Abstract
Bisphenol A is an endocrine disruptor associated with hormone synthesis and reproduction alterations. However, the initiating events underpinning these dysfunctions are still unclear. Here, we address the hypothesis that BPA interferes with the highly evolutionary conserved process of mitochondrial cholesterol transport, a crucial step in steroid hormone biosynthesis, by using the model organism C. elegans. We observed that embryonic lethality and germline apoptosis, hallmarks of BPA’s reproductive toxicity in C. elegans, are fully rescued by low exogenous cholesterol supplementation. We also observed that increasing BPA concentrations proportionally reduced mitochondrial cholesterol levels. Mutants for strl-1 (ortholog of StAR), but not C41G7.9 (TSPO), show reproductive defects similar to BPA’s while BPA exposure in a strl-1 background did not worsen these effects. Finally, cholesterol supplementation rescued these defects for all strl-1 genotype/BPA combinations assessed. Together, these results uncover a novel mechanism underlying BPA’s germline toxicity through the alteration of cholesterol transport.
Keywords: C. elegans, Bisphenol A, reproductive toxicity, cholesterol, germline
INTRODUCTION
Bisphenol A is a chemical widely used in the manufacturing of plastics that is found in a multitude of common consumer products. Exposure to BPA is ubiquitous via food, drinks, dental fillings, the handling of thermal paper receipts and many more [1–4]. Frequent and long-term exposure to BPA renders it universally detectable in human fluids and tissues including in reproductive ones such as ovarian follicular fluids and the placenta [5, 6].
As a well-documented environmental endocrine disrupting compound, exposure to BPA in mammals has been shown to impair the normal function of the endocrine system and to cause reproductive dysfunctions in males and females including decreased sperm count, sperm motility impairment [7], altered folliculogenesis [8–10], and defective meiotic process [11–13] among others [reviewed in [14]]. While BPA appears to act at several independent levels to reduce fertility, BPA’s reproductive dysfunctions are often accompanied by hormonal imbalance [8, 15–20]. An intriguing possibility for BPA’s mode of action of reproduction may be its ability to interfere with the early step of the steroidogenesis pathway, such as the modulation of cholesterol entry into the mitochondria. Steroid hormone synthesis follows a complex pathway by which cholesterol is converted to biologically active steroid hormones through a series of reactions [21]. To initiate steroidogenesis, cholesterol stored in the cytosol needs to be transported to the matrix of the inner mitochondria membrane, where P450 cholesterol side-chain cleavage enzyme (P450scc; CYP11A1) is located [22]. Cross-membrane cholesterol transport is facilitated by transporter proteins localized on the outer mitochondrial membrane. These transporters, which are highly conserved across species, include steroidogenic acute regulatory protein (StAR) and potentially the translocator protein (TSPO), although conflicting sets of evidence of that role exist for the latter [23, 24]. In mammals, StAR is a 37-kDa protein localized on the outer mitochondrial membrane in steroidogenic cells and carries the rate limiting activity in mitochondrial cholesterol delivery [25, 26]. Although the molecular mechanism of StAR-mediated cholesterol transport remains poorly understood, the indispensable role of StAR in cholesterol translocation was well demonstrated by the cytosolic cholesterol accumulation in murine StAR knockout models [27, 28].
The evidence pertaining to the relationship between BPA and cholesterol transport is ambiguous and mostly based on the impact of BPA exposure on StAR expression where a divergence of effects, either reduction or increase in StAR expression, and consequently differing impacts on downstream hormone production, have been reported [29–33]. However, beyond moderate changes in gene expression, the impact of BPA on StAR function and activity, on cholesterol homeostasis, and their significance for reproduction are unknown.
Here, we use the genetic and reproductive model system Caenorhabditis elegans (C. elegans) to elucidate the complex relationship between cholesterol transport and BPA’s reproductive toxicity. Due to the evolutionary conservation of many biological pathways, C. elegans is a common model organism for the study of reproduction (reviewed in [34]) and is increasingly recognized as a valuable model for the study of toxicity responses [35]. We previously showed that the nematode recapitulates key features of germline response to BPA in rodent models including an alteration of double-strand break repair dynamics, aberrant chromosome morphology and division, and an increase in aneuploidy [12, 36]. Here, to investigate the relationship between BPA and cholesterol, we rely on three important advantages of the model. First, unlike mammals who synthesize cholesterol primarily in the liver and intestine, C. elegans lacks the enzyme necessary for de novo synthesis of sterols [37, 38] and is therefore a cholesterol auxotroph that relies on exogenous cholesterol supplementation from its environment [39, 40]. This feature allows us to precisely manipulate the intracellular levels of cholesterol level by modulating the amount of exogenous cholesterol in the culture medium. Second, C. elegans shows a high degree of conservation of cholesterol transporters including StAR and TSPO (Fan and Papadopoulos 2013). Finally, C. elegans also relies on cholesterol-derived hormones to regulate its reproduction and other biological functions. Specifically, cholesterol can be metabolized by DAF-9 (homolog of CYP17A1 in human) to form dafachronic acids, such as Δ4- and Δ7- dafachronic acids, that can bind and activate the nuclear receptor DAF-12 (homolog of human nuclear receptors VDR/LXR) to regulate, among other processes, reproductive development, fat metabolism, and lifespan [41–43].
In this study, we modulated both cholesterol and BPA exogenous levels and uncovered that exogenous cholesterol fully rescues BPA’s negative impacts on the germline and reproduction. In line with this finding, we observed an imbalanced cholesterol distribution between cytosol and mitochondria in the BPA-exposed group. Additionally, by knocking out StAR, we successfully recapitulated the reproductive phenotype of BPA-exposed worms. Furthermore, BPA exposure was unable to further impair the reproduction of StAR mutant worms, suggesting that StAR is crucial for BPA to elicit its toxicity. Together, our results indicate that BPA impairs StAR-dependent mitochondrial cholesterol transport to induce germline dysfunction.
MATERIALS AND METHODS
Animal maintenance
Bristol N2 C. elegans were used as wild type and maintained on the nematode growth medium (NGM) at 20°C. E. Coli OP50 was used as a food source. Strains with mutation in strl-1 (ok3347) I obtained from the Caenorhabditis Genetics Center and C41G7.9 (tm5526) I obtained from the National Bioresource Project were subjected to the same assays as N2 to identify the critical mitochondrial cholesterol transporter that BPA relied on to exhibit its toxicity. For quantitative RT-PCR studies, we used glp-1(bn18) III.
Exposure
Eggs obtained from the sodium hypochlorite treated gravid wild type, strl-1 or C41G7.9 mutant worms were placed on the NGM plates mixed with 0, 100, and 500 μM BPA. These doses were chosen based on previous experiments where a modest (100 μM) or pronounced (500 μM) effect on germline function was observed [36]. The low dose of 100 μM was measured by GC-MS to approximate human physiological levels that approximate organ BPA levels in human follicular fluids, placenta and umbilical cord samples [44–46]. The cholesterol concentrations used in combination with those of BPA were 0, 0.5, and 5 μg/mL cholesterol which correspond to the minimum required for worm growth and reproduction (0.5 μg/mL) and the standard concentration of 5 μg/mL [47]. Exposure lasted from the birth to the end of reproductive periods of tested worms. Ethanol was used as the vehicle and its final medium concentration was 0.1% for all experiments.
Fertility assessment
To assess the fertility damage caused by the exposure, the numbers of eggs laid by each nematode during its entire reproductive period, larvae successfully hatched from eggs, and offspring surviving to adulthood were separately tallied. The percentage of unhatched eggs and the number of living progeny at 4 days, representing embryonic lethality and brood size respectively, were calculated.
Germline Apoptosis assay
Worms between 20 and 24 hrs post L4 stage were incubated in M9 solution with 25 μg/mL of acridine orange (AO) for 2 hours at room temperature [12]. AO specifically labels engulfed apoptotic germline nuclei and emits 525 nm fluorescence after excitation. The total number of apoptotic nuclei in the pachytene zone of the posterior gonad of each worm was counted. Apoptotic index was calculated as the percentage of apoptotic nuclei divided by the total number of nuclei in the pachytene zone.
Germline morphology examination
To examine the exposures caused germline morphological alterations, wholemount nuclei staining was performed on exposed worms. Briefly, adult worms 20–24 hrs post-L4 were firstly fixed by Carnoy’s fixation and then subjected to DAPI staining. Images from the posterior gonad of each worm were captured and analyzed. The numbers of germline nuclei in each meiotic stage were tallied separately.
Mitochondria Isolation and purification
Mitochondria of nematodes were extracted with the use of Mitochondria Isolation Kit (Sigma-Aldrich, Cat. MITOISO1). For each repeat of an exposure group, 2 g of worms was collected, rinsed with M9 solution, incubated with lysis buffer EBA containing trypsin for 20 mins and then homogenized on ice. Lysates were centrifuged at 11,000 g for 10min at 4°C. The precipitates were re-suspended and collected as the crude mitochondria extraction with lysosomes. To purify the collected crude mitochondria, sucrose density gradient ultracentrifugation was performed as described before [48]. Purified mitochondria were collected, re-suspended with PBS buffer and then stored at −80°C. until use.
Cholesterol Assay
The concentration of cholesterol in mitochondria of worms from each exposure group was measured with the use of Amplex™ Red Cholesterol Assay kit (Invitrogen™, Cat. A12216). Briefly, cholesterol ester in the mitochondria was firstly hydrolyzed by cholesterol esterase into cholesterol, which was then oxidized by cholesterol oxidase to yield H2O2. The H2O2 then reacted with Amplex Red reagent in the presence of horseradish peroxidase and yielded highly fluorescent resorufin with emission maxima of approximately 595 nm. The cholesterol level of a sample could be measured based on the fluorescent intensity of resorufin. The protein level of each sample was measured as well with the use of Bio-Rad DC™ Protein Assay Kit to correct the cholesterol level.
Quantitative RT-PCR
qRT-PCR was performed as previously described [12]. For germline enrichment assessment, three samples of 30 worms each were collected in TRIzol for four conditions: glp-1 mutants were maintained at either 15 °C (permissive temperature) or 25 °C (restrictive temperature). For expression of cholesterol transporter families, N2 worms were exposed to ethanol or BPA (500 μM) with or without cholesterol (5 μg/mL). In all cases, the RNA was extracted, and reverse transcription was carried out using Transcriptor Reverse Transcriptase (Roche) according to the manufacturer’s protocol. qPCR was performed using Brilliant II SYBR green QPCR master mix with low Rox (Stratagene), following the manufacturer’s instructions. Each sample was run in duplicate. Sample values were normalized to gpd-1 (GAPDH) for the germline enrichment study and spo-11 for the exposure studies in order to normalize the signal for germline size. The primer pairs used were: C06H2.2 F: ttcaagacgaaagccgttct, R: caggtgtctttcgatgagca; F25H2.6: F: tgtcaaaagccgaaaaatcc, R: ctgtttcccggacaactgat; T28D6.7: F: atcgagcaaaatgggacaag, R: gtcttcgtggcataccgaat; F45H7.2: F: tccgatagttggaggtacgg, R: tttcgactgtctcgtgttgg; K02D3.2: F: gtggttcgagcccaaactta, R: aaatgacgcaattccctttg; F26F4.4: F: tcggagtgagcaaagacaga, R: gaagaaccgccaataggaca; F52F12.7 strl-1: F: ttgtgtcagcgagaatttgg, R: ttcgggattctcaggatttg.
Statistical test and data representation
For all experiments, a one-way ANOVA with Tukey’s multiple comparisons test was used. Unless stated otherwise, the figures represent all data points inside of a box plot where the edges of the boxes are at the 25th and 75th percentiles, the bar at the median and the edge of the whiskers at the minimum and maximum.
RESULTS
Exogenous cholesterol rescues BPA-induced decreased fertility
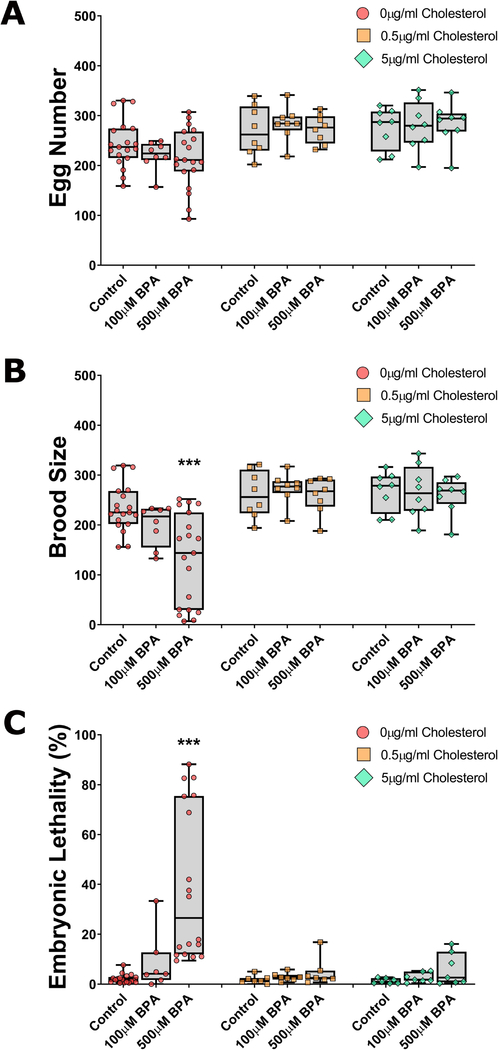
We first examined the impact of two concentrations of BPA (100 and 500 μM) on reproductive features under varying levels of exogenous cholesterol: none, the minimum required for worm growth and reproduction (0.5 μg/mL), and the commonly used concentration of 5 μg/mL [47]. Without cholesterol, worms grow to normal size and are fertile, although they show a slight reduction in brood size. The next generation, which we did not examine here, however, is strongly impacted with severe growth and molting defects and complete sterility [37, 39]. The total number of eggs produced, the percentage of unhatched eggs (embryonic lethality), and the number of progeny successfully reaching adulthood (brood size) were examined per worm. In absence of cholesterol, we observed a BPA concentration-dependent decrease in egg number and brood size, and increase in embryonic lethality, the latter two reaching significance (P<0.001) at the highest concentration of BPA compared to control (Figure 1). This was in line with previous reports [12, 36]. By contrast, in the presence of cholesterol (0.5 μg/mL or 5 μg/mL), no significant difference was observed between the control and the two BPA concentrations for all three fertility measures. These results indicate that BPA-induced reproductive impact is dependent on the absence of exogenous cholesterol.
Figure 1: BPA’s characteristic fertility endpoinds are fully rescued by exogenous cholesterol administration.
Nematodes deprived of cholesterol from L1 stage to adulthood and concomitantly exposed to BPA (either at 100 μM or 500 μM) show a dose-dependent trend towards a reduction in egg number (A) and brood size (B), and an increase in embryonic lethality (C). However, co-administration of cholesterol at either 0.5 μg/mL or 5 μg/mL is sufficient to alleviate these effects. N=8–19 depending on the exposure group. ***P<0.001.
Cholesterol supplementation is sufficient to abrogate BPA-induced germline dysfunction
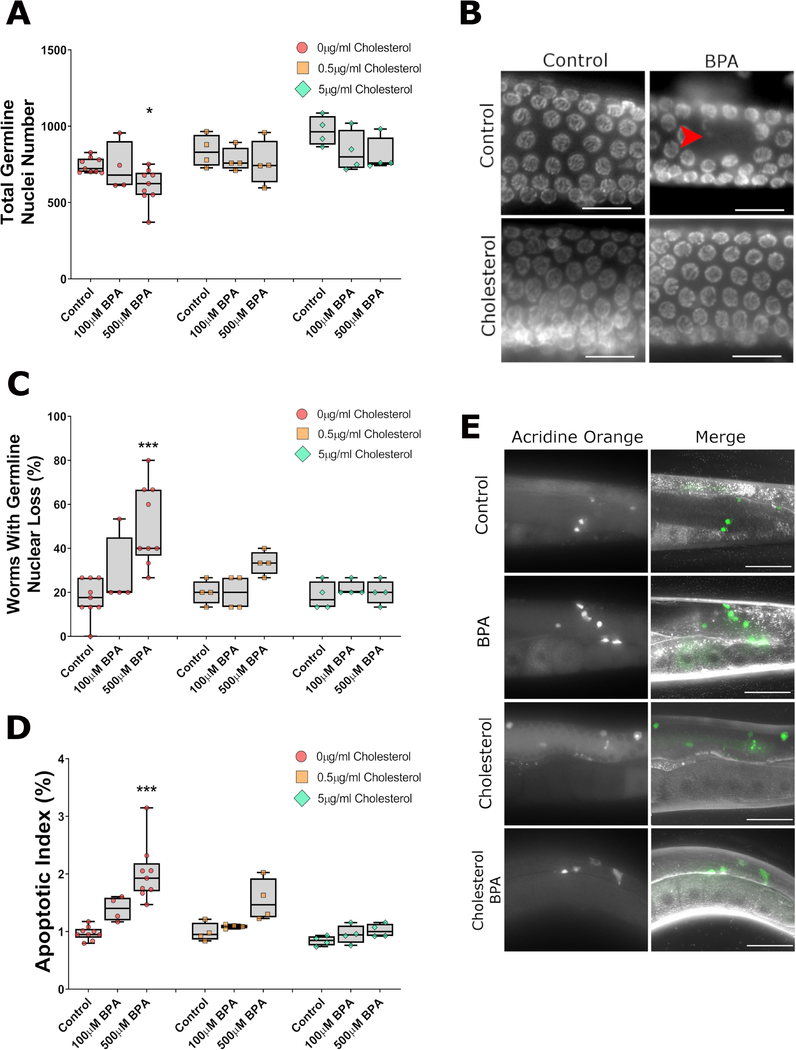
A well-conserved feature of BPA’s impact on reproduction is its alteration of critical meiotic events such as synaptonemal complex formation and meiotic recombination, which result in increased germline apoptosis and nuclear loss [12, 36]. We therefore assessed whether exogenous cholesterol would also rescue these hallmarks of BPA exposure on the germline. In absence of cholesterol, exposure to 500 μM of BPA caused a reduction in the total number of germline nuclei (−16%, P<0.05, Figure 2A) and a corresponding increase in the frequency of morphological abnormalities of the germline such as nuclear gaps, defined as an empty space equivalent to ≥5 nuclei, in the germline (P<0.001, Figure 2B and 2C). These findings were in accordance with the apoptosis assay result as 500 μM BPA doubled the incidence of apoptotic nuclei compared the control (P<0.001, Figure 2D and 2E). By contrast, exposure to 5 μg/mL of cholesterol rescued all defects while the intermediate concentration of cholesterol led to an amelioration of BPA-induced defects but not a full rescue except for the total germline nuclei outcome. Together with the fertility assessment results, these findings suggest that BPA’s impact on the germline can be alleviated by exogenous administration of cholesterol.
Figure 2: BPA-induced germline defects are dependent on the absence of cholesterol.
Examination of germlines 24hrs post-L4 from worms exposed to BPA with or without cholesterol reveals a dependency on cholesterol for the BPA-induced reduction in total number of nuclei in the gonad (A), the increase in the frequency of worms with nuclear loss (i.e. presence of gap larger than 5 nuclei) (B, C), or the increase in apoptotic index (D, E) defined as the proportion of all nuclei undergoing apoptosis as identified by acridine orange staining. BPA and cholesterol concentrations for the panels B and E were 500 μM and 5 μg/mL respectively. ***P<0.001; *P<0.05. The data plotted represents 4–9 independent repeats, each obtained from the averaged values of approximately 5 worms (A), 12 worms (C), and 10 worms (D). Scale bars in B = 10 μm, scale bars in E = 100 μm.
BPA disrupts intracellular cholesterol homeostasis
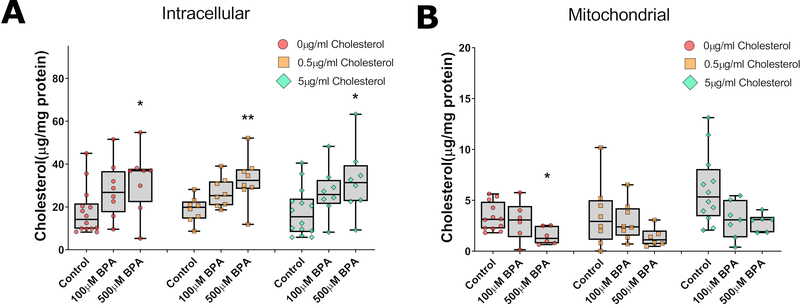
The rescue of BPA’s fertility and germline defects by exogenous cholesterol administration suggests that BPA may alter cholesterol homeostasis. We therefore assessed intracellular cholesterol distribution by measuring the total intracellular and mitochondrial levels of cholesterol under varying BPA concentrations. As shown in Figure 3, the total intracellular cholesterol level was not affected by exogenous cholesterol administration and it remained on average at 19 μg/mg, (normalized to protein level) even under no cholesterol conditions, suggesting a tight regulation of cholesterol intracellular level. By contrast, addition of BPA increased total cholesterol levels reaching significance at 500 μM BPA (1.5-fold increase over control, P<0.05) regardless of the cholesterol level in the media (Fig. 3A). Despite the sharp increase in intracellular cholesterol levels when worms are exposed to BPA, we observed a concentration-dependent decrease in mitochondrial cholesterol level in all groups exposed to BPA. This change was most significant in worms exposed to 500 μM BPA without cholesterol (Fig. 3B). Together, these results indicate that BPA disrupts the levels and distribution of cholesterol inside the cells.
Figure 3: BPA exposure modulates intracellular and mitochondrial cholesterol levels.
Total cholesterol levels (A) or levels measure in purified mitochondria (B) were measured. BPA exposure revealed a concentration-dependent increase in intracellular cholesterol but a decrease in mitochondrial levels. **P<0.01; *P<0.05. N=6–14 depending on the exposure group, each repeat generated from 2 g of worms.
BPA relies on StAR but not TSPO to elicit its reproductive toxicity
If BPA reproductive and germline defects are caused by interfering with cholesterol mitochondrial transport, we would predict that (1) null mutants for the two main proposed cholesterol transporters, namely StAR and TSPO, should phenocopy BPA’s defects, and (2) in the context of these mutant backgrounds, BPA should not lead to a further decrease in fertility or germline function. We therefore exposed wild type (N2) worms as well as strl-1 (StAR) or C41G7.9 (TSPO) mutants to BPA and performed a fertility assessment and germline morphology examination as described above.
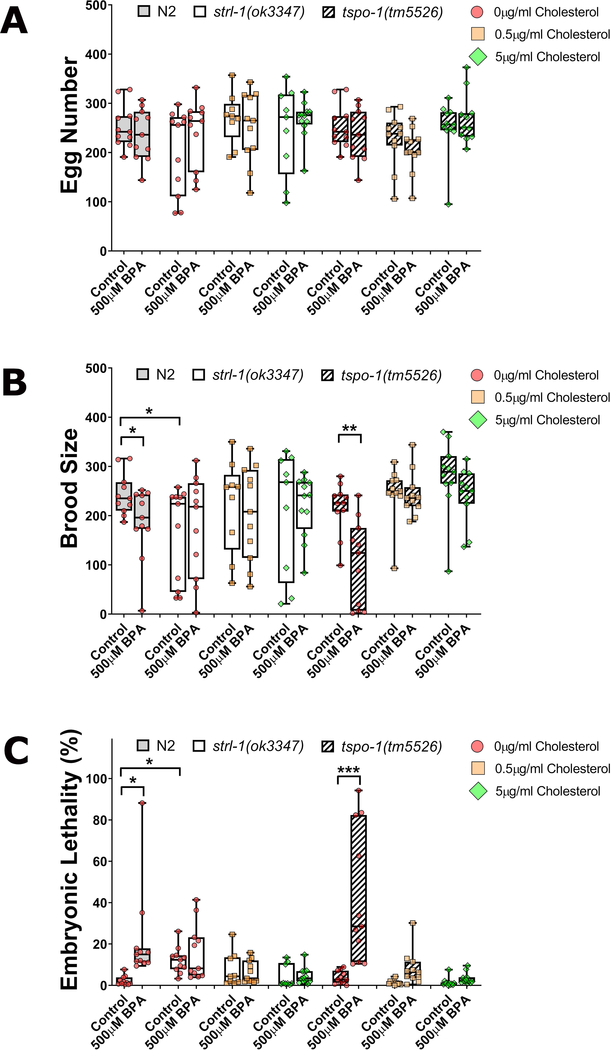
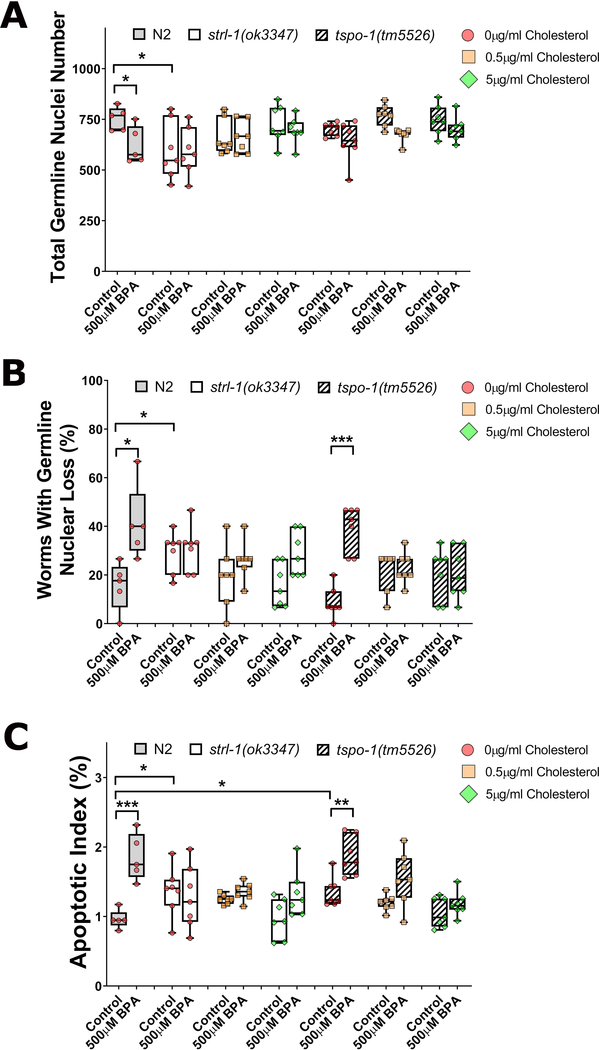
As shown in figure 4, compared to wild type, the overall reproductive ability of StAR mutants was reduced as evidenced by an increased embryonic lethality, and reduced brood size (Figure 4A–C). Furthermore, we also observed germline dysfunctions highly reminiscent to those of BPA’s, including decreased germline nuclei number, an increase in the incidence of germline gaps, and elevated germline apoptosis (Figure 5A–C). All parameters were ameliorated by the addition of cholesterol although the brood size showed a noticeable wide spread. BPA exposure did not worsen any of the fertility or germline parameters observed and showed levels statistically comparable to their unexposed control at all levels of cholesterol tested. These findings indicate that StAR is a crucial mediator for BPA’s elicitation of reproductive toxicity effects.
Figure 4: strl-1 mutants, but not C41G7.9, phenocopy BPA reproductive effects and shows reduced sensitivity to BPA.
Wild type (N2), strl-1(ok3347), or C41G7.9(tm5526) worms were exposed to BPA at the highest concentration 500 μM under varying concentrationss of cholesterol. Egg number (A), brood size (B), and embryonic lethality (C). ***P<0.001; *P<0.05. N=9–12 individual worms were analyzed.
Figure 5: TSPO mutants do not show reproductive or germline phenotypes are sensitive to BPA exposure.
Wild type (N2), strl-1(ok3347), or C41G7.9(tm5526) worms were exposed to BPA at the highest concentration 500 μM under varying concentrationss of cholesterol. total germline nuclei number (A), presence of nuclear loss (B), and apoptotic index (C) were measured. ***P<0.001; **P<0.01; *P<0.05. Values plotted represent the average of approximately 5 (A), 12 (B), and 10 worms (C) over 5 to 7 individual repeats.
In contrast to strl-1 mutants, C41G7.9 mutants did not show reproductive or germline defects at levels significantly different from wild type. In C41G7.9 mutants, BPA exposure led to a significant reduction in brood size and a significant increase in embryonic lethality (P<0.05, Figure 4A–C). In line with these reproductive parameters, the total germline nuclei number was also significantly reduced, and accompanied by an increase in germline apoptosis and gap frequency (Figure 5A–C). These results indicate that StAR, but not TSPO, is required for the elicitation of BPA’s reproductive toxicity.
To test whether BPA exposure may downregulate the level of StAR which may cause reproductive dysfunction, we first assessed whether StAR is indeed expressed in the germline of C. elegans. Since C. elegans’ genome codes for 7 proteins, including STRL-1/StAR, containing a START (StAR-related lipid-transfer domain) and therefore show homology to StAR and are predicted to bind lipids [49], we monitored the expression by qRT-PCR of all seven START domain-containing genes in glp-1 temperature sensitive mutants at permissive and non-permissive temperatures where the germline is mostly absent. This allowed to quickly determine the expression and relative enrichment of all seven members in the germline. As shown in Figure S1, four transporters F25H2.6, F26F4.4, C06H2.2 and strl-1 showed a pronounced germline enrichment. Next, we assessed whether their expression was altered by BPA exposure. All four transcripts were upregulated following BPA exposure compared to control and two transcripts, including strl-1, were reduced following cholesterol co-administration (Figure S2). Together, our results indicate that the change in expression levels of strl-1 is in the opposite direction to the intra-mitochondrial levels of cholesterol, suggesting that strl-1 overexpression might be due to a sensing of reduced cholesterol concentration by the mitochondria and the cell.
Downstream cholesterol derivatives, DA-4 and DA-7, are not sufficient to rescue BPA’s reproductive outcomes
C. elegans relies on cholesterol derivatives named dafachronic acids to regulate reproduction [42]. Since changes in levels and distribution of cholesterol may alter their synthesis and thereby cause the phenotypes observed, we tested two important dafachronic acids, namely DA-4 and DA-7, and assessed whether their administration could rescue the effects of BPA. To test this hypothesis, we co-exposed worms to BPA and a mixture of DA-4 and DA-7 at levels shown to be physiologically relevant for the worm’s growth and reproduction [50]. While cholesterol supplementation was able to rescue BPA’s effect on embryonic lethality, the most sensitive reproductive endpoint in all our experiments, DA-4/DA-7 co-administration was not sufficient in reverting BPA’s effects (Figure S3). These results therefore suggest other cholesterol-derived products, and not DA-4 and DA-7, are involved in the rescue of BPA’s reproductive endpoints.
DISCUSSION
C. elegans relies on cholesterol derivatives to regulate many biological functions, such as development, reproduction and aging [41–43]. In this study, we uncovered that cholesterol supplementation is sufficient to mitigate all BPA-mediated reproductive dysfunctions in C. elegans. We assessed the intracellular cholesterol levels and found a positive association between intracellular cholesterol deregulation and BPA-elicited reproductive damage. We also showed that BPA exposure leads to a reduction of cholesterol mitochondrial levels in whole worms. Furthermore, we showed that mitochondrial cholesterol transporter StAR, but not TSPO, is required for BPA to exhibit its reproductive toxicity. It is worth noting that the doses of BPA used in this study were based on our previous studies [12, 36] in order to generate a range of internal doses in C. elegans which would approximate organ BPA levels in human tissues such as placental [45] and umbilical cord samples [44]. This study is the first one to report that exogenous cholesterol can rescue BPA exposure induced germline dysfunction and restore the fertility of exposed organisms. The study thus identifies a novel mode of action for BPA through interfering with cholesterol homeostasis and therefore also opens the door for the development of remediation approaches.
The impact of BPA on steroidogenesis has been well studied before. Exposure to BPA alters the expression of critical enzymes in hormone biosynthesis [33, 51, 52] and impairs the synthesis of steroid hormones and their precursors in both rodent [33, 53] and human granulosa cells [51]. However, whether mitochondrial cholesterol uptake, the initial step in steroidogenesis, can be affected by BPA has not been comprehensively studied. By measuring the intracellular and mitochondrial cholesterol level of worms exposed to varying concentrations of BPA and cholesterol, we observed a cytosolic cholesterol accumulation caused by BPA exposure. This was evidenced by increased total intracellular cholesterol level but that concomitant with a decrease in mitochondrial cholesterol level. The observed alteration in intracellular cholesterol distribution is consistent with the previous finding that exposure to BPA results in cholesterol sequestration to the perinuclear area in rodent granulosa cells [33]. Interestingly, a similar mitigation of BPA’s toxicity by cholesterol was also observed in this context, as administering cholesterol prevented BPA’s cytotoxicity and restored the ability of exposed cells to produce progesterone, the precursor of steroid hormone [33]. The increased cytosolic cholesterol levels could be explained by BPA’s ability to decrease the expression of ATP-binding cassette transporter ABCA1, which is responsible for the efflux of cholesterol [33]. However, it cannot explain the decreased mitochondrial cholesterol level since ABCA1 deficiency allows for increased mitochondrial cholesterol [54]. Alternatively, in intestinal Caco-2 cells, BPA promotes expression of the Niemann-Pick C1-like 1 genes and consequently cholesterol absorption by the cells also raising intracellular cholesterol levels. However, in all these cases, the impact of BPA on mitochondrial cholesterol levels was not assessed [55]. Thus, our findings point to an evolutionarily-conserved dramatic impact of BPA on the intracellular distribution of cholesterol.
To initiate steroidogenesis, cholesterol needs to be transported to the inner mitochondrial membrane where cholesterol side-chain cleavage enzyme (CYP11A1, P450scc) and 3β-hydroxysteroid dehydrogenase (CYP17A1, 3β-HSD) reside [22]. Homologs of these enzymes carrying the same function have been identified in C. elegans [56, 57], suggesting that cholesterol also needs to be transported in mitochondria for worms to synthesize downstream molecules, including sterol hormones known as dafachronic acids. It had been previously observed that BPA exposure increases the expression of StAR in rat granulosa cells [29, 33] perhaps via BPA’s down-regulation of microRNA let-7 family [58], of which StAR mRNA is a target [59]. However, the increased expression of StAR contrasts with the accompanied progesterone decrease [29, 33]. Here, we observed a cytosolic cholesterol accumulation concomitant to a decreased mitochondrial concentration, indicating that BPA impairs the distribution of cholesterol likely by interfering with the activity of key cholesterol transporters such as StAR. The discrepancy between impaired mitochondrial cholesterol transport and the detected increase in expression of StAR found in our study as well as others [29] suggests that elevated StAR expression could be a consequence of a compensatory mechanisms in response to cholesterol deprivation. It also suggests that assessing StAR mRNA levels may not be informative and that the impact of BPA, and perhaps other environmental toxicants, on the early steps of steroidogenesis should primarily be assessed biochemically.
In summary, our study demonstrates that BPA exposure negatively impacts the efficiency of StAR in mitochondrial cholesterol transport, alters intracellular cholesterol distribution, and thereby impairs the reproduction of an organism. This study not only provides an important mechanism regarding the effect of BPA on steroidogenesis and reproduction, but also highlights the importance of cholesterol in preventing BPA-induced reproductive dysfunction.
Supplementary Material
Figure S1: Germline enrichment of START domain members as assessed by qRT-PCR. Temperature sensitive glp-1(bn18) mutants were raised at either a permissive (15°C) or non-permissive temperature (25°C). At 25°C, the gonadal arms contain very few germline nuclei. The data is represented here as the fold change of levels at 15°C/25°C each first normalized to gpdh-2 levels. qRT-PCR revealed a strong enrichment in the expression of 4 cholesterol transporters including strl-1. ***P<0.001; *P<0.05. N=3 independent experiments of 30 worms each, each reaction performed in duplicates.
Figure S2: Relative change in expression level of seven START domain members following BPA exposure. Wild type worms were exposed to either control (ethanol), BPA (500 μM) or cholesterol (5 μg/mL) +BPA. The expression levels were normalized over spo-11 levels to account for differences in overall germline size. ***P<0.001; **P<0.01. N=3 independent experiments of 30 worms each, each reaction performed in duplicates.
Figure S3: A combination of dafachronic acids Δ4 and Δ7 DA is not sufficient to rescue BPA’s fertility defects. Worms were exposed to either control or BPA (500 μM) alone or in combination with either cholesterol at 0.5 μg/mL or a combination of Δ4 and Δ7 DA at 0.25 μg/mL each. While cholesterol supplementation rescued all fertility endpoints affected by BPA, dafachronic supplementation was not sufficient to alleviate the effects of absence of cholesterol or of BPA exposure. ***P<0.001; *P<0.05. N=4–8 experimental repeats depending on exposure groups.
Acknowledgments
P.A. is supported by R01 NIH/NIEHS ES02748701 and the Burroughs Wellcome Foundation. Y.C. and D.F. received support from the T32 NIH/NIEHS ES015457 Training in Molecular Toxicology. A.H. received support from the R25 NIH/NIEHS ES02550703. The authors declare no competing interests.
REFERENCE
- 1.Hartle JC, Navas-Acien A, and Lawrence RS, The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environ Res, 2016. 150: p. 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hines CJ, et al. , Air, hand wipe, and surface wipe sampling for Bisphenol A (BPA) among workers in industries that manufacture and use BPA in the United States. J Occup Environ Hyg, 2017. 14(11): p. 882–897. [DOI] [PubMed] [Google Scholar]
- 3.Xue J, et al. , Resin-based dental sealants as a source of human exposure to bisphenol analogues, bisphenol A diglycidyl ether, and its derivatives. Environ Res, 2018. 162: p. 35–40. [DOI] [PubMed] [Google Scholar]
- 4.Thayer KA, et al. , Bisphenol A, Bisphenol S, and 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) in Urine and Blood of Cashiers. Environ Health Perspect, 2016. 124(4): p. 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang RP, et al. , Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: A thorough literature review. Sci Total Environ, 2018. 626: p. 971–981. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, et al. , Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci Total Environ, 2018. 626: p. 1494–1501. [DOI] [PubMed] [Google Scholar]
- 7.Richter CA, et al. , In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol, 2007. 24(2): p. 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamez JM, et al. , Exposure to a low dose of bisphenol A impairs pituitary-ovarian axis in prepubertal rats: effects on early folliculogenesis. Environ Toxicol Pharmacol, 2015. 39(1): p. 9–15. [DOI] [PubMed] [Google Scholar]
- 9.Shi M, et al. , Prenatal exposure to bisphenol A analogues on female reproductive functions in mice. Toxicol Sci, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Adewale HB, et al. , Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod, 2009. 81(4): p. 690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brieno-Enriquez MA, et al. , Human meiotic progression and recombination are affected by Bisphenol A exposure during in vitro human oocyte development. Hum Reprod, 2011. 26(10): p. 2807–18. [DOI] [PubMed] [Google Scholar]
- 12.Allard P and Colaiacovo MP, Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc Natl Acad Sci U S A, 2010. 107(47): p. 20405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Susiarjo M, et al. , Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet, 2007. 3(1): p. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siracusa JS, et al. , Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod Toxicol, 2018. 79: p. 96–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu HJ, et al. , Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat Res, 2013. 752(1–2): p. 57–67. [DOI] [PubMed] [Google Scholar]
- 16.El-Beshbishy HA, Aly HA, and El-Shafey M, Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol Ind Health, 2013. 29(10): p. 875–87. [DOI] [PubMed] [Google Scholar]
- 17.Xi W, et al. , Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod Toxicol, 2011. 31(4): p. 409–17. [DOI] [PubMed] [Google Scholar]
- 18.Salian S, Doshi T, and Vanage G, Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology, 2009. 265(1–2): p. 56–67. [DOI] [PubMed] [Google Scholar]
- 19.Salian S, Doshi T, and Vanage G, Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci, 2009. 85(21–22): p. 742–52. [DOI] [PubMed] [Google Scholar]
- 20.Moustafa GG and Ahmed AAM, Impact of prenatal and postnatal exposure to bisphenol A on female rats in a two generational study: Genotoxic and immunohistochemical implications. Toxicol Rep, 2016. 3: p. 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller WL, Molecular biology of steroid hormone synthesis. Endocr Rev, 1988. 9(3): p. 295–318. [DOI] [PubMed] [Google Scholar]
- 22.Farkash Y, Timberg R, and Orly J, Preparation of antiserum to rat cytochrome P-450 cholesterol side chain cleavage, and its use for ultrastructural localization of the immunoreactive enzyme by protein A-gold technique. Endocrinology, 1986. 118(4): p. 1353–65. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj V, Stocco DM, and Tu LN, Minireview: translocator protein (TSPO) and steroidogenesis: a reappraisal. Mol Endocrinol, 2015. 29(4): p. 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan J, et al. , Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci U S A, 2015. 112(23): p. 7261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikonen E, Mechanisms for cellular cholesterol transport: defects and human disease. Physiol Rev, 2006. 86(4): p. 1237–61. [DOI] [PubMed] [Google Scholar]
- 26.Bose HS, Lingappa VR, and Miller WL, The steroidogenic acute regulatory protein, StAR, works only at the outer mitochondrial membrane. Endocr Res, 2002. 28(4): p. 295–308. [DOI] [PubMed] [Google Scholar]
- 27.Caron KM, et al. , Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci U S A, 1997. 94(21): p. 11540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa T, et al. , Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol, 2000. 14(9): p. 1462–71. [DOI] [PubMed] [Google Scholar]
- 29.Zhou W, et al. , Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol, 2008. 283(1–2): p. 12–8. [DOI] [PubMed] [Google Scholar]
- 30.Peretz J, et al. , Bisphenol A impairs follicle growth, inhibits steroidogenesis, and down-regulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medwid S, Guan H, and Yang K, Bisphenol A stimulates steroidogenic acute regulatory protein expression via an unknown mechanism in adrenal cortical cells. J Cell Biochem, 2018. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, et al. , Bisphenol A stimulates differentiation of rat stem Leydig cells in vivo and in vitro. Mol Cell Endocrinol, 2018. 474: p. 158–167. [DOI] [PubMed] [Google Scholar]
- 33.Samardzija D, et al. , Bisphenol A decreases progesterone synthesis by disrupting cholesterol homeostasis in rat granulosa cells. Mol Cell Endocrinol, 2018. 461: p. 55–63. [DOI] [PubMed] [Google Scholar]
- 34.Lesch BJ and Page DC, Genetics of germ cell development. Nat Rev Genet, 2012. 13(11): p. 781–94. [DOI] [PubMed] [Google Scholar]
- 35.Leung MC, et al. , Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci, 2008. 106(1): p. 5–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, et al. , Exposure to the BPA-Substitute Bisphenol S Causes Unique Alterations of Germline Function. PLoS Genet, 2016. 12(7): p. e1006223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurzchalia TV and Ward S, Why do worms need cholesterol? Nat Cell Biol, 2003. 5(8): p. 684–8. [DOI] [PubMed] [Google Scholar]
- 38.Hieb WF and Rothstein M, Sterol requirement for reproduction of a free-living nematode. Science, 1968. 160(3829): p. 778–80. [DOI] [PubMed] [Google Scholar]
- 39.Merris M, et al. , Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: developmental requirement for 4alpha-methyl sterols. J Lipid Res, 2003. 44(1): p. 172–81. [DOI] [PubMed] [Google Scholar]
- 40.Chitwood DJ and Lusby WR, Metabolism of plant sterols by nematodes. Lipids, 1991. 26(8): p. 619–27. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee M, et al. , Dafachronic acid inhibits C. elegans germ cell proliferation in a DAF-12-dependent manner. Dev Biol, 2017. 432(2): p. 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motola DL, et al. , Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell, 2006. 124(6): p. 1209–23. [DOI] [PubMed] [Google Scholar]
- 43.Matyash V, et al. , Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol, 2004. 2(10): p. e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vandenberg LN, et al. , Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health, 2013. 28(1): p. 37–58. [DOI] [PubMed] [Google Scholar]
- 45.Schonfelder G, et al. , Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect, 2002. 110(11): p. A703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandenberg LN, et al. , Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Environ Health Perspect, 2010. 118(8): p. 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stiernagle T, Maintenance of C elegans. WormBook, 2006: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clayton DA and Shadel GS, Purification of mitochondria by sucrose step density gradient centrifugation. Cold Spring Harb Protoc, 2014. 2014(10): p. pdb prot080028. [DOI] [PubMed] [Google Scholar]
- 49.Soccio RE and Breslow JL, StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J Biol Chem, 2003. 278(25): p. 22183–6. [DOI] [PubMed] [Google Scholar]
- 50.Wollam J, et al. , A novel 3-hydroxysteroid dehydrogenase that regulates reproductive development and longevity. PLoS Biol, 2012. 10(4): p. e1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwintkiewicz J, et al. , Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect, 2010. 118(3): p. 400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansur A, et al. , Does BPA alter steroid hormone synthesis in human granulosa cells in vitro? Hum Reprod, 2016. 31(7): p. 1562–9. [DOI] [PubMed] [Google Scholar]
- 53.Peretz J, et al. , Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci, 2011. 119(1): p. 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith B and Land H, Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep, 2012. 2(3): p. 580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng D, et al. , Bisphenol A promotes cholesterol absorption in Caco-2 cells by up-regulation of NPC1L1 expression. Lipids Health Dis, 2017. 16(1): p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dumas KJ, et al. , Functional divergence of dafachronic acid pathways in the control of C. elegans development and lifespan. Dev Biol, 2010. 340(2): p. 605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan J and Papadopoulos V, Evolutionary origin of the mitochondrial cholesterol transport machinery reveals a universal mechanism of steroid hormone biosynthesis in animals. PLoS One, 2013. 8(10): p. e76701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veiga-Lopez A, et al. , Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology, 2013. 154(5): p. 1873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Men Y, et al. , The Steroidogenic Acute Regulatory Protein (StAR) Is Regulated by the H19/let-7 Axis. Endocrinology, 2017. 158(2): p. 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Germline enrichment of START domain members as assessed by qRT-PCR. Temperature sensitive glp-1(bn18) mutants were raised at either a permissive (15°C) or non-permissive temperature (25°C). At 25°C, the gonadal arms contain very few germline nuclei. The data is represented here as the fold change of levels at 15°C/25°C each first normalized to gpdh-2 levels. qRT-PCR revealed a strong enrichment in the expression of 4 cholesterol transporters including strl-1. ***P<0.001; *P<0.05. N=3 independent experiments of 30 worms each, each reaction performed in duplicates.
Figure S2: Relative change in expression level of seven START domain members following BPA exposure. Wild type worms were exposed to either control (ethanol), BPA (500 μM) or cholesterol (5 μg/mL) +BPA. The expression levels were normalized over spo-11 levels to account for differences in overall germline size. ***P<0.001; **P<0.01. N=3 independent experiments of 30 worms each, each reaction performed in duplicates.
Figure S3: A combination of dafachronic acids Δ4 and Δ7 DA is not sufficient to rescue BPA’s fertility defects. Worms were exposed to either control or BPA (500 μM) alone or in combination with either cholesterol at 0.5 μg/mL or a combination of Δ4 and Δ7 DA at 0.25 μg/mL each. While cholesterol supplementation rescued all fertility endpoints affected by BPA, dafachronic supplementation was not sufficient to alleviate the effects of absence of cholesterol or of BPA exposure. ***P<0.001; *P<0.05. N=4–8 experimental repeats depending on exposure groups.