ABSTRACT
Background
Fatty acids are a vital component of human milk. They influence infant neurodevelopment and immune function, and they provide ∼50% of milk's energy content.
Objectives
The objectives of this study were to characterize the composition of human milk fatty acids in a large Canadian birth cohort and identify factors influencing their variability.
Methods
In a subset of the CHILD cohort (n = 1094), we analyzed milk fatty acids at 3–4 mo postpartum using GLC. Individual and total SFAs, MUFAs, and n–3 and n–6 PUFAs were analyzed using SD scores and principal component analysis (PCA). Maternal diet, sociodemographic, health, and environmental factors were self-reported. Single-nucleotide polymorphisms were assessed in the fatty acid desaturase 1 (FADS1-rs174556) and 2 (FADS2-rs174575) genes.
Results
Fatty acid profiles were variable, with individual fatty acid proportions varying from 2- to >30-fold between women. Using PCA, we identified 4 milk fatty acid patterns: “MUFA and low SFA,” “high n–6 PUFA,” “high n–3 PUFA,” and “high medium-chain fatty acids.” In multivariable-adjusted analyses, fish oil supplementation and fatty cold water fish intake were positively associated with DHA and the “high n–3 PUFA” pattern. Mothers carrying the minor allele of FADS1-rs174556 had lower proportions of arachidonic acid (ARA; 20:4n–6). Independent of selected dietary variables and genetic variants, Asian ethnicity was associated with higher linoleic acid (18:2n–6) and total n–3 PUFAs. Ethnic differences in ARA were explained by FADS1 genotype. Maternal obesity was independently associated with higher total SFAs, the “high medium-chain fatty acid” pattern, and lower total MUFAs. Lactation stage, season, study site, and maternal education were also independently associated with some milk fatty acids. No associations were observed for maternal age, parity, delivery mode, or infant sex.
Conclusions
This study provides unique insights about the “normal” variation in the composition of human milk fatty acids and the contributing dietary, genetic, sociodemographic, health, and environmental factors. Further research is required to assess implications for infant health.
Introduction
Maternal nutrition during pregnancy has many short- and long-term effects on the offspring (1, 2). In addition to nutritional factors during pregnancy, the importance of breastfeeding for infant growth and development is widely recognized (3, 4). Yet, substantial variation in these associations is reported across different populations (5–7), reflecting the variable and dynamic nature of human milk. Variations in human milk composition are related to multiple factors, including maternal diet, genetics, lactation stage, breastfeeding practices, maternal and infant health status, and environmental exposures.
Human milk fatty acids are the most variable macronutrient of human milk and account for ∼50% of its energy content (8). Their role in infant physical and cognitive development is well known (9–12) and has stimulated interest in identifying fixed and modifiable factors that influence their composition. Fatty acids in human milk are derived from maternal body stores, endogenous synthesis in the mammary gland, and uptake from maternal plasma (8, 13, 14). Variants in the fatty acid desaturase genes (FADS1, encoding Δ-5 desaturase, and FADS2, encoding Δ-6 desaturase) have been shown to influence long-chain PUFAs (LC-PUFAs) in human milk (15, 16). These Δ-5 and Δ-6 desaturases are important enzymes in the endogenous formation of LC-PUFAs. Maternal nutrition is another key factor that impacts the fatty acids available for uptake from the maternal plasma (13). Thus, both genetic and dietary factors can influence the proportions of milk fatty acids.
In addition to diet and genetics, length of gestation and lactation stage are known to influence milk fatty acid composition (17); however, little is known about the impact of other sociodemographic characteristics, health conditions, and environmental exposures. To our knowledge, these factors have never been studied simultaneously to assess their independent and combined impact on different milk fatty acids and fatty acid patterns. Although plasma fatty acid patterns have been extensively studied during pregnancy (18), less is known about milk fatty acid patterns during lactation. In the current study, we aimed to identify milk fatty acid patterns among over 1000 women from the CHILD birth cohort and comprehensively assess their association with genetic, dietary, sociodemographic, health, and environmental factors.
Methods
Design and study population
This study was embedded in the CHILD study (n = 3455), a population-based birth cohort recruited from 4 sites in Canada (19). Women with singleton pregnancies from Edmonton, Manitoba, Toronto, and Vancouver were enrolled between 2008 and 2012 and remained eligible if they delivered a healthy infant >35 weeks of gestation. This study was performed in a selected subsample of all mothers who breastfed and provided a milk sample at a home visit 3–4 mo postpartum (n = 1200). For the current study, we excluded mothers with missing information on key dietary, health, environmental, or genetic factors of interest (n = 13). In order to meet assumptions of normality in regression analyses, we also excluded dyads with ≥1 milk fatty acid values <4 or >4 SD from the mean (n = 93) (Supplemental Figure 1). Excluding these mothers did not appreciably change the overall distribution or mean proportions of individual fatty acids (Supplemental Table 1). Our final subsample of 1094 mothers included a representative subset of 406 dyads (20) plus 688 additional dyads selected to enrich for maternal and infant chronic health conditions (e.g., asthma, allergies, and obesity). This study was approved by the Human Research Ethics Boards at McMaster University and the Universities of Manitoba, Alberta, and British Columbia and the Hospital for Sick Children.
Maternal diet, sociodemographic, health, and environmental factors
Maternal diet during pregnancy was estimated using an FFQ (21). For this study, we specifically analyzed fatty cold water fish (salmon, mackerel, and bluefish), white fish (sole, halibut, snapper, or cod), and shell fish (shrimp, lobster, crab, and oysters) (converted from servings per week to ounces per day). We also analyzed red meat (beef, pork, veal, lamb, and game), egg, and nuts (nuts and seeds) (ounce equivalents of lean meat). Dietary intakes of fish, meat, egg, and nuts were adjusted for total daily energy intake using the nutrient residual method (22). The total Healthy Eating Index 2010 (HEI-2010) score and individual HEI components (fatty acids and dairy) were also derived and analyzed per quintile increase (23). Fish oil (fish oil or cod liver oil) supplement and daily multivitamin use (yes or no) were self-reported during pregnancy (19). Fish oil supplement use was self-reported again during lactation, at the time of milk collection.
Questionnaires were administered at enrollment in the study (second or third trimester of pregnancy) to collect information about maternal age, ethnicity (classified for the purposes of this analysis as Caucasian, Asian, First Nation, or Other), education (completion of postsecondary degree), parity, marital status, and smoking (19). The study site (Vancouver, Edmonton, Manitoba, or Toronto) and season (spring, summer, fall, or winter) of sample collection were also assessed as indicators of the environment.
Maternal prepregnancy BMI (in kg/m2) was calculated from measured height and self-reported prepregnancy weight, and it was classified as underweight (<18.5), normal weight (18.5 to <25.0), overweight (25.0 to <30.0), and obese (≥30.0). Information on gestational weight gain and health conditions such as gestational diabetes and hypertension, atopy (hay fever, skin allergies, or aeroallergen, drug, insect, food, pet, or other allergies), asthma, and inflammatory bowel disorders was also obtained through standardized questionnaires.
Birth, lactation, and infant factors
Delivery mode, infant sex, gestational age at birth, and birth weight were obtained from medical records. Lactation stage (weeks postpartum), season at milk sample collection (spring, summer, fall, or winter), breastfeeding exclusivity (breast milk only or breast milk supplemented with formula), and mode of breastfeeding (nursing directly at the breast only or including some pumped milk) were documented at the time of sample collection. Infant colds from birth until the time of milk sample collection were reported by parents and classified as any or no colds.
Fatty acid desaturase (FADS) genotypes
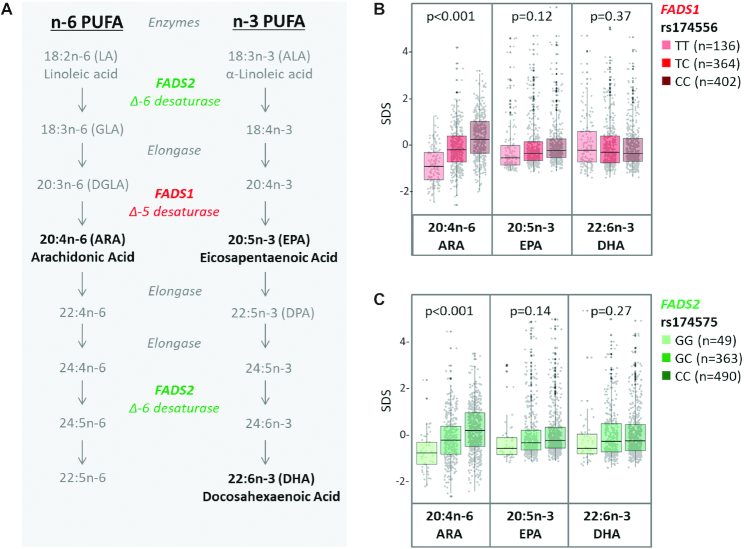
Maternal DNA was isolated from peripheral blood samples collected during pregnancy. Genotyping of single nucleotide polymorphisms (SNPs) was performed using the HumanCoreExome BeadChip (Illumina) comprising >240,000 tagSNPs and >240,000 exome-focused markers. A stringent process of quality control at subject and SNP levels using PLINK version 1.9 was applied. Subjects were omitted who demonstrated low genotype call rates (missingness >0.10), discrepancies with reported sex, were ethnic outliers based on principal component analysis (PCA), or were related as determined by identity by descent. SNPs with missingness >0.10, those that had significant departures from Hardy–Weinberg equilibrium (P < 1 × 10−6), or those that had a minor allele frequency <0.01 were excluded. IMPUTE2 software was used to impute genotypes based on the 1000 Genomes Project reference panel (24), which resulted in >23 million single nucleotide variants of high quality (>0.7). Using this imputed data set, we selected 2 SNPs that were previously reported to influence the endogenous formation of LC-PUFA (25, 26): rs174556, located in the FADS1 gene, and rs174575, located in the FADS2 gene (27) (Figure 1A). Because these 2 SNPs are in linkage disequilibrium, we included only rs174556 in the multivariable analyses.
FIGURE 1.
PUFA metabolism (A) and association of milk PUFAs with FADS genetic variants in the CHILD cohort (B and C) (n = 902). Metabolism of n–6 PUFA and n–3 PUFA involves desaturation steps that compete for the FADS1 and FADS2 enzymes (A). FADS1 (B) and FADS2 (C) genotypes are strongly associated with proportions of n–6 PUFA 20:4n–6 (ARA) but not n–3 PUFAs 20:5n–3 (EPA) or 22:6n–3 (DHA) in milk. Differences in milk fatty acid proportions between FADS1 and FADS2 genotypes were evaluated using ANOVA. *P < 0.05. ARA, arachidonic acid; FADS, fatty acid desaturase; SDS, standard deviation score.
Milk collection and fatty acid measurements
In advance of the 3- to 4-mo postpartum CHILD home visit (median: 15.3 wk; 95% range: 11.3, 28.5 wk), mothers were provided a sterile collection jar and instructed as follows: “One day prior to the home visit, collect breast milk over 2 or more feedings to a volume of at least 10 mL. Once you have started collecting breast milk, keep the container refrigerated” (28). Although mothers were asked to hand express the milk, some reported using a pump. Milk samples were aliquoted aseptically according to a standardized protocol and then stored at –80°C. Total milk lipids were extracted using a modified Folch protocol (29). Samples were processed and analyzed in 3 batches at the University of Alberta by GLC as previously described (30). Fatty acids were identified according to commercial standards GLC-502 and GLC-643 (Nu-Chek Prep; Elysian) and expressed as a relative percentage of total identified fatty acids. These standards were included in each GC batch run for quality assurance between batches to correctly identify and quantify each column run with potential changes in column conditions or peak retention time. The fatty acids analyzed in this study include SFAs: capric acid (10:0), lauric acid (12:0), myristic acid (14:0), palmitic acid (16:0), margaritic acid (17:0), stearic acid (18:0), and arachidic acid (20:0); MUFAs: palmitoleic acid (16:ln–7), oleic acid (18:ln-9), vaccenic acid (18:lc–11); n–3 PUFAs: α-linoleic acid (ALA; 18:3n–3), eicosatetraenoic acid (20:4n–3), EPA (20:5n–3), docosapentaenoic acid (DPA; 22:5n–3), and DHA (22:6n-3); and n–6 PUFAs: linoleic acid (LA; 18:2n–6), γ-linolenic acid (GLA; 18:3n–6), conjugated linoleic acid (CLA; 18:2c–9, t–11), dihomo-γ-linoleic acid (DGLA; 20:3n–6), arachidonic acid (ARA; 20:4n–6), and adrenic acid (22:4n–6). In addition, the following summary measures and ratios were assessed: total SFA, total MUFA, total n–3 PUFA, total n–6 PUFA, n–6:n–3 PUFA ratio, ARA:DHA ratio, ARA:(EPA + DHA) ratio, and (EPA + DPA):DHA ratio.
Statistical analysis
First, we calculated the mean and SD for each fatty acid and used t test and ANOVA to compare mean values according to fish intake and fish oil supplement use. For all subsequent analyses, to enable comparison between effect estimates for different fatty acids, we converted measurements into SD scores so that all fatty acids could be evaluated on a comparable scale (per SD increase). We examined correlation coefficients between fatty acids and used PCA, a dimensionality reduction technique, to transform the 21 correlated fatty acids into a smaller number of uncorrelated principal components (or “fatty acid patterns”) (31). Each component is present in every sample. We retained PCA components with an eigenvalue ≥2 and named the patterns on the basis of high factor loadings >|0.20|. To improve the interpretability of the patterns, we applied a varimax rotation (18).
We used crude (unadjusted) linear regression analyses to explore the associations between dietary, genetic, socio-demographic, health, environmental, birth, and lactation factors (described previously) and human milk fatty acid composition (individual and total fatty acids, fatty acid ratios, and fatty acid patterns defined by PCA). Finally, after accounting for multiple testing, the factors that were significantly associated with multiple fatty acid measures in the unadjusted regression analyses were further investigated in multivariable linear regression models to identify independent determinants of fatty acid composition. To avoid collinearity, some highly correlated variables were excluded from the final models (e.g., fatty acid HEI score, estimated DHA and ARA intake, white fish and shell fish intake, fish oil supplement use during lactation, multivitamin use during pregnancy, and gestational weight gain). For example, fatty cold water fish, white fish, and shell fish intake were highly intercorrelated, and upon mutual adjustment, only fatty cold water fish remained significantly associated with DHA in milk; thus, only fatty cold water fish was retained in the final model. Similarly, fish oil supplementation during pregnancy and lactation were highly correlated, and both were associated with LC-PUFAs in milk. Because supplementation in pregnancy was more common, it was retained in the final model. The multivariable adjusted models were further adjusted for rs174556 FADS genotype. In the final models, we adjusted for batch (to account for any potential variability between milk fatty acid subsets) and excluded dyads with missing data for exposure variables.
In a sensitivity analysis to control for constant-sum constraint (compositional nature of milk fatty acids), proportions data were transformed using the centered log ratio (CLR) method (32), and all of the previously discussed analyses were repeated using the CLR-transformed values. P values < 0.05 were considered statistically significant, after applying false discovery rate correction for multiple testing using the Benjamini–Hochberg method (33). Analyses were performed using SPSS for Windows, version 21.0 (IBM). Figures were generated using the ggcorrplot and ggplot2 packages in R studio software version 3.3.2 (R Foundation for Statistical Computing).
Results
In this subsample of 1094 dyads, the mean ± SD maternal age was 32.9 ± 4.3 y, and the mean gestational age at delivery was 39.1 ± 1.4 wk (Table 1). Approximately half of mothers were primiparous (57%), and the majority were Caucasian (72%). Nearly 80% had a postsecondary education, almost 35% were overweight or obese, and 23% had asthma. The median lactation time at milk sample collection was 15.3 wk (95% range: 11.3, 28.5 wk).
TABLE 1.
Characteristics of mothers and infants from a subset of the CHILD cohort (n = 1094)1
| Values | |
|---|---|
| Maternal characteristics | |
| Age, y | 32.9 ± 4.3 |
| Parity, n (%) | |
| 1 | 618 (56.5) |
| 2 | 340 (31.1) |
| ≥3 | 136 (12.4) |
| Completed postsecondary education, n (%) | 874 (79.9) |
| Marital status, married, n (%) | 1036 (94.7) |
| Ethnicity, n (%) | |
| Asian | 181 (16.5) |
| Caucasian | 792 (72.4) |
| First Nations | 44 (4.0) |
| Other | 75 (6.9) |
| Study site, n (%) | |
| Edmonton | 240 (21.9) |
| Toronto | 284 (26.0) |
| Vancouver | 278 (25.4) |
| Manitoba | 292 (26.7) |
| Prenatal smoking, n (%) | 54 (4.9) |
| Prepregnancy BMI, kg/m2 | 23.1 (18.2, 38.2) |
| Underweight, n (%) | 33 (3.0) |
| Normal weight, n (%) | 634 (58.0) |
| Overweight, n (%) | 239 (21.8) |
| Obese, n (%) | 140 (12.8) |
| Weight gain during pregnancy, kg | 15.4 (4.1, 29.5) |
| BMI at 1 y postpartum, kg/m2 | 23.8 (18.2, 38.9) |
| Maternal diet during pregnancy | |
| Diet quality, HEI-2010 score (max 100) | 73.2 ± 7.9 |
| Fatty acids HEI-2010 score (max 10) | 4.00 ± 2.6 |
| Dairy HEI score (max 10) | 8.39 ± 2.2 |
| Nuts and seed intake,2 oz equivalents/d | 1.10 ± 1.04 |
| Meat intake,2 oz equivalents/d | 1.69 ± 1.30 |
| Egg intake,2 oz equivalents/d | 0.58 ± 0.54 |
| Energy intake, kcal/d | 2015.8 ± 686.1 |
| Fatty cold water fish intake,2 oz/d | 0.25 ± 0.41 |
| Never or 1 time per month | 422 (39.6) |
| ≤ 1–3 times per month | 443 (40.5) |
| 1–6 times per week | 179 (16.4) |
| White fish intake,2 oz/d | 0.19 ± 0.32 |
| Never or 1 time per month | 437 (39.9) |
| ≤ 1–3 times per month | 493 (45.1) |
| 1–6 times per week | 114 (10.4) |
| Shell fish intake,2 oz/d | 0.12 ± 0.26 |
| Never or 1 time per month | 575 (52.6) |
| ≤ 1–3 times per month | 369 (33.7) |
| 1–6 times per week | 100 (9.1) |
| Estimated dietary DHA intake, mg/d | 128.3 ± 128.8 |
| Estimated dietary ARA intake, mg/d | 118.4 ± 86.4 |
| Fish oil supplement use during pregnancy, n (%) | 242 (22.1) |
| Fish oil supplement use during lactation (n = 888), n (%) | 109 (12.3) |
| Multivitamin use during pregnancy, n (%) | 975 (89.1) |
| Maternal health conditions, n (%) | |
| Asthma | 256 (23.4) |
| Atopy3 | 730 (66.7) |
| Gestational diabetes | 95 (8.7) |
| Gestational hypertensive disorders | 31 (2.8) |
| Inflammatory bowel disorders | 25 (2.3) |
| Birth and infant characteristics | |
| Cesarean delivery, n (%) | 284 (26.0) |
| Sex, male, n (%) | 603 (55.1) |
| Gestational age at birth, wk | 39.1 ± 1.4 |
| Birth weight, g | 3453.9 ± 502.5 |
| Colds from birth to 3 mo, n (%) | 483 (44.1) |
| Infant weight at 3 mo, kg | 6.5 ± 1.0 |
| Infant length at 3 mo, cm | 62.7 ± 3.8 |
| Breastfeeding characteristics at sample collection | |
| Lactation time, wk | 15.3 (11.3, 28.5) |
| Exclusive breastfeeding, n (%) | 553 (50.5) |
| Feeding mode, n (%) | |
| Direct breastfeeding only | 429 (39.2) |
| Some pumping | 636 (40.3) |
| Season of milk sampling, n (%) | |
| Spring | 259 (23.7) |
| Summer | 305 (27.9) |
| Fall | 264 (24.1) |
| Winter | 264 (24.1) |
| Maternal SNPs in the FADS1–FADS2 gene cluster (n = 902),4n (%) | |
| Rs174556 (FADS1) | |
| CC | 402 (44.6) |
| CT | 364 (40.4) |
| TT | 136 (15.1) |
| Rs174575 (FADS2) | |
| CC | 490 (54.3) |
| CG | 363 (40.2) |
| GG | 49 (5.4) |
Values are n (%) for categorical variables, means ± SDs for continuous variables with a normal distribution, or medians (95% ranges) for continuous variables with a skewed distribution. ARA, arachidonic acid; FADS, fatty acid desaturase; HEI-2010, Healthy Eating Index 2010; SNP, single nucleotide polymorphism.
Ounce equivalents of lean meat: 1 oz = 28.35 g.
Maternal atopy includes hay fever, skin allergies, or aeroallergen, drug, insect, food, pet, or other allergies at milk collection.
Rs174556 (FADS1) located in CHR 11, position 61580635; Rs174575 (FADS2) located in CHR 11, position 61602003.
Milk fatty acid composition, variation, correlations, and patterns
Overall, the most abundant classes of milk fatty acids were MUFA, ranging from 32% to 56% of total fatty acids (mean: 43.06% ± 3.59%), and SFA, ranging from 25% to 56% (39.75% ± 5.00%). The most abundant individual fatty acids were MUFA 18: ln–9 (oleic acid: 37.05% ± 3.59%) and SFA 16:0 (palmitic acid; 20.90% ± 2.76%) (Table 2). The mean proportions of total n–3 and n–6 PUFAs were 2.39% ± 0.70% and 14.78% ± 3.09%, respectively (Table 2), and the mean ratio of total n–6:n–3 PUFA was 6.53 ± 1.72. The mean proportions of 22:6n–3 (DHA) and 20:4n–6 (ARA) were 0.18% ± 0.12% and 0.38% ± 0.09%, respectively (Table 2). Relative interindividual variation was higher for n–3 PUFAs (5.5-fold variation between mothers with the highest compared with lowest proportions) and n–6 PUFAs (3.8-fold) than for MUFAs (1.7-fold) and SFAs (2.3-fold). The n–3 PUFAs DHA and EPA were among the most variable fatty acids, with 24.7- and 39.0-fold variation, respectively.
TABLE 2.
Human milk fatty acid composition (% total fatty acids) and differences by fish oil supplementatio n during pregnancy in the CHILD cohort (n = 1094)1
| All mothers (n = 1094) | Fish oil supplementation, mean values | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Fold variation | No (n = 839) | Yes (n = 242) | P value | |
| Total SFA, % |
39.75 ± 5.00 | 24.52–55.81 | 2.3 | 39.90 | 39.31 | |
| 10:0 (capric acid) | 0.71 ± 0.30 | 0.01–1.64 | 164.0 | 0.70 | 0.74 | * |
| 12:0 (lauric acid) | 4.80 ± 1.57 | 1.25–10.18 | 8.1 | 4.82 | 4.71 | |
| 14:0 (myristic acid) | 5.97 ± 1.80 | 2.10–12.68 | 6.0 | 6.04 | 5.76 | * |
| 16:0 (palmitic acid) | 20.90 ± 2.76 | 13.22–32.21 | 2.4 | 20.98 | 20.65 | |
| 17:0 (margaric acid) | 0.31 ± 0.08 | 11.60–58.32 | 5.0 | 0.31 | 0.32 | |
| 18:0 (stearic acid) | 6.54 ± 1.31 | 2.56–11.88 | 4.6 | 6.53 | 6.60 | |
| Total MUFA, % | 43.06 ± 3.59 | 32.20–55.99 | 1.7 | 43.04 | 43.09 | |
| 16:ln–7 (palmitoleic acid) | 2.69 ± 0.68 | 0.75–5.25 | 7.0 | 2.70 | 2.65 | |
| 18:ln–9 (oleic acid) | 37.05 ± 3.59 | 26.66–48.55 | 1.8 | 36.97 | 37.23 | |
| 18:l c–11 (vaccenic acid) | 1.62 ± 0.40 | 0.09–3.48 | 38.7 | 1.64 | 1.57 | * |
| Total n–3 PUFA, % | 2.39 ± 0.70 | 0.93–5.07 | 5.5 | 2.34 | 2.57 | ** |
| 18:3n–3 (ALA) | 1.92 ± 0.61 | 0.44–4.38 | 10.0 | 1.90 | 1.99 | ** |
| 20:4n–3 (eicosatetraenoic acid) | 0.08 ± 0.03 | 0.03–0.22 | 7.3 | 0.08 | 0.09 | ** |
| 20:5n–3 (EPA) | 0.08 ± 0.05 | 0.01–0.39 | 39.0 | 0.07 | 0.11 | ** |
| 22:5n–3 (DPA) | 0.13 ± 0.05 | 0.04–0.34 | 8.5 | 0.12 | 0.15 | ** |
| 22:6n–3 (DHA) | 0.18 ± 0.12 | 0.03–0.74 | 24.7 | 0.17 | 0.24 | ** |
| Total n–6 PUFA, % | 14.80 ± 3.09 | 7.14–27.39 | 3.8 | 14.73 | 15.02 | |
| 18:2n–6 (LA) | 13.62 ± 3.01 | 6.17–25.59 | 4.1 | 13.55 | 13.84 | |
| 18:3n–6 (GLA) | 0.10 ± 0.05 | 0.02–0.31 | 15.5 | 0.10 | 0.10 | |
| 18:2c–9, t–11 (CLA) | 0.02 ± 0.01 | 0.01–0.06 | 6.0 | 0.02 | 0.02 | |
| 20:3n–6 (DGLA) | 0.35 ± 0.11 | 0.06–0.74 | 12.3 | 0.35 | 0.34 | |
| 20:4n–6 (ARA) | 0.38 ± 0.09 | 0.15–0.74 | 4.9 | 0.38 | 0.38 | |
| 22:4n–6 (adrenic acid) | 0.04 ± 0.03 | 0.01–0.15 | 15.0 | 0.04 | 0.04 | |
| Ratios | ||||||
| n–6:n–3 PUFA | 6.53 ± 1.72 | 3.19–17.29 | 5.4 | 6.63 | 6.14 | ** |
| ARA:DHA | 2.71 ± 1.47 | 0.44–17.60 | 40.0 | 2.89 | 2.03 | ** |
| ARA:(EPA + DHA) | 1.83 ± 0.80 | 0.29–5.91 | 20.4 | 1.96 | 1.36 | ** |
| (EPA + DPA):DHA | 1.31 ± 0.67 | 0.37–11.85 | 32.0 | 1.33 | 1.21 | * |
Values are mean ± SD percentages of fatty acids (expressed as percentage of total fatty acids). Thirteen participants were missing data for fish oil supplementation. Differences in milk fatty acid proportions between the fish oil supplement groups were evaluated using t test. P *<0.05,** <0.001. ALA, α-linolenic acid; ARA, arachidonic acid; CLA, conjugated linoleic acid; DGLA, dihomo-γ-linoleic acid; DPA, docosapentaenoic acid; GLA, γ-linolenic acid; LA, linoleic acid.
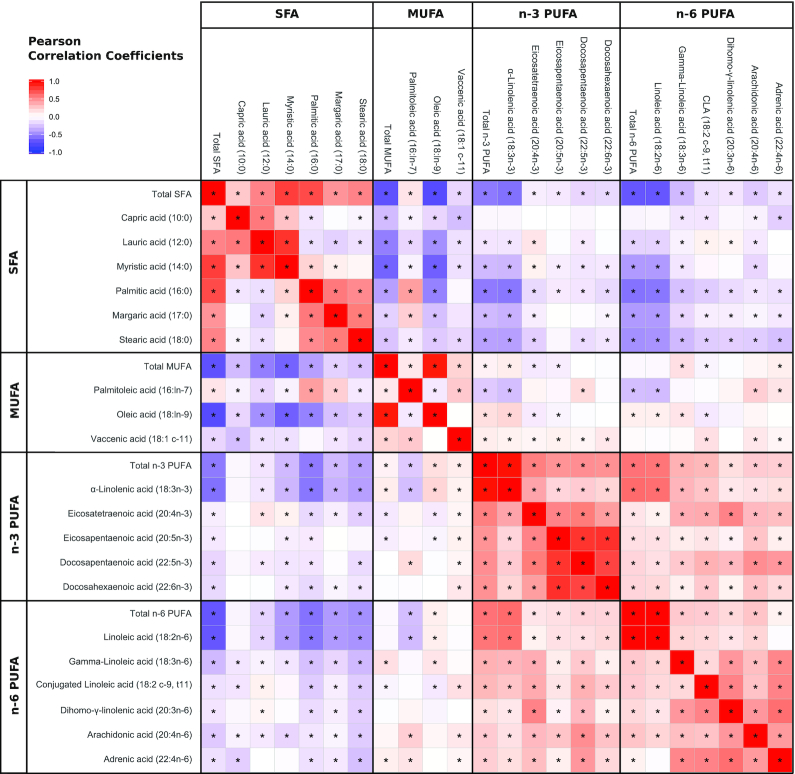
The proportions of n–3 and n–6 PUFAs were positively correlated with each other, with the highest correlation coefficients between 18:3n–3 (ALA) and 18:2n–6 (LA) (r = 0.59, P < 0.001) (Figure 2). SFAs were negatively correlated with MUFAs, n–3 PUFAs, and n–6 PUFAs (r = –0.71, –0.55, and –0.67, respectively; P < 0.01). Some of these correlation coefficients differed when using CLR-transformed fatty acids (Supplemental Figure 2), including notable inverse correlations between n–3 PUFA and n–6 PUFA.
FIGURE 2.
Correlation of human milk fatty acids in the CHILD cohort (n = 1094). Coloring reflects direction and magnitude of the Pearson correlation coefficients. *Correlation is significant at P < 0.05. False discovery rate not applied.
Using PCA, we identified 4 components (or “patterns”) that explained ∼60% of the variation in milk fatty acids: “MUFA and low SFA” (23.3% of variation explained), “high n–6 PUFA” (14.2%), “high n–3 PUFA” (12.0%), and “high medium-chain fatty acids” (fatty acids with <14 carbons; 10.0%). The factor loadings, which describe how strongly each individual fatty acid contributes to each fatty acid pattern, are shown in Table 3.
TABLE 3.
Human milk fatty acid patterns from principal component analysis in the CHILD cohort (n = 1094) 1
| MUFA and low SFA | High n–6 PUFA | High n–3 PUFA | High medium-chain SFA | |
|---|---|---|---|---|
| SFA | ||||
| 10:0 (capric acid) | — | — | — | 0.45 |
| 12:0 (lauric acid) | — | — | — | 0.91 |
| 14:0 (myristic acid) | — | — | — | 0.90 |
| 16:0 (palmitic acid) | –0.83 | — | — | — |
| 17:0 (margaric acid) | –0.78 | — | — | — |
| 18:0 (stearic acid) | –0.76 | — | — | — |
| 20:0 (arachidic acid) | — | 0.66 | — | — |
| MUFA | ||||
| 16:ln–7 (palmitoleic acid) | –0.31 | — | — | — |
| 18:ln–9 (oleic acid) | 0.36 | — | — | –0.73 |
| 18:lc–11 (vaccenic acid) | — | — | — | — |
| n–3 PUFA | ||||
| 18:3n–3 (ALA) | 0.63 | — | 0.25 | — |
| 20:4n–3 (eicosatetraenoic acid) | — | 0.46 | 0.58 | — |
| 20:5n–3 (EPA) | — | — | 0.94 | — |
| 22:5n–3 (DPA) | — | 0.33 | 0.86 | — |
| 22:6n–3 (DHA) | — | — | 0.89 | — |
| n–6 PUFA | ||||
| 18:2n–6 (LA) | 0.68 | — | — | — |
| 18:3n–6 (GLA) | 0.27 | 0.59 | — | — |
| 18:2c-9, t11 (CLA) | — | 0.59 | — | — |
| 20:3n–6 (DGLA) | — | 0.87 | — | — |
| 20:4n–6 (ARA) | — | 0.49 | 0.33 | — |
| 22:4n–6 (adrenic acid) | — | 0.82 | — | — |
| Explained variance, % | 23.3 | 14.2 | 12.0 | 10.0 |
Values are factor loadings from principal component analysis. Only loadings >|0.20| are shown. ALA, α-linolenic acid; ARA, arachidonic acid; CLA, conjugated linoleic acid; DGLA, dihomo-γ-linoleic acid; DPA, docosapentaenoic acid; GLA, γ-linolenic acid; LA, linoleic acid.
Dietary determinants of human milk fatty acids
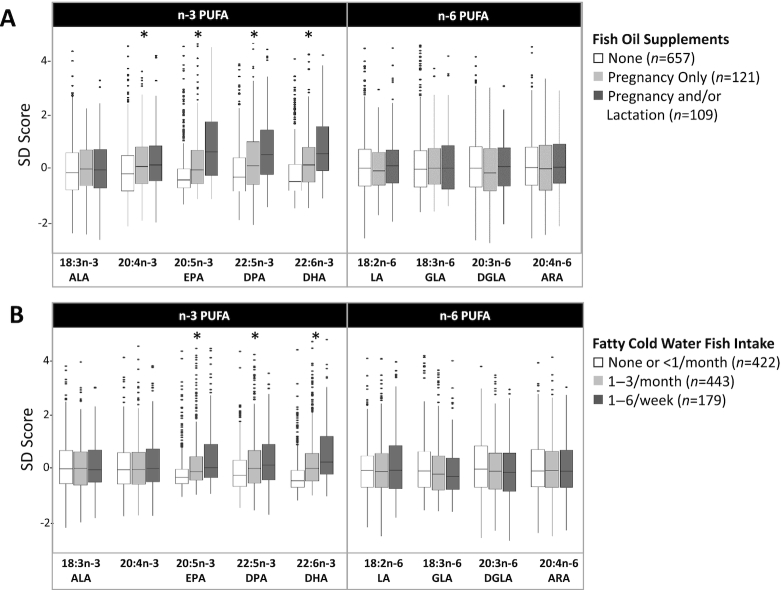
During pregnancy, 78% of mothers reported consuming fish or shell fish, and 22% reported taking a daily fish oil supplement. About 40% of mothers using fish oil during pregnancy continued using this supplement during lactation (12% of all mothers). The mean proportions of n–3 PUFAs were higher in mothers using fish oil supplements, especially if supplementation occurred during lactation. For example, DHA proportions were lowest in mothers who did not use fish oil supplements (0.16%), higher in mothers who used fish oil supplements during pregnancy only (0.23%), and even higher in mothers who used fish oil during pregnancy and lactation (0.27%) (p-for-trend < 0.001; Figure 3A, Supplemental Table 2). In regression models, these differences translate to 0.66 SD (95% CI: 0.52, 0.80) and 0.86 SD (95% CI: 0.66, 1.05) increases in DHA proportions with fish oil supplementation during pregnancy and lactation, respectively (Figure 4).
FIGURE 3.
Association of PUFAs in human milk with fish oil supplement use (A) and fatty cold water fish intake (B) in the CHILD cohort (n = 1094). Long-chain n–3 PUFAs, but not n–6 PUFAs, are associated with fish oil supplement use during pregnancy and lactation and fatty cold water fish intake in a dose-dependent manner. Associations were evaluated using ANOVA and test for trend analysis. *P-for-trend < 0.001. ALA, α-linoleic acid; ARA, arachidonic acid; DGLA, dihomo-γ-linoleic acid; DPA, docosapentaenoic acid; GLA, γ-linolenic acid; LA, linoleic acid; 20:4n–3, eicosatetraenoic acid.
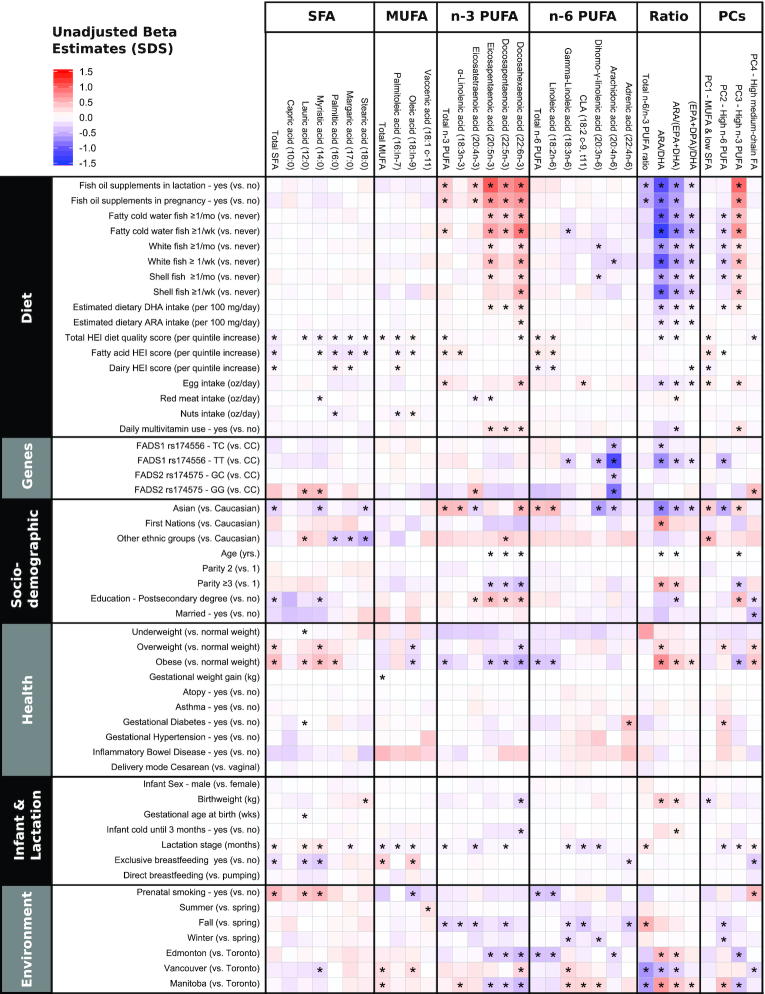
FIGURE 4.
Crude associations of individual factors with human milk fatty acids (n = 1094). Coloring reflects the direction and the magnitude of β estimates from crude linear regression models for each fatty acid (relative proportions converted to SDSs). Ratios are not expressed as SDSs. One ounce = 28.35 g. *P < 0.05 after false discovery rate adjustment for multiple comparisons. ARA, arachidonic acid; CLA, conjugated linoleic acid; DPA, docosapentaenoic acid; FA, fatty acid; HEI, Healthy Eating Index; PC, principal component; SDS, standard deviation score.
Fish oil supplement use during pregnancy was also positively associated with the “high n–3 PUFA” pattern and negatively associated with adrenic acid, the “high n–6 PUFA” pattern, and n–6:n–3 PUFA, ARA:DHA, and ARA:(EPA + DHA) ratios (P< 0.05) (Table 2, Figures 4 and 5). Similar results were observed for white fish, shell fish, and especially fatty cold water fish intake. For example, there was a dose-dependent increase in DHA proportions according to the frequency of fatty cold water fish consumption (Figure 3B), with an estimated 0.62 SD (95% CI: 0.47, 0.76) increase in DHA per 1-ounce increase in estimated daily fatty cold water fish intake. The associations of DHA with fish oil supplement use and fatty cold water fish intake remained significant after adjustment for other dietary, genetic, sociodemographic, and environmental factors and multiple testing [multivariable adjusted βs for DHA: +0.50 SD (95% CI: 0.35, 0.65) for fish oil supplement use and +0.30 SD (95% CI: 0.14, 0.45) per 1-ounce fatty cold water fish intake] (Figure 5). In contrast, SFA, MUFA, and n–6 PUFA were mostly unaffected by fish intake and fish oil supplement use (Table 2, Figures 3 and 4, Supplemental Table 2).
FIGURE 5.
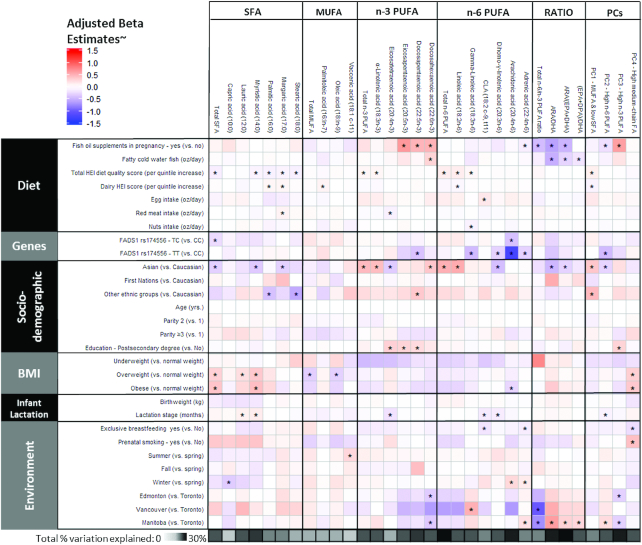
Multivariable adjusted (˜) models of maternal diet, genetic, sociodemographic, health, and environmental predictors of human milk fatty acid composition in the CHILD cohort (n = 806). Coloring reflects the direction and the magnitude of β estimates from multivariable linear regression models for each fatty acid (relative proportions converted to SDSs), with adjustment for batch and all variables shown. Ratios are not expressed as SDSs. One ounce = 28.35 g. *P < 0.05 after false discovery rate adjustment for multiple comparisons. ARA, arachidonic acid; CLA, conjugated linoleic acid; DPA, docosapentaenoic acid; FA, fatty acid; HEI, Healthy Eating Index; PC, principal component; SDS, standard deviation score.
We also investigated other sources of dietary fat and overall diet quality. Dairy, egg, meat, and nut intake during pregnancy were positively associated with a few human milk fatty acids (e.g., palmitic acid, margaric acid, palmitoleic acid, and CLA); however, these associations were relatively weak (Figure 5). A higher HEI-2010 score, reflecting diet quality, was associated with lower individual and total SFA and higher total n–6 PUFA, LA, and GLA. HEI-2010 score was also positively associated with the “MUFA and low SFA” pattern (P < 0.05) (Figure 5).
Similar associations were observed using CLR-transformed fatty acid data (Supplemental Figure 3), except that fish oil supplement use was associated with multiple fatty acids beyond n–3 PUFAs, including some SFAs, MUFAs, and n–6 PUFAs (including ARA), although most of these additional associations attenuated to nonsignificant after adjusting for other factors and multiple testing (Supplemental Figure 4). Overall in the fully adjusted model, the estimated dietary factors explained 0.02–17% of the variance in human milk fatty acids, with the lowest explained variance for SFAs and MUFAs (e.g., 0.02% for capric acid and 0.07% for palmitoleic acid) and the highest explained variance for n–3 PUFAs, including EPA (11%), DHA (15%), ARA:DHA (12%), ARA:(EPA + DHA) (17%), and the “high n–3 PUFA” pattern (12%).
Genetic determinants of milk fatty acids
Mothers carrying the minor allele of rs174556 (C>T; minor allele frequency = 15.1%) in FADS1 had lower proportions of the longer chain n–6 PUFAs (GLA, DGLA, and ARA), scored lower on the “high n–6 PUFA” pattern, and had lower ratios of ARA:DHA and ARA:(EPA + DHA) (Figures 1B and 4). The strongest effect estimates were observed for 20:4n–6 (ARA), which was >1 SD lower among FADS1 homozygotes (–1.23 SD; 95% CI: –1.43, –1.05 for genotype TT compared with CC) and intermediate among heterozygotes (–0.40 SD; 95% CI: –0.53, –0.27 for genotype TC compared with CC). These associations were essentially unchanged in adjusted models (Figure 5). Mothers carrying the minor alleles of rs174575 (G>C; minor allele frequency = 5.4%) in FADS2 also had lower proportions of ARA (Figures 1C and 4). Furthermore, these mothers had higher proportions of SFAs, including 12:0 (lauric acid) and 14:0 (myristic acid); higher proportions of 20:4n–3 (eicosatetraenoic acid); and scored higher on the medium-chain fatty acid pattern (Figure 4).
For most of these genetic associations, similar results were observed using SD scores and CLR-transformed fatty acids, with one notable exception: using CLR-transformed data, we additionally observed lower proportions of EPA and DPA in mothers homozygous for rs174575 (genotype GG) in FADS2 (Supplemental Figure 3). Overall, FADS genotypes explained a substantial proportion of variance in n–6 PUFAs, particularly ARA (17% variance explained), but did not significantly contribute to the observed variance in human milk SFAs, MUFAs, or n–3 PUFAs (range: 0–5%).
Other sociodemographic, health, and environmental determinants of human milk fatty acids
In addition to dietary and genetic factors, we explored other potential determinants of human milk fatty acids, including maternal ethnicity, age, parity, education, health (BMI, gestational weight gain, atopy, gestational diabetes, or hypertensive disorders), environmental (study site, smoking, and season of milk collection), birth (delivery mode, sex, and birth weight), and lactation factors (lactation stage and breastfeeding exclusivity). These were first explored individually in unadjusted (crude) analyses (Figure 4, Supplemental Figure 3) and then considered collectively, along with FADS1 genotypes and dietary factors, in multivariable models (Figure 5, Supplemental Figure 4).
Several associations with ethnicity were observed; some were explained by ethnic variations in FADS SNPs, whereas others were independent of FADS as well as dietary factors. For example, Asian mothers had –0.47 SD (95% CI: –0.65, –0.30 SD) lower ARA proportions compared with Caucasian mothers, but this association was completely attenuated after adjusting for FADS1 genotype (Figure 5) (adjusted β: 0.01 SD; 95% CI: –0.17, 0.21 SD). By contrast, in multivariable adjusted models accounting for genetics and other factors, Asian ethnicity remained independently associated with higher DHA, ALA, and total n–3 PUFA; higher LA and total n–6 PUFA; and lower total SFA, myristic acid, margaric acid, and DGLA. Asians also had lower ARA:DHA and ARA:(EPA + DPA) ratios, scored lower on the “high n–6 PUFA” pattern, and scored higher on the “MUFA and low SFA” pattern (Figure 5). Similar associations were observed when using CLR-transformed data, although not all reached statistical significance (Supplemental Figure 4). Mothers with a postsecondary degree had higher eicosatetraenoic acid, EPA, and DPA proportions and scored higher on the “high n–3 PUFA” pattern compared with mothers without a postsecondary education (Figure 5, Supplemental Figure 4).
Independent of dietary, genetic, and socioeconomic factors, maternal weight status was associated with milk fatty acid composition. Compared with normal-weight mothers, overweight and obese mothers had +0.32 SD (95% CI: 0.16, 0.49 SD) and +0.39 SD (95% CI: 0.18, 0.60 SD) higher total SFA, respectively (Figure 5). Being overweight or obese was also positively associated with the “high medium-chain fatty acid” pattern and negatively associated with total MUFA and ARA proportions (Figure 5). Some of these associations were not replicated using CLR-transformed fatty acids (Supplemental Figure 4). In addition, using CLR-transformed fatty acids, DPA and DHA proportions were lower among obese mothers (Supplemental Figure 4).
Milk collected later in lactation had higher lauric and myristic acid and lower CLA, eicosatetraenoic acid, DGLA, and the “high n–6 PUFA” pattern (P < 0.05 in multivariable models) (Figure 5, Supplemental Figure 4). Exclusive breastfeeding at the time of milk sample collection tended to be associated with lower SFA and higher MUFA (Figure 4), but these relations were not observed in multivariable models (Figure 5). In the multivariable models, exclusive breastfeeding was associated with lower CLA, adrenic acid, and the “high medium-chain fatty acid” pattern. Using CLR-transformed data, both lactation stage and exclusive breastfeeding were independently associated with lower total n–6 PUFA (Supplemental Figure 4). No consistent associations were observed between milk fatty acids and maternal age, parity, health conditions (including atopy, asthma, gestational diabetes and hypertension, and inflammatory bowel disorders), delivery mode (cesarean compared with vaginal), breastfeeding mode (direct compared with pumping), infant birth weight, gestational age, sex, or cold symptoms (Figure 4). Using CLR-transformed data, we observed similar associations (Supplemental Figure 4).
Finally, when considering environmental exposures, some associations with smoking were observed in the unadjusted analyses (Figure 4), but these were largely explained by dietary factors and sociodemographic factors (Figure 5). Notably, in the multivariable adjusted models, mothers in Edmonton and Manitoba had lower DHA proportions compared with mothers in Toronto. In addition, higher ARA and adrenic acid proportions were observed in milk collected in winter compared with spring (Figure 5), although these seasonal patterns differed when using CLR-transformed data (Supplemental Figure 4).
Altogether, these sociodemographic and environmental factors explained 1.4–16% of variance in human milk fatty acids, with the lowest percentage for palmitoleic acid and the highest percentage for ARA:(EPA + DHA) ratio.
Discussion
In this study of 1094 mother–infant dyads, we demonstrated the interindividual variation of human milk fatty acid profiles, identified 4 human milk fatty acid patterns, and assessed the combined impact of maternal genetics and diet on milk fatty acid composition. We further identified novel associations with maternal prepregnancy BMI, education, ethnicity, and environmental factors. This evidence regarding the determinants of milk fatty acid composition has important clinical implications because fatty acids have immediate and long-term effects on infant growth, health, and neurodevelopment (34).
Human milk DHA proportions are low in the CHILD cohort compared with other populations
Previous studies of human milk have shown substantial worldwide variability for some individual fatty acids (35, 36). Across 9 countries, Yuhas et al. (36) found that SFAs and MUFAs were relatively constant, whereas large variations were seen in PUFAs, especially DHA. Our study confirms this variability in milk PUFAs and DHA at the individual level. A systematic review of 65 studies (total n = 2,474, ranging from 5 to 198 mothers per study) showed that worldwide mean DHA proportions were 0.32% ± 0.22% and mean ARA proportions were 0.47% ± 0.13% (35). In our study of 1094 Canadian mothers, the mean values of ARA (0.38% ± 0.09%) were similar to the global average, whereas mean DHA proportions (0.18% ± 0.12%) were lower by ∼50% (35), consistent with the low estimated dietary DHA intake in our population (mean: 128 mg/d; substantially below the recommended amount of 200 mg/d) (37). Notably, 69% of mothers in our study produced milk with DHA proportions <0.2%, which is the minimum proportion required in human milk substitutes according to the World Association of Perinatal Medicine's dietary guidelines (37). However, if the mothers used fish oil supplements during pregnancy and/or lactation, the average breast milk DHA proportions exceeded this minimum recommendation.
Diet and genetics are key determinants of human milk fatty acid composition
Among mothers who consumed fatty cold water fish or took fish oil supplements during pregnancy and/or lactation, we observed higher proportions of n–3 PUFAs and lower ratios of total n–6:n–3 PUFAs, ARA:DHA, and ARA:(DHA + EPA). These findings are consistent with those of a community-based intervention cohort study (38) and several randomized controlled trials in which fish oil supplementation during pregnancy or lactation (39–41) or salmon intake during pregnancy (42) increased human milk EPA and DHA proportions without affecting individual n–6 PUFA proportions, including ARA (42). In our study, when examining the CLR-transformed fatty acids, in the unadjusted analyses, fish oil supplement use was also associated with lower ARA proportions, although in the multivariable analyses, the genetic determinants had a stronger impact on ARA. Our results demonstrate the relevance of maternal fish oil intake during both pregnancy and lactation and suggest short- and long-term effects on PUFA proportions in human milk, consistent with2 different mechanistic pathways: 1) incorporation into body stores during pregnancy for later release into milk during lactation and 2) direct absorption into serum and transfer to milk during lactation.
Among other dietary variables examined, we found that total HEI-2010 score (23) was associated with lower SFAs and higher n–6 PUFA, whereas red meat intake was associated with higher margaric acid. In line with these findings, a study exploring maternal dietary patterns and human milk fatty acids among Chinese mothers showed differences in SFAs and n–6 PUFAs among the 4 dietary patterns that the researchers identified (43).
Our findings confirm previously reported associations of FADS1 and FADS2 SNPs with human milk ARA (25), explaining 17% of the variation in this n–6 PUFA. In addition, we found some novel associations between FADS variants and n–3 PUFAs, which compete with n–6 PUFAs for these desaturase enzymes. Notably, the FADS1 genotype fully explained the ethnic difference in ARA proportions between Asians and Caucasians in our multiethnic cohort. We also replicated the associations of rs174575 in FADS2 with EPA (26), although these were only apparent when using CLR transformation that accounts for the compositional nature of the milk fatty acid data. A few other associations were also observed between FADS1/2 and MUFAs (using CLR-transformed data) and SFAs, which could indicate direct metabolism by these enzymes (44) or reflect indirect effects related to overall fatty acid homeostasis.
Maternal BMI, ethnicity, and environmental factors are associated with milk fatty acid composition
Independent of FADS1 genotype and dietary factors, we observed higher SFA and lower MUFA proportions among overweight and obese mothers, in line with results from studies in South Korea (n = 238) (45) and Finland (n = 100) (46). We observed ethnic differences in fatty acid profiles independent of diet, FADS1 genotypes, and BMI, suggesting that other unmeasured genetic, sociodemographic, or lifestyle factors related to ethnicity may influence milk fatty acid composition, although it is possible that our FFQ was unable to completely capture ethnic differences in diet.
Other sociodemographic and environmental factors, including maternal education, study site, and season of milk sampling, were also associated with human milk fatty acids. These results might reflect ethnicity- or education-related differences in diet that were not captured by our adjustments for key dietary sources of PUFAs, overall diet quality, and fish oil supplement use. We also observed differences across the 4 study sites despite following a standardized protocol for milk collection and processing and analyzing all samples in the same laboratory, suggesting a possible role for other unmeasured site-specific factors, such as climate or lifestyle. Contrary to some previous studies (17, 47), we did not find any associations between milk fatty acids and infant gestational age and sex, maternal age, parity, marital status, or smoking, although we had limited power to address some of these factors. To date, few studies have examined the associations of maternal health conditions and milk fatty acids, suggesting higher milk DHA proportions among mothers with gestational hypertensive disorders and changes in SFAs during maternal infections (48, 49). We did not capture maternal infection data in the CHILD cohort, and we did not find any consistent associations between milk fatty acids and gestational hypertension or diabetes, nor any other maternal health condition we assessed (including asthma, atopy, and inflammatory bowel disorders). However, we had relatively low power to study these outcomes in our generally healthy cohort.
Overall, our study suggests that a combination of diet, genetics, sociodemographic, health (BMI), and environmental factors influence human milk fatty acid patterns. Together, these factors explained ∼25% of the variation observed in n–3 PUFAs (e.g., 26% for DHA, mainly explained by diet, sociodemographic, and environmental factors) and n–6 PUFAs (e.g., 24% for ARA, mainly explained by genetics) and ∼10% of the variation observed in SFAs (e.g., 10% for palmitic acid, mainly diet) and MUFAs (e.g., 9% for oleic acid, mainly BMI). We acknowledge that these estimates do not fully capture the contributions of genetics and diet because we only assessed 2 genetic variants and selected dietary variables. Nevertheless, they provide useful information about the relative importance of different determinants of milk fatty acid composition.
Strengths and limitations
To our knowledge, this is the largest (n = 1094) multiethnic study of human milk fatty acids in a population-based cohort. We comprehensively assessed multiple dietary, genetic, sociodemographic, health, and environmental factors, and we used statistical approaches to correct for multiple testing, reduce data dimensionality, and account for the compositional nature of our data. Using CLR-transformed data, we observed mostly similar but also some different associations. Further research is needed to explore the utility of CLR or other transformations for studying compositional fatty acid data and to compare these against absolute proportions in appropriately collected human milk samples. Limitations of our study include the use of an FFQ, which cannot accurately estimate nutrient intakes, and the reliance on dietary data collected during pregnancy, which may not accurately reflect diet during lactation (50). In addition, we lacked information on total milk fat, calories, and volume and therefore could not determine the total “dose” of milk fatty acids delivered to each infant for comparison against dietary intake recommendations. Finally, we restricted our genetic analyses to known SNPs in the FADS genes; further research is needed to identify other genetic factors influencing milk fatty acids.
In conclusion, in the large multiethnic CHILD cohort, we showed that human milk fatty acid composition is variable and independently associated with several dietary, genetic, sociodemographic, and environmental factors. Together, these factors explained ∼25% of the variation observed in n–3 PUFAs and n–6 PUFAs and ∼10% of the variation observed in SFAs and MUFAs. The contribution of diet and BMI was mainly observed for SFAs, whereas diet and sociodemographic factors contributed to n–3 PUFA variability and FADS genotypes contributed mainly to n–6 PUFA variability. Further research is needed to confirm our findings in other populations, identify additional determinants of human milk fatty acid composition, and understand their collective biological impact on the health and development of breastfed infants.
Supplementary Material
Acknowledgments
We are grateful to all the families who took part in this study and the entire CHILD team, which includes interviewers, nurses, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, and receptionists at the following institutions: McMaster University, University of Manitoba, University of Alberta, University of Toronto, and University of British Columbia. We thank Sue Goruk and Maria Giurgius (University of Alberta) for assisting with analysis of human milk FAs, Hasantha Sinnock (University of Manitoba) for assisting with data cleaning, Jihoon Choi (Queen's University) for extracting the FADS SNPs, Faisal Atakora (University of Manitoba) for providing guidance on statistical analyses and figures, and Sonia Anand and Guillaume Pare for deriving the genotypes used in this article.
The authors’ responsibilities were as follows—KM and MBA: designed and managed the project; TJM, ABB, PJM, SET, MRS, and PS: conceived the CHILD cohort design, managed study recruitment, and oversaw clinical assessments of study participants; DLL: managed the CHILD study database; CJF: oversaw and performed FA analysis of human milk samples; QLD: conducted the genome-wide association study in the CHILD cohort; KM: conducted all the statistical analyses: KM and MBA: interpreted the data and wrote the manuscript: and all authors: provided feedback and read and approved the final manuscript. KM and MBA have full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest related to the study.
Notes
This research was funded by the Manitoba Medical Service Foundation and the Children's Hospital Foundation of Manitoba. The Canadian Institutes of Health Research (CIHR) and the Allergy, Genes and Environment (AllerGen) Network provided core funding for the CHILD Cohort Study. KM received funding from the Canadian Lung Association. This research was supported in part by the Canada Research Chairs Program. MBA holds a Canada Research Chair in the Developmental Origins of Chronic Disease. MRS holds the AstraZeneca chair in Respiratory Epidemiology at McMaster University. These entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supplemental Figures 1–4 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ALA, α-linoleic acid; ARA, arachidonic acid; CLA, conjugated linoleic acid; CLR, centered log ratio; DGLA, dihomo-γ-linoleic acid; DPA, docosapentaenoic acid; FADS, fatty acid desaturase; GLA, γ-linolenic acid; HEI-2010, Healthy Eating Index 2010; LA, linoleic acid; LC-PUFA, long-chain PUFA; PCA, principal component analysis; SNP, single nucleotide polymorphism.
References
- 1. Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–91. [DOI] [PubMed] [Google Scholar]
- 2. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Beer M, Vrijkotte TG, Fall CH, van Eijsden M, Osmond C, Gemke RJ. Associations of infant feeding and timing of linear growth and relative weight gain during early life with childhood body composition. Int J Obes (Lond). 2015;39(4):586–92. [DOI] [PubMed] [Google Scholar]
- 4. Giugliani ER, Horta BL, Loret de Mola C, Lisboa BO, Victora CG. Effect of breastfeeding promotion interventions on child growth: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):20–9. [DOI] [PubMed] [Google Scholar]
- 5. Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162(5):397–403. [DOI] [PubMed] [Google Scholar]
- 6. Victora CG, Barros F, Lima RC, Horta BL, Wells J. Anthropometry and body composition of 18 year old men according to duration of breast feeding: birth cohort study from Brazil. BMJ. 2003;327(7420):901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durmus B, van Rossem L, Duijts L, Arends LR, Raat H, Moll HA, Hofman A, Steegers EAP, Jaddoe VWV. Breast-feeding and growth in children until the age of 3 years: the Generation R study. Br J Nutr. 2011;105(11):1704–11. [DOI] [PubMed] [Google Scholar]
- 8. Koletzko B, Rodriguez-Palmero M, Demmelmair H, Fidler N, Jensen R, Sauerwald T. Physiological aspects of human milk lipids. Early Hum Dev. 2001;65(Suppl):S3–18. [DOI] [PubMed] [Google Scholar]
- 9. Koletzko B, Rodriguez-Palmero M. Polyunsaturated fatty acids in human milk and their role in early infant development. J Mammary Gland Biol Neoplasia. 1999;4(3):269–84. [DOI] [PubMed] [Google Scholar]
- 10. Much D, Brunner S, Vollhardt C, Schmid D, Sedlmeier EM, Bruderl M, Heimberg E, Bartke N, Boehm G, Bader BL et al.. Breast milk fatty acid profile in relation to infant growth and body composition: results from the INFAT study. Pediatr Res. 2013;74(2):230–7. [DOI] [PubMed] [Google Scholar]
- 11. Lassek WD, Gaulin SJ. Maternal milk DHA content predicts cognitive performance in a sample of 28 nations. Matern Child Nutr. 2015;11(4):773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauritzen L, Brambilla P, Mazzocchi A, Harslof LB, Ciappolino V, Agostoni C. DHA effects in brain development and function. Nutrients. 2016;8(1):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Innis SM. Maternal nutrition, genetics, and human milk lipids. Curr Nutr Rep. 2013;2(3):151–8. [Google Scholar]
- 14. Del Prado M, Villalpando S, Elizondo A, Rodriguez M, Demmelmair H, Koletzko B. Contribution of dietary and newly formed arachidonic acid to human milk lipids in women eating a low-fat diet. Am J Clin Nutr. 2001;74(2):242–7. [DOI] [PubMed] [Google Scholar]
- 15. Lattka E, Illig T, Koletzko B, Heinrich J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr Opin Lipidol. 2010;21(1):64–9. [DOI] [PubMed] [Google Scholar]
- 16. Lattka E, Illig T, Heinrich J, Koletzko B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes?. Clin Nutr. 2010;29(3):277–87. [DOI] [PubMed] [Google Scholar]
- 17. Bokor S, Koletzko B, Decsi T. Systematic review of fatty acid composition of human milk from mothers of preterm compared to full-term infants. Ann Nutr Metab. 2007;51(6):550–6. [DOI] [PubMed] [Google Scholar]
- 18. Voortman T, Tielemans MJ, Stroobant W, Schoufour JD, Kiefte-de Jong JC, Steenweg-de Graaff J, van den Hooven EH, Tiemeier H, Jaddoe VWV, Franco OH. Plasma fatty acid patterns during pregnancy and child's growth, body composition, and cardiometabolic health: the Generation R study. Clin Nutr. 2017; 37(3):984–92. [DOI] [PubMed] [Google Scholar]
- 19. Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, Denburg JA, HayGlass KT, Kobor MS, Kollmann TR et al.. The Canadian Healthy Infant Longitudinal Development (CHILD) study: examining developmental origins of allergy and asthma. Thorax. 2015;70(10):998–1000. [DOI] [PubMed] [Google Scholar]
- 20. Azad MB, Robertson B, Atakora F, Becker AB, Subbarao P, Moraes TJ, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR et al.. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J Nutr. 2018;148(11):1733–42. [DOI] [PubMed] [Google Scholar]
- 21. Fred Hutchinson Cancer Research Center. Food frequency questionnaires. Available from: https://sharedresources.fredhutch.org/services/food-frequency-questionnaires-ffq.
- 22. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S.; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 23. Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2015;526:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mychaleckyj JC, Nayak U, Colgate ER, Zhang D, Carstensen T, Ahmed S, Ahmed T, Mentzer AJ, Alam M, Kirkpatrick BD et al.. Multiplex genomewide association analysis of breast milk fatty acid composition extends the phenotypic association and potential selection of FADS1 variants to arachidonic acid, a critical infant micronutrient. J Med Genet. 2018;55(7):459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n–6) and (n–3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr. 2008;138(11):2222–8. [DOI] [PubMed] [Google Scholar]
- 27. Glaser C, Heinrich J, Koletzko B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism. 2010;59(7):993–9. [DOI] [PubMed] [Google Scholar]
- 28. Moraes TJ, Lefebvre DL, Chooniedass R, Becker AB, Brook JR, Denburg J, HayGlass KT, Hegele RG, Kollmann TR, Macri J et al.. The Canadian Healthy Infant Longitudinal Development birth cohort study: biological samples and biobanking. Paediatr Perinat Epidemiol. 2015;29(1):84–92. [DOI] [PubMed] [Google Scholar]
- 29. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 30. Cruz-Hernandez C, Goeuriot S, Giuffrida F, Thakkar SK, Destaillats F. Direct quantification of fatty acids in human milk by gas chromatography. J Chromatogr A. 2013;1284:174–9. [DOI] [PubMed] [Google Scholar]
- 31. Abdi H, Williams LJ. Principal component analysis. Wiley Interdiscip Rev Comput Stat. 2010;2(4):433–59. [Google Scholar]
- 32. Logan CA, Brandt S, Wabitsch M, Brenner H, Wiens F, Stahl B, Marosvolgyi T, Decsi T, Rothenbacher D, Genuneit J. New approach shows no association between maternal milk fatty acid composition and childhood wheeze or asthma. Allergy. 2017;72(9):1374–83. [DOI] [PubMed] [Google Scholar]
- 33. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 34. Innis SM. Polyunsaturated fatty acids in human milk: an essential role in infant development. Adv Exp Med Biol. 2004;554:27–43. [DOI] [PubMed] [Google Scholar]
- 35. Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85(6):1457–64. [DOI] [PubMed] [Google Scholar]
- 36. Yuhas R, Pramuk K, Lien EL. Human milk fatty acid composition from nine countries varies most in DHA. Lipids. 2006;41(9):851–8. [DOI] [PubMed] [Google Scholar]
- 37. Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S et al.. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36(1):5–14. [DOI] [PubMed] [Google Scholar]
- 38. Juber BA, Jackson KH, Johnson KB, Harris WS, Baack ML. Breast milk DHA levels may increase after informing women: a community-based cohort study from South Dakota USA. Int Breastfeed J. 2016;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dunstan JA, Roper J, Mitoulas L, Hartmann PE, Simmer K, Prescott SL. The effect of supplementation with fish oil during pregnancy on breast milk immunoglobulin A, soluble CD14, cytokine levels and fatty acid composition. Clin Exp Allergy. 2004;34(8):1237–42. [DOI] [PubMed] [Google Scholar]
- 40. Makrides M, Neumann MA, Gibson RA. Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. Eur J Clin Nutr. 1996;50(6):352–7. [PubMed] [Google Scholar]
- 41. Boris J, Jensen B, Salvig JD, Secher NJ, Olsen SF. A randomized controlled trial of the effect of fish oil supplementation in late pregnancy and early lactation on the n–3 fatty acid content in human breast milk. Lipids. 2004;39(12):1191–6. [DOI] [PubMed] [Google Scholar]
- 42. Urwin HJ, Miles EA, Noakes PS, Kremmyda LS, Vlachava M, Diaper ND, Perez-Cano FJ, Godfrey KM, Calder PC, Yaqoob P. Salmon consumption during pregnancy alters fatty acid composition and secretory IgA concentration in human breast milk. J Nutr. 2012;142(8):1603–10. [DOI] [PubMed] [Google Scholar]
- 43. Tian HM, Wu YX, Lin YQ, Chen XY, Yu M, Lu T, Xie L. Dietary patterns affect maternal macronutrient intake levels and the fatty acid profile of breast milk in lactating Chinese mothers. Nutrition. 2018;58:83–8. [DOI] [PubMed] [Google Scholar]
- 44. Guillou H, Rioux V, Catheline D, Thibault JN, Bouriel M, Jan S, D'Andrea S, Legrand P. Conversion of hexadecanoic acid to hexadecenoic acid by rat Δ6-desaturase. J Lipid Res. 2003;44(3):450–4. [DOI] [PubMed] [Google Scholar]
- 45. Kim H, Kang S, Jung BM, Yi H, Jung JA, Chang N. Breast milk fatty acid composition and fatty acid intake of lactating mothers in South Korea. Br J Nutr. 2017;117(4):556–61. [DOI] [PubMed] [Google Scholar]
- 46. Makela J, Linderborg K, Niinikoski H, Yang B, Lagstrom H. Breast milk fatty acid composition differs between overweight and normal weight women: the STEPS study. Eur J Nutr. 2013;52(2):727–35. [DOI] [PubMed] [Google Scholar]
- 47. Hahn WH, Song JH, Song S, Kang NM. Do gender and birth height of infant affect calorie of human milk? An association study between human milk macronutrient and various birth factors. J Matern Fetal Neonatal Med. 2017;30(13):1608–12. [DOI] [PubMed] [Google Scholar]
- 48. Dangat KD, Mehendale SS, Yadav HR, Kilari AS, Kulkarni AV, Taralekar VS, Joshi SR. Long-chain polyunsaturated fatty acid composition of breast milk in pre-eclamptic mothers. Neonatology. 2010;97(3):190–4. [DOI] [PubMed] [Google Scholar]
- 49. Gardner AS, Rahman IA, Lai CT, Hepworth A, Trengove N, Hartmann PE, Geddes DT. Changes in fatty acid composition of human milk in response to cold-like symptoms in the lactating mother and infant. Nutrients. 2017;9(9):E1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jia X, Pakseresht M, Wattar N, Wildgrube J, Sontag S, Andrews M, Subhan FB, McCargar L, Field CJ. Women who take n–3 long-chain polyunsaturated fatty acid supplements during pregnancy and lactation meet the recommended intake. Appl Physiol Nutr Metab. 2015;40(5):474–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.