Abstract
The hypothalamic neuropeptide, orexin (or hypocretin), is implicated in numerous physiology and behavioral functions, including affective states such as depression and anxiety. The underlying mechanisms and neural circuits through which orexin modulates affective responses remain unclear. The objective of the present study was to test the hypothesis that the serotonin (5-HT) system of the dorsal raphe nucleus (DRN) is a downstream target through which orexin potentially manifests its role in affective states. Using a diurnal rodent, the Nile grass rat (Arvicanthis niloticus), we first characterized the expression of the orexin receptors OX1R and OX2R in the DRN using in situ hybridization. The results revealed distinct distributions of OX1R and OX2R mRNAs, with OX1R predominantly expressed in the dorsal and lateral wings of the DRN that are involved in affective processes, while OX2R was mostly found in the ventral DRN that is more involved in sensory-motor function. We next examined how the orexin-OX1R pathway regulates 5-HT in the DRN and some of its projection sites using a selective OX1R antagonist SB-334867 (10 mg/kg, i.p.). A single injection of SB-334867 decreased 5-HT-ir fibers within the anterior cingulate cortex (aCgC); five once-daily administrations of SB-334867 decreased 5-HT-ir not only in the aCgC but also in the DRN, oval bed nucleus of the stria terminalis (ovBNST), nucleus accumbens shell (NAcSh), and periaqueductal gray (PAG). HPLC analysis revealed that five once-daily administrations of SB-334867 did not affect 5-HT turnover any of the five sites, although it increased the levels of both 5-HT and 5-HIAA in the NAcSh. These results together suggest that orexinergic modulation of DRN 5-HT neurons via OX1Rs may be one pathway through which orexin regulates mood and anxiety, as well as perhaps other neurobiological processes.
Keywords: orexin, dorsal raphe, serotonin, anxiety, depression
Introduction
Orexin, also known as hypocretin, is a hypothalamic neuropeptide with a well-established role in regulating many important physiological functions including the sleep-wake cycle and energy homeostasis (Siegel, 2004; Tsujino and Sakurai, 2009). The orexin system has also been implicated in regulating mood and anxiety (Nollet and Leman, 2013; Pizza et al., 2014). For example, narcoleptic patients have diminished central orexin levels, and have a higher likelihood of mood and anxiety disorders when compared to the general population or to individuals with other neurological disorders (Fortuyn et al., 2010; Ohayon, 2013; Vourdas et al., 2002). Similarly, lower orexin levels have been reported in patients suffering from major depressive disorders or comorbid depression and anxiety (Brundin et al., 2007a; Brundin et al., 2009; Brundin et al., 2007b; Johnson et al., 2010; Rotter et al., 2011). On the other hand, a positive correlation between orexin and positive emotions has been observed in both dogs and humans (Blouin et al., 2013; Wu et al., 2011). Although there is a clear association between orexin and affective state, the underlying neural pathways through which orexin regulates mood and emotion are not well understood.
One of the potential downstream targets of the orexinergic system in regulating affective state is the dorsal raphe nucleus (DRN), which contains the greatest number of midbrain 5-HTergic neurons and is implicated in depression and anxiety among other functions (Graeff, 1993; Michelsen et al., 2007). Orexin neurons project heavily to the DRN (Nixon and Smale, 2007; Peyron et al., 1998), where orexin peptides induce excitatory responses in vitro (Soffin et al., 2004) and stimulate 5-HT release in vivo (Tao et al., 2006). Manipulating the central 5-HT system is the basis of many popular pharmacotherapies for treating affective disorders, albeit controversial because the underlying mechanisms and effectiveness of such drugs are unclear (Cipriani et al., 2018; Harmer et al., 2017). Regardless, our previous findings in a diurnal rodent model of seasonal affective disorder (SAD) support for a role of the orexin-DRN pathway in regulating mood and anxiety (Deats et al., 2014; Ikeno et al., 2016; Leach et al., 2013).
SAD is a major depressive disorder with a seasonal pattern, in which patients experience recurring depression episodes in fall and winter followed by spontaneous remission in spring and summer (Rosenthal et al., 1984). The symptoms of SAD can be alleviated by bright light therapy, suggesting a causal link between reduced light exposure and depression in winter months. Bright light exposure promotes arousal and wakefulness in diurnal mammals including humans; but induces sleep in in nocturnal ones (Smale et al., 2003). Therefore, a diurnal model is advantageous for understanding the neuropathology of SAD (Yan et al., 2019). Previous work from this laboratory utilized diurnal Nile grass rats (Arvicanthis niloticus) that were housed in a winter-like lighting condition with reduced daytime light intensity (Leach et al., 2013). After four weeks, the animals showed higher depression- and anxiety-like behaviors compared to the controls housed in a summer-like condition with bright light during the day. The increased depression- and anxiety-like behaviors were accompanied by attenuated orexin-ir fibers and fewer 5-HT-ir neurons in the DRN, and a lower density of 5-HT-ir fibers/terminals in the anterior cingulate cortex (aCgC) (Deats et al., 2014; Leach et al., 2013). We also found a functional connection between light, hypothalamic orexin cells and the DRN using the neural activity marker Fos (Adidharma et al., 2012). Bright light exposure increased Fos-ir in both orexin cells and in the DRN, and pretreating grass rats with a selective orexin receptor 1 (OX1R) antagonist, SB-334867, prevented light-induced Fos in the DRN. Furthermore, treating grass rats with SB-334867 led to increased depressive-like behaviors even when the animals were housed in a summer-like bright light condition (Deats et al., 2014). These results collectively suggest that in diurnal grass rats, orexinergic inputs to the DRN underlie light-dependent changes in behavioral paradigms modeling aspects of depression and anxiety. It should be noted that although the distribution of orexin receptors is generally similar between diurnal and nocturnal rodents, there are species-specific expression in brain regions implicated in regulating sleep, emotion and cognition (Ikeno and Yan, 2018). For example, OX1R mRNA has been detected in the caudate putamen and ventral tuberomammillary nucleus only in diurnal grass rats, but not in nocturnal mice (Ikeno and Yan, 2018). Thus, elucidating orexinergic regulation in 5-HT system in diurnal grass rats will contribute to a better understanding on how the two systems interact in humans.
In the present study we first characterized the expression of orexin receptors in the DRN of grass rats. The results revealed a distinct pattern in the distribution of OX1R and OX2R, one that suggests OX1R plays a dominant role in the DRN for modulating affective behaviors. We then assessed 5-HT-ir in the DRN and in several 5-HT neuron projecting sites involved in mood and anxiety, including the aCgC, oval bed nucleus of the stria terminalis (ovBNST), nucleus accumbens shell (NAcSh), and periaqueductal gray (PAG) following either acute (single administration) or subchronic (five daily administrations) orexin receptor antagonism with SB-334867. As discussed above, we previously found that a single administration of SB-334867 increased depression-like behaviors in grass rats housed in a summer-like condition (Deats et al., 2014), and demonstrating changes in the 5-HT system following the same treatment would support it as a downstream target through which orexin regulates affective state. The subchronic paradigm was intended to induce a more sustained attenuation of OX1R-mediated signaling, as in our grass rat SAD model that displays depression-like behaviors when animals are housed under low-intensity daylight (Deats et al., 2014; Leach et al., 2013). Following OX1R antagonism, we measured 5-HT-ir as well as levels of 5-HT and its metabolite 5-HIAA in the DRN and in projecting sites. The results demonstrate that OX1R-mediated signaling regulates the 5-HT system of the DRN, as well as some DRN projection sites, and provide insights into the pathways through which orexin neurons modulate affective functioning in a diurnal species.
Methods
Animals and housing conditions:
Adult male grass rats (Arvicanthis niloticus) were produced from a breeding colony originally established with animals imported from sub-Saharan Africa in 1993 and since maintained at Michigan State University (for details see McElhinny et al., 1997). The animals in the colony were housed in a 12hr light:12hr dark (LD) cycle with food (Prolab 2000 #5P06, PMI Nutrition LLC, MO, USA) and water available ad libitum. The time of lights-on was defined as Zeitgeber time (ZT) 0. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23) and were approved by the Institutional Animal Care and Use Committee of Michigan State University.
Experiment 1: Distribution of orexin receptors in the DRN
To determine the distribution of orexin receptors in the DRN, male grass rats (n = 5) were transcardially perfused with saline followed by 4% paraformaldehyde around midday between ZT5–7. The brains were post-fixed with 4% paraformaldehyde and cryoprotected in 20% sucrose before being processed for in situ hybridization using cRNA probes for OX1R and OX2R mRNA (details below).
Experiment 2: Effects of acute OX1R antagonism on 5-HT immunoreactivity (ir)
Male grass rats (4–6 month old) received a single intraperitoneal (i.p.) injection of either the selective OX1R antagonist, SB-334867 (10 mg/kg, Tocris Biosciences, Bristol, UK), or vehicle (60:40 DMSO/saline, 0.4 ml) at ZT2. This dose was based on our previous study of grass rats (Adidharma et al., 2012; Deats et al., 2014), with effects on their brain and behaviors observed at 3 to 4 hr post-injection. Animals were overdosed with pentobarbital, then transcardially perfused with saline followed by 4% paraformaldehyde at ZT5 (i.e., 3 hr post-injection, n = 6/group). Brains were post-fixed and cryoprotected, and sectioned at 40 μm for immunostaining of 5-HT.
Experiment 3: Effects of subchronic OX1R antagonism on 5-HT-ir, 5-HT content and turnover
Similar to above, male grass rats received an i.p. injection of either the selective OX1R antagonist, SB-334867 (10 mg/kg), or vehicle (60:40 DMSO/saline, 0.4 ml), but once a day for five consecutive days. 24 hours after the last injection, one cohort of animals (n = 6/group) was overdosed with pentobarbital and perfused. Their brains were prepared for immunostaining of 5-HT as in Experiment 2. Another cohort of animals (n = 8–9/group) was injected once a day for five days with SB-334867 or vehicle, overdosed and rapidly decapitated the next day, and the brains used for HPLC analysis of 5-HT system measures in the DRN, aCgC and NAc. Fresh-frozen brains were sliced coronally at 200 μm. Micropunches (0.5-mm diameter) through the sites of interest were made bilaterally and stored at −80° C until being processed for HPLC.
In situ hybridization:
Antisense and sense cRNA probes for OX1R and OX2R were produced and in situ hybridization was performed as described previously (Ikeno and Yan, 2018). In brief, coronal sections (40 μm) containing the DRN were treated with proteinase K and acetic anhydride prior to incubation with DIG-labelled OX1R or OX2R antisense (0.5 μg/ml) or sense probes (0.5 μg/ml) overnight at 60 °C. After a series of washes, sections were treated with RNase A, then incubated in an alkaline phosphatase-conjugated DIG antibody (1:5000, Sigma-Aldrich) for 3 d. Sections were then incubated in NBT/BCIP solution (Roche) overnight at room temperature.
Immunohistochemistry (IHC):
Single-label IHC for 5-HT was conducted using methods similar to those described in our previous study (Leach et al., 2013). Briefly, coronal sections containing the DRN and four of its target regions of interest were rinsed in 0.1 M phosphate buffer before incubation in a primary rabbit antiserum against 5-HT (1:10,000, NT-102 5HTrab, Protos Biotech, NY) at 4°C for 2 days. This antiserum was raised against a formaldehyde 5-HT-hemocyanin conjugate, and specificity was established by abolishment of immunostaining following preabsorption with the immunogen (Chalazonitis et al., 2008). Sections were rinsed in phosphate buffer before incubation in a secondary antibody (biotinylated goat anti-rabbit, 1:1000, Vector lab, CA) at 4° C overnight. Processing was completed with the avidin-biotin-immunoperoxidase technique (VECTASTAIN Elite ABC System, Vector lab, CA) per the manufacturer’s protocol. Finally, the 5-HT containing cell bodies and fibers were stained brown using 3’3-diaminobenzidine (Sigma-Aldrich, St. Louis, MO) as chromogen.
Quantitative analysis of IHC results:
Images of the brain sections were captured using a CCD video camera (CX9000, MBF Bioscience, VM, USA) attached to a light microscope (Nikon Instruments Inc., NY, USA). The camera and microscope settings were kept constant for all images. 5-HT-ir was analyzed in the DRN, NAcSh, ovBNST, aCgC and PAG. Sections containing the brain regions of interest (4–8 sections per region) correspond to the following plates in the rat brain atlas (Paxinos and Watson, 2004): planes 92 to 97 (Bregma −7.08 to −7.68 mm) for DRN, planes 20 to 24 (Bregma 1.56 to 1.08 mm) for NAcSh, planes 30 to 33 (Bregma 0.36 to 0 mm) for ovBNST, planes 14 to 33 (Bregma 2.28 to 0 mm) for aCgC and planes 86 to 93 (Bregma −6.36 to −7.20 mm) for PAG. In the DRN, sections from the rostral two-thirds of the DRN were analyzed, with sections containing 5-HT-ir cell bodies clustered at the center defining the rostral subregions (dorsal, ventrolateral, ventral), while the cell bodies spreading laterally defined the middle DRN (dorsal, ventral, ventrolateral “wings”) (Coomans et al., 2013). Observers blind to the animals’ experimental conditions analyzed the cell number and optical density measurements using NIH Image as described in previous studies (Adidharma et al., 2012; Deats et al., 2014). Student’s t-tests were used to determine differences between animals injected with the OX1R antagonist or vehicle on both the number of 5-HT cell bodies and 5-HT fiber density, with ps < 0.05 indicating statistical significance and effect size estimated using Cohen’s d.
HPLC-EC Analysis of Monoamines:
Biogenic amines were quantified as described elsewhere (Perez et al., 2005; Spieles-Engemann et al., 2010b; Kanaan et al., 2015; Chen et al., 2019). Brain punches were sonicated in 200 μl of antioxidant solution (0.4 N perchlorate, 1.34 mM EDTA, and 0.53 mM sodium metabisulfite), and total protein concentration was determined using BCA assays (Pierce). Samples were centrifuged at 10,000 g for 10 min at 4° C. The supernatant was separated on a 150 × 4.6 mm Microsorb MV C18 100–5 column (Agilent Technologies), and simultaneously examined for 5-hydroxytryptamine (5-HT), 5-hydroxy-indoleacetic acid (5-HIAA), dopamine (DA), homovanillic acid (HVA), and 3,4-dihydroxyphenylacetic acid (DOPAC). Analytes were quantified on a 12-channel coulometric array detector (CoulArray 5200, ESA) attached to an autosampler/solvent delivery system (Waters Alliance 2695) under the following conditions: flow rate of 1 ml/min; detection potentials of 25, 85, 120, 180, 220, 340, 420, 480mV; and scrubbing potential of 750 mV. The mobile phase consisted of 100 mM citric acid, 75 mM Na2HPO4, 80 μM heptanesulfonate monohydrate, pH 4.25, in 11% methanol. Sample values were calculated based on a minimum six-level standard curve of the analytes with quality control samples interspersed within the sample run. Data were expressed in ng/mg protein. Student’s t-tests were used to determine differences between groups of animals injected with the OX1R antagonist or vehicle for each measure, with ps < 0.05 indicating statistical significance and effect size estimated using Cohen’s d.
Results
Distribution of OX1R and OX2R mRNA in the DRN of grass rats
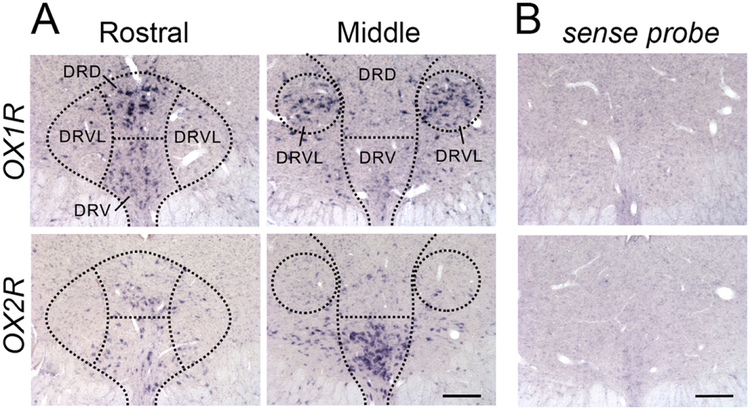
In the DRN, hybridization signals for OX1R and OX2R mRNA showed distinct distribution patterns. Particularly strong signal for OX1R mRNA was detected in the dorsal subregion of the rostral DRN, while there was only moderate expression in the ventral subregion at that level (Fig. 1A). There was also very dense OX1R mRNA in the in the lateral wings of the middle DRN (Fig. 1A). On the other hand, only weak OX2R signal was found in the rostral DRN in either the dorsal and ventral subregions, but very dense OX2R expression was found in the ventral part of the middle DRN (Fig. 1A). A sense probe for OX1R or OX2R mRNA revealed no specific labeling in the DRN (Fig. 1B).
Figure 1:
Distribution of OX1R and OX2R mRNA in the rostral and middle DRN. DRD, DR dorsal; DRV, DR ventral; DRVL, DR ventrolateral wings. Scale bar, 100 μm.
Acute OX1R antagonism increased 5-HT-ir in the DRN and reduced 5-HT-ir fiber density in aCgc
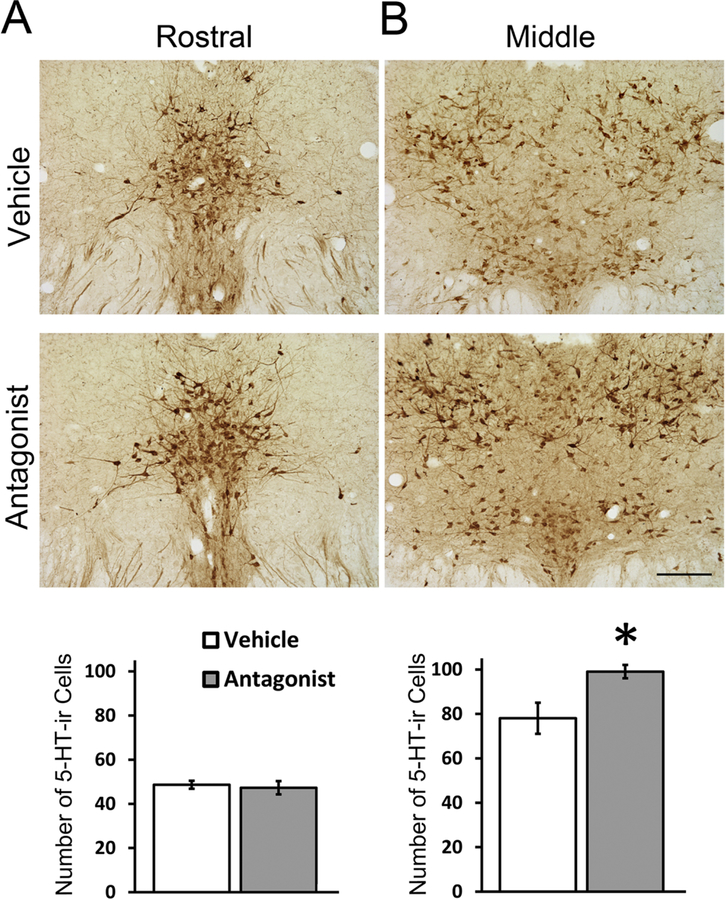
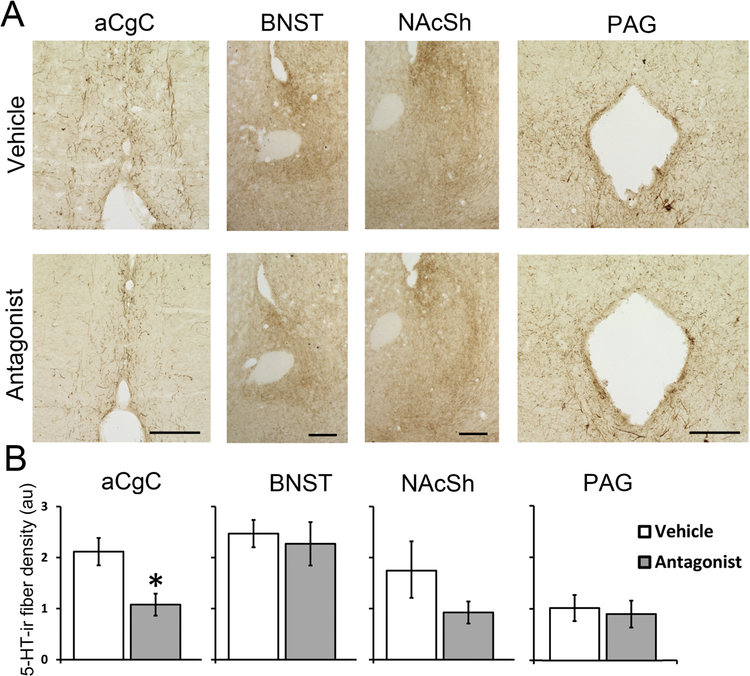
Following a single IP injection of the OX1R antagonist, SB-334867, the numbers of 5-HT-ir neurons in the rostral DRN did not differ between the antagonist- and vehicle-treated groups (Fig. 2A, t8 = −0.38, p = 0.713, Cohen’s d = 0.24), but in the middle DRN, the antagonist-treated group had a small but significantly higher number of 5-HT-ir cells compared to controls (Fig. 2B, t8 = −2.76, p = 0.025, Cohen’s d = 1.75). Antagonist-treated animals also showed significantly lower 5-HT-ir fiber density in the aCgC (Fig. 3, t8 = −3.01, p = 0.017, Cohen’s d = 1.78), but not in the BNST, NAcSh or PAG (ps > 0.05, Cohen’s d = 0.32, 1.75 and 0.0 respectively).
Figure 2:
Representative photomicrographs and quantitative analysis of 5-HT-ir cells in the rostral (A) and middle (B) DRN following a single injection of OX1R antagonist SB-33486 or vehicle. Data are shown as Means ± SEMs (n = 5). *indicates p < 0.05. Scale bar, 100 μm.
Figure 3:
Representative photomicrographs (A) and quantitative analysis (B) of 5-HT-ir fibers in the anterior cingulate cortex (aCgC), oval bed nucleus of the stria terminalis (ovBNST), nucleus accumbens shell (NAcSh), and periaqueductal grey area (PAG) following a single injection of OX1R antagonist SB-334867 or vehicle. Data are shown as Means ± SEMs (n = 5). *indicates p < 0.05. Scale bar, 100 μm.
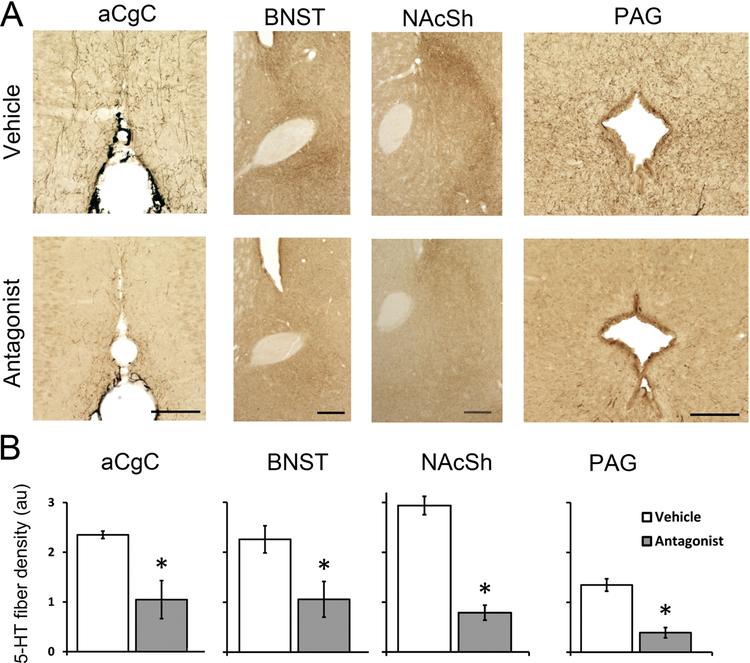
Subchronic OX1R antagonism reduced 5-HT-ir in the DRN and its targets
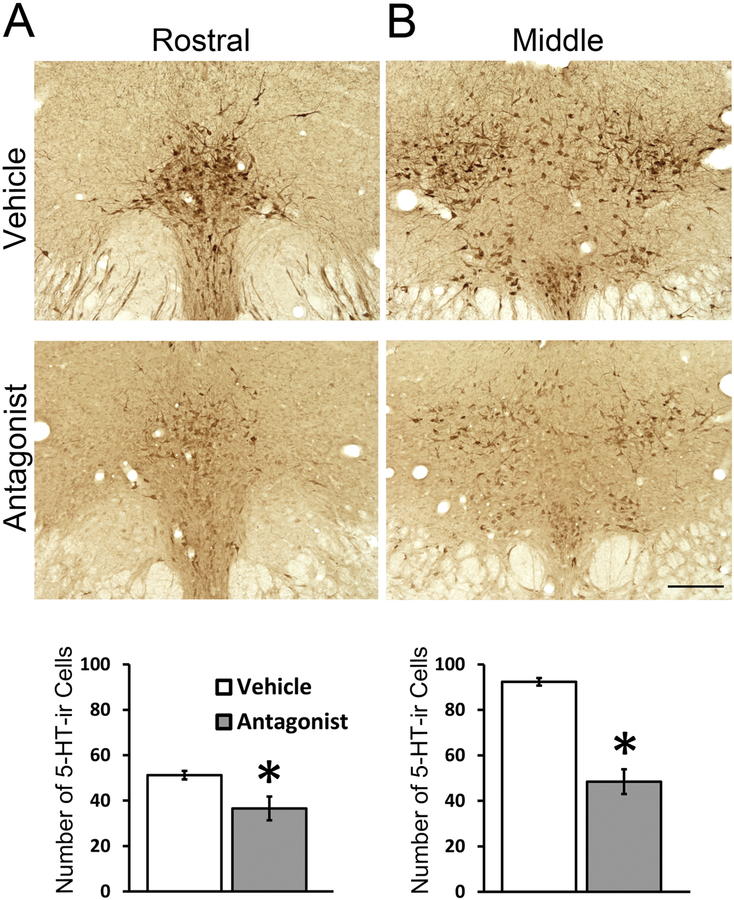
Five daily treatments of SB-334867 significantly reduced the number of 5-HT-ir neurons in the DRN compared to the number found in the DRN of vehicle-treated animals; this was true for both the rostral (Fig. 4A, t9 = 2.85, p = 0.046, Cohen’s d = 1.99) and middle DRN (Fig. 4B, t9 = 8.38, p < 0.001, Cohen’s d = 4.85). SB-334867-treated animals also showed a marked reduction in 5-HT-ir fiber density in the aCgC (t8 = −6.22, p < 0.001, Cohen’s d = 2.26, Cohen’s d = 1.99), BNST (t9 = 3.58, p = 0.006, Cohen’s d = 1.63), NAcSh (t9 = 8.80, p < 0.001, Cohen’s d = 5.32) and PAG (t9 = 5.75, p < 0.001, Cohen’s d = 3.37) (Fig. 5).
Figure 4:
Representative photomicrographs and quantitative analysis of 5-HT-ir cells in the rostral (A) and middle (B) DRN following five daily injections of the selective OX1R antagonist, SB-334867 or vehicle. Data are shown as Means ± SEMs (n = 6 vehicle/n = 5 antagonist). *indicates p < 0.05. Scale bar, 100 μm.
Figure 5:
Representative photomicrographs (A) and quantitative analysis (B) of 5-HT-ir fibers in the anterior cingulate cortex (aCgC), oval bed nucleus of the stria terminalis (ovBNST), nucleus accumbens shell (NAcSh), and periaqueductal grey area (PAG) in animals receiving either 5 daily injection of the OX1R antagonist SB-334867 or vehicle. Data are shown as Means ± SEMs (n = 6 vehicle/n = 5 antagonist). *indicates p < 0.01. Scale bar, 100 μm.
HPLC analysis revealed no significant difference between the SB-334867- and vehicle-treated groups in 5-HT turnover within the three brain sites analyzed (i.e., DRN, aCgC, NAcSh) (Table 1). However, the levels of 5-HT and its metabolite 5-HIAA (normalized by total protein) were higher in the NAcSh of the OXR1 antagonist treated group compared to controls. The antagonist-treated animals also had higher levels of DOPAC and HVA in the NAc, as well as in the DRN. While these measures were all corrected for total protein per sample, it should be noted that total protein per sample measured in the NAc itself was unexpectedly affected by OXR1 antagonist treatment, with total protein lower in the NAc (but not in the DRN or aCgC) of the antagonist-treated group compared to the controls (Table 1).
Table 1:
Intracellular serotonin and dopamine measures indicated by HPLC analysis of the dorsal raphe nucleus (DRN), anterior cingulate cortex (aCgC), and nucleus accumbens (NAc) of male Nile grass rats that received five daily injections of the OXR1 antagonist (SB-334867; Antag) or Vehicle (Veh). Significant differences between groups indicated in bold font.
| 5HIAA (pg/μg protein) | 5-HT (pg/μg protein) | 5HIAA/5-HT | DOPAC (pg/μg protein) | HVA (pg/μg protein) | Total Protein (μg/sample) | ||
|---|---|---|---|---|---|---|---|
| DRN | Veh (Mean ± SEM) | 23.46 ± 1.57 | 9.92 ± 0.76 | 2.42 ± 0.15 | 0.81 ± 0.03 | 1.09 ± 0.03 | 11.48 ± 0.90 |
| Antag (Mean ± SEM) | 26.24 ± 1.41 | 11.15 ± 0.76 | 2.39 ± 0.11 | 0.95 ± 0.06 | 1.26 ± 0.06 | 11.82 ± 0.86 | |
| T-test (p; Cohen’s d) | 0.27; 0.59 | 0.33; 0.52 | 0.91; 0.07 | 0.05; 0.98 | 0.04; 1.14 | 0.81; 0.12 | |
| aCgC | Veh (Mean ± SEM) | 1.59 ± 0.12 | 0.82 ± 0.03 | 1.93 ± 0.14 | 0.26 ± 0.03 | 0.44 ± 0.07 | 57.13 ± 3.46 |
| Antag (Mean ± SEM) | 1.53 ± 0.07 | 0.86 ± 0.03 | 1.79 ± 0.08 | 0.28 ± 0 .03 | 0.41 ± 0.03 | 59.91 ± 2.38 | |
| T-test (p; Cohen’s d) | 0.70; 0.29 | 0.41; 0.54 | 0.34; 0.74 | 0.64; 0.13 | 0.75; 0.21 | 0.51; 0.37 | |
| NAc | Veh (Mean ± SEM) | 2.39 ± 0.15 | 1.43 ± 0.11 | 1.73 ± 0.17 | 1.55 ± 0.11 | 1.58 ± 0.09 | 35.11 ± 2.28 |
| Antag (Mean ± SEM) | 3.40 ± 0.21 | 2.20 ± 0.16 | 1.56 ± 0.06 | 2.61 ± 0.30 | 2.43 ± 0.18 | 22.86 ± 2.17 | |
| T-test (p; Cohen’s d) | 0.00; 1.98 | 0.00; 1.99 | 0.34; 0.49 | 0.01; 1.58 | 0.00; 2.07 | 0.00; 1.92 |
Discussion
The results of the present studies reveal that in diurnal grass rats, orexin-OX1R signaling has a significant impact on the central 5-HT system and therefore likely plays a role in regulating 5-HT-mediated processes, including affective behaviors. This is probably not exclusive to diurnal grass rats because expression of OX1R and OX2R mRNA has also been found in the DRN of laboratory mice and rats (Greco and Shiromani, 2001; Marcus et al., 2001; Mieda et al., 2011; Trivedi et al., 1998), although subregional difference in abundance of each receptor has not been previously reported. In the present study, a closer look of the distribution of each receptor subtype revealed a distinct spatial pattern in the DRN, such that OX1R was predominantly expressed in the dorsal and lateral wings while OX2R was mainly expressed in the ventral subregion (Fig. 1). The DRN subregions are not only defined by the cytoarchitecture and distribution of 5-HT neurons, but also their afferent and efferent projections, together suggesting functional differences (Hale and Lowry, 2011). For example, the dorsal subnucleus and the lateral wings are considered part of the neural system involved in the behavioral and physiological responses related to stress and anxiety, while the ventral subregion regulates sensory-motor functions (Hale and Lowry, 2011). The distinct distribution pattern of OX1R and OX2R mRNA in the DRN suggest that OX1R predominantly influences the dorsal and lateral DRN for orexin’s effects on mood and emotional behaviors in grass rats. This is consistent with existing literature suggesting distinct functions of OX1R and OX2R, including a genetic study that found a significant association between unipolar depression and a polymorphism of OX1R, but not OX2R (Rainero et al., 2011). OX2R has instead been primarily implicated in narcolepsy and catalepsy (Hasegawa et al., 2014; Lin et al., 1999; Willie et al., 2003).
Orexin peptides have been shown to regulate 5-HT neurons in nocturnal laboratory rats (Liu et al., 2002; Soffin et al., 2004; Tao et al., 2006). Both peptides can excite 5-HT neurons directly, as well as indirectly by inhibiting local GABAergic inputs to 5-HT neurons (Liu et al., 2002). The excitatory effects of orexin A can be blocked by the selective OX1R antagonist, SB-334967 (Soffin et al., 2004). Orexins can also induce the release of 5-HT. Infusion of orexin A (30 μM) into the DRN leads to a 2–3-fold increase in local extracellular 5-HT, while orexin B only leads to 20–30% of increase in 5-HT even at a much higher dose (100 μM), suggesting OX1R plays a more important role in regulating DRN 5-HT neurons (Tao et al., 2006). Three hours following a single intraperitoneal injection of an OX1R antagonist we found a small, but statistically significant, increase in the number of 5-HT-ir neurons in the middle portion of the DRN. We also found a significant decrease in 5-HT-ir fiber density in the aCgC when compared to the fiber density found in vehicle-treated animals. Such a seemingly rapid change in immunoreactivity for 5-HT itself (rather than its precursors or metabolizing enzymes) is consistent with the fact that brain SB-334867 concentrations after an IP injection reach peak levels within 30 minutes post-injection and remain high for at least 4 hours (Ishii et al., 2005). In addition, at least one other study found similarly rapid changes in 5-HT immunoreactivity in neural somata and fibers after a single experimental event (Lorenzi and Grober, 2012).
There was no significant effect of a single OX1R antagonist injection on 5-HT-ir fiber density in the other three brain regions examined (BNST, NAcSH or PAG), though, but did so after five daily injections of the OX1R antagonist. These results may collectively suggest that antagonism of orexin-OX1R signaling impedes 5-HTergic output from the DRN to some of its target regions, resulting in accumulation of 5-HT within the somata and less at the terminals. Conversely, the results could indicate that OX1R antagonism causes rapid neurotransmitter release that depletes 5-HT from the fibers. The former case may be more likely, as previous studies have found that damaging raphe serotonergic cells or their ascending projections at least initially reduces rather than increases 5-HT fiber density in numerous forebrain targets (e.g., Holschbach et al., 2018; MacLean and Shipley, 1987; Mayer-Berstein et al., 1997; Zhou and Azmitia, 1986).
Our HPLC analysis of 5-HT turnover after repeated OX1R antagonist injections aimed to address this issue, but we did not find a significant effect on 5-HT turnover in the DRN or in the aCgC and NAcSh. However, levels of both 5-HT and 5HIAA were higher in the NAcSh of the antagonist-treated group, which was unexpected given their lower 5-HT-ir fiber density compared to controls. We do not have a simple explanation for this finding, especially in light of the fact that the amount of total protein in the NAcSh (but not the DRN or aGgC from the very same animals) used to standardize the neurochemical measurements in each sample was unexpectedly lower in the antagonist-treated group than in the controls. Perhaps the reduction in total protein levels in the NAcSh of SB-334867-treated animals reflects an inhibition of orexin-induced neurotrophic factors involved in mesolimbic function (Harada et al., 2013; Winrow et al., 2010; Yamada et al., 2009). In any case, previous work from our group has shown that acute antagonism of OX1R signaling with SB-334867 provokes a depressive phenotype in the forced-swim test in diurnal grass rats (Deats et al., 2014), which may at least partly due to a change in 5-HTergic inputs to the aCgC, as indicated by a reduction in 5-HT-ir fiber density. The aCgC has long been implicated in regulating emotion and mood (Etkin et al., 2011; Pizzagalli, 2011). Patients with unipolar and bipolar depression show reduced neural activity in the aCgC (Drevets et al., 1997), and lesions of the aCgC in rats significantly increase immobility time in a forced swim test, reflecting greater behavioral despair (Bissiere et al., 2006). 5-HT modulates activity of aCgC, with the antidepressant effects of deep brain stimulation in the aCgC nullified by 5-HT depletion (Hamani et al., 2012), and decreased 5-HT transporter binding in the aCgC found after human subjects are treated with bright light therapy (Harrison et al., 2015). Our results show that a single injection of OX1R antagonist significantly reduces 5-HT-ir fibers in the aCgC within 3 hrs, highlighting a potential pathway through which the orexinergic activity can acutely and relatively rapidly regulate affective state. Determining some of the cellular mechanisms through which natural or pharmacological changes in orexin receptor activity can rapidly affect aspects of the 5-HT system that underlie our broader assessments of immunoreactivity and turnover - such as 5-HT cell expression and activity of the TPH2 and AADC enzymes, cell firing rate and pattern, and rates of terminal 5-HT packaging and release - under particular conditions that influence affective state (e.g., different lighting conditions, stress levels, or social environments) would be valuable in future studies.
Following five-days of OX1R antagonism with SB-334867, we found an even more marked change of 5-HT-ir, involving a widespread decrease in the number of 5-HT-ir cells in the DRN and a decrease in 5-HT-ir fiber density not only in the aCgC but also in the NAcSh, BNST and PAG. The latter sites have also been implicated in regulating mood and anxiety (Bennett, 2011; Graeff, 2004; Puig and Gulledge, 2011). For instance, the NAcSh is well known to provide motivational salience to a given stimulus and is tightly linked to the feeling of pleasure, which is a key parameter affected in mood disorders (Ito et al., 2004; Shirayama and Chaki, 2006). 5-HT release in the mesolimbic system is associated with reduced motivated behavior, so one could have expected OX1R antagonism to promote 5-HT signaling (possibly increase 5-HT-ir fiber density in the NAc rather than decrease it). However, optogenetic stimulation of 5-HT release in the NAc does not alone affect responding for saccharine reward in mice (Browne et al., 2019), so perhaps our decrease in 5-HT-ir fiber density is unrelated to orexin’s effects on accumben’s control of affective behaviors. The BNST has been implicated in stress and anxiety, with 5-HT 1A receptor activation associated with an anxiolytic response (Garcia-Garcia et al., 2018; Gomes et al., 2011; Levita et al., 2004) and 2C receptor activity associated with an anxiogenic response (Marcinkiewcz et al., 2016). Furthermore, electrical stimulation of the BNST produces behaviors similar to those caused by a stressful stimulus (Casada and Dafny, 1991). Reduced 5-HT input onto 1A receptors in the BNST may therefore impede the ability to cope with stressful stimuli, generating an anxiogenic response as similarly exhibited by diurnal grass rats treated with OXR1 antagonist or housed in dim light during the daytime (Deats et al., 2014; Ikeno et al., 2016). Lastly, OX1R activation disinhibits the PAG (Ho et al., 2011), which depending on the PAG subregion involved is associated with fearful or anxious behaviors (Brandao et al., 2008; Fendt, 1998; Miller et al., 2010; Morgan and Clayton, 2005), and OX1R receptor antagonism in the PAG can reduce anxiety-related behaviors in laboratory rats (Pourrahimi et al., 2019).
Previous research on our grass rat model of SAD found that dim daylight intensity led to decreased 5-HT-ir fiber density in the DRN, aCgC and PAG; these animals also shows more depression- and anxiety-like behaviors compared to animals housed under conditions of bright daylight intensity (Ikeno et al., 2016; Leach et al., 2013). The dim-daylight animals also had fewer orexin-ir neurons in the hypothalamus and lower orexin-ir fiber density in the DRN compared to controls housed in bright light during the day (Deats et al., 2014). The results from the present study support a causal link between the attenuated OXergic activity and 5-HTergic outputs observed in the grass rat model of SAD, and suggest that the 5-HTergic dorsal raphe is one of the downstream targets for orexin’s modulation of affective behaviors in diurnal grass rats and other animals.
Highlights.
OX1R and OX2R show distinct distribution in the dorsal raphe of grass rats.
OX1R antagonism alters 5-HT-ir within the dorsal raphe and its target sites.
The dorsal raphe is a downstream site of orexin for regulating affective behaviors.
Acknowledgments
This work is supported by NIH grants MH093760 to LY and MH111276 to LY and JSL. The content is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies. The funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- Adidharma W, Leach G, Yan L, 2012. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience 220, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, 2011. The prefrontal-limbic network in depression: Modulation by hypothalamus, basal ganglia and midbrain. Prog Neurobiol 93, 468–487. [DOI] [PubMed] [Google Scholar]
- Bissiere S, McAllister KH, Olpe HR, Cryan JF, 2006. The rostral anterior cingulate cortex modulates depression but not anxiety-related behaviour in the rat. Behav Brain Res 175, 195–199. [DOI] [PubMed] [Google Scholar]
- Blouin AM, Fried I, Wilson CL, Staba RJ, Behnke EJ, Lam HA, Maidment NT, Karlsson KAE, Lapierre JL, Siegel JM, 2013. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Commun 4, 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J, 2008. Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behav Brain Res 188, 1–13. [DOI] [PubMed] [Google Scholar]
- Browne CJ, Abela AR, Chu D, Li Z, Ji X, Lambe EK, Fletcher PJ, 2019. Dorsal raphe serotonin neurons inhibit operant responding for reward via inputs to the ventral tegmental area but not the nucleus accumbens: evidence from studies combining optogenetic stimulation and serotonin reuptake inhibition. Neuropsychopharmacology 44, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Petersen A, Traskman-Bendz L, 2007a. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol 17, 573–579. [DOI] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Traskman-Bendz L, Petersen A, 2009. Increased orexin levels in the cerebrospinal fluid the first year after a suicide attempt. J Affect Disord 113, 179–182. [DOI] [PubMed] [Google Scholar]
- Brundin L, Petersen A, Bjorkqvist M, Traskman-Bendz L, 2007b. Orexin and psychiatric symptoms in suicide attempters. J Affect Disord 100, 259–263. [DOI] [PubMed] [Google Scholar]
- Casada JH, Dafny N, 1991. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull 27, 207–212. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Pham TD, Li Z, Roman D, Guha U, Gomes W, Kan L, Kessler JA, Gershon MD, 2008. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol 509, 474–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR, 2018. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC, Havekes LM, Romijn JA, van Dijk KW, Biermasz NR, Meijer JH, 2013. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J 27, 1721–1732. [DOI] [PubMed] [Google Scholar]
- Deats SP, Adidharma W, Lonstein JS, Yan L, 2014. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience 272, 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR Jr., Todd RD, Reich T, Vannier M, Raichle ME, 1997. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, 1998. Different regions of the periaqueductal grey are involved differently in the expression and conditioned inhibition of fear-potentiated startle. Eur J Neurosci 10, 3876–3884. [DOI] [PubMed] [Google Scholar]
- Fortuyn HA, Lappenschaar MA, Furer JW, Hodiamont PP, Rijnders CA, Renier WO, Buitelaar JK, Overeem S, 2010. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 32, 49–56. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Canetta S, Stujenske JM, Burghardt NS, Ansorge MS, Dranovsky A, Leonardo ED, 2018. Serotonin inputs to the dorsal BNST modulate anxiety in a 5-HT1A receptor-dependent manner. Mol Psychiatry 23, 1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimaraes FS, 2011. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl) 213, 465–473. [DOI] [PubMed] [Google Scholar]
- Graeff FG, 1993. Role of 5-HT in defensive behavior and anxiety. Rev Neurosci 4, 181–211. [DOI] [PubMed] [Google Scholar]
- Graeff FG, 2004. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev 28, 239–259. [DOI] [PubMed] [Google Scholar]
- Greco MA, Shiromani PJ, 2001. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res 88, 176–182. [DOI] [PubMed] [Google Scholar]
- Hale MW, Lowry CA, 2011. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 213, 243–264. [DOI] [PubMed] [Google Scholar]
- Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE, Tescarollo F, Martins U, Covolan L, Nobrega JN, 2012. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry 71, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S, Yamazaki Y, Tokuyama S, 2013. Orexin-A suppresses postischemic glucose intolerance and neuronal damage through hypothalamic brain-derived neurotrophic factor. J Pharmacol Exp Ther 344, 276–285. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Duman RS, Cowen PJ, 2017. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 4, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Tyrer AE, Levitan RD, Xu X, Houle S, Wilson AA, Nobrega JN, Rusjan PM, Meyer JH, 2015. Light therapy and serotonin transporter binding in the anterior cingulate and prefrontal cortex. Acta Psychiatr Scand 132, 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E, Yanagisawa M, Sakurai T, Mieda M, 2014. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest 124, 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, Liao HT, Mackie K, Chiou LC, 2011. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci 31, 14600–14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno T, Deats SP, Soler J, Lonstein JS, Yan L, 2016. Decreased daytime illumination leads to anxiety-like behaviors and HPA axis dysregulation in the diurnal grass rat (Arvicanthis niloticus). Behav Brain Res 300, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno T, Yan L, 2018. A comparison of the orexin receptor distribution in the brain between diurnal Nile grass rats (Arvicanthis niloticus) and nocturnal mice (Mus musculus). Brain Res 1690, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Jeffrey P, Summerfield S, Rodgers RJ, 2005. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res 157, 331–341. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ, 2004. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci 7, 389–397. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A, 2010. A key role for orexin in panic anxiety. Nat Med 16, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach G, Adidharma W, Yan L, 2013. Depression-like responses induced by daytime light deficiency in the diurnal grass rat (Arvicanthis niloticus). PLoS One 8, e57115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG, 2004. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience 128, 583–596. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E, 1999. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98, 365–376. [DOI] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK, 2002. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci 22, 9453–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi V, Grober MS, 2012. Immunohistochemical localization of serotonin in the brain during natural sex change in the hermaphroditic goby Lythrypnus dalli. Gen Comp Endocrinol 175, 527–536. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, Kash TL, 2016. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW, 2007. The dorsal raphe nucleus--from silver stainings to a role in depression. Brain Res Rev 55, 329–342. [DOI] [PubMed] [Google Scholar]
- Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T, 2011. Differential roles of orexin receptor-1 and −2 in the regulation of non-REM and REM sleep. J Neurosci 31, 6518–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Piasecki CC, Peabody MF, Lonstein JS, 2010. GABA(A) receptor antagonism in the ventrocaudal periaqueductal gray increases anxiety in the anxiety-resistant postpartum rat. Pharmacol Biochem Behav 95, 457–465. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC, 2005. Defensive behaviors evoked from the ventrolateral periaqueductal gray of the rat: comparison of opioid and GABA disinhibition. Behav Brain Res 164, 61–66. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L, 2007. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M, Leman S, 2013. Role of orexin in the pathophysiology of depression: potential for pharmacological intervention. CNS Drugs 27, 411–422. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, 2013. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med 14, 488–492. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2004. The Rat Brain in Stereotaxic Coordinates, 5th ed. Elsevier Academic Press, Burlington. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizza F, Magnani M, Indrio C, Plazzi G, 2014. The hypocretin system and psychiatric disorders. Curr Psychiatry Rep 16, 433. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, 2011. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology 36, 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourrahimi AM, Abbasnejad M, Esmaeili-Mahani S, Kooshki R, Raoof M, 2019. Intra-periaqueductal gray matter administration of orexin-A exaggerates pulpitis-induced anxiogenic responses and c-fos expression mainly through the interaction with orexin 1 and cannabinoid 1 receptors in rats. Neuropeptides 73, 25–33. [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT, 2011. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol 44, 449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainero I, Ostacoli L, Rubino E, Gallone S, Picci LR, Fenoglio P, Negro E, Rosso C, De Martino P, De Marchi M, Furlan PM, Pinessi L, 2011. Association between major mood disorders and the hypocretin receptor 1 gene. J Affect Disord 130, 487–491. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA, 1984. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 41, 72–80. [DOI] [PubMed] [Google Scholar]
- Rotter A, Asemann R, Decker A, Kornhuber J, Biermann T, 2011. Orexin expression and promotermethylation in peripheral blood of patients suffering from major depressive disorder. J Affect Disord 131, 186–192. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chaki S, 2006. Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol 4, 277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, 2004. Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol 55, 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA, 2003. Mammalian diurnality: some facts and gaps. J Biol Rhythms 18, 356–366. [DOI] [PubMed] [Google Scholar]
- Soffin EM, Gill CH, Brough SJ, Jerman JC, Davies CH, 2004. Pharmacological characterisation of the orexin receptor subtype mediating postsynaptic excitation in the rat dorsal raphe nucleus. Neuropharmacology 46, 1168–1176. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, McKenna JT, Thakkar MM, Winston S, Strecker RE, McCarley RW, 2006. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience 141, 1101–1105. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM, 1998. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438, 71–75. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T, 2009. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev 61, 162–176. [DOI] [PubMed] [Google Scholar]
- Vourdas A, Shneerson JM, Gregory CA, Smith IE, King MA, Morrish E, McKenna PJ, 2002. Narcolepsy and psychopathology: is there an association? Sleep Med 3, 353–360. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M, 2003. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron 38, 715–730. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Tanis KQ, Reiss DR, Rigby AM, Uslaner JM, Uebele VN, Doran SM, Fox SV, Garson SL, Gotter AL, Levine DM, Roecker AJ, Coleman PJ, Koblan KS, Renger JJ, 2010. Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure. Neuropharmacology 58, 185–194. [DOI] [PubMed] [Google Scholar]
- Wu MF, Nienhuis R, Maidment N, Lam HA, Siegel JM, 2011. Cerebrospinal fluid hypocretin (orexin) levels are elevated by play but are not raised by exercise and its associated heart rate, blood pressure, respiration or body temperature changes. Arch Ital Biol 149, 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Katsuura G, Tatsuno I, Kawahara S, Ebihara K, Saito Y, Nakao K, 2009. Orexins increase mRNA expressions of neurotrophin-3 in rat primary cortical neuron cultures. Neurosci Lett 450, 132–135. [DOI] [PubMed] [Google Scholar]
- Yan L, Lonstein JS, Nunez AA, 2019. Light as a modulator of emotion and cognition: Lessons learned from studying a diurnal rodent. Horm Behav 111, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]