Abstract
It is widely held that social isolation produces higher rates of mortality and morbidity and has deleterious effects on an individual’s sociality. Relatedly, it is widely observed that socially isolated adult rodents display significantly higher levels of aggression when placed in a social situation than do their conspecifics living in social groups. In the following study, we investigated the effects of social isolation on several neurochemical signals that play key roles in the regulation of social behavior in adults. More specifically, we examined the effects of social isolation on vasopressin (AVP) V1a, oxytocin (OT) and serotonin (5-HT)1a receptor binding within the neural circuit controlling social behavior. Male and female Syrian hamsters were housed individually or with two other hamsters for four weeks and were then tested with a same-sex nonaggressive intruder in a neutral arena for five minutes. Social isolation significantly increased aggression in both males and females and altered receptor binding in several brain regions in a sex-dependent manner. For example, V1a receptor binding was greater in socially isolated males in the anterior hypothalamus than it was in any other group. Taken together, these data provide substantial new support for the proposition that the social environment can have a substantial impact on the structural and neurochemical mechanisms regulating social behavior and that the amount and type of social interactions can produce differential effects on the circuit regulating social behavior in a sex-dependent manner.
Keywords: social behavior, sex differences, social experience, dominance, submission, social behavior neural network, anterior hypothalamus, dorsal raphe, bed nucleus of the stria terminalis, medial preoptic area
Introduction
There is considerable evidence that the quality and quantity of social relationships in adulthood can have significant effects on an individual’s health. Indeed, there are data showing that humans and animals with limited social relationships, or who are socially isolated, as adults have higher rates of mortality and morbidity than do those with adaptive social relationships (House et al., 1988; Cacioppo and Hawkley, 2009). The mechanisms underlying how social isolation influences social behavior, and ultimately health, are not well understood but likely involve changes within a variety of neural structures such as the lateral septum, extended amygdala, midbrain, preoptic area, and hypothalamus.
The impact of social isolation on the expression of many different social behaviors has been documented across diverse groups of species. One of the more pronounced effects of social isolation on subsequent social behavior is its ability to increase an individual’s aggressiveness (Brain et al., 1971; Brain, 1972; Stevenson and Rillich, 2013; Scotti et al., 2015; Oliveira et al., 2019). Another effect of social isolation is that it can increase circulating levels of corticoids (Dronjak et al., 2004), suggesting that social isolation is stressful; however, while social isolation has been found to increase circulating corticoids in some studies, these “stress-like” effects have not been observed in others (Ross et al., 2017). Indeed, the effects of social isolation on physiology and behavior may be species- and sex-related (Cacioppo et al., 2015).
The present study was conducted in Syrian hamsters because they are an excellent animal model for preclinical studies relevant to the understanding of primate social behavior. Although the limited amount of field data available suggests that hamsters are not a gregarious species, hamsters display a sophisticated array of social behaviors and skills. Like primates, and unlike many other laboratory rodents, both male and female hamsters establish hierarchical dominance relationships (Drickamer and Vandenbergh, 1973; Drickamer et al., 1973), display rich social, agonistic (Albers et al., 2002), and communicative behaviors (Johnston, 1985), and are capable of complex social discriminations such as the ability to use multiple sensory modalities to discriminate among individuals as well as to identify kin (Todrank et al., 1988; Heth et al., 1998).
Arginine-vasopressin (AVP), oxytocin (OT) and serotonin (5-HT) have been demonstrated to play a significant role in the expression of many different social behaviors in a wide range of species (Terranova et al., 2017; Kelly and Wilson, 2019; Smith et al., 2019). For example, in hamsters, aggression and dominance are regulated by AVP, OT and 5-HT in a sex-dependent manner (Ferris et al., 1997; Gutzler et al., 2010; Terranova et al., 2016). There is also evidence that social experience, including social isolation, can influence key elements of these neurochemical systems (Albers et al., 2006; Albers, 2012; Oliveira et al., 2019). In prairie voles, for example, social isolation can alter the number of OT immunoreactive cells and the number of V1a and OT receptors in specific brain areas, and these effects can be sex-dependent (Grippo et al., 2007; Hiura and Ophir, 2018).
The purpose of the present study was to investigate the effects of social isolation on several neurochemical systems that play a fundamental role in the regulation of social behavior in hamsters. More specifically, we examined whether social isolation results in changes in the number of AVP V1a, OT and 5-HT1a receptors within several regions that are critical in modulating a wide range of social behaviors (Olivier, 2015; Caldwell and Albers, 2016; Johnson and Young, 2017). These studies were conducted in both males and females because there is increasing evidence that AVP, OT and 5-HT have substantial, but very different, roles in the regulation of competitive aggression in males and females (Harmon et al., 2002; Takahashi et al., 2011; Terranova et al., 2016).
Methods
Animals
Adult male and female Syrian hamsters (120–130 g) were purchased from Charles River Laboratories (Wilmington, MA) and were housed in polycarbonate cages (23 × 43× 20 cm) with wire lids, corncob bedding and cotton nesting material. Chow (LabDiet 5001, Purina Mills, Gray Summit, MO) and tap water were available ad libitum, and the colony room was maintained on a 14:10 light:dark cycle as is customary in this species to maintain gonadal patency. Hamsters were housed singly or in groups of three same sex individuals for four weeks before behavioral testing began. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with the standards outlined in the National Institutes of Health Guide for the Care of Use of Laboratory Animals.
Estrous Cycle Monitoring
On each of the 8 days prior to the start of behavioral testing, the estrous cycle was monitored in all females by examining vaginal discharge. Regular estrous cycles are characterized by post-ovulatory vaginal discharge on the day of Estrus which occurs every fourth day of the cycle. Male hamsters were also handled in a manner similar to the females on each of these days to control for any effects of handling.
Behavioral Testing and Scoring
Each hamster was placed in a clean cage containing fresh corncob bedding. A same-sex, nonaggressive, smaller hamster (intruder) was immediately placed into the same cage. All female hamsters were tested on Diestrus 1 of their estrous cycle. All behaviors emited during the 5 min social encounters were scored as described previously (Albers et al., 2002). Briefly, the total duration of four classes of behaviors were scored during the test session: (1) social behavior (stretch, approach, sniff, nose touching, and flank marking); (2) non-social behavior (locomotion, exploration, grooming, nesting, feeding, and sleeping); (3) submissive/defensive behaviors (flight, avoidance, tail up, upright, side defense, full submissive posture, stretch attend, head flag, attempted escape from cage); and (4) aggressive behaviors (upright and side offense, chase and attack, including bite).
Autoradiography
Immediately after behavior testing, hamsters were lightly anesthetized with isoflurane and decapitated. Brains were collected and frozen in dry ice. They were stored at −80°C until they were cut into 20 μm coronal sections using a cryostat. Sections were thaw-mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C until further processing.
V1a and OT Receptors
V1a receptor binding was determined with the I125-labeled linear V1aR antagonist [125I]-Phenylacetyl-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-NH (Perkin-Elmer, Boston, MA). OT receptor binding was determined with the I125-labeled ornithine vasotocin analog Vasotocin, d(CH2)5[Tyr(Me)2,Thr4,Orn8, [125I]Tyr9-NH2] (Perkin-Elmer). For both assays, the tissue was allowed to thaw and dry. It was then fixed in 0.1% paraformaldehyde for 2 min. Slides were then rinsed twice for 10 min each in buffer (50 mM Tris, pH 7.4) and were then incubated in tracer buffer (0.35 mM bacitracin, Sigma-Aldrich, St. Louis, MO; 0.015 mM bovine serum albumin, Sigma-Aldrich, St. Louis, MO; 50 mM I125 linear V1aR antagonist or 50 mM I125 vasotocin analog) for 1 hr. Slides were then rinsed twice for 5 min each and then for 35 min with agitation in buffer (50 mM Tris, 21 mM MgCl). All incubations and washes were performed at room temperature. Finally, the slides were dipped in 4°C deionized water and allowed to dry. The slides and either an I125 or a C14 standard calibration strip (American Radiolabeled Chemicals, St. Louis, MO) were loaded into autoradiography cassettes and exposed to film (Kodak, Rochester, NY) for 3 days (V1a) or 7 days (OT) at room temperature.
5-HT1a Receptors
5-HT1a receptor binding was determined with the H3-labeled 5-HT1a receptor full agonist 8-Hydroxy-DPAT,[Propyl-2,3-ring-1,2,3-3H] (Perkin Elmer). The tissue was allowed to thaw and dry. Slides were then incubated in buffer (50 mM Tris, 120 mM NaCl, 4 mM CaCl2, pH 7.4) for 15 min, followed by a 1 hr incubation in tracer buffer (50 mM Tris, 6 μM H3 5-HT1a agonist). Slides were then rinsed twice for 10 min each in 4°C buffer and then dipped in 4°C deionized water and allowed to dry. The slides and an H3 standard calibration strip (American Radiolabeled Chemicals) were loaded into autoradiography cassettes and exposed to film for 14 weeks.
Autoradiography analysis
Densitometry analysis was performed using Scion Image software (NIH, Bethesda, MD) and a lightbox (Imaging Research, Inc., Ontario, Canada) attached to a camera (Panasonic, Newark, NJ). Standard curves were created using the H3 microscales on the standard calibration strip. For each brain area of interest, three tissue sections located 60 μm apart were analyzed. With the exception of the PVN, a 0.35 mm2 box was placed over the center of each brain area (Tables 1–3), and the optical density was recorded. A 0.26 mm2 box was used to analyze the paraventricular nucleus (PVN). Background binding was subtracted from this measurement. Optical densities were calculated as disintegrating units per min per mg tissue (dpm/mg). Optical densities for each region of interest were averaged within each animal and then averaged within groups. All data were stored and analyzed with Microsoft Excel, Version 14.7.1 and Statistical Package for the Social Sciences (SPSS), Version 23. A two-way analysis of variance (ANOVA) and Tukey post hoc tests were performed to determine if there were differences between male and female and group versus single housed hamsters.
Table 1.
P-values for isolation, sex and interaction effects on V1a receptor densities.
| Brain area | Isolation effect | Sex effect | Interaction effect |
|---|---|---|---|
| LS | p = 0.246 | p = 0.273 | p = 0.614 |
| BNST | p = 0.014* | p = 0.001* | p = 0.830 |
| MPOA | p = 0.413 | p = 0.005* | p = 0.075 |
| AH | p = 0.031* | p = 0.000* | p = 0.044* |
| LH | p = 0.383 | p = 0.975 | p = 0.610 |
| CeA | p = 0.704 | p = 0.313 | p = 0.213 |
| HC | p = 0.661 | p = 0.661 | p = 0.954 |
| PVN | p = 0.602 | p = 0.922 | p = 0.204 |
| PAG | p = 0.105 | p = 0.094 | p = 0.450 |
| DRN | p = 0.300 | p = 0.141 | p = 0.044* |
indicates significant effect
Table 3.
P-values for isolation, sex and interaction effects on 5HT1a receptor densities.
| Brain area | Isolation effect | Sex effect | Interaction effect |
|---|---|---|---|
| LS | p = 0.628 | p = 0.043* | p = 0.377 |
| BNST | p = 0.144 | p = 0.000* | p = 0.012* |
| MPOA | p = 0.377 | p = 0.000* | p = 0.158 |
| AH | p = 0.394 | p = 0.000* | p = 0.600 |
| LH | p = 0.114 | p = 0.589 | p = 0.077 |
| HC | p = 0.235 | p = 0.383 | p = 0.426 |
| PVN | p = 0.168 | p = 0.113 | p = 0.536 |
| PAG | p = 0.102 | p = 0.345 | p = 0.099 |
| DRN | p = 0.750 | p = 0.890 | p = 0.540 |
indicates significant effect
Results
Behavioral Studies
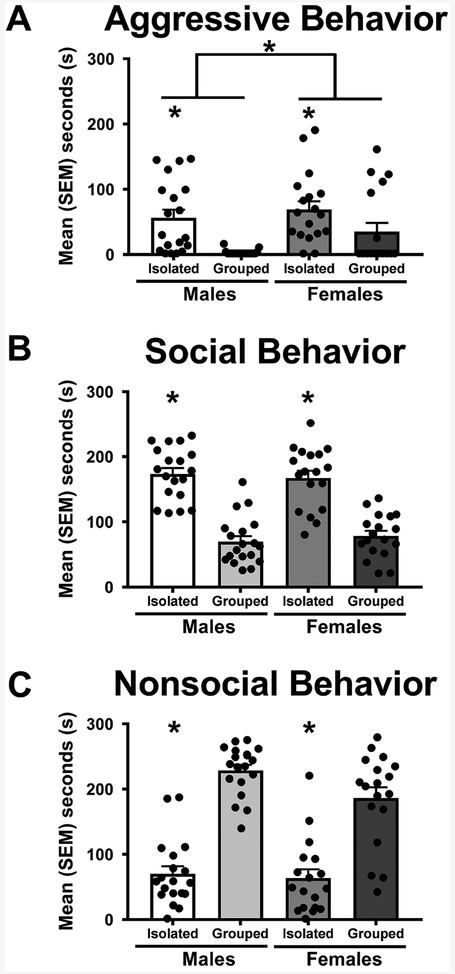
Male and female hamsters that were socially isolated for four weeks were more aggressive than were hamsters that were housed socially [F(1, 73) = 15.96, p < 0.05; η2ρ = 0.19; Figure 1A]. Levels of aggression were similar in socially isolated males and females. Socially housed female hamsters displayed half as much aggression as socially isolated females while socially housed males displayed little or no aggression. As a result, overall, female hamsters were found to be significantly more aggressive than were male hamsters [F(1, 73) = 4.36, p < 0.05; η2ρ = 0.06]. Socially housed male and female hamsters displayed significantly more nonsocial behaviors than did isolated hamsters [F(1, 73) = 123.26, p < 0.05; η2ρ = 0.64; Figure 1B]. In addition, socially isolated male and female hamsters displayed more social behavior than did individuals that were socially housed [F(1, 73) = 109.45, p < 0.05; η2ρ = 0.61; Figure 1C] as well as more flank marking F(1,70) = 8.12, p < 0.05, η2ρ = 0.10.
Figure 1.
Differences in social behaviors between isolated vs group housed hamsters. Mean ± SEM and black dots indicate data from individual hamsters. A. Female hamsters were more aggressive than male hamsters, and socially isolated hamsters were more aggressive than socially housed hamsters. B. Socially housed animals displayed more nonsocial behaviors compared to isolated animals. C. Isolated animals displayed more social behaviors than did socially housed animals. * p<0.05
V1a receptor binding
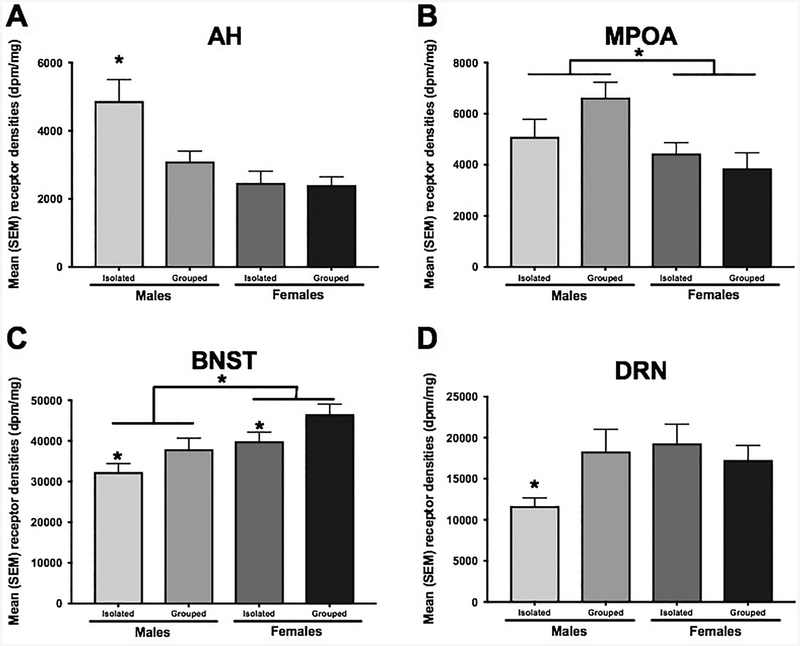
The anatomical distribution of V1a binding was similar to that seen in previous studies conducted in male and female hamsters (Dubois-Dauphin et al., 1990; Ferris et al., 1993; Johnson et al., 1995). V1a binding sites were detectable throughout key regions of the circuitry controlling social behavior (Figure 2). Particularly high V1a binding was found in the lateral septum (LS), bed nucleus of the stria terminalis (BNST) and central amygdala (CeA). Lower to moderate V1a binding was observed in the anterior hypothalamus (AH), medial preoptic area (MPOA), the lateral hypothalamus (LH) and the Ca1 region of the hippocampus (HC). In contrast, little to no V1a binding was identified in the basolateral amygdala (BLA) (Table 1). Perhaps the most interesting finding was that there was a significant interaction between sex and housing conditions in V1a binding within the anterior hypothalamus (AH) [F(1, 68) = 4.22, p < 0.05; η2ρ = 0.06; Figure 3A]. V1a binding was greater in isolated males than in group housed males while no difference in V1a binding was observed between isolated females and group housed females. The higher levels of V1a binding in isolated males led to significantly greater V1a binding in males compared to females in the AH. Significantly higher V1a binding was seen in the MPOA of males compared to females [F(1, 60) = 8.69, p < 0.05; η2ρ = 0.13; Figure 3B]. In the BNST, socially isolated males and females displayed significantly lower V1a binding than did socially housed hamsters [F(1, 70) = 6.44, p < 0.05; η2ρ = 0.09; Figure 3C]. There was also a significant overall sex difference in V1a binding with females having significantly higher levels of V1a binding than did males in the BNST [F(1, 70) = 11.18, p < 0.05; η2ρ = 0.14; Figure 3C]. In the DRN, there was a significant interaction between social isolation and sex with socially isolated males exhibiting less V1a binding than did all other groups [F(1, 42) = 4.32, p < 0.05; η2ρ = 0.09; Figure 3D]. There were no other significant effects of sex or housing condition on V1a binding in any of the other regions examined (Table 1).
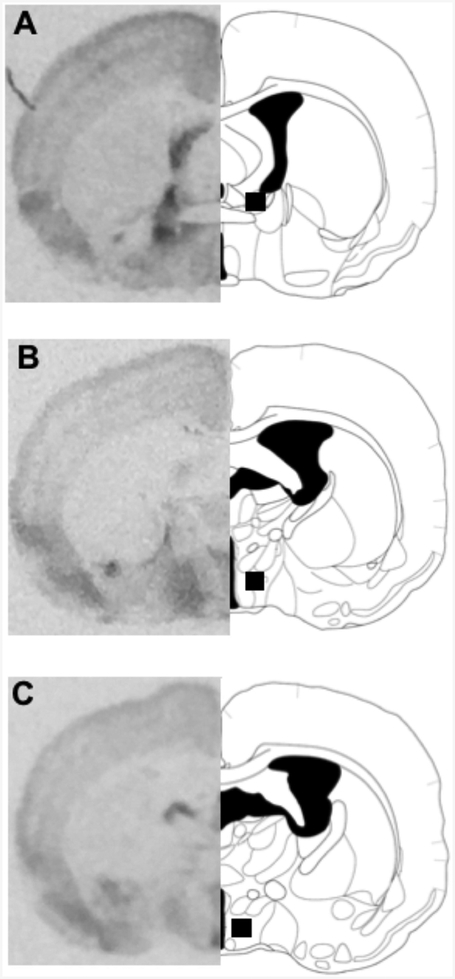
Figure 2.
Representative autoradiograms illustrating receptor binding and brain sites where the binding was quantified (adapted from Morin and Wood, 2001). A. BNST. B. MPOA. C. AH.
Figure 3.
A. Isolated males had a greater V1a receptor binding in the AH compared to isolated females and socially housed males and females. B. Males had a greater receptor binding compared to females in the MPOA. C. In the BNST, males had a greater V1a receptor binding compared to females and socially housed animals had a greater V1a receptor binding than did isolated animals. * p<0.05
Oxytocin receptor (OTR) binding
The overall distribution of OTR binding was similar to that reported previously in male and female hamsters (Dubois-Dauphin et al., 1992). Like previous work, the present study identified high levels of OTR binding within elements of the circuitry controlling social behavior such as the BNST and CeA. In contrast to the prior study where no OTR binding was found in the hypothalamus, the present results found moderate OTR binding in the AH. Females had greater OTR binding than did males in the LH [F(1, 32) = 6.47, p < 0.05; η2ρ = 0.18; Figure 4A], but males had greater OTR binding than did females in the PVN [F(1, 27) = 20.51, p < 0.05; η2ρ = 0.46; Figure 4B]. There was also a significant effect of sex [F(1, 22) = 6.17, p < 0.05; η2ρ =0.28; Figure 4C] and a significant interaction [F(1, 22) = 7.09, p < 0.05; η2ρ = 0.31; Figure 4C] between sex and social isolation in the DRN. There were no other significant effects of sex or housing condition on OTR binding in any of the other regions examined (Table 2).
Figure 4.
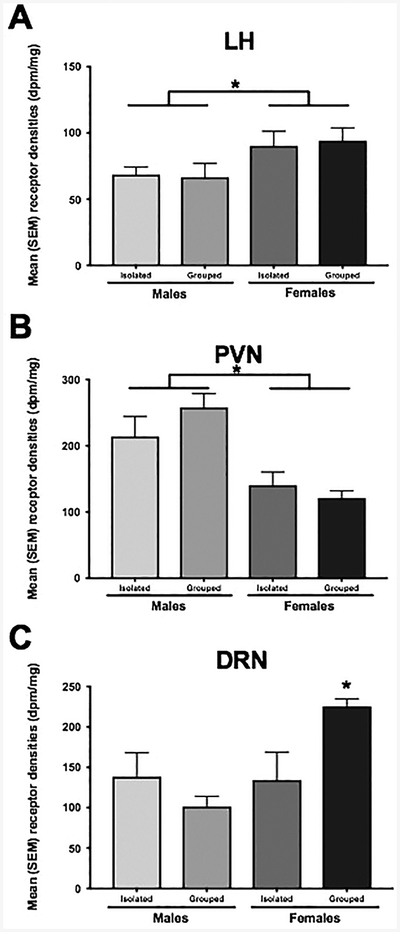
A. Females had a greater OT receptor binding compared to males in the LH, but B. males had a greater binding than females in the PVN. * p<0.05
Table 2.
P-values for isolation, sex and interaction effects on OT receptor densities.
| Brain area | Isolation effect | Sex effect | Interaction effect |
|---|---|---|---|
| LS | p = 0.793 | p = 0.067 | p = 0.439 |
| BNST | p = 0.882 | p = 0.306 | p = 0.940 |
| MPOA | p = 0.900 | p = 0.445 | p = 0.121 |
| AH | p = 0.773 | p = 0.074 | p = 0.464 |
| LH | p = 0.919 | p = 0.017* | p = 0.763 |
| CeA | p = 0.618 | p = 0.533 | p = 0.239 |
| HC | p = 0.541 | p = 0.168 | p = 0.149 |
| BLA | p = 0.133 | p = 0.251 | p = 0.185 |
| PVN | p = 0.600 | p = 0.000* | p = 0.186 |
| PAG | p = 0.082 | p = 0.363 | p = 0.991 |
| DRN | p = 0.283 | p = 0.024* | p = 0.017* |
indicates significant effect
Serotonin 1a receptor binding
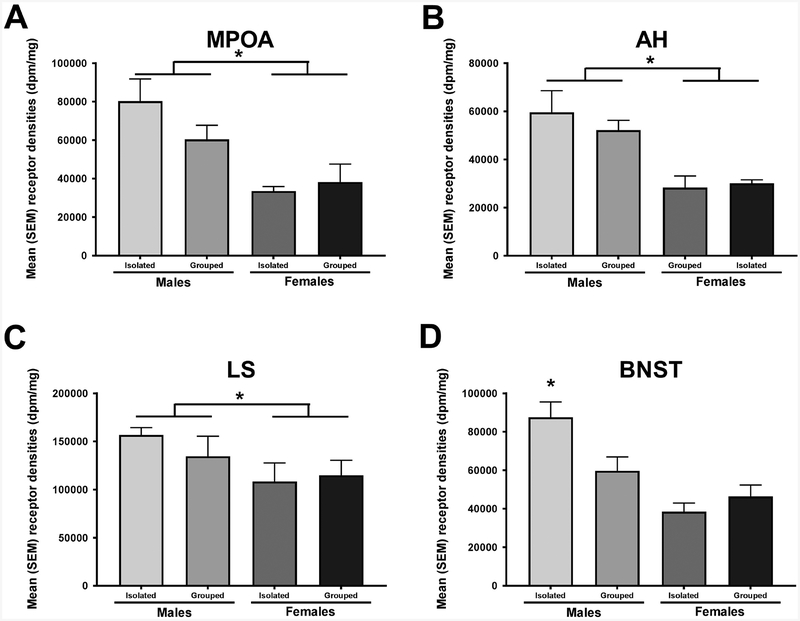
There are only limited data on 5-HT1a binding in Syrian hamsters (Duncan et al., 1999). In the present study, 5-HT1a binding was detectable in most brain areas examined with the exception of the CeA or BLA. In general, the pattern of 5HT-1a binding was similar to that seen previously in male rats (Pazos and Palacios, 1985). Males had higher 5-HT1a binding in the MPOA [F(1, 28) = 16.77, p < 0.05; η2ρ = 0.40; Figure 5A], the AH [F(1, 24) = 25.87, p < 0.05; η2ρ = 0.55; Figure 5B], and the LS [F(1, 31) = 4.51, p < 0.05; η2ρ = 0.14; Figure 5C] than did females. In addition, there was a significant interaction between sex and housing condition in the BNST such that isolated males had greater 5-HT1a binding compared to socially housed males as well as socially isolated and socially housed females [F(1, 32) = 7.24, p < 0.05; η2ρ = 0.20; Figure 5D]. There were no significant effects of sex or housing condition on 5HT1a receptor density in the other regions examined (Table 3).
Figure 5.
Males had a greater 5HT1a receptor binding compared to females in the A. MPOA, B. the AH and C. the LS D. Isolated males had a greater 5HT1a receptor binding compared to isolated females and socially housed males and females. * p<0.05
Discussion
As expected, socially isolated adult males and females displayed significantly more aggression than did hamsters housed in social groups. Interestingly, however, social housing reduced aggression only by about 50% in females while aggression was almost completely eliminated in socially housed males. Recent studies in male and female rats found that social isolation for seven weeks significantly increased aggression in both sexes by a similar amount when compared to socially housed rats (Oliveira et al., 2019). In the present study, socially housed male and female hamsters spent significantly more time expressing nonsocial behaviors than did socially isolated hamsters, and hamsters housed in social isolation spent significantly more time socially interacting than did hamsters housed in groups. The differences in the time spent socially interacting between the socially isolated and socially housed hamsters may be the result of differences in the rewarding properties of those interactions. Other studies have found that rodents housed in social isolation find brief social interactions more rewarding than do rodents housed in social groups (Douglas et al., 2004; Matthews et al., 2005).
Four weeks of social isolation resulted in alterations in the density of V1a, OT and 5-HT1a receptor binding within specific brain regions involved in the regulation of social behavior when compared with the receptor binding observed in socially housed hamsters. The effects of housing conditions were observed most prominently on V1a receptor binding. In the BNST, social isolation appeared to reduce the number of V1a receptors in both male and female hamsters. In contrast, in the AH social isolation appeared to increase the number of V1a receptors, although this effect occurred primarily in males. Comparison of the present data with a previous study on the effects of social isolation and social interaction in male hamsters suggests that the amount as well as the nature of the social interaction can have differential effects on the expression of aggression and V1a receptor binding (Albers et al., 2006). In the earlier study, social experience was manipulated in a very different way than in the present study. Instead of simply housing hamsters in groups as in the present study, social experience was provided giving singly housed male hamsters social interactions with a small, nonaggressive male for 30 min/week for three weeks. Aggression in the males that engaged in social interaction for 30 min/week for three weeks was then significantly lower when compared to hamsters in complete social isolation for three weeks. Interestingly, however, although aggression was significantly higher in the socially isolated group, when hamsters were provided social interactions for 30 min/week for three weeks, a substantial amount of aggression was found to occur. Thus, the amount and type of social interaction appears to determine the extent to which aggressiveness is reduced by social interaction.
The amount and type of social interaction also appears to have differential effects on V1a receptor binding. In the present study, males and females housed socially for four weeks exhibited significantly more V1a receptor binding in the BNST than did hamsters that were socially isolated. In contrast, no differences were observed in V1a receptor binding in the BNST in the previous study wherein male hamsters were allowed to socially interact for 90 min over three weeks (Albers et al., 2006). In the previous study, however, this brief amount of social interaction resulted in higher V1a receptor binding in the PVN and LH and lower V1a binding in the CeA compared with males housed in total social isolation. No similar, site-specific effects on V1a receptor binding between social isolation and social housing were observed in the present study. Interestingly, V1a receptor binding was significantly lower in the AH of the socially housed group in the present study as well as in the previous study where only 90 min of social interaction was provided. Thus, the inhibitory effects of social stimulation on V1a receptor number in the AH appears to be quite robust and reproducible over a variety of conditions. The higher V1a receptors seen in socially isolated males may explain the higher levels of aggression observed as the result of social isolation. Indeed, injection of AVP into the AH significantly increases aggression in socially isolated males and in males trained to fight but not in males that are socially housed (Ferris et al., 1997; Huhman et al., 1998; Caldwell and Albers, 2004).
The effects of social experience on OTR and 5-HT1a receptor binding were more limited than that seen for V1a binding. The only effects of social experience on OTR binding were in the DRN where socially housed females displayed greater binding than did socially housed and socially isolated males and socially isolated females. This is in contrast to other rodents, such as rats, where socially housed females have higher OTR binding in the posterior BNST and nucleus accumbens (NAc) compared with isolated females (Oliveira et al., 2019). In terms of 5-HT1a receptor binding, the only effects of social experience were seen in greater 5-HT1a binding in the BNST in socially isolated males compared to all other groups.
The data of the present study add to the increasing body of evidence that the social environment can alter the functioning of neurochemical circuits containing AVP, OT or 5-HT1a in a variety of species. For example, in female rats, social housing results in higher OTR binding in the posterior BNST and nucleus accumbens compared with isolated females (Oliveira et al., 2019), and in prairie voles social isolation significantly increases OT receptor mRNA in the hypothalamus (Pournajafi-Nazarloo et al., 2013). Socially isolated male and female rats have lower levels of V1a receptor binding in the LH and dentate gyrus, although no differences were observed in several other brain regions (Oliveira et al., 2019). Social isolation also modulates 5-HT neurotransmission in a variety of brain sites (Malick and Barnett, 1976; Bibancos et al., 2007; Sargin et al., 2016; Liu et al., 2019). Social experiences other than the withdrawal of social interaction (i.e., social isolation) can also have substantial effects on the expression of these circuits. For instance, pair bonding in male prairie voles results in higher V1a receptor binding in the AH and MPOA than that seen in sexually naïve males that are not in a pair bond (Winslow et al., 1993; Gobrogge et al., 2009). The larger number of V1a receptors in the AH may also explain the higher levels of aggression (i.e., mate guarding) seen in pair-bonded male voles compared to males that are not in a pair bond. Indeed, how social stimuli regulate the expression of V1a and other receptors likely differs across species. Even within species, however, high levels of plasticity in the expression these receptors have been observed in response to different social conditions at a variety of development stages (Prounis et al., 2015; Hiura and Ophir, 2018; Prounis et al., 2018). The structural changes that can occur in the neural circuitry regulating social behavior in response to dynamic changes in the social environment may tune these circuits in order to provide more adaptive behavior in response to new social challenges.
In the present study, sex differences in V1a, OT and 5-HT1a receptor binding were observed in several different brain regions. For example, V1a and 5-HT1a binding was greater in the AH and MPOA in males compared to females. Previous studies in rodents have also identified sex differences in V1a, OT and 5-HT1a receptors in various brain sites (Fischette et al., 1983; Zhang et al., 1999; Albers, 2015; Dumais and Veenema, 2016), although the brain regions wherein sex differences in the number of these receptors are observed differs across species. The present study also demonstrates that the effects of social experience on receptor number can be sex-dependent. Because gonadal hormones can alter V1a, OT and 5-HT1a receptor binding, sex differences in the circulating levels of these hormones could underlie at least some of the sex differences (Johnson, 1992; Johnson et al., 1995; Simon et al., 1998). In the present study all males and females were gonadally intact and, to reduce variability and the effects of hormonal cyclicity, all females were studied during the diestrous phase of the estrous cycle.
Many of the brain sites wherein we observed the effects of social isolation and sex on receptor binding are part of the circuitry involved in controlling aggression. While this complex circuitry is not well understood, particularly in females, the present data may provide some clues as to how aggression is regulated (Delville et al., 2000; Terranova et al., 2017). The AH is a critical site for the control of aggression in males and females, and AVP- and 5-HT containing projections into the region are involved in aggression and dominance (Terranova et al., 2016). In males, the AH is likely involved in mediating at least some of the aggression-promoting effects of social isolation through an up-regulation of V1a receptors in this region. Another relevant finding is that V1a receptor binding was significantly lower in the DRN in isolated males compared to all other groups. Activation of V1a receptors in the DRN increases the activity of 5-HT neurons through a glutamate-dependent mechanism (Rood and Beck, 2014). Because the DRN provides about half of the 5-HT innervation to the AH and a reduction in 5-HT release in the AH would increase aggression, the decrease in V1a receptor binding in the DRN by social isolation could contribute to the aggression-promoting effects of social isolation (van de Kar and Lorens, 1979; Delville et al., 2000). Another interesting finding from the present study was that social isolation significantly reduced V1a receptor binding in the BNST. Although AVP has been found to increase male aggression in several brain sites, AVP release in the BNST in male rats is negatively correlated with aggression (Veenema et al., 2010). Thus, if activation of V1a receptors by AVP in the BNST reduces aggression, then the reduction in V1a receptor binding in the BNST could contribute to the increase in aggressiveness of males and females housed in social isolation.
In socially housed females, OTR binding in the DRN was higher than in socially isolated females or males. Consistent with these data, studies in female rats have found that knocking down OTRs in the DRN increases female aggression toward a male intruder (Grieb et al., 2018). Other studies have found that activation of OTRs can increase the release of 5-HT within the raphe, which can in turn inhibit the activity of 5-HT neurons (Yoshida et al., 2009). Given the substantial 5-HT containing projections from the DRN to the AH (van de Kar and Lorens, 1979; Delville et al., 2000), increased OTR binding in the DRN might decrease 5-HT output from the DRN to the AH, thereby decreasing aggression. These data suggest that the lower OTR binding in the DRN may play a role in reducing aggression in socially housed females.
In conclusion, the mechanisms underlying the ability of social experience to alter an individual’s subsequent social behavior are not well understood. The present study, along with other data, demonstrates that the amount and type of social interactions can produce differential effects on the circuit regulating social behavior in a sex-dependent manner. Indeed, the present data illustrate the plasticity that can occur in three of the major receptors that have key roles in the regulation of social behavior in response to social experience, V1a, OT and 5HT1a receptors.
Social isolation increases aggression in males and females
Social isolation can alter V1a, OT and 5-HT1a receptor binding
Social isolation can influence receptor binding in a sex-dependent manner
Funding and Disclosure
This work was supported by NIH grants MH109302 and MH110212 to HEA, and funds from the Brains and Behavior Program at Georgia State University. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE (2012). The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav 61(3), 283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Albers HE (2015). Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol 36, 49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Dean A, Karom MC, Smith D, and Huhman KL (2006). Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Res 1073–1074, 425–430. doi: 10.1016/j.brainres.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Albers HE, Huhman KL, and Meisel RL (2002). “Hormonal Basis of Social Conflict and Communication,” in Hormones, Brain and Behavior, eds. Pfaff D, Arnold AP, Etgen A, Fahrbach SE & Rubin RT. (Amsterdam: Academic Press; ), 393–433. [Google Scholar]
- Bibancos T, Jardim DL, Aneas I, and Chiavegatto S (2007). Social isolation and expression of serotonergic neurotransmission-related genes in several brain areas of male mice. Genes Brain Behav 6(6), 529–539. doi: 10.1111/j.1601-183X.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- Brain PF (1972). Effects of isolation/grouping on endocrine function and fighting behavior in male and female golden hamsters. (Mesocricetus auratus Waterhouse). Behav Biol 7(3), 349–357. [DOI] [PubMed] [Google Scholar]
- Brain PF, Nowell NW, and Wouters A (1971). Some relationships between adrenal function and the effectiveness of a period of isolation in inducing intermale aggression in albino mice. Physiol Behav 6(1), 27–29. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Capitanio JP, and Cole SW (2015). The neuroendocrinology of social isolation. Annu Rev Psychol 66, 733–767. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, and Hawkley LC (2009). Perceived social isolation and cognition. Trends Cogn Sci 13(10), 447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, and Albers HE (2004). Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Hormones and Behavior 46(4), 444–449. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, and Albers HE (2016). Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors. Curr Top Behav Neurosci 27, 51–103. doi: 10.1007/7854_2015_390. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, and Ferris CF (2000). Neural connections of the anterior hypothalamus and agonistic behavior in golden hamster. Brain Behav Evol 55(2), 53–76. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, and Spear LP (2004). Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol 45(3), 153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, and Vandenbergh JG (1973). Predictors of social dominance in the adult female golden hamster (Mesocricetus auratus). Anim Behav 21(3), 564–570. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Vandenbergh JG, and Colby DR (1973). Predictors of dominance in the male golden hamster (Mesocricetus auratus). Anim Behav 21(3), 557–563. [DOI] [PubMed] [Google Scholar]
- Dronjak S, Gavrilovic L, Filipovic D, and Radojcic MB (2004). Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav 81(3), 409–415. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pevet P, Barberis C, Tribollet E, and Dreifuss JJ (1992). Localization of binding sites for oxytocin in the brain of the golden hamster. Neuroreport 3(9), 797–800. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pevet P, Tribollet E, and Dreifuss JJ (1990). Vasopressin in the brain of the golden hamster: The distribution of vasopressin binding sites and of immunoreactivity to the vasopressin-related glycopeptide. Journal of Comparative Neurology 300, 535–548. [DOI] [PubMed] [Google Scholar]
- Dumais KM, and Veenema AH (2016). Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol 40, 1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Short J, and Wheeler DL (1999). Comparison of the effects of aging on 5-HT7 and 5-HT1A receptors in discrete regions of the circadian timing system in hamsters. Brain Res 829(1–2), 39–45. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Grzonka Z, Luber-Narod J, and Insel TR (1993). An iodinated vasopressin (V1) antagonist blocks flank marking and selectively labels neural binding sites in golden hamsters. Physiol Behav 54(4), 737–747. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH Jr., Koppel G, Perry KW, Fuller RW, and Delville Y (1997). Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. Journal of Neuroscience 17(11), 4331–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischette CT, Biegon A, and McEwen BS (1983). Sex differences in serotonin 1 receptor binding in rat brain. Science 222(4621), 333–335. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, and Wang Z (2009). Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A 106(45), 19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, et al. (2007). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32(8–10), 966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, and Albers HE (2010). Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus). Eur.J.Neurosci 31(9), 1655–1663. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Huhman KL, Moore TO, and Albers HE (2002). Oxytocin inhibits aggression in female Syrian hamsters. J Neuroendocrinol 14(12), 963–969. [DOI] [PubMed] [Google Scholar]
- Heth G, Todrank J, and Johnston RE (1998). Kin recognition in golden hamsters: evidence for phenotype matching. Anim Behav 56(2), 409–417. doi: 10.1006/anbe.1998.0747. [DOI] [PubMed] [Google Scholar]
- Hiura LC, and Ophir AG (2018). Interactions of sex and early life social experiences at two developmental stages shape nonapeptide receptor profiles. Integr Zool 13(6), 745–760. doi: 10.1111/1749-4877.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, and Umberson D (1988). Social relationships and health. Science 241(4865), 540–545. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Jasnow AM, Janicki MM, Mickley NC, and Albers HE (Year). “Vasopressin (AVP) dose-dependently increases flank marking but not aggressive behavior in Syrian hamsters”), 1927. [Google Scholar]
- Johnson AE (1992). The regulation of oxytocin receptor binding in the ventromedial hypothalamic nucleus by gonadal steroids. Ann N Y Acad Sci 652, 357–373. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Barberis C, and Albers HE (1995). Castration reduces vasopressin receptor binding in the hamster hypothalamus. Brain Res 674(1), 153–158. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, and Young LJ (2017). Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev 76(Pt A), 87–98. doi: 10.1016/j.neubiorev.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE (1985). “Communication,” in The Hamster: Reproduction and Behavior, ed. Seigel HI. (New York: Plenum Press; ), 121–149. [Google Scholar]
- Kelly AM, and Wilson LC (2019). Aggression: Perspectives from social and systems neuroscience. Horm Behav. doi: 10.1016/j.yhbeh.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun Y, Zhao X, Kim JY, Luo L, Wang Q, et al. (2019). Enhancement of Aggression Induced by Isolation Rearing is Associated with a Lack of Central Serotonin. Neurosci Bull. doi: 10.1007/s12264-019-00373-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malick JB, and Barnett A (1976). The role of serotonergic pathways in isolation-induced aggression in mice. Pharmacol Biochem Behav 5(1), 55–61. doi: 10.1016/0091-3057(76)90288-4. [DOI] [PubMed] [Google Scholar]
- Matthews TJ, Abdelbaky P, and Pfaff DW (2005). Social and sexual motivation in the mouse. Behav Neurosci 119(6), 1628–1639. doi: 10.1037/0735-7044.119.6.1628. [DOI] [PubMed] [Google Scholar]
- Oliveira VEM, Neumann ID, and de Jong TR (2019). Post-weaning social isolation exacerbates aggression in both sexes and affects the vasopressin and oxytocin system in a sex-specific manner. Neuropharmacology. doi: 10.1016/j.neuropharm.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Olivier B (2015). Serotonin: a never-ending story. Eur J Pharmacol 753, 2–18. doi: 10.1016/j.ejphar.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Pazos A, and Palacios JM (1985). Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res 346(2), 205–230. [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Kenkel W, Mohsenpour SR, Sanzenbacher L, Saadat H, Partoo L, et al. (2013). Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides 43, 20–26. doi: 10.1016/j.peptides.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Prounis GS, Foley L, Rehman A, and Ophir AG (2015). Perinatal and juvenile social environments interact to shape cognitive behaviour and neural phenotype in prairie voles. Proc Biol Sci 282(1819). doi: 10.1098/rspb.2015.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prounis GS, Thomas K, and Ophir AG (2018). Developmental trajectories and influences of environmental complexity on oxytocin receptor and vasopressin 1A receptor expression in male and female prairie voles. J Comp Neurol 526(11), 1820–1842. doi: 10.1002/cne.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, and Beck SG (2014). Vasopressin indirectly excites dorsal raphe serotonin neurons through activation of the vasopressin1A receptor. Neuroscience 260, 205–216. doi: 10.1016/j.neuroscience.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AP, Norvelle A, Choi DC, Walton JC, Albers HE, and Huhman KL (2017). Social housing and social isolation: Impact on stress indices and energy balance in male and female Syrian hamsters (Mesocricetus auratus). Physiol Behav 177, 264–269. doi: 10.1016/j.physbeh.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargin D, Oliver DK, and Lambe EK (2016). Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels. Elife 5. doi: 10.7554/eLife.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti MA, Carlton ED, Demas GE, and Grippo AJ (2015). Social isolation disrupts innate immune responses in both male and female prairie voles and enhances agonistic behavior in female prairie voles (Microtus ochrogaster). Horm Behav 70, 7–13. doi: 10.1016/j.yhbeh.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NG, Cologer-Clifford A, Lu SF, McKenna SE, and Hu S (1998). Testosterone and its metabolites modulate 5HT1A and 5HT1B agonist effects on intermale aggression. Neurosci Biobehav Rev 23(2), 325–336. [DOI] [PubMed] [Google Scholar]
- Smith CJW, DiBenedictis BT, and Veenema AH (2019). Comparing vasopressin and oxytocin fiber and receptor density patterns in the social behavior neural network: Implications for cross-system signaling. Front Neuroendocrinol 53, 100737. doi: 10.1016/j.yfrne.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PA, and Rillich J (2013). Isolation associated aggression--a consequence of recovery from defeat in a territorial animal. PLoS One 8(9), e74965. doi: 10.1371/journal.pone.0074965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, de Almeida RM, and Miczek KA (2011). Brain serotonin receptors and transporters: initiation vs. termination of escalated aggression. Psychopharmacology (Berl) 213(2–3), 183–212. doi: 10.1007/s00213-010-2000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JI, Ferris CF, and Albers HE (2017). Sex Differences in the Regulation of Offensive Aggression and Dominance by Arginine-Vasopressin. Front Endocrinol (Lausanne) 8, 308. doi: 10.3389/fendo.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JI, Song Z, Larkin TE 2nd, Hardcastle N, Norvelle A, Riaz A, et al. (2016). Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc Natl Acad Sci U S A 113(46), 13233–13238. doi: 10.1073/pnas.1610446113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todrank J, Heth G, and Johnston RE (1988). Kin recognition in golden hamsters: evidence for knship odours. Anim.Behav 56(377), 386. [DOI] [PubMed] [Google Scholar]
- van de Kar LD, and Lorens SA (1979). Differential serotonergic innervation of individual hypothalamic nuclei and other forebrain regions by the dorsal and median midbrain raphe nuclei. Brain Res 162(1), 45–54. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, and Neumann ID (2010). Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav 58(2), 273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, and Insel TR (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365(6446), 545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, et al. (2009). Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci 29(7), 2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, and Rubinow DR (1999). Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience 94(1), 251–259. [DOI] [PubMed] [Google Scholar]