar-sweetened beverage intake. J Am Diet Assoc. 2011;111(6):874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davy BM, Jahren AH, Hedrick VE, You W, Zoellner JM. Influence of an intervention targeting a reduction in sugary beverage intake on the δ(13)C sugar intake biomarker in a predominantly obese, health-disparate sample. Public Health Nutr. 2017;20(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farooq M, Fontana J, Sazonov E. A novel approach for food intake detection using electroglottography. Physiol Meas. 2014;35:739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hedrick VE, Davy BM, Wilburn GA, Jahren AH, Zoellner JM. Evaluation of a novel biomarker of added sugar intake (δ 13C) compared with self-reported added sugar intake and the Healthy Eating Index-2010 in a community-based, rural US sample. Public Health Nutr. 2016;19(3):429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hedrick V, Zoellner J, Jahren A, Woodford N, Bostic J, Davy B. A dual-carbon-and-nitrogen stable isotope ratio model is not superior to a single-carbon stable isotope ratio model for predicting added sugar intake in southwest Virginian adults. J Nutr. 2015;145(6):1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nash S, Kristal A, Bersamin A, Hopkins S, Boyer B, O'Brien D. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup'ik study population. J Nutr. 2013;143(2):161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuhnle G, Joosen A, Kneale C, O'Connell T. Carbon and nitrogen isotopic ratios of urine and faeces as novel nutritional biomarkers of meat and fish intake. Eur J Nutr. 2013;52:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel PS, Cooper AJ, O'Connell TC, Kuhnle GG, Kneale CK, Mulligan AM, Luben RN, Brage S, Khaw KT, Wareham NJ et al.. Serum carbon and nitrogen stable isotopes as potential biomarkers of dietary intake and their relation with incident type 2 diabetes: the EPIC-Norfolk study. Am J Clin Nutr. 2014;100(2):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petzke K, Boeing H, Klaus S, Metges C. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr. 2005;135:1515–20. [DOI] [PubMed] [Google Scholar]
- 25. Yun HY, Lampe JW, Tinker LF, Neuhouser ML, Beresford SA, Niles KR, Mossavar-Rahmani Y, Snetselaar LG, Van Horn L, Prentice RL et al.. Serum nitrogen and carbon stable isotope ratios meet biomarker criteria for fish and animal protein intake in a controlled feeding study of a Women's Health Initiative Cohort. J Nutr. 2018;148(12):1931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bjerregaard P, Larsen C, Dahl-Petersen I, Buchardt B. Stable isotopes of carbon and nitrogen as markers of dietary variation among sociocultural subgroups of Inuit in Greenland. Am J Hum Biol. 2017;29(5):e23018. [DOI] [PubMed] [Google Scholar]
- 27. O'Brien DM, Thummel KE, Bulkow LR, Wang Z, Corbin B, Klejka J, Hopkins SE, Boyer BB, Hennessy TW, Singleton R. Declines in traditional marine food intake and vitamin D levels from the 1960s to present in young Alaska Native women. Public Health Nutr. 2017;20(10):1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schoeller D, Minagawa M, Slater R, Kaplan I. Stable isotopes of carbon, nitrogen and hydrogen in the contemporary North American human food web. Ecology of Food and Nutrition. 1986;18(3):159–70. [Google Scholar]
- 29. Huelsemann F, Koehler K, Braun H, Schaenzer W, Flenker U. Human dietary δ15N intake: representative data for principle food items. Am J Phys Anthropol. 2013;152(1):58–66. [DOI] [PubMed] [Google Scholar]
- 30. Nash SH, Bersamin A, Kristal AR, Hopkins SE, Church RS, Pasker RL, Luick BR, Mohatt GV, Boyer BB, O'Brien DM. Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr. 2012;142:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Farquhar G, Ehleringer J, Hubick K. Carbon isotope discrimination and photosynthesis. Ann Rev Plant Physiol Mol Biol. 1989;40:503–37. [Google Scholar]
- 32. Jahren A, Saudek C, Yeung E, Kao W, Kraft R, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr. 2006;84(6):1380–4. [DOI] [PubMed] [Google Scholar]
- 33. Jahren A, Kraft R. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc Natl Acad Sci. 2008;105(46):17855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McConnel M, Olson D. Sugar and Sweeteners Outlook. In: USDA, editor: Economic Research Service; 2018. [Google Scholar]
- 35. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26(suppl 1):s33–50. [DOI] [PubMed] [Google Scholar]
- 36. Tataranni P, Ravussin E.. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–4. [DOI] [PubMed] [Google Scholar]
- 37. Reinhardt M, Piaggi P, DeMers B, Trinidad C, Krakoff J. Cross calibration of two dual-energy X-ray densitometers and comparison of visceral adipose tissue measurements by iDXA and MRI. Obesity. 2017;25(2):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macko SA, Engel MH, Andrusevich V, Lubec G, O'Connell TC, Hedges RE. Documenting the diet in ancient human populations through stable isotope analysis of hair. Phil Trans R Soc Lond B. 1999;354(1379):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harrell FJ, Lee K, Mark D. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. [DOI] [PubMed] [Google Scholar]
- 40. Martinez del Rio C, Carleton S. How fast and how faithful: the dynamics of isotopic incorporation into animal tissues. J Mammal. 2012;93(2):353–9. [Google Scholar]
- 41. Nash S, Kristal A, Boyer B, King I, Metzgar J, O'Brien D. Relation between stable isotope ratios in human red blood cells and hair: implications for using nitrogen isotope ratio of hair as a biomarker of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr. 2009;90(6):1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nash SH, Kristal AR, Hopkins SE, Boyer BB, O'Brien DM. Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a Yup'ik study population. J Nutr. 2014;144(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Minagawa M. Reconstruction of human diet from σ13C and σ15N in contemporary Japanese hair: a stochastic method for estimating multi-source contribution by double isotopic tracers. Appl Geochem. 1992;7(2):145–58. [Google Scholar]
- 44. Nardoto GB, Silva S, Kendall C, Ehleringer JR, Chesson LA, Ferraz ESB, Moreira MZ, Ometto JP, Martinelli LA. Geographical patterns of human diet derived from stable-isotope analysis of fingernails. Am J Phys Anthropol. 2006;131(1):137–46. [DOI] [PubMed] [Google Scholar]
- 45. Valenzuela LO, O'Grady SP, Enright LE, Murtaugh M, Sweeney C, Ehleringer JR. Evaluation of childhood nutrition by dietary survey and stable isotope analyses of hair and breath. Am J Hum Biol. 2018;30(3):e23103. [DOI] [PubMed] [Google Scholar]
- 46. Yeung E, Saudek C, Jahren A, Kao W, Islas M, Kraft R, Coresh J, Anderson CA. Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol. 2010;172(9):1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McCue M, Smith A, McKinney R, Rewald B, Pinshow B, McWilliams S. A mass balance approach to identify and compare differential routing of 13C-labeled carbohydrates, lipids, and proteins in vivo. Physiol Biochem Zool. 2011;84(5):506–13. [DOI] [PubMed] [Google Scholar]
- 48. Newsome SD, Wolf N, Peters J, Fogel ML. Amino acid δ13C analysis shows flexibility in the routing of dietary protein and lipids to the tissue of an omnivore. Integr Comp Biol. 2014;54(5):890–902. [DOI] [PubMed] [Google Scholar]
- 49. Wolf N, Newsome S, Peters J, Fogel M. Variability in the routing of dietary proteins and lipids to consumer tissues influences tissue‐specific isotopic discrimination. Rapid Commun Mass Spectrom. 2015;29(15):1448–56. [DOI] [PubMed] [Google Scholar]
- 50. Choy K, Nash S, Kristal A, Hopkins S, Boyer B, O'Brien D. The carbon isotope ratio of alanine in red blood cells is a new candidate biomarker of sugar-sweetened beverage intake. J Nutr. 2013;143(6):878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cook C, Alvig A, Liu Y, Schoeller D. The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. J Nutr. 2010;140(2):333–7. [DOI] [PubMed] [Google Scholar]
- 52. Hülsemann F, Koehler K, Wittsiepe J, Wilhelm M, Hilbig A, Kersting M, Braun H, Flenker U, Schänzer W. Prediction of human dietary δ(15)N intake from standardised food records: validity and precision of single meal and 24-h diet data. Isotopes Environ Health Stud. 2017;53(4):356–67. [DOI] [PubMed] [Google Scholar]
- 53. Thiagarajan P, Prchal J.. Erythrocyte Turnover. In: Kaushansky K, Lichtman MA, Prchal JT, Levi MM, Press OW, Burns LJ et al., Williams Hematology, 9 ed New York, NY: McGraw-Hill Education; 2015. [Google Scholar]
- 54. Petzke K, Lemke S.. Hair protein and amino acid 13C and 15N abundances take more than 4 weeks to clearly prove influences of animal protein intake in young women with a habitual daily protein consumption of more than 1 g per kg body weight. Rapid Commun Mass Spectrom. 2009;23(16):2411–20. [DOI] [PubMed] [Google Scholar]
- 55. Huelsemann F, Flenker U, Koehler K, Schaenzer W. Effect of a controlled dietary change on carbon and nitrogen stable isotope ratios of human hair. Rapid Commun Mass Spectrom. 2009;23(16):2448–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.