Abstract
Purpose:
To determine the relationship between systemic factors and radial peripapillary capillary (RPC) vessel density (VD) in healthy African American (AA) participants of the African American Eye Disease Study.
Design:
A population-based, cross-sectional study.
Methods:
4135 eyes from 2127 AA participants aged 40 years and older in Inglewood, California, were imaged for 6×6mm optic disc scans on a spectral-domain optical coherence tomography angiography (OCTA) device. Of these, 1029 eyes from 1029 participants who met the inclusion and exclusion criteria were analyzed, including only one eye per participant. Custom software was used to quantify RPC VD. Multivariate linear regression was used to identify systemic factors associated with RPC VD with a significance level set at 0.05. The contribution of each variable to the final model was estimated with the magnitude of standardized regression coefficients (SRC). The fit of the final model was measured by the R2.
Results:
Average RPC VD was 0.346±0.045. Controlling for signal strength, the systemic variables in the final multivariate model associated with reduced RPC VD were older age (β=−0.0123 per decade; SRC=−0.2733; p=<0.0001), male sex (β=−0.0067; SRC=−0.0716; p=0.0060), and longer diabetes duration (β=−0.0022 per 5 years; SRC=−0.0527; p=0.0427). The model R2 was 0.3689.
Conclusions:
Age, sex, and systemic influences, such as diabetes duration, need to be considered when assessing changes in RPC VD in glaucoma and other ocular diseases. Longitudinal studies are needed to investigate whether reduced RPC VD and the factors that affect it are associated with an increased risk of developing glaucomatous nerve damage.
INTRODUCTION
Glaucoma is a group of progressive, multifactorial diseases characterized by deterioration and loss of retinal ganglion cells and their axons comprising the retinal nerve fiber layer (RNFL).1,2 It is among the leading causes of irreversible loss of vision worldwide, affecting nearly 80 million people by 2020.3 Previous studies have reported that vascular risk factors, including systemic hypertension, acute hypotension, vasospasm, antihypertensive medication, autonomic dysregulation, and focal arteriolar narrowing may play a significant role in the development and progression of glaucoma by compromising ocular blood flow (OBF). 2,4–8 While many studies9–14 have demonstrated that reduced vessel density (VD) in the radial peripapillary capillary (RPC) layer measured from optical coherence tomography angiography (OCTA) is associated with glaucomatous nerve damage, we still do not understand whether vascular changes within the RPC lead to subsequent optic nerve damage.
Several epidemiological studies have confirmed that people of African descent, and specifically African Americans (AAs), carry a greater burden of both glaucoma and systemic vascular disease compared to other ethnic populations.3,15–19 For example, Quigley and Broman have reported that approximately 4.39% of Africans over 40 years of age are to be affected by glaucoma by 2020, which is the highest prevalence among populations worldwide.3 Tielsch et al. have also reported the prevalence of glaucoma ranging from 1.23% among AAs aged 40 through 49 years to 11.26% among AAs aged 80 years and older, as compared to 0.92% to 2.16% among whites in the corresponding age groups.15 The Eye Diseases Prevalence Research Group have estimated the age-adjusted prevalence of glaucoma, specifically primary open angle glaucoma (POAG), in AAs to be up to three times higher than in whites.16 Moreover, the prevalence of vascular diseases and diabetes, which are potential risk factors for glaucoma, in AAs has been reported in the literature to be disproportionately higher than in other populations.17–19
There have been limited reports that examined the direct effects of systemic factors, including age, sex, hypertension, and diabetes, on peripapillary VD.20–24 The African American Eye Disease Study (AFEDS) is a population-based, cross-sectional ocular epidemiology study of AAs, ages 40 and older, residing in and around Inglewood, California. We hypothesized that the presence of some systemic characteristics is associated with reduction of peripapillary VD and thus greater risk of glaucoma. The purpose of the current study was to determine the relationship of peripapillary VD in AAs with systemic factors, including age, sex, diagnosis of hypertension, presence and duration of diabetes mellitus, hemoglobin A1c, body mass index (BMI), blood pressure (BP), use of antihypertensive medication, history of heart failure or stroke, and smoking status, and signal strength (SS).
METHODS
Study Population
Self-identified AAs, ages 40 years and older, residing within 30 census tracts in and around Inglewood, California were recruited for the population-based, cross-sectional study. The research protocol was approved by the institutional review board of the University of Southern California Health Sciences. The study complied with the Health Insurance Portability and Accountability Act of 1996 and was carried out in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects following an explanation of the nature and intent of the study through a computer-assisted in-home interview.
Clinical Assessment
The clinical examination included a clinical questionnaire, measurements of height, weight, waist-to-hip ratio, pulse rate, BP, random blood glucose, serum hemoglobin A1c, and OCTA imaging (CIRRUS™ HD-OCT 5000 with AngioPlex® OCT Angiography; ZEISS, Dublin, CA). 6×6mm optic disc scans of the peripapillary region in the RPC layer were obtained and collected by trained imaging technicians beginning in February 2016. A total of 350×350 A scans with spacing of approximately 17.14 µm were taken for the 6×6mm OCTA scans. Systemic information collected from the questionnaire and the clinical exam included age, sex, diagnosis of hypertension, BP, BMI, hemoglobin A1c, diagnosis and duration of diabetes, use of antihypertensive medications, history of heart failure or stroke, and smoking status.
The diagnoses of ocular diseases, including glaucoma and diabetic retinopathy, were based on assessment by an ophthalmologist, as explained previously25. Healthy participants in the study had clinical exam results that ranged from normal to glaucoma suspect and had non-glaucomatous optic discs, according to the onsite ophthalmologist. Exclusion criteria included: diagnoses of glaucoma, vision-threatening diabetic retinopathy (defined as severe non-proliferative diabetic retinopathy (NPDR), PDR, and diabetic macular edema), and other relevant ocular disease to reduce RPC VD variation from ocular disease; SS < 7/10; and poor image quality from motion artifacts, media opacities, such as vitreous floaters, or decentration based on a standardized image quality grading algorithm. One eye from each subject was included in the study. When both eyes met the study criteria, the right eye was selected.
OCTA Image Analysis
An automated segmentation software (CIRRUS 11.0; ZEISS) was used to detect the boundaries of retinal layers and create two-dimensional OCTA en face images of the RPC layer, which extends from the inner limiting membrane to the posterior surface of the RNFL. A custom quantification software with an interactive interface in MATLAB (R2017a; MathWorks Inc, Natick, MA) was used to quantify the RPC VD, as described previously26. The en face OCTA images were converted to binary vessel maps using a method combining global thresholding, Hessian filter, and adaptive threshold (Figure 1). The avascular area within the optic nerve head was selected to establish the baseline background noise for global thresholding and then was excluded from quantification. Large vessels of more than 32 µm were also removed from quantification. Vessel density (VD) was defined as the unitless ratio of total sum area of white pixels to total sum area of all pixels in the binary vessel map and provided information about both medium-sized retinal blood vessels and capillaries.
Figure 1.
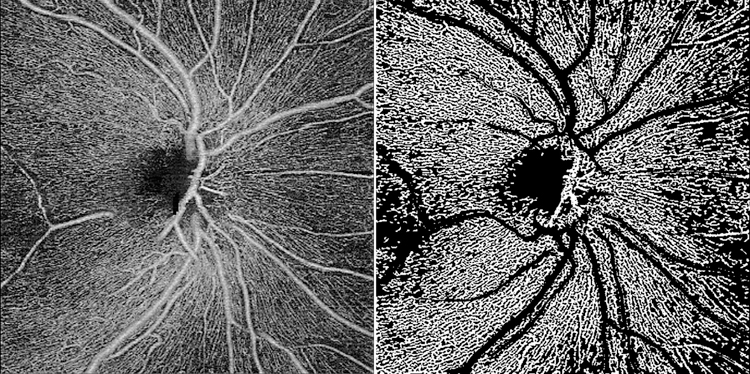
(left) 6×6mm OCTA en face image centered on optic nerve head and (right) binarized image, excluding large vessels, of a representative healthy African American eye.
Statistical Analysis
Mean, standard deviation (SD), and frequency were calculated for the systemic variables. Univariate linear regression analysis with VD as the dependent variable and systemic variables as the independent variables was performed to calculate the degree of change in VD by every one-unit of change in each systemic variable and assess the strength of each association.
Multivariate linear regression analysis was based on a conceptual model of VD and involved a stepwise selection of the independent variables to be included in the final model for RPC VD. The contribution of each independent variable to the final model was estimated by the magnitude of standardized regression coefficients (SRC). The fit of the final model was measured by the R2. Significance level was set at p<0.05 for all analyses.
Locally weighted scatterplot smoothing (LOWESS)27 was used to generate plots with smooth fit lines to examine the non-linear relationships between the significant independent systemic variables included in the final multivariate model and VD, adjusting for sex and SS. SAS 9.4 (Cary, NC) and Microsoft Excel 2016 (Redmond, WA) were used for all data analyses.
RESULTS
Of 4135 eyes from 2127 participants who received OCTA imaging, 1455 eyes from 1029 participants met our inclusion and exclusion criteria. 1029 eyes from 1029 participants were analyzed, including only one eye per participant. The flowchart of the exclusion and inclusion criteria is shown in Figure 2.
Figure 2.
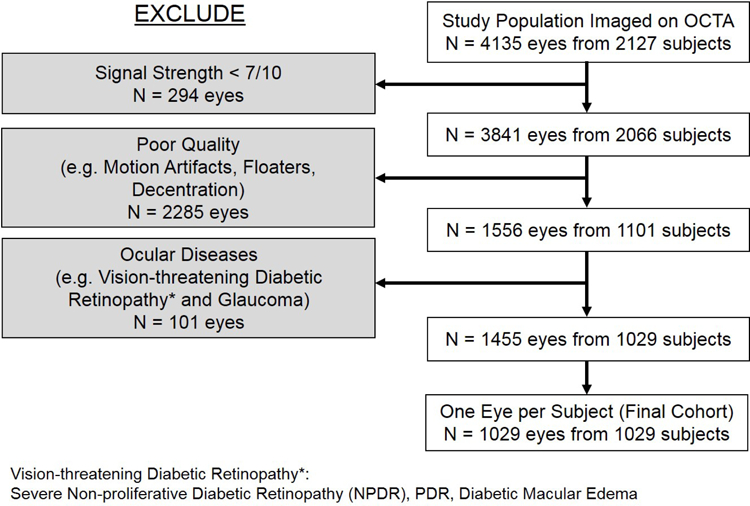
The flowchart of the inclusion and exclusion criteria for OCTA scans.
Frequency distribution of the candidate systemic variables and univariate analysis in healthy AAs are presented in Table 1. Candidate variables were age, sex, diagnosis of hypertension, BP, BMI, hemoglobin A1c, diagnosis and duration of diabetes, use of antihypertensive medications, history of heart failure or stroke, smoking status, and SS. Univariate linear regression analysis of the systemic factors showed significant association of reduced RPC VD with older age (β = −0.0017 per year; p = <0.0001), male sex (β = −0.0092; p = 0.0016), diagnosis of hypertension (β = −0.0126; p = <0.0001), higher systolic BP (SBP) (β = −0.0003 per mmHg; p = 0.0001), higher hemoglobin A1c (β = −0.0033; p = 0.0088), diagnosis of diabetes (β = −0.0118; p = 0.0009), and longer duration of diabetes (β = −0.0009 per year; p = 0.0008). Reduced RPC VD was also significantly associated with lower SS (β = 0.0291; p = <0.0001). Average RPC VD was 0.346 ± 0.045.
Table 1.
Frequency Distribution of Systemic Variables and Univariate Linear Regression with Vessel Density in the Healthy Participants of the African American Eye Disease Study
| Variables | N | Mean or Frequencya | Betab | P-Valuesb |
|---|---|---|---|---|
| Age (years) | 1029 | 58 ± 10 | −.0017 | <.0001c |
| Sex (Male) | 1029 | 369 (36) | −.0092 | 0.0016c |
| Diagnosis of Hypertension | 1029 | 611 (59) | −.0126 | <.0001c |
| Systolic Blood Pressure (mmHg) | 1026 | 130 ± 20 | −.0003 | 0.0001c |
| Diastolic Blood Pressure (mmHg) | 1026 | 80 ± 12 | −.0001 | 0.5556 |
| Body-Mass Index (kg/m2) | 1021 | 30.2 ± 7.0 | 0.0003 | 0.1295 |
| Hemoglobin A1c (%) | 960 | 6.0 ± 1.1 | −.0033 | 0.0088c |
| Diagnoses of Diabetes | 968 | 197 (20) | −.0118 | 0.0009c |
| Diabetes Duration (years) | 958 | 1.7 ± 5.2 | −.0009 | 0.0008c |
| Taking Blood Pressure Medications | 498 | 436 (88) | −.0107 | 0.0852 |
| History of Heart Failure or Stroke | 995 | 54 (5) | −.0057 | 0.3585 |
| History of Smoking | 988 | 430 (44) | −.0052 | 0.0706 |
| Signal Strength | 1029 | 9.3 ± 0.8 | 0.0291 | <.0001c |
| Vessel Density | 1029 | 0.346 ± 0.045 | - | - |
All data listed as mean ± standard deviation or frequency (percent).
Beta coefficients and p-values calculated from univariate linear regression with vessel density.
P ≤ 0.05 considered significant.
In the multivariate analysis, age, sex, diabetes duration, and SS were determined to be independently and significantly affecting RPC VD (Table 2). Controlling for SS (β = 0.0248; SRC = 0.4695; p = <0.0001), older age (β = −0.0123 per decade; SRC = −0.2733; p = <0.0001), male sex (β = −0.0067; SRC = −0.0716; p = 0.0060), and longer diabetes duration (β = −0.0022 per 5 years; SRC = −0.0527; p = 0.0427) were found to be significantly associated with reduced RPC VD in the final model. The model R2 was 0.3689.
Table 2.
Multivariate Modela Assessing Systemic Determinants of Radial Peripapillary Capillary Vessel Density in Healthy Participants of the African American Eye Disease Study
| Variable | Parameter Estimates (95% CI) | SRC | P Valuesb |
|---|---|---|---|
| Age (per 10 years) | −0.0123 (−0.0147, −0.0010) | −0.2733 | <.0001 |
| Male Sex | −0.0067 (−0.0114, −0.0019) | −0.0716 | 0.0060 |
| Diabetes Duration (per 5 years) | −0.0022 (−0.0044, −0.0001) | −0.0527 | 0.0427 |
Model controlled for signal strength. Model R2 was 0.3689.
P ≤ 0.05 considered significant.
CI = Confidence Interval; SRC = Standardized Regression Coefficient
Figure 3 shows the LOWESS plot for predicted values of RPC VD plotted against age in years. There is reduced VD for each increasing year of age. Figure 4 presents the LOWESS plot for predicted values of RPC VD plotted against diabetes duration in years (where 15+ years is one cluster). There is reduced VD for each increasing year of diabetes duration.
Figure 3.
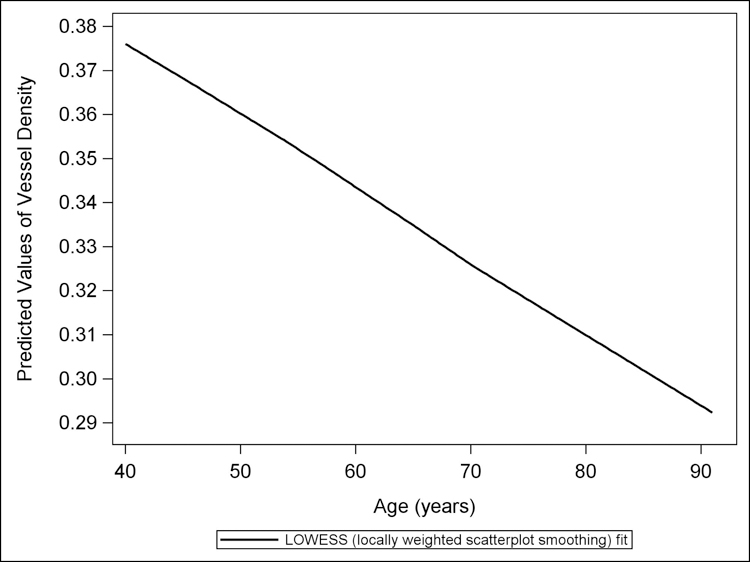
The LOWESS plot of predicted values of vessel density, from a multivariate linear regression model controlling for sex, diabetes duration, and signal strength, versus age. Reduction in peripapillary vessel density is seen with increasing age in healthy African Americans.
Figure 4.
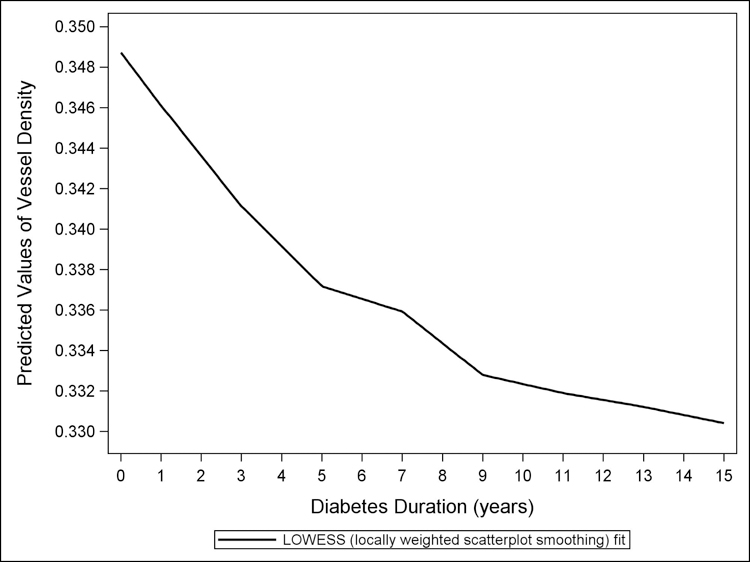
The LOWESS plot of predicted values of vessel density, from a multivariate linear regression model controlling for age, sex, and signal strength, versus diabetes duration. Reduction in peripapillary vessel density is seen with longer diabetes duration in healthy African Americans.
In supplemental univariate analysis of 501 eyes from 501 participants with OCTA scans of only 10/10 signal strength, reduced RPC VD had significant association with older age (β = −0.0011 per year; p = <0.0001), male sex (β = −0.0071; p = 0.0438), diagnosis of hypertension (β = −0.0075; p = 0.0209), higher SBP (β = −0.0002 per mmHg; p = 0.0197), higher hemoglobin A1c (β = −0.0034; p = 0.0471), and longer duration of diabetes (β = −0.0008 per year; p = 0.0129). Average RPC VD was 0.369 ± 0.036. In the multivariate analysis of this cohort, age and male sex were determined to be independently and significantly affecting RPC VD. Older age (β = −0.0115; SRC = −0.3084; p = <0.0001) and male sex (β = −0.0089; SRC = −0.1138; p = 0.0078) were found to be significantly associated with reduced RPC VD in this model. The model R2 was 0.1027.
DISCUSSION
To our knowledge, AFEDS is the only population-based, and possibly the largest, OCTA study to date. In the current study of healthy AA eyes aged 40 and over, we assessed the systemic determinants of RPC VD, a measure beginning to be used as a clinical marker for glaucoma disease. We found that older age, male sex, and longer diabetes duration were independent predictors of reduced RPC VD. This data highlights the importance of recognizing the influence of systemic factors on peripapillary perfusion measurements and offers a potential pathophysiologic mechanism for the previous observation that diabetes increases risk for glaucoma, as will be discussed below.
This population-based study provides strong evidence of the association of reduced RPC VD with increasing age. Significant reduction in RPC VD was reported previously in a smaller study of healthy eyes of subjects beyond 60 years of age.20 On the other hand, other small studies showed no association of age with RPC VD in healthy participants, though they used smaller measurement areas and a different method of calculating the vessel parameters.21,22 One of those studies21 examined 52 people ranging from 19 to 63 years, thus it may not have had the power and older age range to demonstrate any significant association with RPC VD. Additionally, it calculated the RPC VD from an annulus with a 3.4mm circle diameter, which may also have limited the study’s ability to detect age-related changes. A second study22 included large vessels in its VD calculation, which may have reduced the sensitivity for detecting age-related changes20. The significant association between older age and reduced RPC VD seen in the current study is also supported by the well-established relationship between age and thickness of the RNFL, the retinal layer that the RPC is supplying.28–33 These studies reported that mean RNFL thickness decreases approximately 1.5 to 3.8 µm per decade in the healthy population,28–32 with worsening over ages 75 years or older (−5.6 µm per decade)33. Based on the current study, we estimate that RPC VD decreases by 0.0123 per decade in the healthy population. Additionally, reduction of major retinal artery and vein caliber34–36 and increase in retinal vessel wall thickness37 have been found to be associated with age and corroborate the decrease in the retinal microcirculation with older age, as found in the current study.
While one may have expected female sex to be associated with reduced VD due to disproportionately higher prevalence of glaucoma and blindness among women in the general population4,38, we found male sex to be associated with reduced RPC VD. This result agrees with the smaller study from India by Rao et al.22, where male sex was shown to be significantly associated with lower peripapillary VD. However, another study on healthy individuals and using the same imaging software reported that sex has no significant effect on RPC density.21 Similarly, in studies examining the association of RNFL thickness and sex, there have been conflicting results on whether male sex is associated with thinner RNFL, controlling for age.28,39–41 Interestingly, previous studies have shown increased prevalence of atherosclerosis and resulting systemic vascular diseases among men42, so it is possible that the reduction in RPC VD among males may be related to this sex difference in vascular pathology. While we do not yet know if reduced RPC VD is a risk factor for glaucoma, it is plausible that the reduced RPC VD among healthy AA males seen in this study may explain the higher prevalence of POAG among AA male individuals, as reported in a previous study16. Additional research is needed to verify these relationships.
We found longer diabetes duration to be independently associated with reduced RPC VD. Previous studies reported no significant relationship between diabetes duration and RPC VD but significant association of diagnosis of diabetes with reduced RPC VD in participants without severe diabetic retinopathy.23,24,43 However, these studies had smaller sample sizes, which may not have been powered to represent all disease durations, and used different quantification algorithms.23,24 The result from our supplemental analysis of OCTA scans with signal strength of only 10/10, limiting the sample size to 501 eyes, also showed no significant relationship between diabetes duration and RPC VD, further emphasizing the importance of sample size in having the power to detect this relationship. Other studies reported duration of diabetes to be associated with loss of the ganglion cell layer44 and RNFL45. Furthermore, Rodrigues et al. demonstrated potential coupling of RNFL thinning and peripapillary VD reduction in diabetic participants with no or minimal DR, as compared to healthy participants, thus supporting the idea that peripapillary neurovascular changes may occur early on in the course of DR.24 These findings along with our results further suggest that microvascular damage by diabetes, which leads to impairment in vascular autoregulation and reduced blood flow to the retina and optic nerve, starts early on and increases with diabetes duration.6,23,24,43,46,47 Moreover, they may indicate that subtle changes and damage in the microcirculation can be detected in the RPC simply with the duration of systemic diabetes and prior to the onset of significant ocular involvement.23,24,43 Also, it is interesting that while we found hemoglobin A1c, a measure of short-term glucose control, to be significantly correlated to VD, it was not significant in the final model, suggesting that RPC VD may be more associated with damage by chronic, prolonged disease rather than the more acute state of the diabetes.
It is interesting that all variables related to hypertension, including diagnosis of hypertension, BP, and use of anti-hypertensive medication, were not significant in the final multivariate model. This finding is supported by prior smaller studies.22,48 For example, Rao et al. reported no significant association of hypertension with RPC VD in healthy participants.22 Furthermore, Liu et al. reported no significant association of BP parameters with peripapillary VD in both healthy and glaucoma groups.48 On the other hand, findings from previous studies31,32 that examined the association of systemic hypertension and RNFL thickness have been mixed. For instance, Mauschitz et al.31 reported that systemic hypertension is associated with reduced RNFL thickness in European population, but Cheung et al.32 reported no significant association of systemic factors, including BP, in non-glaucomatous Chinese subjects. Furthermore, the Baltimore Eye Survey49 reported that systemic hypertension may even have different effects on glaucoma depending on the age group. It suggested that hypertension has a protective effect on glaucoma in younger subjects, ages less than 60 years, but an adverse effect on older subjects, ages more than 70 years. It was thought that younger patients have good ocular perfusion pressure and OBF without chronic vessel damage from hypertension, but older patients have atherosclerosis, narrowed vessels, and increased resistance, which may all reduce OBF.49 However, this was not verified in the current study of peripapillary VD in our AA population. Longitudinal studies examining the association of duration of hypertension and RPC VD is needed to clarify the relationship between increased BP and reduced VD in glaucoma.
The greatest contributor of the final model of RPC VD was SS. We found that RPC VD increases with higher SS, which agrees with the findings of previous studies22,50,51, and thus controlled for in our final model analysis. Rao et al. examined the effect of SS on RPC VD in healthy eyes and found that higher SS was significantly associated with greater VD.22 Moreover, while Venugopal et al. examined the association of SS with RPC VD in both glaucoma and healthy patients, the same trend was shown.50,51 Overall, these results emphasize the importance of controlling for SS, even when using a SS cutoff point of 7 or more, as suggested by the manufacturer52, in analyses for OCTA parameters.
This study has several limitations. First, our study population consists of self-identified African Americans. While the ancestry markers for African heritage could be assessed in the future, we currently do not have access to this data. Future studies including ancestry markers would allow for a more well-defined study population. Second, our data on systemic factors, including diagnosis and duration of diabetes, are largely based on self-report, which may include bias and inaccuracy. Furthermore, measurements taken during the clinical exam provide only a snapshot of the participants’ medical condition at the time of imaging. For example, severity of hypertension over many years cannot be determined by clinical diagnosis of hypertension itself, blood pressure measurement readings from a single day, or use of antihypertensive medication at the time. Additionally, since early capillary dropout and changes in the peripapillary vessel morphology may be present before progression of ocular diseases, including glaucoma46 and DR43, our results may not reflect the true strengths of the association of each systemic factor with RPC VD in the healthy population. Overall, longitudinal studies should further examine the association of duration of hypertension and other vascular diseases with VD in the normal eyes.
In conclusion, out of 11 systemic factors, older age, male sex, and longer diabetes duration were independently associated with reduced RPC VD. Our results suggest that age, sex, and systemic influences, such as diabetes duration, need to be considered when assessing changes in RPC VD in glaucoma and other ocular diseases. Additionally, SS must be considered when assessing OCTA parameters. Longitudinal studies are needed to further investigate whether these factors affecting RPC VD are associated with an increased risk of developing glaucomatous nerve damage.
Supplementary Material
Acknowledgements:
The African American Eye Disease Study Group, University of Southern California, Los Angeles, CA: Rohit Varma, MD, MPH; Roberta McKean-Cowdin, PhD; Mina Torres, MS; Alicia Fairbrother-Crisp, MPH; Farzana Choudhury MBBS, MS, PhD; Xuejuan Jiang, PhD; Bruce Burkemper, PhD, MS; Tengiz Adamashvili; Carlos Lastra, MD; Elizabeth Corona; YuPing Wang, COT; Jacqueline Douglass, Jaimie Barrera; Judith Linton.
Funding/Support:
National Institutes of Health Grants (Bethesda, MD; K23EY027855-01, GMR; U10EY023575, RV; K08EY027006, AHK), American Glaucoma Society Young Clinician Scientist Grant (San Francisco, CA; GMR), unrestricted grant to the USC Department of Ophthalmology from Research to Prevent Blindness (New York, NY), and Carl Zeiss Meditec (Dublin, CA; SD-OCTA device). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Parts of the data in the current manuscript were previously presented as a paper at the Association for Research in Vision and Ophthalmology Annual Meeting 2019.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
Ali Fard is an employee for Carl Zeiss Meditec (Dublin, CA). Amir H. Kashani is a consultant for Carl Zeiss Meditec. Ruikang K. Wang is a consultant of Carl Zeiss Meditec and holds a patent on the SD-OCTA device from Carl Zeiss Meditec. The remaining authors declare no financial disclosure.
References:
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363(9422):1711–20. [DOI] [PubMed] [Google Scholar]
- 2.McMonnies CW. Glaucoma history and risk factors. J Optom 2017;10(2):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90(3):262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi J, Kook MS. Systemic and ocular hemodynamic risk factors in glaucoma. Biomed Res Int 2015;2015:141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology 2000;107(7):1287–93. [DOI] [PubMed] [Google Scholar]
- 6.Flammer J, Orgül S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res 1998;17(2):267–89. [DOI] [PubMed] [Google Scholar]
- 7.Papastathopoulos KI, Jonas JB. Follow up of focal narrowing of retinal arterioles in glaucoma. Br J Ophthalmol 1999;83(3):285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang JC, Konduru R, Zhang X, et al. Relationship among visual field, blood flow, and neural structure measurements in glaucoma. Invest Ophthalmol Vis Sci 2012;53(6):3020–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter GM, Sylvester B, Chu Z, et al. Peripapillary microvasculature in the retinal nerve fiber layer in glaucoma by optical coherence tomography angiography: focal structural and functional correlations and diagnostic performance. Clin Ophthalmol 2018;12:2285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CL, Zhang A, Bojikian KD, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography–based microangiography. Invest Ophthalmol Vis Sci 2016;57(9):475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung JK, Hwang YH, Wi JM, Kim M, Jung JJ. Glaucoma diagnostic ability of the optical coherence tomography angiography vessel density parameters. Curr Eye Res 2017;42(11):1458–67. [DOI] [PubMed] [Google Scholar]
- 12.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology 2017;124(5):709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HS, Liu CH, Wu WC, Tseng HJ, Lee YS. Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci 2017;58(9):3637–45. [DOI] [PubMed] [Google Scholar]
- 14.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology 2016;123(12):2498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA 1991;266(3):369–74. [PubMed] [Google Scholar]
- 16.Friedman DS, Wolfs RC, O’Colmain BJ. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol 2004;122(4):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Racette L, Wilson MR, Zangwill LM, Weinreb RN, Sample PA. Primary open-angle glaucoma in blacks: a review. Surv Ophthalmol 2003;48(3):295–313. [DOI] [PubMed] [Google Scholar]
- 18.Jamerson K, DeQuattro V. The impact of ethnicity on response to antihypertensive therapy. Am J Med 1996;101(3):22S–32S. [DOI] [PubMed] [Google Scholar]
- 19.Ford ME, Tilley BC, McDonald PE. Social support among African-American adults with diabetes, Part 2: A review. J Natl Med Assoc 1998;90(7):425–32. [PMC free article] [PubMed] [Google Scholar]
- 20.Pinhas A, Linderman R, Mo S, et al. A method for age-matched OCT angiography deviation mapping in the assessment of disease-related changes to the radial peripapillary capillaries. PLoS One 2018;13(5):e0197062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansoori T, Sivaswamy J, Gamalapati JS, Agraharam SG, Balakrishna N. Measurement of radial peripapillary capillary density in the normal human retina using optical coherence tomography angiography. J Glaucoma 2017;26(3):241–6. [DOI] [PubMed] [Google Scholar]
- 22.Rao HL, Pradhan ZS, Weinreb RN, et al. Determinants of peripapillary and macular vessel densities measured by optical coherence tomography angiography in normal eyes. J Glaucoma 2017;26(5):491–7. [DOI] [PubMed] [Google Scholar]
- 23.Cao D, Yang D, Yu H, et al. Optic nerve head perfusion changes preceding peripapillary retinal nerve fibre layer thinning in preclinical diabetic retinopathy. Clin Exp Ophthalmol 2019;47(2):219–25. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues TM, Marques JP, Soares M, et al. Peripapillary neurovascular coupling in the early stages of diabetic retinopathy. Retina 2018. [DOI] [PubMed]
- 25.McKean-Cowdin R, Fairbrother-Crisp A, Torres M, et al. The African American Eye Disease Study: Design and Methods. Ophthalmic Epidemiol 2018;25(4):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu Z, Lin J, Gao C, et al. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J Biomed Opt 2016;21(6):066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleveland WS, Grosse E. Computational methods for local regression. Stat Comput 1991;1:47–62. [Google Scholar]
- 28.Khawaja AP, Chan MP, Garway-Heath DF, et al. Associations with retinal nerve fiber layer measures in the EPIC-Norfolk Eye Study. Invest Ophthalmol Vis Sci 2013;54(7):5028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology 2007;114(6):1046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girkin CA, McGwin G Jr, Sinai MJ, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology 2001;118:2403–2408. [DOI] [PubMed] [Google Scholar]
- 31.Mauschitz MM, Bonnemaijer PW, Diers K, et al. Systemic and ocular determinants of peripapillary retinal nerve fiber layer thickness measurements in the European Eye Epidemiology (E3) population. Ophthalmology 2018:1526–36. [DOI] [PubMed]
- 32.Cheung CY, Chen D, Wong TY, et al. Determinants of quantitative optic nerve measurements using spectral domain optical coherence tomography in a population-based sample of non-glaucomatous subjects. Invest Ophthalmol Vis Sci 2011;52(13):9629–35. [DOI] [PubMed] [Google Scholar]
- 33.Rougier MB, Korobelnik JF, Malet F, et al. Retinal nerve fibre layer thickness measured with SD-OCT in a population-based study of French elderly subjects: the Alienor study. Acta Ophthalmol 2015;93(6):539–45. [DOI] [PubMed] [Google Scholar]
- 34.Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci 2003;44(11):4644–50. [DOI] [PubMed] [Google Scholar]
- 35.Leung H, Wang JJ, Rochtchina E, et al. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci 2003; 44(7):2900–4. [DOI] [PubMed] [Google Scholar]
- 36.Kawasaki R, Wang JJ, Rochtchina E, et al. Cardiovascular risk factors and retinal microvascular signs in an adult Japanese population: the Funagata Study. Ophthalmology 2006;113(8):1378–84. [DOI] [PubMed] [Google Scholar]
- 37.Muraoka Y, Tsujikawa A, Kumagai K, et al. Age- and hypertension-dependent changes in retinal vessel diameter and wall thickness: an optical coherence tomography study. Am J Ophthalmol 2013; 156(4):706–14. [DOI] [PubMed] [Google Scholar]
- 38.Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol 2010;21(2):91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alasil T, Wang K, Keane PA, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma 2013;22(7):532–41. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann EM, Schmidtmann I, Siouli A, et al. The distribution of retinal nerve fiber layer thickness and associations with age, refraction, and axial length: the Gutenberg health study. Graefes Arch Clin Exp Ophthalmol 2018;256(9):1685–93. [DOI] [PubMed] [Google Scholar]
- 41.Lamparter J, Schmidtmann I, Schuster AK, et al. Association of ocular, cardiovascular, morphometric and lifestyle parameters with retinal nerve fibre layer thickness. PLoS One 2018;13(5):e0197682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fairweather D Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol 2014;8(Suppl 3):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vujosevic S, Muraca A, Vet Gatti et al. Peripapillary microvascular and neural changes in diabetes mellitus: An OCT-Angiography Study. Invest Ophthalmol Vis Sci 2018;59(12):5074–81. [DOI] [PubMed] [Google Scholar]
- 44.van Dijk HW, Verbraak FD, Kok PH, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci 2010;51(7):3660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi R, Guo Z, Wang F, Li R, Zhao L, Lin R. Alterations in retinal nerve fiber layer thickness in early stages of diabetic retinopathy and potential risk factors. Curr Eye Res 2018;43(2):244–53. [DOI] [PubMed] [Google Scholar]
- 46.Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology 2015;122(1):72–8. [DOI] [PubMed] [Google Scholar]
- 47.Chopra V, Varma R, Francis BA, et al. Type 2 diabetes mellitus and the risk of open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology 2008;115(2):227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Jia Y, Takusagawa HL, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol 2015;133:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma: a population-based assessment. Arch Ophthalmol 1995;113(2):216–21. [DOI] [PubMed] [Google Scholar]
- 50.Venugopal JP, Rao HL, Weinreb RN, et al. Repeatability of vessel density measurements of optical coherence tomography angiography in normal and glaucoma eyes. Br J Ophthalmol 2018;102(3):352–7. [DOI] [PubMed] [Google Scholar]
- 51.Venugopal JP, Rao HL, Weinreb RN, et al. Repeatability and comparability of peripapillary vessel density measurements of high-density and non-high-density optical coherence tomography angiography scans in normal and glaucoma eyes. Br J Ophthalmol 2018:0:1–6. [DOI] [PubMed] [Google Scholar]
- 52.C. Z. M. I. Cirrus HD-OCT User Manual 4th ed. Dublin CA: Carl Zeiss Meditec; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


