Abstract
Purpose
Psychological stress exists widely in modern society and results in the disruption of testicular tight junctions, germ cell apoptosis, and the disorder of fertility hormones and even causes infertility. Ghrelin (GHRL), a 28-amino acid peptide secreted mainly by the stomach and pancreas, has been reported to alleviate male reproductive injury through inhibiting apoptosis. However, whether GHRL has a beneficial effect on psychological stress-induced testicular injury and the possible mechanisms remain poorly understood.
Methods
Male mice were immobilized in Decapicone bags for 3 h daily for 14 days treated with or without GHRL (i.p. 100 mg/kg body weight). Body weight and testicular weight were measured. Histological alterations and apoptosis were examined by H.E. staining and TUNEL staining, respectively. The expression of endoplasmic reticulum (ER) stress markers, inflammatory cytokines, Toll-like receptor 4 (TLR4), and nuclear factor-κB (NF-κB) in the testes was investigated.
Results
Exposure to stress caused testicular histological alterations, an elevation of the Johnsen score, and germ cell apoptosis, while GHRL partially alleviated the adverse effects. The expression of ER stress marker proteins, including GRP78, CHOP, ATF6, p-JNK, and XBP-1, was upregulated in the stress group; however, GHRL treatment significantly suppressed the activation of ER stress in the testes. GHRL also inhibited the expression of TNF-α, IL-1β, IL-6, IL-10, TLR4, and NF-κB.
Conclusions
GHRL alleviated testicular injury induced by ER stress and inflammation which is associated with the TLR4/NF-κB signaling pathway, and these findings may provide a novel strategy for preventing and treating reproductive dysfunction.
Keywords: Ghrelin, Endoplasmic reticulum stress, Inflammatory cytokines, TLR4/NF-κB pathway, Testicular injury
Introduction
Male infertility is becoming an increasingly common problem; it accounts for approximately 15% of the global prevalence of infertility, which represents at least 30 million infertile men worldwide [1, 2]. The testes are a vital target organ of male infertility resulting from multiple environmental factors, such as chronic psychological stress associated with a fast-paced lifestyle [3, 4]. Psychological stress results in the disturbance of testicular tight junctions, germ cell apoptosis, the disorder of fertility hormones, and a decrease in seminal quality, resulting in infertility [4].
Previous studies have suggested that endoplasmic reticulum (ER) stress is involved in testicular injury [5, 6]. Under ER stress conditions, many specific molecular chaperone proteins are involved in preventing misfolded or unfolded protein aggregation. Glucose-regulated protein78 (GRP78), as the main ER chaperone, regulates the activation of three proteins, namely, pancreatic ER kinase (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6), which are located in the ER transmembrane and act as major signaling elements [7, 8]. These stressors further activate the gene transcription of downstream genes, such as C/EBP homologous protein (CHOP), activating transcription factor 4 (ATF4), and c-Jun N-terminal kinase (JNK), and initiate cellular apoptosis upon prolonged ER stress [9]. Previous studies have demonstrated that ER stress results in cellular apoptosis and triggers inflammation in various diseases by regulating specific genes [10, 11]. For example, many elements, such as CHOP, JNK, PERK, IRE1, ATF6, and caspases, are involved in the regulation of apoptosis upon ER stress [12–14].
Ghrelin (GHRL), a natural ligand for the growth hormone secretagogue, is secreted by specific cells of the gastric mucosa and pancreas and operates as an orexigenic stomach hormone in response to nutrient restriction [15–17] and signal changes in the metabolic status to the brain [18]. GHRL has two main molecular forms, namely acylated GHRL and unacylated GHRL. Acylated GHRL is widely present in plasma and related to the regulation of GH secretion and energy metabolism [19, 20]. Acylated GHRL has already been shown to have multiple in vivo and in vitro biological functions, including influencing the immune system and metabolic process [21–23]. Unacylated GHRL can control local inflammatory responses to bacterial infection [24] and improve chronic kidney disease and skeletal muscle injury by inhibiting inflammatory signals and oxidative stress [25, 26]. Furthermore, acylated GHRL prevents umbilical endothelial cells from undergoing apoptosis by inhibiting the activation of the nuclear factor-κB (NF-κB) pathway [27, 28].
Many studies have shown that GHRL has a beneficial effect on spermatogenesis and reproductive organ development and programming [29–31]. Previous studies have indicated that GHRL protects testes against oxidative stress-induced damage, including decreased seminiferous tubule diameter and increased apoptosis and spermatogenesis cycle [32, 33]. Similarly, GHRL alleviates differentiating spermatogonia injury caused by ionizing radiation through stimulating the spermatogenic recovery and testosterone (T) secretion [34]. Furthermore, GHRL has a therapeutic effect on infertility induced by cryptorchid and testicular torsion by alleviating testicular damage and anti-oxidative activity and improving spermatogenesis in chemical drug-induced mouse models [35–37]. Psychological stress is common in modern society and results in damage to testicular cells and causes infertility [38, 39]. However, whether GHRL has a beneficial effect on psychological stress-induced testicular injury and the possible mechanisms remain poorly understood.
The current study was performed to investigate whether exogenous GHRL administration can attenuate testicular injury in a psychological stress model and the possible mechanisms. Here, we demonstrated that GHRL protected the testes from psychological stress-induced apoptosis and ER stress and inhibited inflammation through the TLR4/NF-κB pathway.
Materials and methods
Ethical approval
All animal care and use procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the Committee for the Update of the Guide for the Care and Use of Laboratory Animals and the Institute for Laboratory Animal Research and were approved by Jining Medical University (Jining, China).
Chemicals and drugs
Ghrelin (031-31) was obtained from Phoenix Pharmaceuticals (Belmont, CA, USA). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). The TUNEL kit was purchased from Promega (Madison, WI, USA). Primary antibodies against GRP78 (3183, rabbit polyclonal) and CHOP (5554, rabbit monoclonal) were obtained from Cell Signaling Technology (Beverley, MA, USA), and antibodies against β-actin (sc-47778, mouse monoclonal), XBP-1 (sc-8015, rabbit polyclonal), goat anti-rabbit (sc-2004), and goat anti-mouse (sc-2005) secondary antibodies were obtained from Santa Cruz Biotechnology (CA, USA). Antibodies against p-JNK (ab47337, rabbit polyclonal) and ATF6 (ab122897, mouse monoclonal) were obtained from Abcam Inc. (MA, USA). All the other reagents were of analytical grade.
Animals and experimental treatment
Adult BALB/c mice (7–8 weeks old, 20–25 g) were purchased from the Hubei Provincial Center for Disease Control and Prevention (Wuhan, China). The animals were provided free access to sterile standard mouse chow and water and were kept under a 12-h light:12-h dark cycle with controlled humidity and temperature (20–22 °C). All the animals were housed in polypropylene cages (three per cage) and were provided adequate bedding.
After acclimation for one week, all the animals were divided into the following 3 groups (12 mice in each group): control, stress, and GHRL + S. The mice in the stress and control groups were given 100 μL of saline by intraperitoneal (i.p.) injection every day. The mice in the stress group were immobilized in Decapicone bags (Braintree Scientific Company, Braintree, MA, USA) and placed in individual cages in a fume hood for 3 h daily for 14 days [40]. The mice in the GHRL + S group were injected with GHRL (100 mg/kg body weight) i.p. before they were immobilized in the Decapicone bags. The body weight of each mouse was measured prior to sacrifice. The testes were immediately excised and weighed after the experimental mice were sacrificed using diethyl ether anesthesia 24 h after the last stress treatment.
Testicular histology detection
The left testes were fixed in 4% paraformaldehyde and then embedded in paraffin to produce approximately 5-μm-thick serial sections using a microtome. At least two non-serial sections were stained with hematoxylin and eosin (H.E.) using standard procedures for morphological analysis. Each specimen was evaluated using the Johnsen score (JS) [41].
Terminal dUTP nick-end labeling staining
For the detection of apoptosis, paraffin-embedded sections were stained by the terminal dUTP nick-end labeling (TUNEL) technique using an in situ apoptosis detection kit according to the manufacturer’s protocols. The images were scanned and analyzed using a panoramic slide scanner (3D-Histech, Budapest, Hungary).
Quantitative real-time PCR
Total RNA from testicular tissues was extracted by using TRIzol regent (Invitrogen Life Technologies, Carlsbad, CA, USA), and the concentration was determined by a Nanodrop spectrophotometer (Thermo Fisher, Wilmington, USA). An equal amount (1 μg) of RNA from each sample was reverse transcribed into cDNA using the PrimeScript Reagent Kit with gDNA Eraser (TaKaRa, Dalian) and oligo (dT) primers according to the manufacturer’s protocol. Quantitative real-time PCR was performed by using SYBR® Premix Ex Taq™ II (Takara, Dalian, China) in a 20 μL reaction volume, which included 2 μL of cDNA and 10 μM primer. The thermocycling condition used was as follows: 1 cycle, 95 °C for 1 min; 40 cycles, 95 °C for 20 s (denaturation), 60 °C for 30 s, and 72 °C for 20 s. Quantitative real-time PCR analysis was conducted with the Roche LC480 II Light-cycler. The mRNA levels were determined using the 2−ΔΔCt method and standardized to the levels of β-actin.
Western blot analysis
Testis tissues (~ 50 mg) were homogenized in radio immune precipitation (RIPA) buffer supplemented with protease and phosphatase inhibitors. The protein concentration of each supernatant was measured by using the Bradford’s method. Equal amounts of denatured proteins were separated by electrophoresis on 10% SDS-PAGE gels according to standard protocols. The proteins were electrophoretically transferred onto polyvinylidene difluoride membranes (Hybond-P PVDF, Amersham Biosciences, Little Chalfont, UK), which were blocked for 1 h at room temperature in TBS-Tween-20 containing 5% skim milk. Western blot analysis was performed using primary antibodies (GRP78, CHOP, p-JNK, XBP-1, ATF6, all diluted 1:1000) and incubated with HRP-conjugated anti-rabbit and anti-mouse secondary antibodies (diluted 1:1000). Immunoreactivity was detected by enhanced chemiluminescence reagents (Pierce, Rockford, IL) according to the manufacturer’s protocol. The relative integrated density of each protein band was digitized by ImageJ 2× (1.48 v, National Institutes of Health, USA).
Statistical analysis
The results are presented as the mean ± SEM. The significance of the difference was analyzed by one-way analysis of variance (ANOVA) followed by post-hoc Duncan’s multiple-range test using GraphPad Prism 8 software (https://www.graphpad.com/scientific-software/prism). The results were considered statistically significant at P < 0.05.
Results
GHRL treatment ameliorates changes in testicular weight and histology caused by stress
To evaluate the effect of GHRL on stress-induced testicular dysfunction, organic parameters were recorded after the mice were killed. Stress treatment resulted in a marked reduction in testicular injury. As shown in Table 1, the testicular weights and relative testis weights (the ratios of testicular weight/body weight) of the mice in the stress-treated group were 7.57 and 13.72% lower, respectively, compared to those of the mice in the control group. However, the reduction was partially reversed after the administration of GHRL (100 mg/kg).
Table 1.
The effect of GHRL on the weight of body and reproductive organs in stress-induced mice
| Weight | Control | Stress | GHRL + S |
|---|---|---|---|
| Body (g) | 27.11 ± 0.53a* | 21.03 ± 0.36b | 22.37 ± 0.30c |
| Testis (mg) | 182.32 ± 2.73a | 168.51 ± 17.29b | 195.26 ± 6.31c |
| Epididymis (mg) | 2.30 ± 0.08 | 2.15 ± 0.29 | 2.52 ± 0.08 |
| Testis/BW (%) | 7.36 ± 0.68a | 6.35 ± 0.47b | 7.88 ± 0.33a |
| Epididymis/BW (%) | 62.50 ± 1.77a | 46.98 ± 6.19b | 56.71 ± 1.99a |
All data were expressed as means ± SEM. n = 12
*P < 0.05 indicated statistical significance with different letters
We further examined whether GHRL inhibits stress-induced morphological changes in the testes using H.E. staining (Fig. 1). Normal histology was observed in the control group. In the stress group, morphological result showed severe vacuolation and apoptotic germ cells in the seminiferous tubules. However, histological injury of the seminiferous tubules was markedly ameliorated by exogenous GHRL treatment. The Johnsen score was significantly lower in the stress group than in the control group, while GHRL treatment inhibited the decrease in Johnsen score compared to that in the stress group (Fig. 1).
Fig. 1.
Effect of GHRL on stress-induced histopathological change in testes. Testicular cross sections were stained with Hematoxylin and eosin (H.E.). Original magnification: ×200. In the stress group, mice showed severe vacuolation (arrows), exudative germ cells (asterisks) and atrophy of spermatogenic cells in the seminiferous tubules compared to the mice in the control group, however, GHRL treatment significantly attenuated adverse effect induced by stress (P < 0.05). A significant decrease in Johnsen score was detected in stress group (P < 0.05), whereas the effect was partly reversed when compared to GHRL group (P < 0.05). All results are expressed as mean ± SEM, n = 4. *P < 0.05 indicated statistical significance with different letters
GHRL treatment ameliorates stress-induced germ cell apoptosis
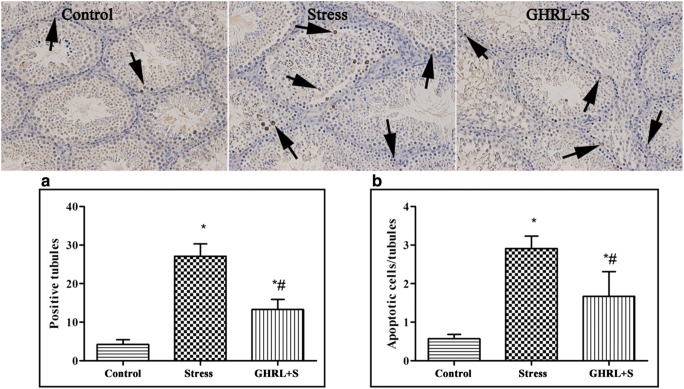
We further examined germ cell apoptosis in the mice testes using the TUNEL assay (Fig. 2). TUNEL results revealed that stress led to severe germ cellular apoptosis. Although the percentage of positively stained seminiferous tubules increased significantly when compared to that in the control group (P < 0.05), GHRL treatment significantly attenuated the rate of apoptosis induced by stress (P < 0.05) (Fig. 2a). Similarly, GHRL prevented the increase in TUNEL-positive cells per seminiferous tube induced by stress (P < 0.05) (Fig. 2b).
Fig. 2.
Effect of GHRL on stress-induced testicular germ cell apoptosis. Original magnification: 200×. Apoptotic germ cell was showed by TUNEL staining (brown). Arrow showed apoptotic germ cells in seminiferous tubules. (a) The analysis of percentage of the number of seminiferous tubules showing TUNEL-signal. (b) The average of TUNEL-positive germ cells in tubules. In the stress group, the mice showed a significant increase in the number of seminiferous tubules and average of TUNEL-positive germ cells in the tubules compared to the mice in the control group; however, GHRL treatment significantly attenuated the rate of apoptosis induced by stress (P < 0.05). All results are expressed as mean ± SEM, n = 4. *P < 0.05 indicated significantly different with the control group; #P < 0.05 indicated significantly different with the stress group
The effect of GHRL on stress-induced ER stress
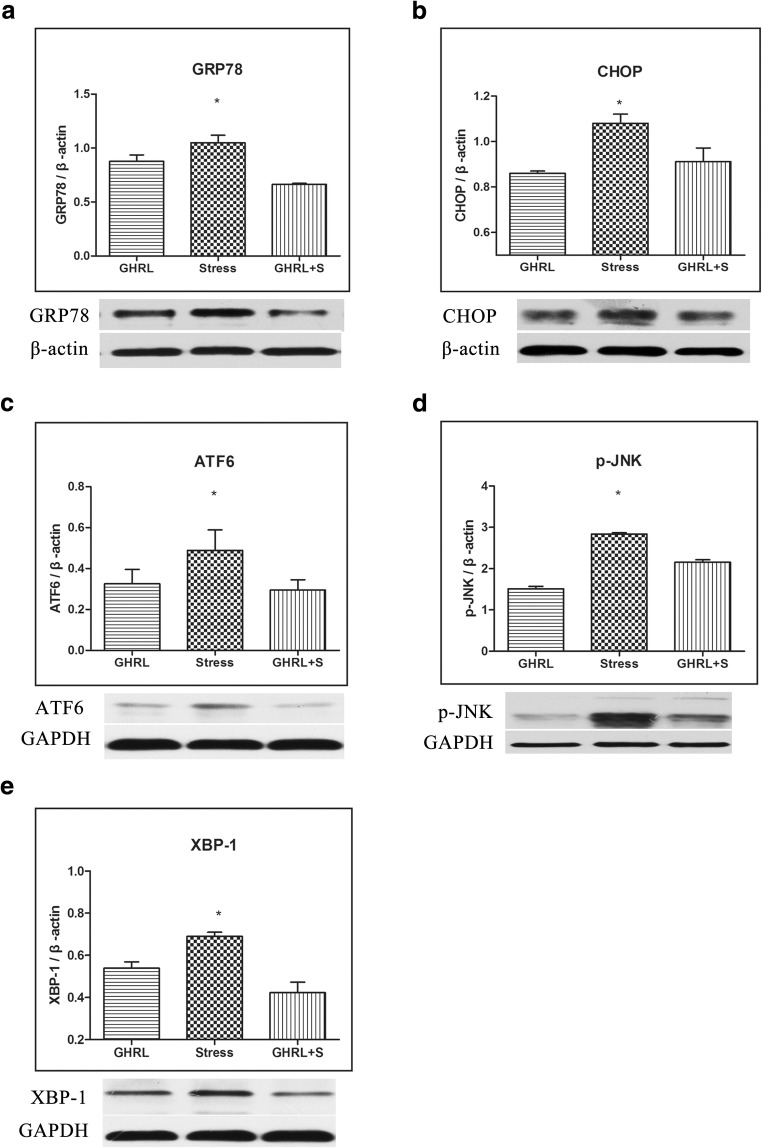
To explore the possible mechanisms by which GHRL protects the testes against apoptosis, we investigated the ability of GHRL to alleviate ER stress (Fig. 3). The results showed that the expression of ER stress marker, including GRP78, CHOP, ATF6, p-JNK, and XBP-1 proteins, was significantly upregulated in the stress group when compared with the control group; however, this effect was partly reversed upon pretreatment with GHRL (P < 0.05). These results suggested that the effect of GHRL on apoptosis may be associated with ER stress.
Fig. 3.
Effect of GHRL on stress-induced ER stress in testes. (a–e) The expression levels of GRP78, CHOP, ATF6, p-JNK, and XBP-1 in the testes tissue were determined by Western blot with specific antibodies as indicated. Stress significantly increased the expression of ER stress marker, including GRP78, CHOP, ATF6, p-JNK, and XBP-1, when compared with the control group; however, this effect was partly reversed upon pretreatment with GHRL. The relative protein levels of all the genes were quantified by ImageJ 2×. All data were expressed as means ± SEM, n = 4. *P < 0.05 as compared with the controls
GHRL inhibits the inflammatory response and TLR4/NF-κB pathway
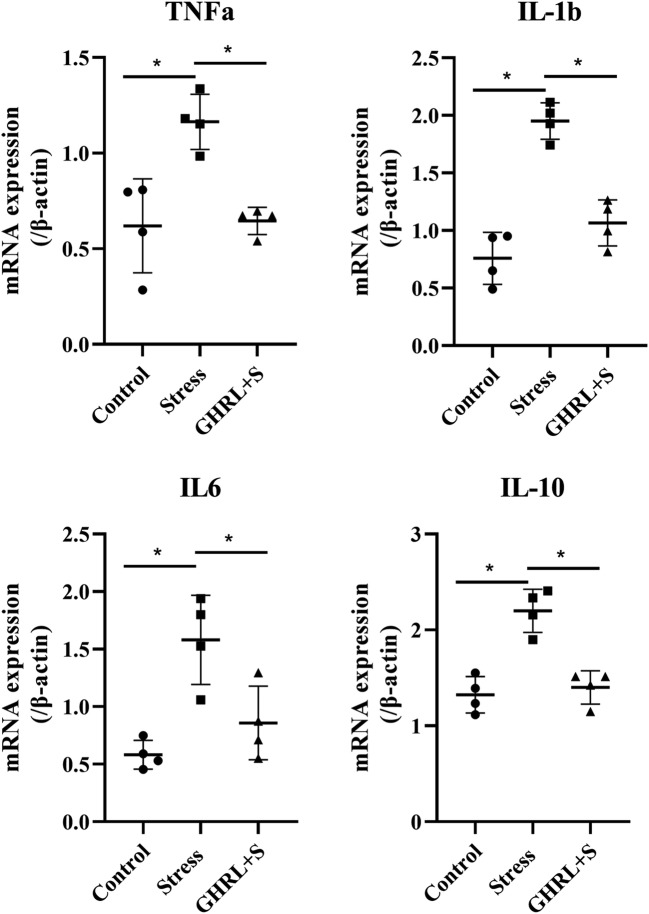
We investigated whether GHRL inhibits the expression of inflammatory cytokines induced by stress and the possible mechanisms of this action (Fig. 4). Stress induced the upregulation of TNF-α, IL-1β, IL-6, and IL-10 levels, but GHRL significantly attenuated this effect (P < 0.05). Similarly, compared to stress, GHRL significantly inhibited the upregulation of TLR4 and NF-κB (Fig. 5). All of the results suggested that GHRL inhibited the expression of pro-inflammatory cytokines through the TLR4/NF-κB pathway.
Fig. 4.
Effect of GHRL on the expression of inflammatory cytokines in mice testes. The relative expressions of TNF-α, IL-1β, IL-6, and IL-10 were determined by q-PCR. The quantitative data was given and calculated by dividing β-actin mRNA expression. The expression of TNF-α, IL-1β, IL-6, and IL-10 was significantly increased in the stress group compared to that in the control group (P < 0.05), while GHRL treatment ameliorated this effect (P < 0.05). All data were expressed as means ± SEM of four animals in each group. *P < 0.05 indicated significantly different between groups
Fig. 5.
Effect of GHRL on the TLR4/NF-κB pathway. (a) The expression level of TLR4 and NF-κB in the testis tissue was determined by Western blot with specific antibodies. (b) Quantitative data of expression in all groups. Stress significantly increased the expression of TLR4 and NF-κB, while GHRL treatment ameliorated this effect. The values represent mean ± S.E.M. of four animals in each group. *P < 0.05 indicated significantly different with the control group and GHRL + S group
Discussion
Psychological stress existed widely in the fast-paced lifestyle of modern society. Epidemiological investigation has showed that 25% of the American population reports high stress and that 50% can identify a major stressful event in the previous decades [42]. Chronic psychological stress increases the rate of health problems, results in a significant decline in the quality of semen, and even causes infertility [43]. Therefore, it is important to explore novel therapeutics to minimize the influence of stress.
Immobilization is commonly adopted to simulate psychological stress, and it results in germ cell apoptosis and a disorder in testosterone secretion [38, 39]. However, it is unknown whether ER stress is associated with immobilization stress-induced male reproductive injury. In the current study, we found that ER stress was involved in testicular injury induced by immobilization stress, as determined by the expression of major marker proteins of the ER stress response in an immobilization mouse model. When Sertoli or Leydig cells are under oxidative stress or chemical stress, many partially folded and/or unfolded proteins accumulate and aggregate in the ER lumen and then induce ER stress when the capacity of the ER to deal with the disturbance is surpassed [44, 45]. ER stress then activates the specific unfolded protein response (UPR), which enhances the capacity of ER to deal with misfolded and/or unfolded proteins by regulating transcription and translation [46]. Currently, at least three functionally distinct components of the UPR have been directly associated with the occurrence of ER stress, specifically, an initial decrease in general protein synthesis in the organelle, the promotion of protein folding via ER-resident chaperones (e.g., GRP78), and the degradation of accumulating misfolded proteins [12, 47, 48]. Furthermore, ER stress accompanied by inflammation has been associated with testicular injury, such as testosterone synthesis and secretion.
GHRL, a protective reproductive autocrine/paracrine molecule that participates in the regulation of testicular function, has been reported to protect male reproductive function from injury suppressing degenerative effects following testicular hyperthermia, reducing the spermatogenesis cycle following experimental varicocele, and reversing reproductive dysfunction in high fat diet-fed rats [49–51]. The molecular mechanisms of these protective effects are associated with the enhancement of anti-oxidative stress ability and the inhibition of cell apoptosis [35, 36, 52]. However, whether GHRL has a beneficial effect on psychological stress-induced testicular injury and the possible mechanisms remain poorly understood. The current study first focused on whether GHRL alleviates the adverse effects of psychological stress-induced reproductive injury in an immobilization stress model and the role of GHRL in ER stress and the inflammatory response. Previous reports have indicated that GHRL exerts anti-apoptotic effects in various cells and an ischemia/reperfusion (I/R) model [53–55]. Furthermore, GHRL has been shown to have a protective effect against oxidative stress and to contribute to a decrease in microvascular endothelial cell apoptosis [56].
Similarly, GHRL reduces CCl4-induced hepatocellular injury and cell apoptosis by decreasing the activation of nuclear factor κB (NF-κB), as assessed by p65 nuclear translocation in humans [57]. Furthermore, it has been reported that desacyl GHRL protects cardiomyocytes against doxorubicin-induced cardiac fibrosis and apoptosis, most likely through ERK1/2 phosphorylation and the PI3K/Akt pathway, which is a GHSR-independent mechanism [58]. The mechanism by which GHRL inhibits apoptosis has not yet been fully elucidated, but it may be associated with the activation of the PI3K/Akt signaling pathway [59–61]. Although increasing evidence has demonstrated that GHRL has an effect on the inflammatory response and oxidative stress, the possible mechanism by which GHRL affects anti-inflammatory and anti-oxidative stress ability is unclear.
Previous studies have shown that GHRL inhibits lipopolysaccharide-induced inflammatory cytokines in mouse kidneys; however, no mechanism was reported in the study [52]. A similar study reported that GHRL prevents germ cells from undergoing apoptosis and proinflammatory cytokine production in I/R of rat testes; however, the possible mechanism remains unknown [62]. In the present study, we first reported that the ability of GHRL to inhibit inflammation and apoptosis may be associated with the suppression of TLR4/NF-κB in the testes in an immobilization stress mouse model. Some studies have revealed that GHRL is implicated in improving immunocyte reactions and the inflammatory environment, by inhibiting the production of various pro-inflammatory cytokines, including TNF-a, IL1β, IL6, and IL8 in human endothelial cells [63], monocytes, and T cells [64]. Previous evidence has increasingly suggested that ER stress enhances the inflammatory response through ERK1/2, PI3K/AKT, and NF-κB activation [63]. Similarly, previous studies have demonstrated that GHRL inhibits the inflammatory response by activating the ERK1/2 and AKT signaling pathways in various cell models [59, 65] and alleviating ER stress-induced inflammation through MAPK and NF-κB activation [66]. Furthermore, some other signaling pathways are involved in the ER stress-induced inflammatory response [67].
Conclusion
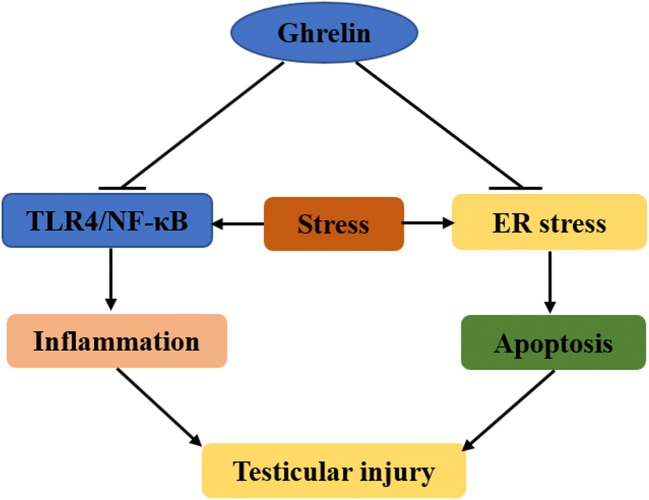
In conclusion, our results revealed that GHRL reduced ER stress induced by stress. GHRL protected the testes against the stress-induced inflammatory response, which was associated with promoting the TLR4/NF-κB signaling pathway (Fig. 6). GHRL may be a potential benefit treatment for male reproductive dysfunction caused by emotional stress.
Fig. 6.
Schematic diagram of the therapeutic effects of GHRL against testicular injury induced by stress
Funding information
This study was supported by the National Natural Science Foundation Cultivation Project of Jining Medical University (JYP201725) and the Fund for Teachers’ Research of Jining Medical University (JY2017KJ029).
Compliance with ethical standards
The authors declare that there are no conflicts of interest that influenced this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agarwal A, Ahmad G, Sharma R. Reference values of reactive oxygen species in seminal ejaculates using chemiluminescence assay. J Assist Reprod Genet. 2015;32:1721–1729. doi: 10.1007/s10815-015-0584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alahmar AT. Role of oxidative stress in male infertility: an updated review. J Hum Reprod Sci. 2019;12:4–18. doi: 10.4103/jhrs.JHRS_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhongade MB, Prasad S, Jiloha RC, Ray PC, Mohapatra S, Koner BC. Effect of psychological stress on fertility hormones and seminal quality in male partners of infertile couples. Andrologia. 2015;47:336–342. doi: 10.1111/and.12268. [DOI] [PubMed] [Google Scholar]
- 4.Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. 2018;16:10–20. doi: 10.1016/j.aju.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karna KK, Choi BR, You JH, Shin YS, Soni KK, Cui WS, Lee SW, Kim CY, Kim HK, Park JK. Cross-talk between ER stress and mitochondrial pathway mediated adriamycin-induced testicular toxicity and DA-9401 modulate adriamycin-induced apoptosis in Sprague-Dawley rats. Cancer Cell Int. 2019;19:85. doi: 10.1186/s12935-019-0805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi W, Guo Z, Yuan R. Testicular injury attenuated by rapamycin through induction of autophagy and inhibition of endoplasmic reticulum stress in streptozotocin-induced diabetic rats. Endocr Metab Immune Disord Drug Targets. 2019;19:665–675. doi: 10.2174/1871530319666190102112844. [DOI] [PubMed] [Google Scholar]
- 7.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors role of ATP biding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 8.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 9.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 10.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 13.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 14.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 16.Hayashida T, Murakami K, Mogi K, Nishihara M, Nakazato M, Mondal MS, Horii Y, Kojima M, Kangawa K, Murakami N. Ghrelin in domestic animals: distribution in stomach and its possible role. Domest Anim Endocrinol. 2001;21:17–24. doi: 10.1016/s0739-7240(01)00104-7. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi H, Kurose Y, Suzuki Y, Kojima M, Yamaguchi T, Yoshida Y, Ogino M, Hodate K, Azuma Y, Sugino T. Ghrelin differentially modulates the GH secretory response to GHRH between the fed and fasted states in sheep. Domest Anim Endocrinol. 2009;37:55–60. doi: 10.1016/j.domaniend.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 19.Martín-Pastor M, De Capua A, Alvarez CJP, Díaz-Hernández MD, Jiménez-Barbero J, Casanueva FF, Pazos Y. Interaction between ghrelin and the ghrelin receptor (GHS-R1a), a NMR study using living cells. Bioorg Med Chem. 2010;18:1583–1590. doi: 10.1016/j.bmc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Murtuza MI, Isokawa M. Endogenous ghrelin-O-acyltransferase (GOAT) acylates local ghrelin in the hippocampus. J Neurochem. 2018;144:58–67. doi: 10.1111/jnc.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Churm R, Caplin S, Barry J, Davies JS, Stephens JW, Prior SL. Acyl-ghrelin mediated lipid retention and inflammation in obesity-related type 2 diabetes. Mol Cell Endocrinol. 2019;481:8–13. doi: 10.1016/j.mce.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Fang C, Xu H, Guo S, Mertens-Talcott SU, Sun Y. Ghrelin signaling in immunometabolism and inflamm-aging. Adv Exp Med Biol. 2018;1090:165–182. doi: 10.1007/978-981-13-1286-1_9. [DOI] [PubMed] [Google Scholar]
- 23.Qu R, Chen X, Hu J, Fu Y, Peng J, Li Y, Chen J, Li P, Liu L, Cao J, Wang W, Qiu C, Guo L, Vasilev K, Chen J, Zhou G, Li W, Zhao Y. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci Rep. 2019;9:1348. doi: 10.1038/s41598-018-38174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yılmaz G, Kırzıoğlu FY, Doğuç DK, Koçak H, Orhan H. Ghrelin levels in chronic periodontitis patients. Odontology. 2014;102:59–67. doi: 10.1007/s10266-012-0100-3. [DOI] [PubMed] [Google Scholar]
- 25.Gortan Cappellari G, Zanetti M, Vinci P, Guarnieri G, Barazzoni R. Unacylated ghrelin: a novel regulator of muscle intermediate metabolism with potential beneficial effects in chronic kidney disease. J Ren Nutr. 2017;27:474–477. doi: 10.1053/j.jrn.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Gortan Cappellari G, Semolic A, Ruozi G, Vinci P, Guarnieri G, Bortolotti F, Barbetta D, Zanetti M, Giacca M, Barazzoni R. Unacylated ghrelin normalizes skeletal muscle oxidative stress and prevents muscle catabolism by enhancing tissue mitophagy in experimental chronic kidney disease. FASEB J. 2017;31:5159–5171. doi: 10.1096/fj.201700126R. [DOI] [PubMed] [Google Scholar]
- 27.Han K, Wang Q-Y, Wang C-X, Luan S-Y, Tian W-P, Wang Y, Zhang R-Y. Ghrelin improves pilocarpine-induced cerebral cortex inflammation in epileptic rats by inhibiting NF-κB and TNF-α. Mol Med Rep. 2018;18:3563–3568. doi: 10.3892/mmr.2018.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu R, Chen X, Wang W, Qiu C, Ban M, Guo L, Vasilev K, Chen J, Li W, Zhao Y. Ghrelin protects against osteoarthritis through interplay with Akt and NF-κB signaling pathways. FASEB J Off Publ Fed Am Soc Exp Biol. 2018;32:1044–1058. doi: 10.1096/fj.201700265R. [DOI] [PubMed] [Google Scholar]
- 29.Torres PJ, Luque EM, Ponzio MF, Cantarelli V, Diez M, Figueroa S, Vincenti LM, Carlini VP, Martini AC. The role of intragestational ghrelin on postnatal development and reproductive programming in mice. Reprod Camb Engl. 2018;156:331–341. doi: 10.1530/REP-18-0192. [DOI] [PubMed] [Google Scholar]
- 30.Martins AD, Sá R, Monteiro MP, Barros A, Sousa M, Carvalho RA, Silva BM, Oliveira PF, Alves MG. Ghrelin acts as energy status sensor of male reproduction by modulating Sertoli cells glycolytic metabolism and mitochondrial bioenergetics. Mol Cell Endocrinol. 2016;434:199–209. doi: 10.1016/j.mce.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Kheradmand A, Dezfoulian O, Alirezaei M, Rasoulian B. Ghrelin modulates testicular germ cells apoptosis and proliferation in adult normal rats. Biochem Biophys Res Commun. 2012;419:299–304. doi: 10.1016/j.bbrc.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Asadi N, Kheradmand A, Gholami M, Moradi FH. Effect of ghrelin on the biochemical and histopathology parameters and spermatogenesis cycle following experimental varicocele in rat. Andrologia. 2018;50:e13106. doi: 10.1111/and.13106. [DOI] [PubMed] [Google Scholar]
- 33.Hazrati A., Salimnejad R., Alipour M. R., Mirzaei Bavil F., Alihemmati A. Protective effect of ghrelin on testicular damages caused by chronic hypoxia in rats: A histopathological study. Andrologia. 2018;50(4):e12989. doi: 10.1111/and.12989. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Zeng Y, Zhao J, Zhu C-J, Hou W-G, Zhang S. Upregulation and nuclear translocation of testicular ghrelin protects differentiating spermatogonia from ionizing radiation injury. Cell Death Dis. 2014;5:e1248. doi: 10.1038/cddis.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi E, Boekelheide K, Sigman M, Hall SJ, Hwang K. Ghrelin modulates testicular damage in a cryptorchid mouse model. PLoS One. 2017;12:e0177995. doi: 10.1371/journal.pone.0177995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarac M, Bakal U, Tartar T, Kuloglu T, Yardim M, Artas G, Aydin S, Kazez A. Ghrelin and NUCB2/Nesfatin-1 expression in unilateral testicular torsion-induced rats with and without N-acetylcysteine. Cell Mol Biol (Noisy-le-grand) 2017;63:40–45. doi: 10.14715/cmb/2017.63.7.7. [DOI] [PubMed] [Google Scholar]
- 37.Salimnejad R., Soleimani Rad J., Mohammad Nejad D., Roshangar L. Effect of ghrelin on total antioxidant capacity, lipid peroxidation, sperm parameters and fertility in mice against oxidative damage caused by cyclophosphamide. Andrologia. 2017;50(2):e12883. doi: 10.1111/and.12883. [DOI] [PubMed] [Google Scholar]
- 38.Hou G, Xiong W, Wang M, Chen X, Yuan T-F. Chronic stress influences sexual motivation and causes damage to testicular cells in male rats. J Sex Med. 2014;11:653–663. doi: 10.1111/jsm.12416. [DOI] [PubMed] [Google Scholar]
- 39.Nargund VH. Effects of psychological stress on male fertility. Nat Rev Urol. 2015;12:373–382. doi: 10.1038/nrurol.2015.112. [DOI] [PubMed] [Google Scholar]
- 40.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnsen Svend G. Testicular Biopsy Score Count – A Method for Registration of Spermatogenesis in Human Testes: Normal Values and Results in 335 Hypogonadal Males. Hormone Research in Paediatrics. 1970;1(1):2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 42.Oken BS, Chamine I, Wakeland W. A systems approach to stress, stressors and resilience in humans. Behav Brain Res. 2015;282:144–154. doi: 10.1016/j.bbr.2014.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arun S, Burawat J, Sukhorum W, Sampannang A, Maneenin C, Iamsaard S. Chronic restraint stress induces sperm acrosome reaction and changes in testicular tyrosine phosphorylated proteins in rats. Int J Reprod Biomed Yazd Iran. 2016;14:443–452. [PMC free article] [PubMed] [Google Scholar]
- 44.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2003;18:575. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 45.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 46.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 47.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozkanli S, Basar MM, Selimoglu S, Erol B, Ozkanli O, Nurili F, Kahraman S. The ghrelin and orexin activity in testicular tissues of patients with idiopathic non-obstructive azoospermia. Kaohsiung J Med Sci. 2018;34:564–568. doi: 10.1016/j.kjms.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asadi N, Kheradmand A, Gholami M, Moradi FH. Effect of ghrelin on the biochemical and histopathology parameters and spermatogenesis cycle following experimental varicocele in rat. Andrologia. 2018;50:e13106. doi: 10.1111/and.13106. [DOI] [PubMed] [Google Scholar]
- 51.Dallak M. Unacylated ghrelin stimulates steroidogenesis in lean rats and reverses reproductive dysfunction in high fat diet-fed rats. Syst Biol Reprod Med. 2019;65:129–146. doi: 10.1080/19396368.2018.1523971. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Bansal S, Falk S, Ljubanovic D, Schrier R. Ghrelin protects mice against endotoxemia-induced acute kidney injury. Am J Physiol Ren Physiol. 2009;297:F1032–F1037. doi: 10.1152/ajprenal.00044.2009. [DOI] [PubMed] [Google Scholar]
- 53.Andreis PG, Malendowicz LK, Trejter M, Neri G, Spinazzi R, Rossi GP, Nussdorfer GG. Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett. 2003;536:173–179. doi: 10.1016/s0014-5793(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 54.Waseem T, Duxbury MS, Ito H, Rounds J, Gonzalez JE, Lautz D, et al. Ghrelin: a novel humoral mediator of intestinal adaptation. Gastroenterology. 2004;126:A136.
- 55.Guney Y, Turkcu UO, Hicsonmez A, Andrieu MN, Kurtman C. Ghrelin may reduce radiation-induced mucositis and anorexia in head-neck cancer. Med Hypotheses. 2007;68:538–540. doi: 10.1016/j.mehy.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 56.Shimada T, Furuta H, Doi A, Ariyasu H, Kawashima H, Wakasaki H, Nishi M, Sasaki H, Akamizu T. Des-acyl ghrelin protects microvascular endothelial cells from oxidative stress-induced apoptosis through sirtuin 1 signaling pathway. Metabolism. 2014;63:469–474. doi: 10.1016/j.metabol.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Moreno M, Chaves JF, Sancho-Bru P, Ramalho F, Ramalho LN, Mansego ML, Ivorra C, Dominguez M, Conde L, Millán C. Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology. 2010;51:974–985. doi: 10.1002/hep.23421. [DOI] [PubMed] [Google Scholar]
- 58.Pei XM, Yung BY, Yip SP, Ying M, Benzie IF, Siu PM. Desacyl ghrelin prevents doxorubicin-induced myocardial fibrosis and apoptosis via the GHSR-independent pathway. Am J Physiol-Endocrinol Metab. 2014;306:E311–E323. doi: 10.1152/ajpendo.00123.2013. [DOI] [PubMed] [Google Scholar]
- 59.Granata R, Settanni F, Trovato L, Destefanis S, Gallo D, Martinetti M, Ghigo E, Muccioli G. Unacylated as well as acylated ghrelin promotes cell survival and inhibit apoptosis in HIT-T15 pancreatic β-cells. J Endocrinol Investig. 2006;29:RC19–RC22. doi: 10.1007/BF03347367. [DOI] [PubMed] [Google Scholar]
- 60.Miao Y, Xia Q, Hou Z, Zheng Y, Pan H, Zhu S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2007;359:795–800. doi: 10.1016/j.bbrc.2007.05.192. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Ying B, Shi L, Fan H, Yang D, Xu D, Wei Y, Hu X, Zhang Y, Zhang X. Ghrelin inhibit cell apoptosis in pancreatic β cell line HIT-T15 via mitogen-activated protein kinase/phosphoinositide 3-kinase pathways. Toxicology. 2007;237:194–202. doi: 10.1016/j.tox.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Taati M, Moghadasi M, Dezfoulian O, Asadian P, Zendehdel M. Effects of ghrelin on germ cell apoptosis and proinflammatory cytokines production in ischemia-reperfusion of the rat testis. Iran J Reprod Med. 2015;13:85–92. [PMC free article] [PubMed] [Google Scholar]
- 63.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-κB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 64.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chung H, Chung H-Y, Bae CW, Kim C-J, Park S. Ghrelin suppresses tunicamycin- or thapsigargin-triggered endoplasmic reticulum stress-mediated apoptosis in primary cultured rat cortical neuronal cells. Endocr J. 2011;58:409–420. doi: 10.1507/endocrj.k10e-396. [DOI] [PubMed] [Google Scholar]
- 66.Wu L, Wang D, Xiao Y, Zhou X, Wang L, Chen B, Li Q, Guo X, Huang Q. Endoplasmic reticulum stress plays a role in the advanced glycation end product-induced inflammatory response in endothelial cells. Life Sci. 2014;110:44–51. doi: 10.1016/j.lfs.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 67.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–264. doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]