Abstract
Human endometrium has a key role in implantation process. The measurement of endometrial thickness is the most commonly used in clinical practice. Managing patients with thin endometrium still represents a major challenge for clinicians. The objective of this systematic review was to investigate all available interventions to improve endometrial thickness (EMT) in women with history of thin endometrium undergoing fresh or frozen-thawed embryo transfers (ET). We performed a comprehensive search of relevant studies from January 1978 to February 2018. The different strategies were categorized as hormonal, vascular, and growth factor approaches and specifically analyzed according to the type of ET. Thirty-one studies were included. Overall, quality of the evidence ranged from very low to moderate, with only few randomized controlled trials that support the use of either GnRH analogues in fresh ET or sildenafil in frozen ET for enhancing endometrial growth. Besides, intensified estradiol administration is a common approach that might improve EMT in frozen ET. The present review evidences the paucity of reliable data regarding the efficiency of different interventions aiming at increasing EMT before fresh or frozen-thawed ET. Robust and high-quality randomized controlled trials are still needed before guidelines can be established.
Keywords: Endometrial thickness, Embryo transfer, Pregnancy, In vitro fertilization
Introduction
Since assisted reproductive technology (ART) has been developed, clinicians and researchers aimed to improve their procedures with a unique goal: increasing the live birth rate. Endometrial receptivity is a critical feature for pregnancy achievement. How to characterize optimal endometrium before embryo replacement remains an open question [1]. Several tools for endometrial evaluation have been investigated [2]; among them, ultrasound assessment of endometrial thickness (EMT) is widely used as a routine prognosis factor for pregnancy. Thin endometrium not only implicates lower pregnancy rate [3, 4] but also seems to be associated with adverse perinatal outcomes [5], miscarriages [6], or abnormal placentation [7]. EMT below 7 mm is the most frequently reported cutoff to define a thin endometrium [8–12]. The prevalence of thin endometrium varies across published studies: it ranges from 2.4% [12] to 8.5% [13]. Several therapeutic alternatives have been explored to improve EMT and subsequent chances of live birth, but thin endometrium remains a challenging situation for clinicians.
We report in this systematic review all interventions that have been investigated specifically in women with thin endometrium undergoing either a fresh or frozen-thawed ET. The treatment options were classified in three main approaches, corresponding to physiological pathways or mechanisms involved in normal endometrial growth: (i) “hormonal” treatments, (ii) “vascular” treatments, and (iii) “growth factor” treatments.
Material and methods
Literature search
In order to embrace an overview of the present literature about thin endometrium, a first exhaustive search on thin endometrium was achieved over a 10-year period (January 2007 to May 2017). Secondly, a complementary search focused on therapeutic strategies for thin endometrium over a 40-year period (January 1978 to February 2018) to answer our question about EMT improvement in thin endometrium population during an ET cycle.
Studies were identified by searching multiple literature databases, including PubMed, Embase, and Cochrane Library, and clinical trial registers (ClinicalTrials.gov and EudraCT). The searches were limited to publications in English involving human subjects.
For the first search, a combination of Medical Subject Headings (MeSH) and text words was used to generate two subsets of citations, one including studies involving thin endometrium and the second including studies of in vitro fertilization, intracytoplasmic sperm injection, intrauterine insemination, and embryo transfer. The two subsets of citations were then combined with “AND” generating a set of citations relevant to the research question. Strategies for electronic search of database were the following combined search terms: ((“embryo transfer” [MeSH Terms] OR (“embryo” [All Fields] AND “transfer” [All Fields]) OR “embryo transfer” [All Fields]) OR (“iui” [All Fields] OR (intrauterine [All Fields] AND (“insemination” [MeSH Terms] OR “insemination” [All Fields]))) OR (“in vitro fertilisation” [All Fields] OR (“fertilisation” [All Fields] AND “vitro” [All Fields]) OR “fertilisation in vitro” [All Fields] OR (“vitro” [All Fields] AND “fertilisation” [All Fields]) OR “fertilization in vitro” [MeSH Terms] OR (“fertilization” [All Fields] AND “vitro” [All Fields]) OR “fertilization in vitro” [All Fields] OR (“vitro” [All Fields] AND “fertilization” [All Fields]) OR “in vitro fertilization” [All Fields] OR “ivf” [All Fields]) OR (“sperm injections, intracytoplasmic” [MeSH Terms] OR (“sperm” [All Fields] AND “injections” [All Fields] AND “intracytoplasmic” [All Fields]) OR “intracytoplasmic sperm injections” [All Fields] OR (“sperm” [All Fields] AND “injections” [All Fields] AND “intracytoplasmic” [All Fields]) OR “sperm injections, intracytoplasmic” [All Fields] OR “icsi” [All Fields])) AND ((Thin [All Fields] AND (“endometrium” [MeSH Terms] OR “endometrium” [All Fields])) OR ((“endometrium” [MeSH Terms] OR “endometrium” [All Fields]) AND thickness [All Fields]) OR (endometrial [All Fields] AND thickness [All Fields])).
A targeted search on therapeutic strategies was carried out using the first search strategy combined with the following search terms (and their variants): “therapeutics,” “granulocyte colony-stimulating factor,” “aspirin,” “human chorionic gonadotropin,” “sildenafil citrate,” “oestrogen,” “antioxidant,” “vitamin E,” “alpha-tocopherol,” “pentoxifylline,” and “nifedipine.”
Study selection
Abstracts of scientific meetings and conference proceedings were not considered. Two authors (T.A. and M.G.) performed an initial screening of the title and abstract of all articles and clinical studies to exclude citations deemed irrelevant by both observers. The full texts of potentially relevant articles were retrieved and assessed for inclusion by three review authors (J.R., C.S., and N.R.). Any disagreement or uncertainty was resolved by discussion among reviewers to reach a consensus. The quality of studies was judged using a modified version of the grid reading of the French National Authority for Health. The grid was based on three major criteria: selection of subjects, comparability of study groups, and assessment of EMT. Studies included in our systematic review met 3 mandatory criteria: (i) patients included in the study had a present or past history of thin endometrium; (ii) patients included in the study were planned for an ET; and (iii) a treatment aiming at improving endometrial thickness was assessed.
Data extraction
Data were extracted from included articles by all five reviewers, using a data extraction form developed for the present review. No investigator was contacted in case of missing or obscure data. The following data were extracted: authors, year of publication, title, study design, definition of thin endometrium, inclusion criteria, treatment, mode of administration, adjuvant treatment, number of subjects in each group, main outcome, secondary outcomes, results in each group, and conclusion.
Results
Study selection and characteristics
In total, 1268 articles were collected from the PubMed (n = 625), Embase (n = 322), and Cochrane (n = 321) databases. Then, after duplicates and congress abstract removal, 865 abstracts were screened. Full texts of 151 studies were assessed in details. One hundred sixteen studies did not reach inclusion criteria and 4 were either reviews or meta-analysis. Thirty-one studies were finally included in this systematic review. Fifty-six studies recorded in ClinicalTrial.gov and EudraCT were retrieved but were not included in final analysis due to missing conclusive data (Fig. 1).
Fig. 1.
Flow diagram of study selection
Selected studies were carefully analyzed and subsequently classified according to the mechanism proposed to overcome thin endometrium.
Hormonal approach
Hormones, especially estrogen, play a key role during follicular phase for proper endometrial growth [14, 15]. Hence, several hormonal strategies have been promoted to overcome thin endometrium. Eight studies had tested hormonal treatments such as estradiol administration adjustment [7, 16–19], hCG priming in the follicular phase [20, 21], or GnRH agonist during luteal phase [22]. Baseline characteristics of included studies are reported in Table 1 and treatment protocols are illustrated in Fig. 2.
Table 1.
Baseline characteristics of studies included in hormonal approaches
| Case | Control | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Design | EMT cutoff (mm) | n | Control | n | EMT Outcomes | Case (mm) | Controls (mm) | p |
| Estradiol: frozen embryo transfer (HRT) | |||||||||
| Dmowski et al. 1997 | Prospective historical control | < 10 | 13 | Self-controlled with different estradiol regimens | 10 | Max EMT prior to pg admin | 11.7 ± 1.8 | 8.7 ± 1.5 | < 0.0001 |
| Check et al. 2004 | Prospective randomized (2 treatments) | < 8 | 9 | Sildenafil, 25 mg four times daily, from D3 to D9 of HRT | 7 | Mean EMT | 7.14 ± 0.26 | 6.44 ± 0.38 | ns |
| Chen MJ et al., 2006 | Prospective, not randomized, controlled study | < 8 | 10 | Self-controlled with fresh embryo transfer with thin EMT | 23 | Mean EMT | 8.7 ± 0.9 | 6.9 ± 0.06 | 0.031 |
| Zolghadri et al. 2014 | Prospective, randomized, controlled | < 3 | 30 | 20 μg vaginal tablet | 30 | EMT d16 of HRT | 5.93 ± 0.38 | 6.74 ± 0.32 | < 0.001 |
| Liao et al. 2014 | Retrospective cohort study | < 8 | 178 | Oral estradiol valerate tablet (2 to 8 mg) | 69 | EMT increase | 1.9 ± 1.1 | 1.5 ± 0.9 | < 0.01 |
| hCG: frozen embryo transfer (HRT) | |||||||||
| Papanikolaou et al. 2013 | Prospective not randomized self-controlled | < 6 | 17 | Same patients, preceding treatment cycle | 17 | EMT | 6.0 (4.2–8.6) | 5.2 (3.4–6.0) | 0.008 |
| Davar et al., 2016 | Prospective, not controlled, cohort study | < 7 | 28 | NA | NA | EMT after hCG | 7.85 ± 0.52 | NA | NA |
| GnRH agonist, fresh embryo transfer | |||||||||
| Qublan et al. 2008 | Prospective randomized, controlled | < 7 | 60 | Placebo | 60 | EMT after treatment | 8.92 ± 1.6 | 7.12 ± 0.45 | < 0.01 |
EMT, endometrial thickness; HRT, hormonal replacement therapy; NA, non-available; ns, not significant
Fig. 2.
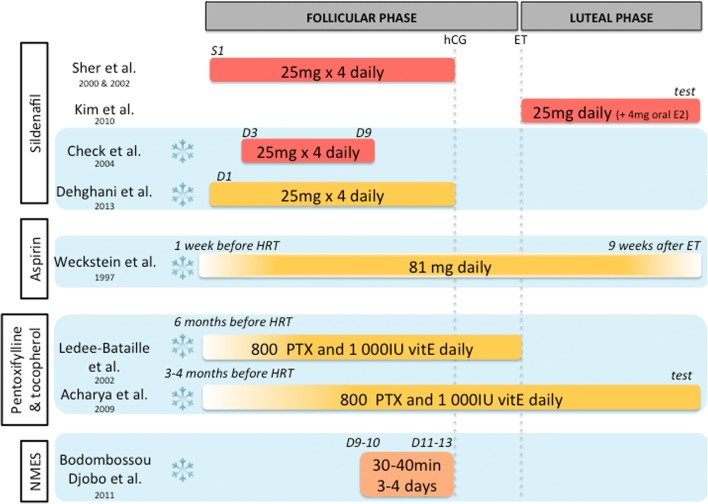
Hormonal therapeutic options for thin endometrium before an embryo transfer. Blue background with frozen flake identifies FET cycle. Timing for treatment introduction and ending is specified (D, day; i.e., D2, 2nd day of menstrual cycle). Administration route is represented: subcutaneous in yellow, vaginal in red, and oral in dark yellow. ET, embryo transfer; OR, oocyte retrieval
Estradiol administration adjustment
Prior to ovulation, estrogen plays an essential role in endometrial regeneration and growth [14]. Three strategies were studied in thin endometrium population during HRT to enhance EMT:
Increase in estradiol administration duration and dose. A prospective trial reported a significant EMT increase after an extended estrogen therapy for 14 to 82 days (mean value 30 days), with significantly higher pregnancy rate than in the control group (38.5% vs. 4.3%, P = 0.016) [7]. Most patients had a thin endometrium after an endometrial curettage or uterine surgery.
Change in administration route. While oral route for estradiol administration is widely prescribed owing to its simple and well-tolerated use, parenteral routes may increase estradiol (E2) serum concentration through bypassing of first-pass hepatic metabolism [23]. In particular, vaginal route results in higher serum E2 concentrations and its greater efficiency of E2 delivery to the endometrium makes it a good option when achieving adequate EMT with oral route is challenging [24]. A retrospective study compared extended oral estrogen administration and additional vaginal estrogen administration in inadequate endometrial patients on day 13 of HRT [17]. Vaginal E2 administration was associated with higher increase in EMT but failed to show improvements in pregnancy rates. However, a prospective trial using 4 mg of additional E2 given vaginally did not observe improvement in EMT [16]. Pregnancy outcomes were not reported. Besides, the use of subcutaneous E2 pellets in donor oocyte recipients with inadequate endometrial response was shown to significantly increase EMT in a shorter period (13.1 ± 3.9 days versus 21.1 ± 4.9 days, p < 0.001) [18]. However, these results need to be interpreted with caution as thin endometrium cutoff was set to 10 mm in this study. To the best of our knowledge, there was no additional study comparing other administration route (transdermal, intramuscular) for estradiol in patients with thin endometrium.
Modification of molecule type. A randomized controlled trial (RCT) compared the effects of vaginal synthetic estrogen (Vagifem®) and vaginal natural estrogen (Premarin®) on the EMT of patients with refractive endometrium. EMT was measured on the 14th day of drug administration. EMT in the synthetic estrogen Vagifem group was significantly higher than that in the natural estrogen Premarin group [19].
hCG priming in the follicular phase
hCG is a glycopeptide secreted by syncitiotrophoblasts from the implanting conceptus to support progesterone production by the corpus luteum. hCG has numerous local and systemic functions; it appears to be fundamental in the initial dialogue between the endometrium and the blastocyst during the peri-implantation period [25, 26]. Nonetheless, hCG/LH receptors have been observed as early as the follicular phase in the endometrial epithelium and might modulate endometrial differentiation and angiogenesis [27]. Based on these findings, subcutaneous hCG injection during the follicular phase of HRT cycles was evaluated in patients with repeated thin endometrium. A significant increase in EMT was reported, with 17% of treated patients reaching an EMT above 7 mm. Nine out of 17 patients, with at least two prior implantation failures with top embryos, were pregnant, and 7 finally delivered [20]. Similarly, a prospective non-controlled study included women with previous failed IVF cycles due to thin endometrium and candidate for frozen-thawed ET with HRT. A significant increase in EMT after hCG priming was observed and 5 patients out of 28 were pregnant [21]. However, this needs to be interpreted with caution as hCG during follicular phase at higher doses has also been shown to adversely affect EMT and receptivity in oocyte recipients with normal EMT [28] and is thus not recommended [29].
GnRH agonists in the luteal phase
GnRH agonists are synthetic peptides that are structurally analogous to natural GnRH, released on a pulsatile mode by the hypothalamus. When chronically administrated, they inhibit normal pituitary-gonadal function and are widely prescribed in daily reproductive medicine practice. As GnRH agonist use in the luteal phase has been suggested to improve embryonic developmental potential, the impact of a single administration of GnRH agonist at the time of implantation in oocyte recipients was explored and reported improved implantation rate and live birth rate [30]. However, the methodology of subsequent studies on the topic is heterogeneous; thus, the addition of GnRH agonist to progesterone for luteal support seems promising but cannot be recommended in the general population [31, 32]. Several GnRH analogue injections during luteal phase for patients with a thin endometrium at the time of oocyte pick-up significantly improved implantation and clinical pregnancy rates (22/60 versus 8/60 in the control group, p < 0.01). Furthermore, treated patients from this RCT displayed a significant increase in EMT compared with the placebo group [22].
“Vascular” approach
Several treatments improving uterine blood flow have been suggested for thin endometrium management. In fact, high blood flow impedance of uterine radial artery was reported in thin endometrium [33]. One could hence expect to improve EMT by increasing uterine blood flow. Nine studies had focused on a vascular approach and proposed treatment of thin endometrium with sildenafil citrate [16, 34–37], aspirin [38], pentoxifylline-tocopherol [39, 40], or electrostimulation [41]. Baseline characteristics of included studies are reported in Table 2, and treatment protocols are illustrated in Fig. 3.
Table 2.
Baseline characteristics of studies included in vascular approaches
| Case | Control | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Design | EMT cutoff (mm) | n | Control | n | EMT outcomes | Case (mm) | Controls (mm) | p |
| Sildenafil: fresh embryo transfer | |||||||||
| Sher and Fisch 2000 | Prospective, cross-over self-controlled | < 8 | 4 | Previous cycle (self-control) | 4 | EMT | 11, 10, 12, 8 | NA | |
| Sher and Fisch 2002 | Retrospective cohort analysis | < 9 | 105 | NA | NA | EMT |
73/105 (EMT ≥ 9) 32/105 (EMT < 9) |
NA | NA |
| Kim 2010 | Prospective, randomized, controlled | < 8 | 21 | No additional treatment | 27 | EMT | 6.8 ± 1.4 | 7.4 ± 1.4 | ns |
| Sildenafil: frozen embryo transfer (HRT) | |||||||||
| Check et al. 2004 | Prospective, randomized, controlled | < 8 | 9 | 2 mg estradiol twice daily from Day 2 | 7 | EMT | 6.44 ± 0.38 | 7.14 ± 0.26 | ns |
| Dehghani et al. 2013 | Prospective, randomized, controlled | < 8 | 40 | HRT only | 40 | EMT | 9.8 | 8.0 | p < 0.0001 |
| Aspirin: frozen embryo transfer (HRT) | |||||||||
| Weckstein et al. 1997 | Prospective, randomized, controlled | < 8 | 15 | HRT only | 13 | EMT increase | 1.6 ± 1.15 | 0.9 ± 0.8 | ns |
| Pentoxifylline and tocopherol: frozen embryo transfer (HRT) | |||||||||
| Lédée-Bataille et al. 2002 | Prospective, not controlled | < 7 | 18 | NA | NA | EMT increase | 1.3 ± 1 | NA | |
| Acharya et al. 2009 | Retrospective analysis | < 6 | 20 | NA | NA | EMT before and after treatment | 4.37 ± 1.5 and 6.05 ± 1.83 | NA | < 0.001 |
| Neuromuscular electrical stimulation and biofeedback therapy: frozen embryo transfer (HRT) | |||||||||
| Bodombossou-Djobo et al. 2011 | Prospective cohort study | < 7 | 20 | Refused treatment | 21 | EMT | 7.93 ± 1.42 | 6.78 ± 0.47 | 0.002 |
EMT, endometrial thickness; HRT, hormonal replacement therapy; NA, non-available; ns, not significant
Fig. 3.
Vascular therapeutic options for thin endometrium before an embryo transfer. Blue background with frozen flake identifies FET cycle. Timing for treatment introduction and ending is specified (D, day; S, stimulation day; i.e., D1, 1st day of menstrual cycle). Administration route is represented: vaginal in red, oral in dark yellow, and electrostimulation in orange. ET, embryo transfer; HRT, hormonal replacement therapy
Sildenafil
Sildenafil citrate is a selective phosphodiesterase type 5 inhibitor that enhances nitric oxide–mediated vasodilatation by blocking cGMP hydrolysis. Sher and Fisch first published the use of sildenafil to treat patients with recurrent thin endometrium, undergoing a fresh embryo transfer. They observed an improvement in uterine blood flow and in EMT [34]. Such result was confirmed in a larger cohort study a few years later, with 45% of pregnancy rate [35]. Somehow, previous endometritis was observed to decrease the response to sildenafil [35]. Three RCT [16, 36, 37] investigated the potential benefit of sildenafil on endometrial growth and subsequent pregnancy with conflicting results. A single RCT reported no significant improvement in EMT and pregnancy rate after sildenafil and valerate estradiol administration during the luteal phase following fresh ET [37], unlike Sher and Fisch who administrated sildenafil during the follicular phase and until ovulation trigger. Similarly, in HRT cycles, a second RCT failed to observe any improvement in EMT nor uterine blood flow [16]. At least, a third RCT including a larger number of HRT cycles reported enhanced EMT with triple-line endometrial pattern in 77.5% of patients versus 30% in the control group (p < 0.001). In addition, chemical pregnancy rate was higher in the sildenafil group, although not significantly [36].
Aspirin
Low-dose aspirin is believed to improve uterine blood flow [42]. Although no benefit has been shown in unselected IVF patients [43, 44], low-dose aspirin administration could be helpful for patients with thin endometrium. Indeed, a RCT explored the effect of aspirin in oocyte recipients with refractory endometrium receiving HRT [38]. Patients treated with aspirin showed significantly higher implantation rate (24% versus 9%, p < 0.05). However, this was not associated with a demonstrable increase in EMT.
Pentoxifylline and tocopherol
Pentoxifylline is a non-specific phosphodiesterase inhibitor with vasodilating effect and that improves the flow properties of blood by decreasing its viscosity. Alpha-tocopherol (or vitamin E) is an antioxidant reinforcing pentoxifylline action when administrated together and acting as well as a vasodilator itself. Two observational studies with a small number of patients explored the impact of the association of pentoxifylline and tocopherol during 3 to 6 months [39, 40] and reported improved EMT and pregnancies [39, 40].
Neuromuscular electrical stimulation and biofeedback therapy
Pelvic floor neuromuscular electrical stimulation (NMES) is commonly used in urology and gynecology and obstetrics during pregnancy and post-partum period, but not in reproductive medicine. A prospective cohort study suggested that repeated NMES could be applied to patients with refractory endometrium undergoing an HRT cycle for a frozen ET, as it significantly increased EMT [41]. Stimulating uterine smooth muscle contraction might enhance uterine blood flow, leading to improved peripheral tissue trophicity.
Growth factor approach
Various growth factors are involved in endometrial growth. The past decade has been marked by a growing interest in their use in reproductive medicine [45]. Hence, 19 studies proposed the use of intrauterine instillation of growth factor treatments such as G-CSF [46–58] or platelet-rich plasma [59–61]. Baseline characteristics of included studies are reported in Table 3 and treatment protocols are illustrated in Fig. 4.
Table 3.
Baseline characteristics of studies included in growth factor approaches
| Case | Control | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Design | EMT cutoff (mm) | n | Control | n | EMT outcomes | Case (mm) | Controls (mm) | p |
| G-CSF: fresh embryo transfer | |||||||||
| Gleicher et al. 2013 | Prospective, not controlled, cohort study | < 7 | 21 | NA | NA | EMT on ET day | 9.3 ± 2.1 | NA | NA |
| Kunicki et al. 2014 | Prospective, not controlled, cohort study | < 7 | 37 | NA | NA | EMT 72 h after G-CSF | 8.42 ± 1.7 | NA | NA |
| Tehraninejad et al. 2015 | Prospective, not randomized, self-controlled | < 6 | 15 | Preceding cycle canceled for thin endometrium | 15 | EMT 5 days after GCSF | 7.12 ± 0.84 | 5.18 ± 0.41 | NA |
| Lee et al. 2016 | Prospective, not controlled, cohort study | < 8 | 50 | NA | NA | EMT on hCG day and on ET day | From 7.2 ± 0.6 to 8.5 ± 1.5 | NA | NA |
| Sarvi et al. 2017 | Prospective, controlled, randomized | < 6 | 15 | Placebo on the same day | 13 | EMT on dET | 9.1 ± 1.5 | 6.9 ± 1.1 | < 0.001 |
| G-CSF: frozen embryo transfer | |||||||||
| Gleicher et al. 2011 | Prospective, not controlled, case reports | < 7 | 4 | NA | NA | EMT on ET day | 10.2/7.3/9.1/8.1 | NA | NA |
| Li et al., 2014 |
Retrospective, case/control (cycles) |
≤ 7 | 40 | Refused G-CSF | 80 | EMT after G-CSF | 6.51 ± 0.6 | 6.26 ± 1.4 | ns |
| Shah et al. 2014 | Prospective, not controlled, cohort study | < 8 | 117 | NA | NA | EMT increase | 3.24 ± 0.86 | NA | NA |
| Eftekhar et al. 2014 | Prospective, not randomized, controlled | < 7 | 34 | Refused G-CSF | 34 | EMT on day of progesterone | 7.91 ± 0.55 | 8.23 ± 0.82 | 0,1 |
| Xu et al. 2015 | Prospective retrospective control group | < 7 | 27 | FET despite thin EMT | 52 | EMT | 8.4 ± 2 | 6.5 ± 0.5 | NA |
| Mishra et al. 2016 | Prospective, not controlled, cohort study | < 7 | 35 | NA | NA | EMT | 6.58 ± 0.84 | NA | NA |
| Check et al. 2016 | Prospective, not controlled, case reports | < 5 | 3 | NA | NA | EMT > 6 mm | 0% | NA | NA |
| Kunicki et al. 2017 | Prospective, not randomized, controlled | < 7 | 29 | Refused G-CSF | 33 | EMT after G-CSF | 7.90 (6.5–8.7) | 6.9 (6.0–7.7) | 0.01 |
| Platelet rich plasma: frozen embryo transfer (HRT) | |||||||||
| Chang et al. 2015 | Prospective, not controlled, cohort study | < 7 | 5 | NA | NA | Nb of patients with EMT > 7 mm after infusion | 5/5 | ||
| Zadehmodarres et al. 2017 | Prospective, not controlled, cohort study | < 7 | 10 | NA | NA | Nb of patients with EMT > 7 mm after infusion | 10/10 | ||
| Tandulwadkar et al. 2017 | Prospective, not controlled, cohort study | < 7 | 68 | NA | NA | Mean EMT before/after infusion | 5 mm/7.2 mm | ||
EMT, endometrial thickness; HRT, hormonal replacement therapy; Nb, number; NA, non-available; ns, not significant
Fig. 4.
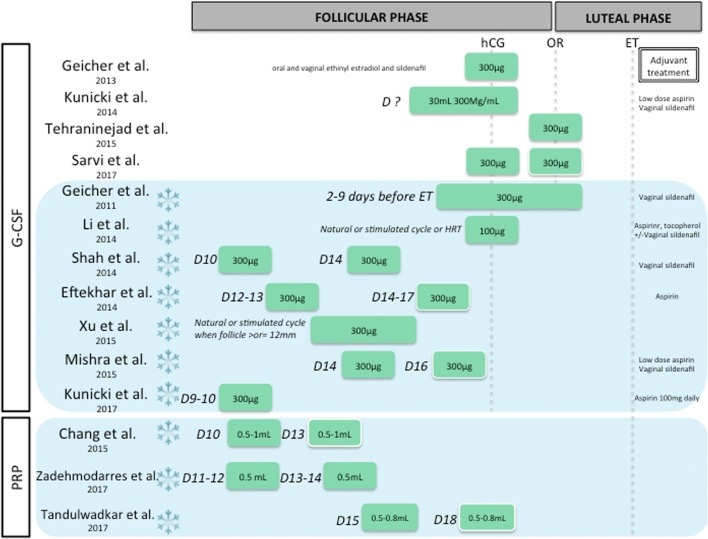
Growth factor therapeutic options for thin endometrium before an embryo transfer. Blue background with frozen flake identifies FET cycle. Days of intrauterine perfusions (D, day; i.e., D12–13, 12th or 13th day of cycle) and total dose of growth factors are specified. Administration route is represented: intrauterine perfusion in green, with a white border when facultative. ET, embryo transfer; HRT, hormonal replacement therapy; OR, oocyte retrieval
Granulocyte colony-stimulating factor
Granulocyte colony-stimulating factor (G-CSF) is a glycoprotein produced by numerous tissues, involved not only in hematopoiesis but also in endometrial growth [62]. It was hence hypothesized to improve the success rates in women with recurrent thin endometrium. G-CSF intrauterine infusion was first successfully described in 2011 by Gleicher et al., in four patients undergoing frozen-thawed ET with HRT [51]. Authors reported noteworthy EMT expansion to at least 7 mm within approximately 48 h from G-CSF intrauterine infusion in patients previously resistant to conventional treatment with estrogen and vasodilators. All four patients conceived. In 2013, the same research group conducted another prospective observational cohort study in fresh IVF cycle including 21 infertile women with an EMT < 7 mm on the day of hCG administration and refractory to traditional treatments with estradiol, sildenafil citrate, and/or beta-blockers, and EMT significantly increased [46]. This innovative remedy applied to small group of patients demonstrated encouraging results.
Several studies subsequently evaluated the effectiveness of G-CSF in women with thin endometrium in stimulated IVF cycles with fresh ET [47–50]. All of them reported an increase in EMT. Only one randomized controlled study, using saline solution as a control, showed significant EMT increase [50]. They observed an enhanced implantation rate after G-CSF (10.3% vs. 5.4%, p = 0.001); however, clinical pregnancy rate was not significantly higher, probably due to small sample size.
Similarly, numerous studies assessed the effectiveness of G-CSF in women with thin endometrium in frozen-thawed ET [52–58]. None of them was randomized; four were controlled (mainly with a group of patients refusing G-CSF infusion). Only one study reported a significant increase in EMT; however, clinical pregnancy rate was not improved with 5 pregnancies among 29 patients in the G-CSF group versus 5/33 in the control group [58].
Intrauterine infusion of platelet-rich plasma
Platelet-rich plasma (PRP) is an autologous concentrate of platelets suspended in a small amount of plasma after centrifugation to which a platelet activator is added in order to activate the clotting cascade, leading to release of numerous cytokines and growth factors. This provides a local environment that is thought to improve regeneration and is applied in various tissues [63].
An observational study by Chang et al., published in 2015, first evaluated the role of autologous PRP in thin endometrium in five patients undergoing frozen ET cycles. The ET increased 48 to 72 h after PRP infusion in all the patients and reached > 7 mm on the day of progesterone administration. All five patients were pregnant [59].
More recently, two cohort studies evaluated the role of intrauterine infusion of autologous PRP on EMT and pregnancy, in women undergoing frozen ET cycles with suboptimal endometrial pattern. The authors reported an EMT increase and 5 patients out of 10 were pregnant [60]. Moreover, a significant increase in endometrium vascularity was described [61].
Since our systematic review of the literature, four additional studies (including one randomized trial) were published regarding the impact of PRP on endometrial growth for thin lining [64–67]. Their results suggest that PRP infusion could statistically increase the EMT in thin endometrium patients. This needs to be confirmed by large randomized and controlled trials.
Discussion and perspectives
In the present systematic review, we expose the evidence found in the literature about therapeutic alternatives for patients with thin endometrium undergoing a fresh or frozen-thawed ET. We specifically focused on studies including patients with a present or past history of thin endometrium. Most studies have methodological limitations such as small sample of patients, etiology for thin endometrium not specified, retrospective analysis, and non-randomized or uncontrolled trial. Moreover, their primary endpoints varied from one study to the other and would not always be the EMT but could also focus on implantation rate or clinical pregnancy rate.
As illustrated in Figs. 2, 3, and 4, treatment prescriptions between studies were not homogeneous with various doses, mode of administration, and adjuvant treatment. Furthermore, in frozen-thawed ET, HRT cycles were readily subject to miscellaneous protocols. Thus, the tremendous lack of solid evidence impedes our efforts towards election of optimal treatment in ET cycles with thin endometrium. We summarized in Fig. 5 available strategies disclosed in literature after rating them according to the degree of published evidence.
Fig. 5.
Summary of proposed strategies in case of thin endometrium according to fresh or frozen embryo transfer. In dark purple, treatments with low to moderate evidence. In light purple with dotted border, strategies with unclear effect. In pale purple without border, interventions with no evidence of benefit
Few studies explored potential interventions to enhance endometrial growth before fresh ET. On one hand, injection of GnRH agonist during luteal phase appears of interest suggesting a direct effect of GnRH agonists on the endometrium [22]; however, this particular publication needs to be validated by additional studies. On the other hand, sildenafil citrate administration and G-CSF infusion in fresh cycles cannot be recommended in common practice, owing to non-significant or conflicting results.
Most studies evaluated interventions during FET and especially with HRT protocol, probably because freeze-all strategy became more popular over the past years [68]. First, intensifying estradiol administration might improve EMT in FET and HRT cycles with either extended estradiol treatment [7] or switch to parenteral routes [17]. Second, sildenafil administration is associated with encouraging outcomes on EMT and endometrial pattern [36]; however, heterogeneity in sildenafil administration in the few published studies might be owing to conflicting results. Third, the numerous studies assessing G-CSF infusion in FET cycles reported contradictory results on EMT. Two recent meta-analyses inquired about the interest of G-CSF infusion in patients with thin endometrium [69, 70] and reported increased implantation and biochemical pregnancy rates although no statistical significance in increasing EMT was detected. Somehow, these results should be interpreted with caution because of the small sample size and limited methodological strength of each study. As a matter of fact, only one study was properly controlled with a placebo and RCT are lacking.
How to stimulate endometrial growth remains unsolved. Regenerative medicine might develop into a useful tool for enhancing endometrial growth. Endometrial cell reconstruction relies on different sources of stem cells such as mesenchymal stem cells in the endometrium, bone marrow–derived stem cells, or even human embryonic stem cells [71]. It appears as a promising therapeutic option for patients with endometrial atrophy or Asherman’s syndrome wishing to conceive [72]. One pregnancy has been successfully obtained after such endometrial regeneration [73]. This could be an interesting option to explore in patients with thin endometrium and a normal uterine cavity.
Thin endometrium: a problematic issue in the medical literature
Definition and cutoff
As mentioned, there is no clear definition of a “thin” endometrium in the literature. The most frequently reported cutoff for EMT to be thin is 7 mm [8–12] but some authors also used a cutoff of 6 mm [40, 74] or 8 mm [6, 75]. Conversely, in some studies about thin endometrium, ET was canceled if EMT was below 6 mm [76], and we might hypothesize that patients with very thin endometrium were never transferred. It is hence extremely challenging to base our investigation on such heterogeneous population.
Furthermore, EMT is an ultrasound measurement, therefore subject to intra- and interobserver variability. Interobserver variability was estimated to be about 1 mm ± 0.8 mm [77]. Although reproducibility has been shown to be satisfying [78], such variability in the measurement requires prudent interpretation in terms of clinical relevance and careful clinical decisions.
Integrating several imaging parameters might enhance our competence in pregnancy prediction [79]. Hence, future studies should focus on validating the most effective combination for endometrial receptivity assessment through ultrasound criteria such as endometrial pattern [80] or endometrial volume [81, 82] as well as electing the best timing for such evaluation [83].
Previous controversy about thin endometrium and pregnancy rate
In the past, studies exploring the relationship between EMT and pregnancy outcomes had conflicting conclusions, and some authors even questioned the clinical relevance of EMT. A meta-analysis from 2014 [12] inquiring about the impact of EMT on IVF outcome indicated that thin endometrium < 7 mm was an infrequent situation and that it should not differ embryo replacement as live birth rate was not significantly reduced (OR 0.38 (95% CI 0.09–1.5)). For some authors, EMT appears not to be a good predictor of pregnancy [84–86]. This is consistent with some case reports disclosing pregnancy on very thin endometrium, below 4 mm [87–90]. The association between EMT and live birth rate might be non-linear [13] but thin endometrium appears to have a good negative predictive value [91]. While the influence of endometrial thickness on IVF outcomes was previously debated, recent papers over the past decade based on several thousands of embryo transfers clearly asserted by now the negative impact of thin endometrium on both pregnancy rates and obstetrical outcomes [4, 6, 13, 92–94], especially during fresh ET cycles [91].
Conclusion
At present, treatment of thin endometrium remains challenging for clinicians, as lack of solid evidence impedes support of one therapeutic option versus the other. Well-designed and adequately powered RCT are required to evaluate the most appropriate approach to improve EMT and subsequent pregnancy and live birth rates in women with thin endometrium. As described in this review, multiple treatments are available, based on various mechanisms. We should probably enrich our endometrial investigation with a more functional approach. This would allow identification of individualized physiopathological pathway involved in this abnormal endometrial growth, so as to subsequently offer personalized treatment.
Acknowledgments
We want to thank Elisangela Arbo-Jouhaud for organizing our workshop group meetings and for her guidance. We are also grateful to Monitoring Force, especially Solene Languille and Bernadette Darne, for their assistance in literature search and for their advice.
Authors’ contributions
T.A. and M.G. were involved in the conception of the review.
N.R., J.R., and C.S. contributed to data collection and extraction, as well as drafting the article.
All authors analyzed and interpreted the data.
T.A. and M.G. had a critical revision of the manuscript.
All authors approved the final version to be published.
Funding information
This research was financially supported by Gedeon Richter France who provided methodological support through Monitoring Force.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miravet-Valenciano JA, Rincon-Bertolin A, Vilella F, Simon C. Understanding and improving endometrial receptivity. Curr Opin Obstet Gynecol. 2015;27:187–192. doi: 10.1097/GCO.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 2.von Grothusen C, Lalitkumar S, Boggavarapu NR, Gemzell-Danielsson K, Lalitkumar PG. Recent advances in understanding endometrial receptivity: molecular basis and clinical applications. Am J Reprod Immunol N Y N 1989. 2014;72:148–157. doi: 10.1111/aji.12226. [DOI] [PubMed] [Google Scholar]
- 3.Momeni M, Rahbar MH, Kovanci E. A meta-analysis of the relationship between endometrial thickness and outcome of in vitro fertilization cycles. J Hum Reprod Sci. 2011;4:130–137. doi: 10.4103/0974-1208.92287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu KE, Hartman M, Hartman A, Luo Z-C, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33:1883–1888. doi: 10.1093/humrep/dey281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung K, Coutifaris C, Chalian R, Lin K, Ratcliffe SJ, Castelbaum AJ, Freedman MF, Barnhart KT. Factors influencing adverse perinatal outcomes in pregnancies achieved through use of in vitro fertilization. Fertil Steril. 2006;86:1634–1641. doi: 10.1016/j.fertnstert.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Yuan X, Saravelos SH, Wang Q, Xu Y, Li T-C, Zhou C. Endometrial thickness as a predictor of pregnancy outcomes in 10787 fresh IVF-ICSI cycles. Reprod BioMed Online. 2016;33:197–205. doi: 10.1016/j.rbmo.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Chen M-J, Yang J-H, Peng F-H, Chen S-U, Ho H-N, Yang Y-S. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006;23:337–342. doi: 10.1007/s10815-006-9053-1. [DOI] [PubMed] [Google Scholar]
- 8.El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 2008;89:832–839. doi: 10.1016/j.fertnstert.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Kumbak B, Erden HF, Tosun S, Akbas H, Ulug U, Bahçeci M. Outcome of assisted reproduction treatment in patients with endometrial thickness less than 7 mm. Reprod BioMed Online. 2009;18:79–84. doi: 10.1016/s1472-6483(10)60428-2. [DOI] [PubMed] [Google Scholar]
- 10.Bozdag G, Esinler I, Yarali H. The impact of endometrial thickness and texture on intracytoplasmic sperm injection outcome. J Reprod Med. 2009;54:303–311. [PubMed] [Google Scholar]
- 11.Okohue JE, Onuh SO, Ebeigbe P, Shaibu I, Wada I, Ikimalo JI, et al. The effect of endometrial thickness on in vitro fertilization (IVF)-embryo transfer/intracytoplasmic sperm injection (ICSI) outcome. Afr J Reprod Health. 2009;13:113–121. [PubMed] [Google Scholar]
- 12.Kasius A, Smit JG, Torrance HL, Eijkemans MJC, Mol BW, Opmeer BC, Broekmans FJM. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:530–541. doi: 10.1093/humupd/dmu011. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro VC, Santos-Ribeiro S, De Munck N, Drakopoulos P, Polyzos NP, Schutyser V, et al. Should we continue to measure endometrial thickness in modern-day medicine? The effect on live birth rates and birth weight. Reprod BioMed Online. 2018;36:416–426. doi: 10.1016/j.rbmo.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Groothuis PG, Dassen HHNM, Romano A, Punyadeera C. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007;13:405–417. doi: 10.1093/humupd/dmm009. [DOI] [PubMed] [Google Scholar]
- 15.Hapangama DK, Kamal AM, Bulmer JN. Estrogen receptor β: the guardian of the endometrium. Hum Reprod Update. 2015;21:174–193. doi: 10.1093/humupd/dmu053. [DOI] [PubMed] [Google Scholar]
- 16.Check JH, Graziano V, Lee G, Nazari A, Choe JK, Dietterich C. Neither sildenafil nor vaginal estradiol improves endometrial thickness in women with thin endometria after taking oral estradiol in graduating dosages. Clin Exp Obstet Gynecol. 2004;31:99–102. [PubMed] [Google Scholar]
- 17.Liao X, Li Z, Dong X, Zhang H. Comparison between oral and vaginal estrogen usage in inadequate endometrial patients for frozen-thawed blastocysts transfer. Int J Clin Exp Pathol. 2014;7:6992–6997. [PMC free article] [PubMed] [Google Scholar]
- 18.Dmowski WP, Michalowska J, Rana N, Friberg J, McGill-Johnson E, DeOrio L. Subcutaneous estradiol pellets for endometrial preparation in donor oocyte recipients with a poor endometrial response. J Assist Reprod Genet. 1997;14:139–144. doi: 10.1007/BF02766129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zolghadri J, Haghbin H, Dadras N, Behdin S. Vagifem is superior to vaginal Premarin in induction of endometrial thickness in the frozen-thawed cycle patients with refractory endometria: a randomized clinical trial. Iran J Reprod Med. 2014;12:415–420. [PMC free article] [PubMed] [Google Scholar]
- 20.Papanikolaou EG, Kyrou D, Zervakakou G, Paggou E, Humaidan P. Follicular HCG endometrium priming for IVF patients experiencing resisting thin endometrium. A proof of concept study. J Assist Reprod Genet. 2013;30:1341–1345. doi: 10.1007/s10815-013-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davar R, Miraj S, Farid MM. Effect of adding human chorionic gonadotropin to frozen thawed embryo transfer cycles with history of thin endometrium. Int J Reprod Biomed Yazd Iran. 2016;14:53–56. [PMC free article] [PubMed] [Google Scholar]
- 22.Qublan H, Qublah H, Amarin Z, Al-Qudah M, Al-Quda M, Diab F, et al. Luteal phase support with GnRH-a improves implantation and pregnancy rates in IVF cycles with endometrium of <or=7 mm on day of egg retrieval. Hum Fertil (Camb) 2008;11:43–47. doi: 10.1080/14647270701704768. [DOI] [PubMed] [Google Scholar]
- 23.Paulson RJ. Hormonal induction of endometrial receptivity. Fertil Steril. 2011;96:530–535. doi: 10.1016/j.fertnstert.2011.07.1097. [DOI] [PubMed] [Google Scholar]
- 24.Tourgeman DE, Slater CC, Stanczyk FZ, Paulson RJ. Endocrine and clinical effects of micronized estradiol administered vaginally or orally. Fertil Steril. 2001;75:200–202. doi: 10.1016/s0015-0282(00)01640-x. [DOI] [PubMed] [Google Scholar]
- 25.Perrier d’Hauterive S, Berndt S, Tsampalas M, Charlet-Renard C, Dubois M, Bourgain C, Hazout A, Foidart JM, Geenen V. Dialogue between blastocyst hCG and endometrial LH/hCG receptor: which role in implantation? Gynecol Obstet Investig. 2007;64:156–160. doi: 10.1159/000101740. [DOI] [PubMed] [Google Scholar]
- 26.Licht P, Russu V, Wildt L. On the role of human chorionic gonadotropin (hCG) in the embryo-endometrial microenvironment: implications for differentiation and implantation. Semin Reprod Med. 2001;19:37–47. doi: 10.1055/s-2001-13909. [DOI] [PubMed] [Google Scholar]
- 27.Licht P, von Wolff M, Berkholz A, Wildt L. Evidence for cycle-dependent expression of full-length human chorionic gonadotropin/luteinizing hormone receptor mRNA in human endometrium and decidua. Fertil Steril. 2003;79(Suppl 1):718–723. doi: 10.1016/s0015-0282(02)04822-7. [DOI] [PubMed] [Google Scholar]
- 28.Prapas N, Tavaniotou A, Panagiotidis Y, Prapa S, Kasapi E, Goudakou M, Papatheodorou A, Prapas Y. Low-dose human chorionic gonadotropin during the proliferative phase may adversely affect endometrial receptivity in oocyte recipients. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2009;25:53–59. doi: 10.1080/09513590802360769. [DOI] [PubMed] [Google Scholar]
- 29.Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2010;1:CD006359. [DOI] [PubMed]
- 30.Tesarik J, Hazout A, Mendoza C. Enhancement of embryo developmental potential by a single administration of GnRH agonist at the time of implantation. Hum Reprod. 2004;19:1176–1180. doi: 10.1093/humrep/deh235. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira JBA, Baruffi R, Petersen CG, Mauri AL, Cavagna M, Franco JG. Administration of single-dose GnRH agonist in the luteal phase in ICSI cycles: a meta-analysis. Reprod Biol Endocrinol. 2010;8:107. doi: 10.1186/1477-7827-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;7:CD009154. [DOI] [PMC free article] [PubMed]
- 33.al MI et. Pathophysiologic features of “thin” endometrium. - PubMed - NCBI [Internet]. [cited 2019 Mar 29]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/?term=Pathophysiologic+features+of+%22thin%22+endometrium.
- 34.Sher G, Fisch JD. Vaginal sildenafil (Viagra): a preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum Reprod. 2000;15:806–809. doi: 10.1093/humrep/15.4.806. [DOI] [PubMed] [Google Scholar]
- 35.Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78:1073–1076. doi: 10.1016/s0015-0282(02)03375-7. [DOI] [PubMed] [Google Scholar]
- 36.Dehghani Firouzabadi R, Davar R, Hojjat F, Mahdavi M. Effect of sildenafil citrate on endometrial preparation and outcome of frozen-thawed embryo transfer cycles: a randomized clinical trial. Iran J Reprod Med. 2013;11:151–158. [PMC free article] [PubMed] [Google Scholar]
- 37.Kim KR. J Womens Med. ed Hee Sun Lee; 2010.
- 38.Weckstein LN, Jacobson A, Galen D, Hampton K, Hammel J. Low-dose aspirin for oocyte donation recipients with a thin endometrium: prospective, randomized study. Fertil Steril. 1997;68:927–930. doi: 10.1016/s0015-0282(97)00330-0. [DOI] [PubMed] [Google Scholar]
- 39.Lédée-Bataille N, Olivennes F, Lefaix J-L, Chaouat G, Frydman R, Delanian S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum Reprod. 2002;17:1249–1253. doi: 10.1093/humrep/17.5.1249. [DOI] [PubMed] [Google Scholar]
- 40.Acharya S, Yasmin E, Balen AH. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies--a report of 20 cases. Hum Fertil Camb Engl. 2009;12:198–203. doi: 10.3109/14647270903377178. [DOI] [PubMed] [Google Scholar]
- 41.Bodombossou-Djobo MMA, Zheng C, Chen S, Yang D. Neuromuscular electrical stimulation and biofeedback therapy may improve endometrial growth for patients with thin endometrium during frozen-thawed embryo transfer: a preliminary report. Reprod Biol Endocrinol. 2011;9:122. doi: 10.1186/1477-7827-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wada I, Hsu CC, Williams G, Macnamee MC, Brinsden PR. The benefits of low-dose aspirin therapy in women with impaired uterine perfusion during assisted conception. Hum Reprod. 1994;9:1954–1957. doi: 10.1093/oxfordjournals.humrep.a138366. [DOI] [PubMed] [Google Scholar]
- 43.Dentali F, Ageno W, Rezoagli E, Rancan E, Squizzato A, Middeldorp S, et al. Low-dose aspirin for in vitro fertilization or intracytoplasmic sperm injection: a systematic review and a meta-analysis of the literature. J Thromb Haemost. 2012;10:2075–2085. doi: 10.1111/j.1538-7836.2012.04886.x. [DOI] [PubMed] [Google Scholar]
- 44.Khairy M, Banerjee K, El-Toukhy T, Coomarasamy A, Khalaf Y. Aspirin in women undergoing in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2007;88:822–831. doi: 10.1016/j.fertnstert.2006.12.080. [DOI] [PubMed] [Google Scholar]
- 45.Giudice LC. Growth factors and growth modulators in human uterine endometrium: their potential relevance to reproductive medicine. Fertil Steril. 1994;61:1–17. doi: 10.1016/s0015-0282(16)56447-4. [DOI] [PubMed] [Google Scholar]
- 46.Gleicher N, Kim A, Michaeli T, Lee H-J, Shohat-Tal A, Lazzaroni E, Barad DH. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum Reprod. 2013;28:172–177. doi: 10.1093/humrep/des370. [DOI] [PubMed] [Google Scholar]
- 47.Kunicki M, Łukaszuk K, Woclawek-Potocka I, Liss J, Kulwikowska P, Szczyptańska J. Evaluation of granulocyte colony-stimulating factor effects on treatment-resistant thin endometrium in women undergoing in vitro fertilization. Biomed Res Int. 2014;2014:913235. doi: 10.1155/2014/913235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tehraninejad E, Davari Tanha F, Asadi E, Kamali K, Aziminikoo E, Rezayof E. G-CSF intrauterine for thin endometrium, and pregnancy outcome. J Family Reprod Health. 2015;9:107–112. [PMC free article] [PubMed] [Google Scholar]
- 49.Lee D, Jo JD, Kim SK, Jee BC, Kim SH. The efficacy of intrauterine instillation of granulocyte colony-stimulating factor in infertile women with a thin endometrium: a pilot study. Clin Exp Reprod Med. 2016;43:240–246. doi: 10.5653/cerm.2016.43.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarvi F, Arabahmadi M, Alleyassin A, Aghahosseini M, Ghasemi M. Effect of increased endometrial thickness and implantation rate by granulocyte colony-stimulating factor on unresponsive thin endometrium in fresh in vitro fertilization cycles: a randomized clinical trial. Obstet Gynecol Int. 2017;2017:3596079. doi: 10.1155/2017/3596079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gleicher N, Vidali A, Barad DH. Successful treatment of unresponsive thin endometrium. Fertil Steril. 2011;95:2123.e13–2123.e17. doi: 10.1016/j.fertnstert.2011.01.143. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Pan P, Chen X, Li L, Li Y, Yang D. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program. Reprod Sci Thousand Oaks Calif. 2014;21:381–385. doi: 10.1177/1933719113497286. [DOI] [PubMed] [Google Scholar]
- 53.Shah J, Gangadharan A, Shah V. Effect of intrauterine instillation of granulocyte colony-stimulating factor on endometrial thickness and clinical pregnancy rate in women undergoing in vitro fertilization cycles: an observational cohort study. 2014;3:100-106.
- 54.Eftekhar M, Sayadi M, Arabjahvani F. Transvaginal perfusion of G-CSF for infertile women with thin endometrium in frozen ET program: a non-randomized clinical trial. Iran J Reprod Med. 2014;12:661–666. [PMC free article] [PubMed] [Google Scholar]
- 55.Xu B, Zhang Q, Hao J, Xu D, Li Y. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reprod BioMed Online. 2015;30:349–358. doi: 10.1016/j.rbmo.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Check JH, Choe JK, Summers-Chase D. Failure to increase the thickness of thin endometria with intrauterine infusion of granulocyte colony stimulating factor (G-CSF) Clin Exp Obstet Gynecol. 2016;43:332–333. [PubMed] [Google Scholar]
- 57.Mishra VV, Choudhary S, Sharma U, Aggarwal R, Agarwal R, Gandhi K, Goraniya N. Effects of granulocyte colony-stimulating factor (GCSF) on persistent thin endometrium in frozen embryo transfer (FET) cycles. J Obstet Gynaecol India. 2016;66:407–411. doi: 10.1007/s13224-015-0775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunicki M, Łukaszuk K, Liss J, Skowrońska P, Szczyptańska J. Granulocyte colony stimulating factor treatment of resistant thin endometrium in women with frozen-thawed blastocyst transfer. Syst Biol Reprod Med. 2017;63:49–57. doi: 10.1080/19396368.2016.1251505. [DOI] [PubMed] [Google Scholar]
- 59.Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8:1286–1290. [PMC free article] [PubMed] [Google Scholar]
- 60.Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017;21:54–56. doi: 10.5935/1518-0557.20170013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tandulwadkar SR, Naralkar MV, Surana AD, Selvakarthick M, Kharat AH. Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: a pilot study. J Hum Reprod Sci. 2017;10:208–212. doi: 10.4103/jhrs.JHRS_28_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen JR, Witz CA, Schenken RS, Tekmal RR. A potential role for colony-stimulating factor 1 in the genesis of the early endometriotic lesion. Fertil Steril. 2010;93:251–256. doi: 10.1016/j.fertnstert.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24:173–182. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- 64.Kim H, Shin JE, Koo HS, Kwon H, Choi DH, Kim JH. Effect of autologous platelet-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: a pilot study. Front Endocrinol. 2019;10:61. doi: 10.3389/fendo.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang Y, Li J, Wei L-N, Pang J, Chen J, Liang X. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine (Baltimore) 2019;98:e14062. doi: 10.1097/MD.0000000000014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol. 2018;57:810–813. doi: 10.1016/j.tjog.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Liu L, Mou S, Zhao H, Fang J, Xiang Y, et al. Investigation of platelet-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J Cell Biochem. 2018. [DOI] [PubMed]
- 68.Wong KM, van Wely M, Mol F, Repping S, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev. 2017;3:CD011184. doi: 10.1002/14651858.CD011184.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Mo S, Chen Y. The effect of G-CSF on infertile women undergoing IVF treatment: a meta-analysis. Syst Biol Reprod Med. 2017;63:239–247. doi: 10.1080/19396368.2017.1287225. [DOI] [PubMed] [Google Scholar]
- 70.Xie Y, Zhang T, Tian Z, Zhang J, Wang W, Zhang H, et al. Efficacy of intrauterine perfusion of granulocyte colony-stimulating factor (G-CSF) for infertile women with thin endometrium: a systematic review and meta-analysis. Am J Reprod Immunol. 1989;2017:78. doi: 10.1111/aji.12701. [DOI] [PubMed] [Google Scholar]
- 71.Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 72.Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, Palmero J, Remohí J, Pellicer A, Simón C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31:1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 73.Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci. 2011;4:43–48. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papanikolaou EG, Pados G, Grimbizis G, Bili E, Kyriazi L, Polyzos NP, Humaidan P, Tournaye H, Tarlatzis B. GnRH-agonist versus GnRH-antagonist IVF cycles: is the reproductive outcome affected by the incidence of progesterone elevation on the day of HCG triggering? A randomized prospective study. Hum Reprod. 2012;27:1822–1828. doi: 10.1093/humrep/des066. [DOI] [PubMed] [Google Scholar]
- 75.Jimenez PT, Schon SB, Odem RR, Ratts VS, Jungheim ES. A retrospective cross-sectional study: fresh cycle endometrial thickness is a sensitive predictor of inadequate endometrial thickness in frozen embryo transfer cycles. Reprod Biol Endocrinol. 2013;11:35. doi: 10.1186/1477-7827-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta P, Banker M, Patel P, Joshi B. A study of recipient related predictors of success in oocyte donation program. J Hum Reprod Sci. 2012;5:252–257. doi: 10.4103/0974-1208.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spandorfer SD, Arrendondo-Soberon F, Loret de Mola JR, Feinberg RF. Reliability of intraobserver and interobserver sonographic endometrial stripe thickness measurements. Fertil Steril. 1998;70:152–154. doi: 10.1016/s0015-0282(98)00101-0. [DOI] [PubMed] [Google Scholar]
- 78.Delisle MF, Villeneuve M, Boulvain M. Measurement of endometrial thickness with transvaginal ultrasonography: is it reproducible? J Ultrasound Med Off J Am Inst Ultrasound Med. 1998;17:481–484-486. doi: 10.7863/jum.1998.17.8.481. [DOI] [PubMed] [Google Scholar]
- 79.Hershko-Klement A, Tepper R. Ultrasound in assisted reproduction: a call to fill the endometrial gap. Fertil Steril. 2016;105:1394–1402.e4. doi: 10.1016/j.fertnstert.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 80.Chen S-L, Wu F-R, Luo C, Chen X, Shi X-Y, Zheng H-Y, Ni YP. Combined analysis of endometrial thickness and pattern in predicting outcome of in vitro fertilization and embryo transfer: a retrospective cohort study. Reprod Biol Endocrinol. 2010;8:30. doi: 10.1186/1477-7827-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zollner U, Specketer M-T, Dietl J, Zollner K-P. 3D-endometrial volume and outcome of cryopreserved embryo replacement cycles. Arch Gynecol Obstet. 2012;286:517–523. doi: 10.1007/s00404-012-2332-4. [DOI] [PubMed] [Google Scholar]
- 82.Joo J-K, Jeong J-E, Kim C-W, Lee K-S. Quantitative assessment of endometrial volume and uterine vascularity and pregnancy outcome in frozen-thawed embryo transfer cycles. J Reprod Med. 2016;61:133–138. [PubMed] [Google Scholar]
- 83.Dechaud H, Bessueille E, Bousquet P-J, Reyftmann L, Hamamah S, Hedon B. Optimal timing of ultrasonographic and Doppler evaluation of uterine receptivity to implantation. Reprod BioMed Online. 2008;16:368–375. doi: 10.1016/s1472-6483(10)60598-6. [DOI] [PubMed] [Google Scholar]
- 84.Zhao J, Zhang Q, Wang Y, Li Y. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod BioMed Online. 2014;29:291–298. doi: 10.1016/j.rbmo.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 85.Coelho Neto MA, Martins WP, Lima MLS, Barbosa M a P, Nastri CO, Ferriani RA, et al. Ovarian response is a better predictor of clinical pregnancy rate following embryo transfer than is thin endometrium or presence of an endometrioma. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2015;46:501–505. doi: 10.1002/uog.14884. [DOI] [PubMed] [Google Scholar]
- 86.Kinay T, Tasci Y, Dilbaz S, Cinar O, Demir B, Haberal A. The relationship between endometrial thickness and pregnancy rates in GnRH antagonist down-regulated ICSI cycles. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2010;26:833–837. doi: 10.3109/09513590.2010.487591. [DOI] [PubMed] [Google Scholar]
- 87.Remohí J, Ardiles G, García-Velasco JA, Gaitán P, Simón C, Pellicer A. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod. 1997;12:2271–2276. doi: 10.1093/humrep/12.10.2271. [DOI] [PubMed] [Google Scholar]
- 88.Sundström P. Establishment of a successful pregnancy following in-vitro fertilization with an endometrial thickness of no more than 4 mm. Hum Reprod. 1998;13:1550–1552. doi: 10.1093/humrep/13.6.1550. [DOI] [PubMed] [Google Scholar]
- 89.Check JH, Cohen R. Live fetus following embryo transfer in a woman with diminished egg reserve whose maximal endometrial thickness was less than 4 mm. Clin Exp Obstet Gynecol. 2011;38:330–332. [PubMed] [Google Scholar]
- 90.Cruz F, Bellver J. Live birth after embryo transfer in an unresponsive thin endometrium. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2014;30:481–484. doi: 10.3109/09513590.2014.900747. [DOI] [PubMed] [Google Scholar]
- 91.Zhang T, Li Z, Ren X, Huang B, Zhu G, Yang W, et al. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: a retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine (Baltimore) 2018;97:e9689. doi: 10.1097/MD.0000000000009689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma N-Z, Chen L, Dai W, Bu Z-Q, Hu L-L, Sun Y-P. Influence of endometrial thickness on treatment outcomes following in vitro fertilization/intracytoplasmic sperm injection. Reprod Biol Endocrinol. 2017;15:5. doi: 10.1186/s12958-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holden EC, Dodge LE, Sneeringer R, Moragianni VA, Penzias AS, Hacker MR. Thicker endometrial linings are associated with better IVF outcomes: a cohort of 6331 women. Hum Fertil Camb Engl. 2018;21:288–293. doi: 10.1080/14647273.2017.1334130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang R, Cai L, Xiong F, Chen J, Yang W, Zhao X. The effect of endometrial thickness on the day of hCG administration on pregnancy outcome in the first fresh IVF/ICSI cycle. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2016;32:473–476. doi: 10.3109/09513590.2015.1132304. [DOI] [PubMed] [Google Scholar]