Abstract
Purpose
To investigate whether the ability of human spermatozoa to decondense in vitro in the presence of heparin (Hep) and glutathione (GSH) is related to assisted reproduction (ART) success.
Methods
Cross-sectional pilot study involving male partners of 129 infertile couples undergoing ICSI with (45) or without (84) donor oocytes at two infertility clinics in CABA, Argentina, between October 2012 and December 2013. In vitro decondensation kinetics with Hep and GSH and DNA fragmentation (TUNEL) were determined on the same sample used for ICSI. The possible relationship of decondensation parameters (maximum decondensation and decondensation velocity) and TUNEL values with ART success was evaluated.
Results
Embryo quality correlated positively with decondensation velocity (D60/D30) (Spearman’s correlation, p < 0.05). According to D60/D30 values, patients were classified as slow decondensers (SlowD) (n = 68) or fast decondensers (FastD) (n = 61). Embryo quality was better in FastD (unpaired t test, p < 0.05). FastD and SlowD were subdivided according to use of donor oocytes. Among SlowD, biochemical and clinical pregnancy rates per transfer were significantly higher in donor (n = 19) vs. in non-donor (n = 31) cycles (Fisher’s exact test, p < 0.05). TUNEL values were not related to embryo quality, but no clinical pregnancies or live births were achieved in TUNEL+ SlowD (n = 7).
Conclusion
Decondensation kinetics of human spermatozoa in vitro with Hep and GSH could be related to embryo quality and ART success.
Keywords: Sperm decondensation, ART success, Heparin, DNA fragmentation, Human spermatozoa
Introduction
Decondensation of sperm chromatin is the first visible change in the fertilizing spermatozoon upon entry into the ooplasm and a prerequisite for pronuclear formation and syngamy. As such, the ability to undergo adequate nuclear decondensation could be a measure of sperm fertilizing ability [1, 2].
There is ample evidence in the literature suggesting that alterations in sperm chromatin condensation during spermiogenesis are related to alterations in chromatin decondensation in the ooplasm which, in turn, could compromise early embryonic development [3, 4]. Furthermore, DNA abnormalities have been linked to abnormal chromatin packaging, and it has been suggested that DNA damage is the main cause of implantation failure in embryos derived from healthy eggs fertilized by sperm with chromosome defects [5].
Even though the last two decades have seen a significant increase in the number of ART procedures, particularly ICSI, there has been no net improvement in healthy term pregnancy [6]. Use of ICSI has been increasing, not necessarily by indication but because of a generalized perception among clinicians and patients that there is no safety issue and because fertilization can be achieved in cases where IVF would be unsuccessful. However, what matters to a couple undergoing ART is healthy live birth, which has not improved, thus stressing the need to better male diagnosis and optimize sperm sample procurement.
Given that ICSI bypasses interaction of spermatozoa with the egg vestments prior to entry into the ooplasm, interest has been placed in developing strategies to evaluate the functionality of the sperm nucleus. Several different techniques have been proposed as measures of chromatin status and DNA fragmentation, but relationship between them and ART success is still controversial [7–13].
In our laboratory, we have been studying human sperm decondensation in vitro in the presence of heparin (Hep) and glutathione (GSH) for several years [14–17]. GSH is the protamine disulfide bond reducer in vivo [18], and Hep is a structural and biological analogue of heparan sulfate (HS). HS is present in murine, human, and bovine oocytes and, as proposed by our laboratory, probably involved in mammalian sperm decondensation in vivo [15–17, 19].
Preliminary data from our laboratory suggest that the ability of human spermatozoa to decondense in vitro in the presence of Hep and GSH is not the same in all infertile patients [20]. It is a known fact that about 10% of ICSI cycles fail to generate embryos and, among these, around 15% still have a condensed sperm head inside the oocyte. Male patients whose spermatozoa are not able to decondense inside the oocyte would not benefit from ICSI [2, 21].
The aim of this study was to analyze sperm decondensation in vitro in the male partners of infertile couples undergoing ART and determine whether it is related in any way to various measures of ART success, especially live birth rate.
Materials and methods
All chemicals and reagents used were from Sigma Chemical Co (St. Louis, MO) unless otherwise stated.
Subject selection
Infertile patients (n = 129) undergoing ICSI at two infertility clinics in Buenos Aires, Argentina, during the period comprised between October 2012 and December 2013, following informed consent, entered the study sequentially, regardless of infertility diagnosis. The only requirement was that the sperm sample used for ICSI had an enough number of spermatozoa for in vitro decondensation assessment, thus excluding severely oligozoospermic male patients from the study. Couples used either their own oocytes (n = 84) or donated oocytes (n = 45).
Sperm in vitro decondensation and DNA fragmentation were determined on the same sample used for ICSI.
Normozoospermic [22] semen specimens to be used as internal control were obtained under informed consent from normal healthy volunteers (n = 5). Donor data were kept confidential.
This study was approved by CEPI (Ethics Committee of Hospital Italiano de Buenos Aires) and IBYME Ethics Committee.
Patient semen sample processing for ICSI
Semen samples were collected by masturbation after 36–48 h of abstinence, allowed to liquefy and processed within 1 h of collection. Motile sperm were separated using a dual-gradient density solution (ISolate®, Irvine Scientific Cat. No. 99264). The remaining pellet was washed by centrifugation with 3 mL of modified human tubal fluid medium (HTF) with gentamicine–HEPES (Irvine Scientific, Cat. No. 90126) containing 5% human serum albumin solution (Irvine Scientific, Cat. No. 9988).
Donor semen sample processing
Semen samples were collected by masturbation after 36–48 h of abstinence, allowed to liquefy and processed within 1 h of collection. Samples were washed twice by centrifugation at 300×g for 10 min in HTF (HTF 4.6 mM KCl; 0.37 mM KH2PO4; 90.7 mM NaCl; 1.3 mM MgSO4; 2.78 mM glucose; 1.6 mM CaCl2·2H2O; 23.8 mM NaHCO3; 3.38 mM sodium pyruvate and 80.2 mM sodium lactate) supplemented with 0.3% bovine serum albumin (BSA). The remaining pellet was overlaid with 1 mL of fresh HTF containing 2.6% BSA (HTF-26B), and sperm were allowed to swim up for 90 min at 37 °C in an atmosphere of 5% CO2 in air. Subsequently, highly motile spermatozoa were incubated in capacitating conditions for 18 h in HTF-26B at 37 °C in an atmosphere of 5% CO2 in air, at a concentration of 5–10 × 106 mL−1.
DNA fragmentation
On the day following oocyte insemination, a 10-μL aliquot of spermatozoa from the same sample used for ICSI was smeared on a poly-l-lysine-coated microscope slide to assess DNA fragmentation by TdT (terminal deoxynucleotidyl transferase)-mediated dUDP nick-end labeling (TUNEL). All TUNEL assessments were performed at the Andrology Laboratory, Hospital Italiano de Buenos Aires (HIBA), using the “in situ Cell Death Detection Kit Fluorescein” (Roche), according to the manufacturer’s instructions. Briefly, samples were fixed in 1% paraformaldehyde solution (Cicarelli Laboratorios, San Lorenzo, Santa Fe, Argentina) for 10 min, washed with PBS, and permeabilized with 0.1% Triton X-100 (Calbiochem) in 0.1% sodium citrate (Calbiochem) for 10 min at room temperature. Subsequently, slides were incubated for 1 h at 37 °C in TUNEL reaction-mixture label solution and terminal transferase solution and washed twice with PBS during 5 min. DNA damage was quantified under a fluorescence microscope (Nikon Eclipse E400) and 5000 cells were evaluated in each sample. The cutoff value for TUNEL positivity was > 20%, as routinely used by the HIBA Andrology Laboratory.
Sperm decondensation assay
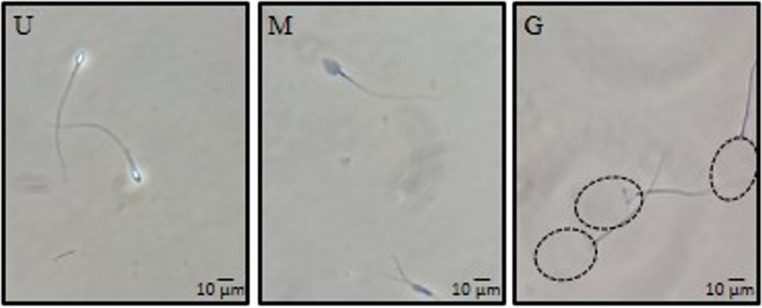
On the day following oocyte insemination, unused spermatozoa were washed once by centrifugation at 300×g in HTF-26B medium and incubated overnight at 37 °C in HTF-26B in an atmosphere of 5% CO2 in air. Subsequently, spermatozoa were decondensed as previously described [14, 23], based on the original decondensation technique introduced by Reyes et al. [24]. Briefly, 3–5 × 106 spermatozoa were incubated in HTF-26B with 46 μM Hep (Grade I-A: from porcine intestinal mucosa; Sigma Cat No. H-3393) and 10 mM GSH (γ-Glu-Cys-Gly; Sigma Cat No. G-4251) in a 0.3-mL final volume, for 1 h at 37 °C in an atmosphere of 5% CO2 in air. After fixation in 2.5% glutaraldehyde, the percentage of decondensed spermatozoa was determined by phase contrast in a Nikon Eclipse E200 microscope at × 400 magnification, using the scoring criteria previously described [14, 23, 25]. Spermatozoa were classified as unchanged (U), moderately decondensed (M), or grossly decondensed (G), according to refringence, granular aspect, and size of the nucleus. Unchanged spermatozoa are bright and do not have an enlarged nucleus; Moderately decondensed cells are no longer refringent, but dark and slightly enlarged; Grossly decondensed heads are very large, granular, gray in color, and almost translucent (Fig. 1). The percentage of decondensed spermatozoa was calculated as the sum of the percentages of M and G shapes, %(M + G). Every assay was run in duplicates and at least 200 cells were evaluated in each sample. Two decondensation parameters were defined: %(M + G), % decondensation after 60 min of incubation, as a measure of maximum decondensation achieved; and D60/D30, ratio of % decondensation at 60 and 30 min of incubation, which is a measure of sperm decondensation velocity.
Fig. 1.
Different stages of nuclear decondensation observed under phase-contrast microscopy following in vitro exposure of spermatozoa to 40 μM Hep + 10 mM GSH as described in “Materials and methods.” Spermatozoa were classified as unchanged (U), moderately decondensed (M), or grossly decondensed (G), according to refringence, granular aspect, and size of the nucleus (× 400)
Oocyte donation
Metaphase II (MII) oocytes were obtained under informed consent from healthy young donor women, under 30 years old, enrolled in the oocyte donor program at PROCREARTE. Similarly to patients undergoing ART, oocyte donors were tested for syphilis, hepatitis B and C, HIV types 1 and 2, and HTLV types 1 and 2.
ICSI procedure
Women were hormonally stimulated beginning with 225 IU/day recombinant FSH (rFSH) for 3 days. As from day 4, two to four HMG ampoules (Human Menopausal Gonadotropin), containing 75 IU FSH and 75 IU LH, were administered. Follicular development was monitored by vaginal ultrasound using a 5-MHz transvaginal probe. When at least two follicles measured ≥ 14 mm mean diameter, patients received 1 ampoule GnRH antagonist (cetrorelix)/day. When at least two follicles measured ≥ 18 mm mean diameter, 250 μg hCGr (Ovidrel, Serono; subcutaneous injection) or 10,000 IU hCG (Gonacor, Ferring; intramuscular injection) was administered. Transvaginal follicular aspiration was performed 34–36 h after hCG and oocytes were retrieved at the embryology laboratory.
Shortly after oocyte retrieval (3–5 h), cumulus cells and corona radiata were removed by transferring oocytes into modified HTF-HEPES medium containing 1 mg/mL hyaluronidase for up to 1 min and pipetting through pipettes of decreasing diameter.
Oocytes that had extruded the first polar body (MII stage) and MI stage oocytes were microinjected with a single spermatozoon introduced across the zona pellucida into the ooplasm [26]. Fertilization (presence of pronuclei) was assessed 16–18 h after injection and embryo cleavage status recorded 24 h later.
Embryo quality score
On day 3 following oocyte insemination, embryo quality was assessed and a number from 1 to 4 was assigned to each embryo, 1 for best quality and 4 for worst. As a measure of overall embryo quality for each patient, an Embryo Quality Score (ES) was calculated as the weighted average of the number of embryos in each category.
Statistical analysis
Data analysis was performed for the overall patient population and for patients subdivided into two groups, according to whether they used their own or donated oocytes. Populations were characterized by their mean ± SD (Gaussian distribution) or median and interquartile range (non-Gaussian distribution), as required. Comparisons between two groups were performed by unpaired Student’s or Mann-Whitney’s test, as needed. Multiple comparisons were performed by ANOVA followed by Tukey’s multiple comparison test. Correlations between sperm parameters (DNA fragmentation and decondensation parameters) and ART outcome measures (% fertilization, % cleavage, and embryo quality score) were assessed by Pearson’s or Spearman’s correlation coefficients, as needed. Contingency tables were analyzed by Fisher’s exact test. Differences were considered significant when p < 0.05.
Results
ICSI success rate
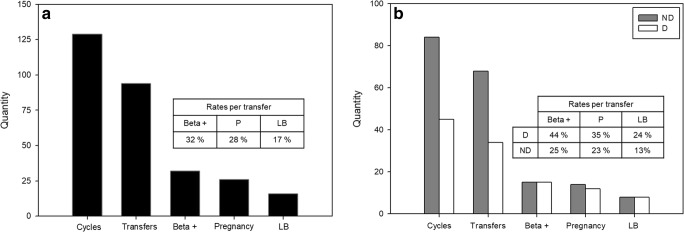
Median overall fertilization rate in the patient population studied was 80% (interquartile range 67–100, n = 129 cycles); 94 cycles resulted in embryo transfer, with a mean of 2 embryos per transfer. Biochemical and clinical pregnancy rates were 32% (30/94) and 28% (26/94) per transfer, respectively, and live birth rate was 17% (16/94) per transfer (Fig. 2a).
Fig. 2.
ICSI results for the overall patient population (panel a, n = 129) and for donor (D; n = 45, white bars) and non-donor (ND; n = 84, gray bars) cycles (panel b). Beta +, clinical pregnancy, and live birth rates (LB) are expressed per transfer. There were no significant differences in ICSI success measures (Fisher’s exact test) between donor and non-donor cycles
Donor oocytes were used in 45 out of 129 cycles; 34/45 donor and 60/84 non-donor cycles resulted in embryo transfer. Most donor cycles that did not result in transfer (8/11, 73%) did so because of a decision to cryopreserve embryos, whereas in 15/24 (60%) non-donor cycles, no viable embryos were available for transfer. Fertilization and cleavage rates were similar in both groups: median 83% (IQ = 75–100) and 80% (IQ = 62–93) fertilization in donors and non-donors, respectively; median 100% (IQ = 100–100) cleavage in both donors and non-donors (Fisher’s exact test, NS). Biochemical pregnancy, clinical pregnancy, and live birth rates per transfer were slightly higher for donor cycles but differences were not significant (Fisher’s exact test, NS) (Fig. 2b). Female patient age was 42.9 ± 0.6 and 37.3 ± 0.5 for patients using donor and non-donor oocytes, respectively (unpaired Student’s test, p < 0.0001). Oocyte donors were always under 30 years old, a rare situation among patients using their own oocytes (3/84).
Fertilized embryos were assigned a quality score as described in “Materials and methods.” Mean ES were 2.5 ± 0.1, 2.6 ± 0.1, and 2.4 ± 0.1 for the overall patient population, non-donor cycles, and donor cycles, respectively. These values were not significantly different from one another (ANOVA + Tukey’s test, NS).
Sperm DNA fragmentation
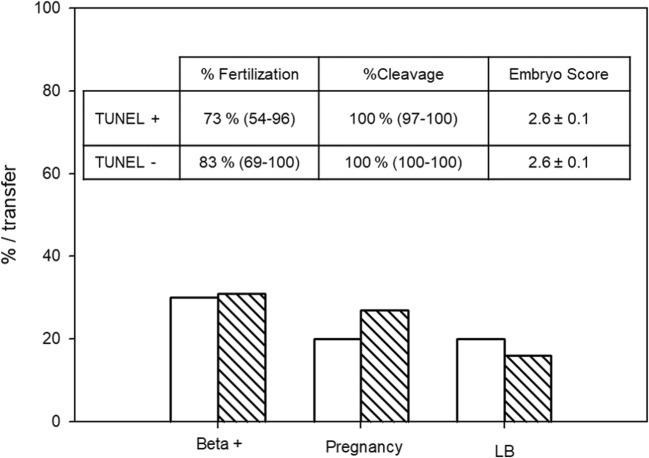
TUNEL assay was performed on spermatozoa obtained from 100 patients and resulted in a median of 9% (interquartile range 4–18). Considering a cutoff value of 20% as stated in “Materials and methods,” 16 patients were classified as TUNEL positive and 84 as TUNEL negative. Cycles involving TUNEL+ patients resulted in 73% (median; IQ = 54–96) fertilization rate, 100% cleavage rate (median; IQ = 97–100), 10 transfers, 3 biochemical pregnancies (30% per transfer), 2 clinical pregnancies (20% per transfer), and 2 live births (20% per transfer). These values were not significantly different (Fisher’s exact test, p = 1) from the corresponding values obtained for cycles involving TUNEL− patients: 83% (median; IQ = 69–100) fertilization rate, 100% cleavage rate (median; IQ = 100–100), 64 transfers, 20 biochemical pregnancies (31% per transfer), 17 clinical pregnancies (27% per transfer), and 2 live births (17% per transfer) (Fig. 3). ES was also similar between both groups: 2.6 ± 0.1 and 2.6 ± 0.3 for TUNEL− and TUNEL+ patients, respectively.
Fig. 3.
ICSI results and sperm DNA fragmentation. Patients were classified as TUNEL positive (TUNEL+, n = 16, solid bars) or TUNEL negative (TUNEL−, n = 88, striped bars) according to a cutoff value of 20%, as stated in “Materials and methods.” Fertilization and cleavage rates are expressed as median and interquartile range; ES is expressed as mean and SEM. Beta +, clinical pregnancy, and live birth rates (LB) are expressed per transfer. There were no significant differences between both groups in any of the parameters analyzed (Mann-Whitney, unpaired Student’s, or Fisher’s exact tests, as needed)
There was a low correlation of TUNEL values with fertilization rate (Spearman’s r = − 0.2049, p = 0.0317) but not with cleavage rate (Spearman’s r = − 0.1910, p = 0.065) or embryo quality as determined by ES values (Spearman’s r = 0.1875, p = 0.8647).
In vitro sperm decondensation
In vitro sperm decondensation kinetics was determined on an aliquot of every semen specimen used for ICSI (Fig. 4). This figure clearly shows that some patients achieved maximum decondensation following 30 min of incubation while others did not. Two decondensation parameters were calculated as described in “Materials and methods”: maximum sperm decondensation %(M + G) and decondensation velocity (D60/D30). Median %(M + G) was 23% (IQ = 18–33) and median D60/D30 was 1.6 (IQ = 1.2–2.3). These two parameters did not correlate with each other (Spearman’s r = 0.1185, p = 0.1618) nor with TUNEL (Spearman’s r = − 0.089, p = 0.3503 for D60/D30) or fertilization rate (Spearman’s r = − 0.081, p = 0.345 for D60/D30) or cleavage rate (Spearman’s r = − 0.0304, p = 0.7514 for D60/D30). However, sperm decondensation velocity did correlate with ES (Spearman’s r = 0.2408, p = 0.0153).
Fig. 4.
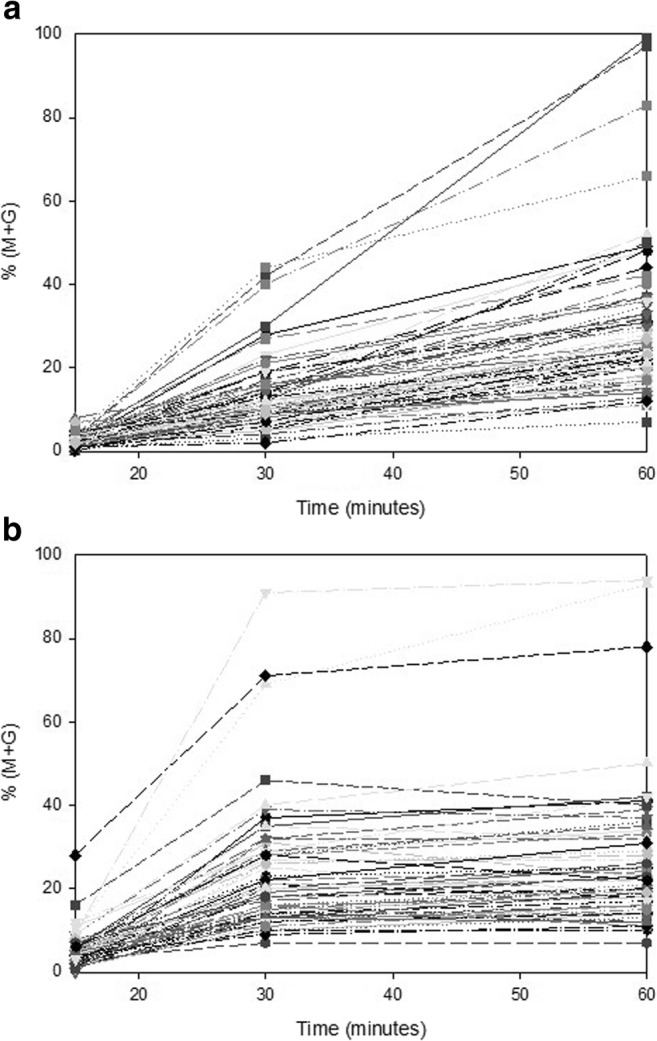
In vitro sperm decondensation kinetics. In vitro sperm decondensation kinetics for each individual patient was determined following incubation in Hep and GSH for 15, 30, and 60 min, as described in “Materials and methods.” Two decondensation parameters were calculated: maximum sperm decondensation %(M + G) after 60 min incubation and decondensation velocity as the ratio of % decondensation following 60 and 30 min of incubation (D60/D30). Panel a individual decondensation kinetics for slow decondensers. Panel b individual decondensation kinetics for fast decondensers
Sperm decondensation kinetics was also determined in a small group (n = 5) of healthy normal donors. Median %(M + G) was 34% (IQ = 29–41) and median D60/D30 was 1.2 (IQ = 1.1–1.3). Contrary to patient spermatozoa, donor spermatozoa always achieved almost maximum decondensation following 30 min of incubation. A cutoff value of 1.50 for normal D60/D30 was determined as mean + 2SD (x̅ ± SD = 1.20 ± 0.15) of donor values using the first specimen obtained from each donor.
Patients were then divided into two groups according to their D60/D30. Those with values greater than 1.5 were considered SlowD (n = 68), and those with values lower than or equal to 1.5 were classified as FastD (n = 61). As expected, decondensation velocity was significantly lower (Mann-Whitney’s test, p < 0.0001) in FastD (median 1.2 (IQ 1.7–2.7) vs. 2.2 (IQ 1.1–1.4)) than in slow decondensers, but maximum nuclear decondensation was similar (Mann-Whitney’s test, p = 0.1309) in both groups: 22% (IQ = 17–30) and 24% (IQ = 19–32) for FastD and SlowD, respectively. Semen concentration, % motility, and % morphology were similar for fast and slow decondensers (unpaired t test, p = 0.322, 0.357, and 0.256, respectively).
TUNEL and ICSI results in fast and slow decondensers
The percentage of TUNEL+ patients was similar among FastD and SlowD: 18% vs. 14%, respectively (Fisher’s exact test, p = 0.5925). Median TUNEL values were also similar (Mann-Whitney’s test, p = 0.0739) in both groups: 10% (IQ = 5–18.5) and 7% (IQ = 4–12) for FastD and SlowD, respectively.
Fertilization rate was similar (Mann-Whitney’s test, p = 0.0739) in FastD and SlowD: median 80% (IQ = 67–100) vs. 80% (IQ = 67–91), and median cleavage rate was 100% in both groups. However, overall ES was better in FastD, as evidenced by a significantly lower ES: mean ES = 2.3 ± 0.1 in FastD vs. 2.6 ± 0.1 in SlowD (unpaired t test, p = 0.0459) (Fig. 5).
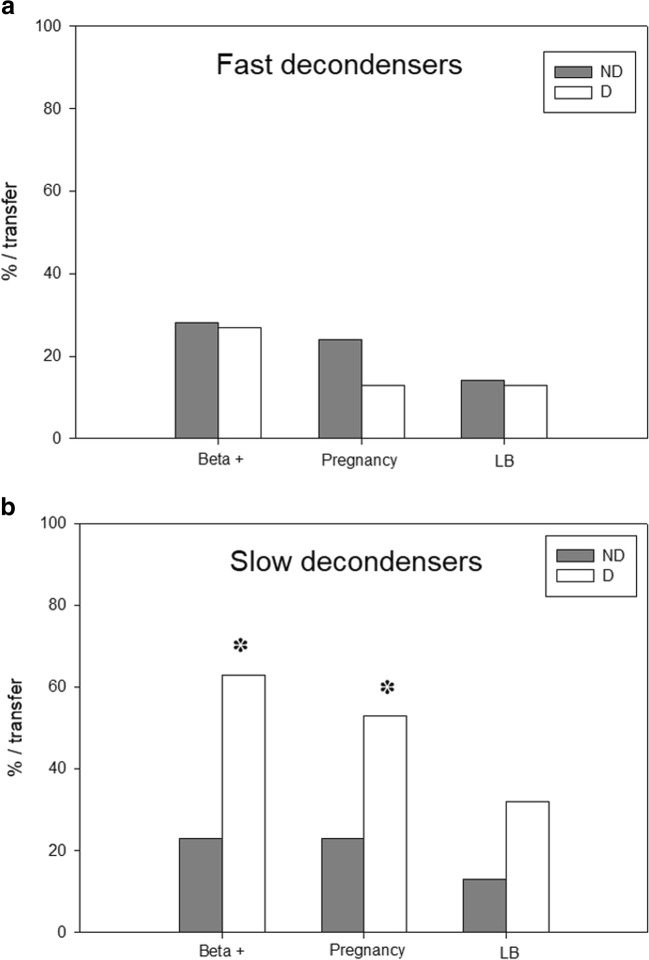
Fig. 5.
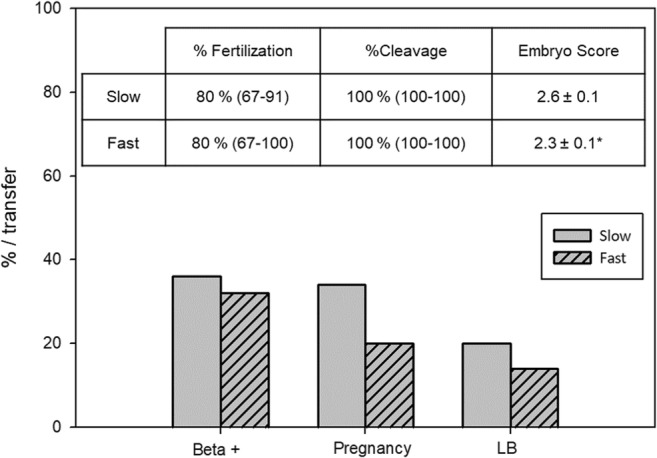
ICSI results and sperm decondensation velocity. Patients were classified as FastD (F; n = 61, striped bars) or SlowD (S; n = 68, solid bars) according to their D60/D30 value, as described in “Results.” F = D60/D30 < 1.5; S = D60/D30 ≥ 1.5. Fertilization and cleavage rates are expressed as median and interquartile range; ES is expressed as mean and SEM. Beta +, clinical pregnancy, and live birth rates (LB) are expressed per transfer. *p < 0.05, unpaired Student’s t test. No significant differences between both groups in the rest of parameters analyzed (Mann-Whitney, unpaired Student’s, or Fisher’s exact tests, as needed)
Biochemical and clinical pregnancy rates per transfer and live birth rate per transfer were similar in FastD and SlowD (Fig. 5): 27% vs. 36% (Fisher’s exact test, p = 0.3854), 20% vs. 34% (Fisher’s exact test, p = 0.17), and 14% vs. 20% (Fisher’s exact test, p = 0.58), respectively.
FastD and SlowD were subdivided into groups according to whether they underwent non-donor or donor cycles. Figure 6 depicts ICSI success rates in non-donor and donor cycles for FastD (panel a) and SlowD (panel b). Biochemical and clinical pregnancy and live birth rates per transfer were similar between non-donor (n = 29 transfers) and donor (n = 15 transfers) groups for FastD (panel a). Semen concentration, % motility, and % morphology were similar for FastD undergoing non-donor or donor cycles (unpaired t test, p = 0.845, 0.060, and 0.752, respectively). As expected, female patient’s age was significantly higher in couples using donor oocytes (unpaired t test, p < 0.0001).
Fig. 6.
ICSI results in donor and non-donor cycles in FastD (panel a) and SlowD (panel b). FastD and SlowD were subdivided into groups according to whether they underwent non-donor (ND, gray bars) or donor (D, white bars) cycles. Beta +, clinical pregnancy, and live birth rates (LB) are expressed per transfer. *p < 0.05, Fisher’s exact test
Contrarily, when SlowD were analyzed, biochemical and clinical pregnancy rates were significantly higher in donor (n = 19 transfers) cycles (Fisher’s exact test, p = 0.0164 and 0.0371, respectively). Live birth rate, though almost three times as high in donor (32%) vs. non-donor (n = 31 transfers, 13%) cycles, was not significantly different (Fisher’s exact test, p = 0.1505). Semen concentration, % motility, and % morphology were similar for SlowD undergoing non-donor or donor cycles (unpaired t test, p = 0.410, 0.227, and 0.248, respectively). As expected, female patient’s age was significantly higher in couples using donor oocytes (unpaired t test, p < 0.0001).
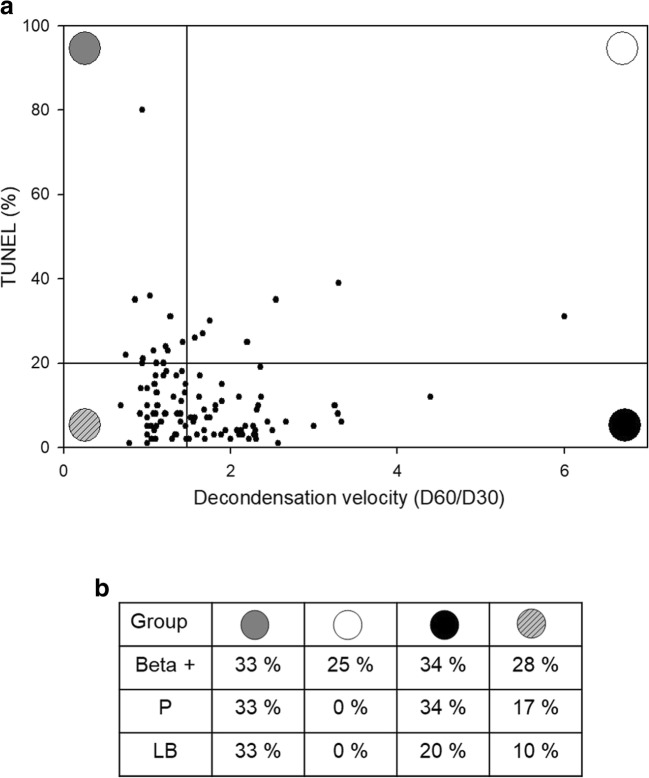
Individual patients were analyzed taking into consideration both decondensation velocity and TUNEL results. Figure 7a shows individual patient values for TUNEL and D60/D30 plotted against each other. Four groups of patients could be identified based on cutoff values for each parameter: (i) TUNEL+ FastD (n = 9), (ii) TUNEL+ SlowD (n = 7), (iii) TUNEL− FastD (n = 40), and (iv) TUNEL− SlowD (n = 44). Biochemical and clinical pregnancy and live birth rates per transfer were calculated for each group and are depicted in Fig. 7b. Even though there were no significant differences in ICSI success measures between patient groups (Fisher’s exact test, p > 0.05), those patients having abnormal values in both parameters, i.e., group (ii) TUNEL+ SlowD, showed their ICSI success rates impaired compared with the others. Accordingly, this was the only group of patients in which no clinical pregnancies or live births were achieved.
Fig. 7.
Panel a TUNEL and D60/D30 values in individual patients. Individual patient values for TUNEL and D60/D30 were plotted against each other. Four groups of patients were identified based on cutoff values for each parameter: (i) TUNEL+ FastD (n = 9, upper left quadrant), (ii) TUNEL+ SlowD (n = 7, upper right quadrant), (iii) TUNEL− FastD (n = 40, lower left quadrant), and (iv) TUNEL− SlowD (n = 44, lower right quadrant). Panel b ICSI success data for patient groups defined in panel a. Beta +, clinical pregnancy (P), and live birth rates (LB) are expressed per transfer. There were no significant differences in ART measures between patient groups (Fisher’s exact test). Gray circle: TUNEL+ FastD. White circle: TUNEL+ SlowD. Striped circle: TUNEL− FastD. Black circle: TUNEL− SlowD
Discussion
To date, despite numerous attempts at improving gamete function testing, decision-making in ART is still an arduous process for both physicians and patients. Undoubtedly, given the complex nature of the reproductive process, no single test of gamete function will ever be able to predict ART success [27, 28]. Thus, from an andrologist’s standpoint, and in an attempt to better understand the causes underlying male infertility [29], the need arises to evaluate as comprehensively as possible sperm function, addressing different aspects of sperm physiology.
In the ICSI era, as a consequence of technically bypassing the interaction of the spermatozoon with the egg vestments, only events taking place after entry into the ooplasm have acquired significance. Accordingly, special attention has been given to evaluation of the functionality of the sperm nucleus. Sperm nuclear and chromatin abnormalities have been found to be detrimental to normal fertilization, embryo development, implantation, and pregnancy [5, 11–13, 30]. A number of diagnostic assays have been developed to evaluate chromosomal abnormalities and DNA fragmentation in semen samples of patients undergoing ART, such as chromomycin A3 binding, in situ nick translation, terminal deoxynucleotidyl transferase (TUNEL), comet, sperm chromatin structure assays, protamine ratio analysis, sperm chromosomal aneuploidy evaluation, and sperm chromatin dispersion assay [13, 31, 32].
Our laboratory has been devoted to the study of human and murine sperm decondensation in vitro in the presence of Hep and GSH for several years. Sperm decondensation in vitro in the presence of Hep and GSH represents a physiological way of inducing sperm decondensation, as both reagents have been proposed as decondensing agents in vivo and concentrations used in the in vitro assay are in the physiological range [14, 23]. Preliminary data obtained more than a decade ago evaluating in vitro sperm decondensation kinetics in a small group of infertile patients (20), revealed that the Hep/GSH decondensation assay distinguished two subgroups of patients, i.e., slow and fast decondensers, and that belonging to one group or the other could not be predicted by conventional semen analysis.
The primary aim of the present study was to analyze whether the in vitro sperm decondensing ability of male partners of infertile couples undergoing ART is related to ART success. Several studies in the literature have addressed the subject of sperm chromatin condensation and decondensation; however, there is scarcity of data on the association between these parameters and ongoing pregnancy [11, 33]. Additionally, because of its widespread use to evaluate sperm nucleus functionality in our clinical setting, we evaluated whether DNA fragmentation determined by TUNEL was related to both sperm decondensing ability and ART success.
In vitro sperm decondensation parameters, i.e., maximum decondensation and decondensation velocity, did not correlate with each other or with % fertilization or cleavage, but correlated positively with embryo quality score. These results suggest that even though abnormal decondensation may not impair fertilization and cleavage, a negative effect on the embryo that may be evidenced later during development, cannot be ruled out [34].
Evaluation of sperm decondensation kinetics in the present study allowed us once again to differentiate fast decondensers who, just as normal fertile donors, achieve maximum decondensation after 30 min from slow decondensers, who need to be incubated further up to 60 min to allow for maximum decondensation.
Fertilization and cleavage rates were similar in both groups, but embryo quality was better in fast decondensers, once again suggesting a possibly delayed negative effect on embryo development. Male subfertility has been indeed associated to failures in sperm chromatin condensation. Pregnancy rates increase with sperm samples showing normal chromatin condensation, and a greater proportion of spermatozoa with normal chromatin compaction has been associated to an increase in early embryo cleavage rate [35–37].
Surprisingly, biochemical and clinical pregnancy rates and live birth rate were similar in slow and fast decondensers. However, when considering whether donor oocytes had been used in the procedure, biochemical and clinical pregnancy rates were similar among donor and non-donor cycles in fast decondensers, but in slow decondensers, both parameters were significantly improved in donor cycles. Undoubtedly, the better quality of donor oocytes, obtained from young donors, could at least in part account for this difference. It is well known that the ability of the oocyte to repair sperm DNA damage [38, 39] diminishes with age. Live birth rate, though three times higher in donor cycles, was not significantly different, probably because of the small number of patients in each subgroup. These results suggest that being a slow decondenser, a negative characteristic from our standpoint because it differs from healthy volunteers, could be a drawback for ART outcome if oocyte quality is not adequate to successfully compensate for a subfertile spermatozoon.
There is still controversy in the literature on the importance of the decondensation process for ART success. While some authors sustain that ICSI outcome is not influenced by chromatin condensation [40], others suggest that not only ICSI but also IUI results are affected by sperm decondensing ability [37]. The idea that being a slow decondenser might have a negative effect on ART outcome became evident once again in this paper when taking into consideration DNA fragmentation results. TUNEL values did not correlate with decondensation parameters, suggesting that both assays are measuring different aspects of sperm nuclear function. Moreover, median TUNEL values were similar in fast and slow decondensers, and so was the percentage of TUNEL-positive patients in both groups. However, upon simultaneous consideration of decondensation velocity (fast/slow) and TUNEL (+/−) values for each patient individually, no clinical pregnancies and thus live births were observed in slow+ subjects. These results suggest that the combination of two abnormal values of sperm nuclear function, i.e., DNA fragmentation (TUNEL+) and decondensation kinetics (slow decondensation), may impair ART success. Due to the small number of patients in this group (n = 7), it was not possible to compare results between those who used donor (n = 5) and non-donor (n = 2) oocytes, though it is tempting to speculate that even good quality donor oocytes might not be able to compensate very severe sperm defects.
It is noteworthy that TUNEL results did not correlate either with cleavage rate or with embryo quality and that TUNEL + and TUNEL− groups had similar pregnancy and live birth rates, adding to the already existing controversy on the usefulness of TUNEL as a measure of sperm fecundity in ART [7, 41]. However, the present results suggest that the combination of TUNEL with in vitro nuclear decondensation, which would provide a more comprehensive evaluation of the functionality of the sperm nucleus, might be of help in the assessment of ART outcome. Patients who, in addition to an abnormally high sperm DNA fragmentation, show an abnormally low sperm decondensation velocity seem to have their chances of success severely impaired.
Undoubtedly, we must not forget that successful reproduction involves not only a functional spermatozoon but also a functional oocyte as well. Thus, we cannot expect to predict ART outcome looking at sperm functionality alone. Nevertheless, the development of novel sperm function assays should help us pinpoint defects of sperm function and, in turn, rule out their contribution to pregnancy failure. The in vitro sperm decondensation assay used in this study is easily performed, reproducible, cost-effective and with a solid physiological basis, important attributes to be considered when looking at the possibility of its use in a clinical setting.
In summary, even though this is a pilot study and validation of these results in a larger cohort of patients is mandatory, this paper is the first to present evidence that evaluating the ability of human spermatozoa to decondense in vitro with Hep and GSH could be a valuable diagnostic tool in the Andrology Laboratory and in the process of decision-making when choosing an ART procedure.
Author’s contributions
CG, MYC, and VJ contributed substantially to acquisition, analysis, and interpretation of data corresponding to in vitro sperm decondensation. RR and LR contributed substantially to acquisition, analysis, and interpretation of data corresponding to DNA fragmentation assessment and ART performance. GRV supervised all clinical procedures. GRV, LC, JCC, VJ, and MR contributed substantially to conception and design, data analysis, and interpretation of data and drafting of the article. All authors contributed substantially to drafting and critical revision of the article and approved the final version submitted for publication.
Funding information
This work was supported by CONICET grant BID PICT 2012-2489 and by a grant from Fundación Bigand, Buenos Aires, Argentina. MYC and CG are doctoral fellows, CONICET, Argentina.
Compliance with ethical standards
This study was approved by CEPI (Ethics Committee of Hospital Italiano de Buenos Aires) and IBYME Ethics Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Esterhuizen AD, Franken DR, Becker PJ, Lourens JGH, Muller II, Van Rooyen LH. Defective sperm decondensation: a cause for fertilization failure. Andrologia. 2002;34:1–7. doi: 10.1046/j.0303-4569.2001.00423.x. [DOI] [PubMed] [Google Scholar]
- 2.Razavi S, Nasr-Esfahani MH, Mardani M, Mafi A, Moghdam A. Effect of human sperm chromatin anomalies on fertilization outcome post-ICSI. Andrologia. 2003;35:238–243. doi: 10.1046/j.1439-0272.2003.00566.x. [DOI] [PubMed] [Google Scholar]
- 3.Hamamah S, Fignon A, Lansac J. The effect of male factors in repeated spontaneous abortion: lesson from in vitro fertilization and intracytoplasmic sperm injection. Hum Reprod Update. 1997;3:393–400. doi: 10.1093/humupd/3.4.393. [DOI] [PubMed] [Google Scholar]
- 4.Saxena P, Misro MM, Chaki SP, Chopra K, Roy S, Nandan D. Is abnormal sperm function an indicator among couples with recurrent pregnancy loss? Fertil Steril. 2008;90:1854–1858. doi: 10.1016/j.fertnstert.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Steger K, Wilhelm J, Konrad L, Satlf T, Greb R, Diemer T, Kliesch S, Bergmann N, Weidner W. Both protamine-1 to protamine-2 mRNA ratio and Bcl2 mRNA content in testicular spermatids and ejaculated spermatozoa discriminate between fertile and infertile men. Hum Reprod. 2008;23:11–16. doi: 10.1093/humrep/dem363. [DOI] [PubMed] [Google Scholar]
- 6.Boulet SL, Mehta A, Kissin D, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015;313:255–263. doi: 10.1001/jama.2014.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, Schill WB, Kruger TF. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod BioMed Online. 2003;7:477–484. doi: 10.1016/S1472-6483(10)61893-7. [DOI] [PubMed] [Google Scholar]
- 8.Muriel L, Garrido N, Fernández JL, Remohí J, Pellicer A, de los Santos MJ, Meseguer M. Value of the sperm deoxyribonucleic acid fragmentation level, as measured by the sperm chromatin dispersion test, in the outcome of in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2006;85:371–383. doi: 10.1016/j.fertnstert.2005.07.1327. [DOI] [PubMed] [Google Scholar]
- 9.Lin MH, Lee RKK, Li SH, Lu CH, Sun FJ, Hwu YM. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection but might be related to spontaneous abortion rates. Fertil Steril. 2008;90:352–359. doi: 10.1016/j.fertnstert.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–831. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 11.Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–1126. doi: 10.1016/j.fertnstert.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 12.Tarozzi N, Nadalini M, Stronati A, Bizzaro D, Dal Prato L, Coticchio G, Borini A. Anomalies in sperm chromatin packaging: implications for assisted reproduction techniques. Reprod BioMed Online. 2009;18:486–495. doi: 10.1016/S1472-6483(10)60124-1. [DOI] [PubMed] [Google Scholar]
- 13.Palermo GD, Neri QV, Cozzubbo T, Rosenwaks Z. Perspectives on the assessment of human sperm chromatin integrity. Fertil Steril. 2014;102:1508–1517. doi: 10.1016/j.fertnstert.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Romanato M, Regueira E, Cameo MS, Baldini C, Calvo L, Calvo JC. Further evidence on the role of heparan sulfate as protamine acceptor during the decondensation of human spermatozoa. Hum Reprod. 2005;20:2784–2789. doi: 10.1093/humrep/dei124. [DOI] [PubMed] [Google Scholar]
- 15.Romanato M, Julianelli V, Zappi M, Calvo L, Calvo JC. The presence of heparan sulfate in the mammalian oocyte provides a clue to human sperm nuclear decondensation in vivo. Hum Reprod. 2008;23:1145–1150. doi: 10.1093/humrep/den028. [DOI] [PubMed] [Google Scholar]
- 16.Julianelli VL, Farrando B, Alvarez Sedó C, Calvo L, Romanato M, Calvo JC. Heparin enhances protamine disulfide bond reduction during in vitro decondensation of human spermatozoa. Hum Reprod. 2012;27:1930–1938. doi: 10.1093/humrep/des139. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez MC, Sedo CA, Julianelli VL, Romanato M, Calvo L, Calvo JC, Fontana VA. Dermatan sulfate synergizes with heparin in murine sperm chromatin decondensation. Syst Biol Reprod Med. 2013;59:82–90. doi: 10.3109/19396368.2012.756952. [DOI] [PubMed] [Google Scholar]
- 18.Perreault SD. Regulation of sperm nuclear reactivation during fertilization. In Bavister BD, Cummins J, Roldan ERS (eds). Fertilization in Mammals. Norwell: Serono Symposia USA; 1990. pp 285–296.
- 19.Canel NG, Bevacqua RJ, Hiriart MI, Rabelo NC, de Almeida Camargo LS, Romanato M, de Calvo LP, Salamone DF. Sperm pretreatment with heparin and L-glutathione, sex-sorting, and double cryopreservation to improve intracytoplasmic sperm injection in bovine. Theriogenology. 2017;93:62–70. doi: 10.1016/j.theriogenology.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Gaubeca-Klix E, Marin-Briggiler C, Cameo M and Calvo L. In vitro sperm nuclear decondensation in the presence of heparin/glutathione distinguishes two subgroups of infertile patients not identified by conventional semen analysis. In: Treatment of infertility: the new Frontiers (abstract book). Boca Raton, FL, 1998: Abstract PO-51.
- 21.Bergere M, Selva J, Baud M, Volante M, Martin B, Hugues JN, Olivennes F, Frydman R, Auroux M. Chromosome 18 analysis by fluorescence in situ hybridization (FISH) in human blastomeres of abnormal embryos after in vitro fertilization (IVF) attempt. Prenat Diagn. 1995;15:835–841. doi: 10.1002/pd.1970150908. [DOI] [PubMed] [Google Scholar]
- 22.WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO Press, World Health Organization; 2010.
- 23.Romanato M, Cameo MS, Bertolesi G, Baldini C, Calvo JC, Calvo L. Heparan sulphate: a putative decondensing agent for human spermatozoa in vivo. Hum Reprod. 2003;18:1868–1873. doi: 10.1093/humrep/deg354. [DOI] [PubMed] [Google Scholar]
- 24.Reyes R, Rosado A, Hernández O, Delgado NM. Heparin and glutathione: physiological decondensing agents of human sperm nuclei. Gamete Res. 1989;23:39–47. doi: 10.1002/mrd.1120230105. [DOI] [PubMed] [Google Scholar]
- 25.Bedford JM, Bent MJ, Calvin H. Variations in the structural character and stability of the nuclear chromatin in morphologically normal human spermatozoa. J Reprod Fertil. 1973;33:19–29. doi: 10.1530/jrf.0.0330019. [DOI] [PubMed] [Google Scholar]
- 26.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 27.Brown DB, Merryman DC, Rivnay B, Houserman VL, Long CA, Honea KL. Evaluating a novel panel of sperm function tests for utility in predicting intracytoplasmic sperm injection (ICSI) outcome. J Assist Reprod Genet. 2013;30:461–477. doi: 10.1007/s10815-013-9960-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavitha P, Malini SS. Positive association of sperm dysfunction in the pathogenesis of recurrent pregnancy loss. J Clin Diagn Res. 2014;8:OC07–OC10. doi: 10.7860/JCDR/2014/9109.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barratt CLR, De Jonge CJ, Sharpe R. Man up’: the importance and strategy for placing male reproductive health Centre stage in the political and research agenda. Hum Reprod. 2018;33(4):541–545. doi: 10.1093/humrep/dey020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Occhio MJ, Hengstberger KJ, Johnston SD. Biology of sperm chromatin structure and relationship to male fertility and embryonic survival. Anim Reprod Sci. 2007;101:1–7. doi: 10.1016/j.anireprosci.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Erenpreiss J, Spano M, Erenpreisa J, Bungum M, Giwercman A. Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl. 2006;8:11–29. doi: 10.1111/j.1745-7262.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 32.Evgeni E, Charalabopoulos K, Asimakopoulos B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J Reprod Infertil. 2014;15:2–14. [PMC free article] [PubMed] [Google Scholar]
- 33.Menezo Y, Clement P, Amar E. Evaluation of sperm DNA structure, fragmentation and decondensation: an essential tool in the assessment of male infertility. Transl Androl Urol. 2017;6:S553–S556. doi: 10.21037/tau.2017.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cebesoy FB, Aydos K, Unlu C. Effect of sperm chromatin damage on fertilization ratio and embryo quality post-ICSI. Arch Androl. 2006;52:397–402. doi: 10.1080/01485010600666953. [DOI] [PubMed] [Google Scholar]
- 35.Depa-Martynow M, Kempistry B, Lianeri M, Jagodizinski PP, Jedrzejczak P. Association between fertilin B, protamines 1 and 2 and sperm specific linker histone H-1 like protein mRNA levels, fertilization ability of human spermatozoa and the quality of preimplantation embryos. Folia Histochem Cytobiol. 2007;45:79–85. [PubMed] [Google Scholar]
- 36.Kazerooni T, Asadi N, Jadid L, Kazerooni M, Ghanadi A, Ghaffarpasand F, Kazerooni Y, Zolghadr J. Evaluation of sperm’s chromatin quality with acridine orange test, chromomycin A3 and aniline blue staining in couples with unexplained recurrent abortion. J Assist Reprod Genet. 2009;26:591–596. doi: 10.1007/s10815-009-9361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irez T, Sahmay S, Ocal P, Goymen A, Senol H, Erol N, Kaleli S, Guralp O. Investigation of the association between the outcomes of sperm chromatin condensation and decondensation tests, and assisted reproduction techniques. Andrologia. 2015;47:438–447. doi: 10.1111/and.12286. [DOI] [PubMed] [Google Scholar]
- 38.Meseguer M, Santiso R, Garrido N, García-Herrero S, Remohí J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95:124–128. doi: 10.1016/j.fertnstert.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 39.Jin J, Pan C, Fei Q, Ni W, Yang X, Zhang L, Huang X. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril. 2015;103:910–916. doi: 10.1016/j.fertnstert.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Karydis S, Asimakopoulos B, Papadopoulos N, Vakalopoulos I, Al-Hasani S, Nicolettos N. ICSI outcome is not associated with the incidence of spermatozoa with abnormal chromatin condensation. In Vivo. 2005;19:921–925. [PubMed] [Google Scholar]
- 41.Simon L, Emery BR, Carrell DT. Review: diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Practice & Research Clinical Obstetrics and Gynaecology. 2017;44:38–56. doi: 10.1016/j.bpobgyn.2017.07.003. [DOI] [PubMed] [Google Scholar]