Abstract
Background
The effects of sedative and anesthetic agents on sympathetic nerve activity (SNA) are poorly understood.
Objective
To determine the effects of commonly used sedative and anesthetic agents on SNA in ambulatory dogs and humans.
Methods
We implanted radiotransmitters in 6 dogs to record stellate ganglion nerve activity (SGNA), subcutaneous nerve activity (ScNA) and blood pressure (BP). After recovery, we injected dexmedetomidine (3 μg/kg), morphine (0.1 mg/kg), hydromorphone (0.05 mg/kg) and midazolam (0.1 mg/kg) in different days. We also studied 12 human patients (10 males, 68.0±9.1 years old) undergoing cardioversion for atrial fibrillation with propofol (0.77±0.18 mg/kg) or methohexital (0.65 mg/kg) anesthesia. Skin sympathetic nerve activity (SKNA) and electrocardiogram were recorded during the study.
Results
SGNA and ScNA were significantly suppressed immediately after administration of dexmedetomidine (p=0.000, 0.000), morphine (p=0.011, 0.014) and hydromorphone (p=0.000, 0.012), along with decreased BP and heart rate (HR) (p<0.001 for each). Midazolam had no significant effect on SGNA and ScNA (p=0.248, 0.149). However it increased HR (p=0.015) and decreased BP (p=0.004) in ambulatory dogs. In patients undergoing cardioversion, bolus of propofol administration significantly suppressed SKNA (from 1.11 ±0.25 μV to 0.77±0.15 μV, p=0.001) and the effects lasted for at least 10 min after the final cardioversion shock. Methohexital decreased chest SKNA from 1.59±0.45 μV to 1.22±0.58 μV (p=0.000) and arm SKNA from 0.76±0.43 μV to 0.55±0.07 μV (p=0.001). The effects lasted for at least 10 min after the cardioversion shock.
Conclusion
Propofol, methohexital, dexmedetomidine, morphine and hydromorphone suppressed, but midazolam had no significant effects on SNA.
Keywords: Skin sympathetic nerve activity, stellate ganglion nerve activity, anesthetic agents, cardioversion, dexmedetomidine, morphine, hydromorphone, midazolam, propofol, methohexital
Anesthetic and sedative agents are frequently used during electrophysiological studies. Their use may reduce arrhythmia inducibility, increase the need to use isoproterenol or hemodynamic support.1–3 While anesthetic agents are known to exert significant effects on synapses and axons of mammalian sympathetic ganglia,4 the quantitative effects of anesthetic agents on sympathetic nerve activity (SNA) of ambulatory animals or human patients remain unclear. Stellate ganglion (SG) is known to be an important source of cardiac sympathetic innervation. The SG nerve activity (SGNA) is important in arrhythmogenesis and blood pressure control.5, 6 The subcutaneous sympathetic nerve activity (ScNA) recorded in ambulatory dogs closely correlate with the SGNA.7 Both SGNA and ScNA are associated with spontaneous onset of ventricular tachycardia in a canine model.8 Subsequently we developed a new method (neuECG) to simultaneously record electrocardiogram (ECG) and superficial skin sympathetic nerve activity (SKNA) using conventional ECG patch electrodes in dogs and in humans.9, 10 The availability of these recording methods provided us with an opportunity to directly determine the effects of anesthetic and sedative agents on SNA. The purpose of the present study was to test the hypothesis that commonly used intravenous anesthetic and sedative agents can suppress the SNA in normal ambulatory dogs and in humans with atrial fibrillation (AF) undergoing direct current cardioversion.
Methods
Animal experiments were performed with approval of the Institutional Animal Care and Use Committee of the Indiana University and conformed to the Guide for the Care and Use of Laboratory Animals. Human studies were performed with approval of the Institutional Review Board of the Indiana University. All human subjects gave written informed consent to participate in the study.
Animal studies
Surgical preparation
Six adult mongrel dogs (four males and two females, weighing 25 to 35 kg) underwent sterile surgeries with isoflurane inhalation anesthesia. A D70-CCTP radiotransmitter manufactured by the Data Sciences International (DSI, St. Paul, MN, USA) was implanted to record left SGNA, left thoracic ScNA and the intravascular BP from the descending aorta. The dogs were allowed to recover for two weeks before the commencement of baseline ambulatory recording.
Injection of anesthetic and sedative agents
All agents were given by single intravenous injection during the daytime. We tested only one drug per day to allow complete washout before the next drug was injected. The dosages were based those reported in the literature. 1) Dexmedetomidine: dosage for sedation when combined with inhalation anesthesia was 2 μg/kg for dogs.11 Based on our experience, 3 μg/kg is an effective dose for sedation in dogs when used alone. 2) Morphine: 0.2 mg/kg was used for heavy sedation in dogs undergoing thoracic skin incisions.12 However our target was moderate sedation and 0.1 mg/kg was adequate. 3) Hydromorphone: 0.05 mg/kg bolus injection was selected based on literature.13 4) Midazolam: 0.2 mg/kg was used as co-induction agent for dogs.14 We used 0.1 mg/kg injection to achieve medium sedation without depressing respiration. The implanted radiotransmitter has a sampling rate limited to 1,000 Hz. Their recordings from SG and subcutaneous space was high pass filtered at 150 Hz to display SGNA and ScNA, respectively. The same electrical signals from the subcutaneous electrodes were then low pass filtered at 100 Hz to display ECG.
Clinical studies
We performed an observational study in 12 patients with persistent AF undergoing cardioversion as part of their standard care. There were 10 men and 2 women with an average age 68.0 ± 9.1 years and an average body mass index of 32.5 ± 7.5 kg/m2. Among them, 11 were given propofol and 1 was given methohexital prior to cardioversion. We performed two channels of neuECG recordings using methods describe in a previous report.9 A pair of bipolar electrodes was placed on the left and right subclavian area to simulate the ECG Lead 1. A second pair of bipolar electrodes was placed on the right upper arm. ECG and SKNA were simultaneously recorded by a ME6000 recorder (Biomation, Almonte, Ontario, Canada) with a sampling rate of 10,000/s starting at least 5 min before sedative administration and continued until 10 min after cardioversion.
Statistical Analysis
The nerve activity recordings were analyzed using custom-written software. For dogs, nerve activities were quantified by integrating the absolute value of the filtered signal over 20-s windows (iSKNA). The integrated nerve activities were then divided by the total number of samples in each window (20,000) to calculate the average SGNA (aSGNA) and average ScNA (aScNA). The BP waveforms were analyzed by the software for automatic detection of systolic BP (SBP) and diastolic BP (DBP). For clinical studies, the electrical signals were band pass filtered from 500–1000 Hz to display nerve activities. The same signals were then band pass filtered between 0.5 to 150 Hz to display surface ECG. The aSKNA was the integrated nerve activity over 5-s windows divided by 50,000, which is the total number of samples within that window. Unless otherwise indicated, all quantitative data are expressed as mean ± standard deviation. Paired t-tests were used to compare the means. Repeated measures ANOVA was performed individually to compare overall differences among different time points after the injection of each agents. Statistical analysis was performed using IBM SPSS Statistics 24 (SPSS Inc, Chicago, IL, USA). Two-sided p ≤ .05 was considered significant.
Results
Effect of anesthetic/sedative agents on sympathetic nerve activity in dogs
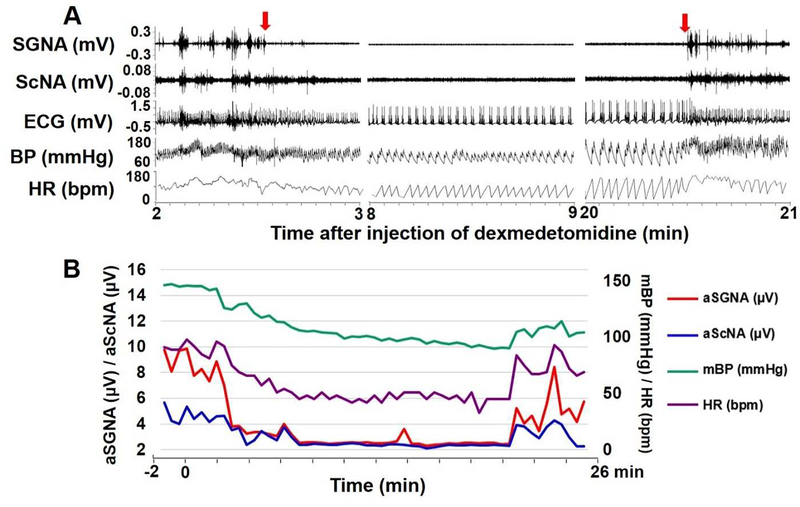
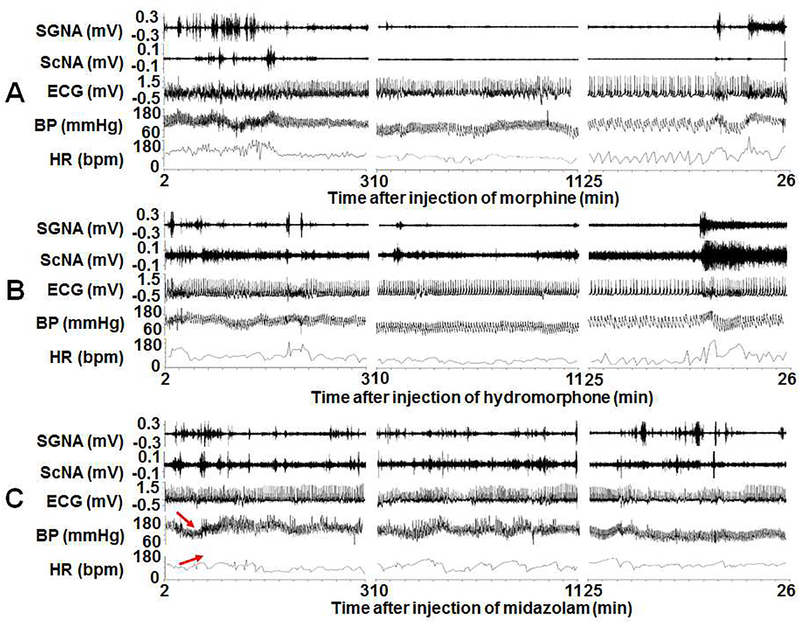
The dogs became quiet and lied down after all drug injections, but they remained conscious without respiratory suppression. Figure 1A shows a typical example of recording showing the effects of dexmedetomidine on nerve activity. Dexmedetomidine eliminated all SGNA and most ScNA bursts about 2 min after injection. There was a continuous reduction of BP and HR before they recovered 20-min later. Figure 1B shows the continuous 28-min mean values of SGNA, ScNA, mBP and HR in this same dog. Figure 2 shows the typical effects of morphine (Figure 2A), hydromorphone (Figure 2B) and midazolam (Figure 2C). Both morphine and hydromorphone decreased SGNA and ScNA about 2 min after the injection, along with the reduction of BP and HR. The nerve activity, BP and HR recovered about 25 min after injection. In comparison, little change of SNA was observed after midazolam injection. Notably midazolam injection was transiently followed by decreased BP but increased HR, suggesting that baroreflexes were intact immediately after injection.
Figure 1:
Effect of dexmedetomidine on sympathetic nerve activities in one dog. A shows three 1-min tracings of SGNA, ScNA, ECG, BP and HR on the 2nd, 8th and 20th minute after injection. Dexmedetomidine reduced the overall nerve activity within 2 min after injection (the first red arrow). There was a reduction of BP and HR, along with increased HR variability. The nerve activity, BP and HR recovered to baseline level about 20 min later (the second red arrow). B shows a 28-min continuous recording of aSGNA, aScNA, mean arterial BP (mBP) and HR in this dog. 0 on the X axis marks the time of dexmedetomidine injection. aSGNA and aScNA decreased significantly about 2 min after injection, and recovered towards baseline level about 20 min later. BP and HR showed a similar trend.
Figure 2:
The SGNA, ScNA, ECG, BP and HR recordings after injection of morphine (A), hydromorphone (B) and midazolam (C) in a typical dog. X axis shows the time after injection in minutes. Morphine and hydromorphone decreased both SGNA and ScNA, accompanied with the decrease of BP and HR. Midazolam had very little effect on SNA but slightly decreased BP and elevated HR immediately after injection (red arrows).
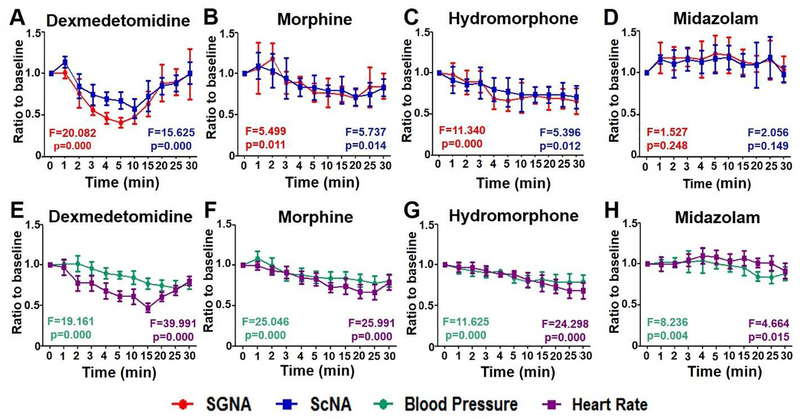
Figure 3 summarizes the effects of drugs on all dogs studied. The ordinate shows the ratios of SGNA, ScNA, BP and HR to baseline. After injection of dexmedetomidine, SGNA decreased to 76±17% of baseline in 2 min (p=0.027) and to a maximum of 41±6% of baseline at 5 min (p=0.005). ScNA also decreased to 75±12% of baseline in 3-min (p=0.04). That is about 1-min later than the reduction of SGNA. The maximum reduction was to 58±12% of baseline at 10 min after injection (p=0.032). The SGNA recovered to 90±16% of baseline at about 25 min after injection (p=0.063) while ScNA recovered to 101±13% of baseline at about 30 min after injection (p=0.952) (Figure 2A). HR showed parallel reduction to 78±10% of baseline at about 2 min after injection (p=0.008). BP decreased significantly at about 4 min after injection to 90±7% of baseline (p=0.028) (Figure 2E). After morphine injection, the SGNA reduced to 76±17% (p=0.046) at 5 min after injection, then partially recovered to 85±15% (p=0.100) at 30 min after injection. ScNA reduced to 79±6% (p=0.001) at 10 min after injection, then partially recovered to 83±10% (p=0.036) at 30 min after injection (Figure 3B). The SGNA and ScNA after hydromorphone injection continued to decline and reached 66±16% (p=0.013) and 71±14% (p=0.016) of baseline at 30 min after injection (Figure 3C). Midazolam had no significant effects on SGNA or ScNA. At 30 min after injection, the SGNA was 104±15% (p=0.565) of the baseline while ScNA was 98±9% (p=0.939) of baseline (Figure 3D). The HR and BP changes paralleled the changes of SGNA and ScNA (Figures 3E–H). Dexmedetomidine had the greatest effects on the maximal reduction of SGNA (by 40.4±5.4%; p<0.001), ScNA (by 55.7±7.8%; p<0.001), HR (by 49.8±5.6%; p<0.001) and mBP (by 69.5±7.3%; p=0.045) among all drugs studied. Repeated measures ANOVA was also performed individually to compare overall differences among different time points. The F values and p values are shown in each panel.
Figure 3:
Effects of dexmedetomidine, morphine, hydromorphone and midazolam on SNA (A to D), BP and HR (E to H) within 30 min after injection. Red, blue, green and purple lines refer to SGNA, ScNA, BP and HR, respectively. X axis is the time after injection. Y axis is the ratio compared to baseline in each time point. Repeated measures ANOVA was performed individually to compare overall differences among different time points. The F values and p values are shown in each panel.
Effect of anesthetic/sedative agents on sympathetic nerve activity in humans
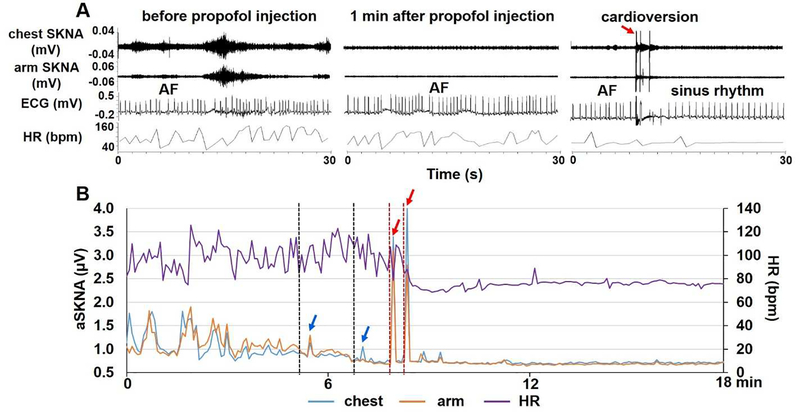
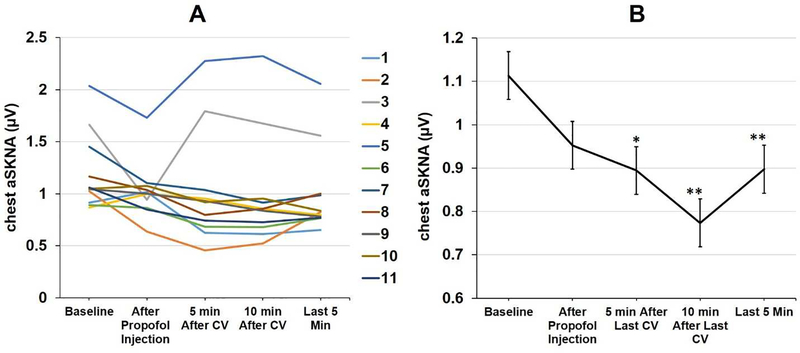
The demographics and clinical characteristics of all 12 patients were shown in Table 1. Figure 4 shows the effect of propofol on sympathetic nerve activities in a patient who underwent cardioversion. Figure 4A shows three 30-s tracings of chest SKNA, arm SKNA, ECG and BP before propofol injection, 1 min after propofol injection and after cardioversion. SKNA was suppressed after the administration of propofol, but briefly increased by cardioversion in this and all patients studied. ECG shows AF before and after propofol injection, and turns to sinus rhythm after cardioversion. Figure 4B shows aSKNA and HR over an 18-min period. The black dotted lines show the timing of propofol injection (50 mg each time). There was transient elevation of SKNA (blue arrows) probably because of pain on propofol injection. That was followed by SKNA suppression. Cardioversion (red dotted lines) were followed by large transient SKNA elevation (red arrows). Propofol suppressed SKNA for at least 10 min. The average chest SKNA at different time point of all 11 patients are shown in Figure 5A. There were large differences of baseline SKNA as well as the responses to propofol. All patients converted to sinus rhythm except one (patient 1) who had a high baseline SKNA that was only briefly suppressed by propofol. Of the 11 patients recorded, bolus of propofol administration significantly reduced aSKNA from 1.11 ±0.25 μV to 0.89±0.36 μV 5 min after injection (p<0.05) and to 0.77±0.14 μV 10 min after injection (p<0.01) (Figure 5B).
Table 1.
Patient characteristics
| Propofol (N=11) | Methohexital (N=1) | |
|---|---|---|
| Age | 68.5 ± 9.3 | 62.0 |
| Gender (M/F) | 9/2 | 1/0 |
| Body weight (kg) | 100.1 ± 27.6 | 153.5 |
| Body mass index (kg/m2) | 31.5 ± 6.8 | 44.5 |
| Dosage (mg/kg) | 0.77 ± 0.18 | 0.65 |
| Persistent atrial fibrillation | 7 | 0 |
| Paroxysmal atrial fibrillation | 3 | 1 |
| Atrial flutter | 1 | 0 |
| Restore to sinus rhythm | 10 | 1 |
| Previous ablation | 7 | 0 |
| ICD implanted previously | 3 | 0 |
| Coronary artery disease | 4 | 0 |
| Congenital heart defect | 1 | 0 |
| Sleep apnea | 1 | 1 |
Figure 4.
Effects of propofol on SNA in a patient undergoing cardioversion. A shows 30-s tracings of chest SKNA, arm SKNA, ECG and BP before propofol injection, 1 min after propofol injection and after cardioversion. Propofol decreased SKNA on both chest and arm leads. ECG shows atrial fibrillation (AF) before and after propofol injection, and turns to sinus rhythm after cardioversion (red arrow). Note that there was transient elevation of the chest and arm SKNA immediately after cardioversion. B shows an 18-min continuous recording of aSKNA and HR. SKNA increased shortly (blue arrows) after each dose of propofol injection (50 mg at the black dotted lines) because of injection pain. The SKNA then progressively decreased. Cardioversion shocks (red line segments) induced large SKNA bursts (red arrows) followed by continued quiescence of SKNA. The propofol suppressed SKNA for at least 10 min.
Figure 5:
Effects of Propofol and cardioversion on average SKNA in all 11 patients. A shows average chest SKNA of each patient. Patient 1 did not restore sinus rhythm after cardioversion and manifested highest SKNA amongst all. Whether or not the high SKNA contributed to cardioversion failure remains unknown. B shows the average level of SKNA in these patients. * p<0.05; ** p<0.01 vs. baseline.
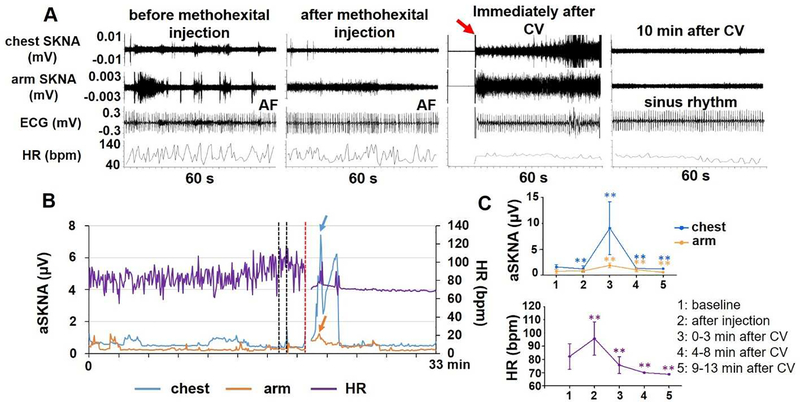
One patient (male, 62 years old) received methohexital (100 mg total) before cardioversion. Figure 6 shows the effect of methohexital on SKNA and HR in this patient. Panel A shows that methohexital decreased SKNA. Cardioversion shock transiently increased SKNA. ECG shows AF before and after methohexital injection, and turns to sinus rhythm after cardioversion. Panel B shows a 33-min continuous recording of aSKNA and HR. Methohexital injections (50 mg and 30 mg each) are shown by the black lines. Panel C shows the mean values of SKNA and HR in different time segments in this patient. Methohexital decreased chest aSKNA from 1.59±0.45 μV to 1.22±0.58 μV (p<0.001) and arm aSKNA from 0.76±0.43 μV to 0.55±0.07 μV (p=0.001). The effects lasted for at least 10 min after the cardioversion shock.
Figure 6.
Effect of methohexital on SKNA in one patient who underwent cardioversion (CV, marked by red arrow). A shows 60-s tracings of chest SKNA, arm SKNA, ECG and HR before methohexital injection, after injection, immediately after and 10 min after cardioversion. B shows a 33-min continuous recording of aSKNA and HR. SKNA decreased after methohexital injection (50 and 30 mg each at the time indicated by the black line segments). Cardioversion (red line segment) induced large and transient SKNA elevation (blue and orange arrows). C shows the mean values of SKNA and HR in different time segments in this patient. ** p<0.01 vs. baseline.
Discussion
We observed in normal ambulatory dogs and humans the effects of widely used anesthetic/sedative agents on SNA. Dexmedetomidine and opioids (morphine and hydromorphone) significantly suppress the SNA in dogs. Among them, the dexmedetomidine seems to have the most profound effects. Propofol and methohexital significantly suppress SKNA in humans, but the duration of SKNA suppression varies among different patients. These findings indicate that anesthetic/sedative agents commonly used in the electrophysiological laboratories have variable effects on the SNA.
Direct SNA recordings in animal models
Monitoring the sympathetic tone using commercially available devices rely on measures such as BP and HR, which may provide conflicting information on sympathetic tone during anesthesia.15 It is highly desirable to develop methods to directly measure the SNA during surgical operation or electrophysiological procedures. We reported that in canine models, the SNA recorded from the thoracic skin closely correlates with the SGNA in ambulatory dogs.7 We also showed that the SNA recorded with standard ECG patch electrodes (SKNA) can be used to measure sympathetic tone in humans.9 However, no data are available to compare the simultaneous responses of SGNA and ScNA to anesthetic/sedative agents. The results of the present study documented that the SGNA and the ScNA responded similarly to drug administration, supporting the use of skin SNA to estimate cardiac sympathetic tone during anesthesia and sedation.
Effects of dexmedetomidine
Dexmedetomidine, a specific and selective a2 adrenoceptor agonist, reduces SNA to multiple tissues and vascular beds.16 Our results confirmed that dexmedetomidine suppresses SNA, HR and BP in ambulatory dogs. These results are consistent with previous microneurography studies in humans. In that study, the authors used microneurography techniques to show that cocaine increased while dexmedetomidine decreased the skin SNA, BP and HR.17 The same group of authors also showed that opposite to what was observed in the skin SNA responses, the muscle SNA was suppressed by the elevated BP during cocaine administration.18 The discrepancies between skin and muscle SNA have also been reported in a study of white coat hypertension.19 In the present study, we found that the SKNA and SGNA in ambulatory dogs were well correlated and responded similarly to anesthetic agents. Because skin sympathetic nerves originate primarily from the ipsilateral SG,20 these data suggest that skin SNA may serve as a useful tool in monitor cardiac sympathetic tone during anesthesia and sedation. This information may be helpful in guiding the anesthesia/sedation during electrophysiological procedures.
Effects of opioids and midazolam on SNA in dogs
Opioid receptors are ubiquitously present in the heart, vasculature, and ganglia. In previous studies in dogs, opioid agents decreased HR, BP, norepinephrine and epinephrine plasma concentrations due to decreased sympathetic outflow measured by renal SNA.21 However, morphine and fentanyl also cause unspecific histamine release-related vasodilatation that reduces BP.22 Therefore, reduction of BP after drug administration does not necessary indicate a reduction of SNA. In fact, microneurography studies showed no acute effects of fentanyl (a potent synthetic opioid) on muscle SNA in humans.23 Chronic mu-opioid receptor stimulation markedly decreases the muscle SNA and its response to hypotension.24 However, those studies did not measure the skin SNA.
We were not able to find microneurography studies on midazolam. However, anxiolytic therapy with alprazolam (a benzodiazepine) increases muscle SNA and HR not only in patients with panic disorder but also in healthy controls.25 In the present study we observed that midazolam had no significant effects on sympathetic tone, but slightly decreased BP about 10 min after intravenous injection. The mechanism of reduction in BP after midazolam may be explained by decreased in systemic vascular resistance and myocardial contractility.26
Effects of propofol and methohexital on human SKNA
Propofol has significant ion channel effects and has been reported to be both antiarrhythmic and proarrhythmic.27 However, its use is associated with a low incidence of ventricular arrhythmias.28 Propofol inhibits the muscle SNA, HR, BP and decreases the baroreflex sensitivity.29 When used to control the pressor response during surgery, the vasodilating effect of propofol overrides the neural vasoconstriction induced by surgery, and a further inhibition of the cardiac baroreflex is observed. We were not able to find the effects of propofol on skin SNA in the literature. The results of our study indicate that propofol is a potent inhibitor of SKNA although the duration and magnitudes of inhibition varied among patients. There is a heterogeneity in the patient cohort. Structural heart diseases may be associated with higher basal levels of SNA, resulting in more resistance to propofol suppression. Among susceptible patients, the SNA suppression effects might prevent the arrhythmia induction during electrophysiological studies. Methohexital is also a muscle SNA inhibitor.30 Only one patient in our study received methohexital. In this patient, there was a significant reduction of SKNA and HR and the effects lasted more than 10 min.
The anti-arrhythmic effects of anesthesia agents
Anesthesia has been used for temporary arrhythmia control. Thoracic epidural anesthesia can be effective for cardiac arrhythmia control and is useful for short-term management of ventricular tachycardia storm.31 Selective mu opioid receptor agonists, such as fentanyl or morphine, increase the ventricular fibrillation threshold in dogs with coronary artery occlusion.32 A meta-analysis including 1295 patients in 9 studies concluded that dexmedetomidine is associated with a lower incidence of ventricular arrhythmia after elective cardiac surgery.33 Methohexital minimizes the possibility of potentially life-threatening cardiac arrhythmias during electroconvulsive therapy compared with thiamylal or thiopental.34 On the other hand, midazolam, which does not suppress SKNA, does not alter the inducibility of reentrant tachycardia nor have they shown to affect the sinoatrial node, refractory periods of atrioventricular conduction, or accessory pathways.35 We propose that the SNA suppression effects might play a role in the mechanism by which anesthetic/sedative agents suppress cardiac arrhythmias.
Implications to clinical and translational research
The existing techniques of studying SNA in humans have certain limitations. Heart rate variability analyses do not have enough temporal resolution to study the SNA and BP on a beat-to-beat basis. Microneurography recordings have sufficient temporal resolution to document an association between SNA and BP. However, because of the difficulties in maintaining sustained impalement, it is impractical to use microneurography techniques for continuous SNA monitoring during electrophysiological studies or surgery. We propose that neuECG recordings can provide useful information by simultaneously measuring ECG and SKNA during anesthesia and sedation. These real time data might be useful in guiding anesthesia during electrophysiological procedures.
Study limitations
This study is limited by the single dose of each agent used and the lack of information about the relative level of sedation achieved by each of those agents. Therefore, the effects of these agents on SNA after long term administration remain unknown. In addition, no nociceptive stimulus was performed to determine if SNA suppression could be reversed in the presence of pain. We did not measure parasympathetic nerve activity during the study. Therefore, the effects of these agents on parasympathetic activity remains unknown.
Conclusions
We found that dexmedetomidine, morphine and hydromorphone suppressed, while midazolam had no significant effects on SGNA and skin SNA in dogs. Propofol and methohexital suppresses SKNA in humans. neuECG recordings might be a useful tool in monitoring SNA during anesthesia and sedation.
Acknowledgement
We thank Chris Corr, Nicole Courtney and David Adams for their assistance.
Source of Funding: This study was supported by NIH Grants R42DA043391, TR002208-01, R01 HL139829, a Medtronic-Zipes Endowment of the Indiana University and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Footnotes
Disclosures: Johnson Wong and Thomas Everett have equity interest in Arrhythmotech, LLC. Other authors do not have conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tirotta CF, Nguyen T, Fishberger S, et al. Dexmedetomidine use in patients undergoing electrophysiological study for supraventricular tachyarrhythmias. Paediatr Anaesth January 2017;27:45–51. [DOI] [PubMed] [Google Scholar]
- 2.Gerstein NS, Young A, Schulman PM, Stecker EC, Jessel PM. Sedation in the Electrophysiology Laboratory: A Multidisciplinary Review. Journal of the American Heart Association June 13 2016;5:pii: e003629. doi: 003610.001161/JAHA.003116.003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nof E, Reichlin T, Enriquez AD, et al. Impact of general anesthesia on initiation and stability of VT during catheter ablation. Heart Rhythm November 2015;12:2213–2220. [DOI] [PubMed] [Google Scholar]
- 4.Larrabee MG, Posternak JM. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol March 1952;15:91–114. [DOI] [PubMed] [Google Scholar]
- 5.Hellyer J, George Akingba A, Rhee KS, et al. Autonomic nerve activity and blood pressure in ambulatory dogs. Heart Rhythm February 2014;11:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014;114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson EA, Rhee KS, Doytchinova A, et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. J Cardiovasc Electrophysiol 2015;26:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doytchinova A, Patel J, Zhou S, et al. Subcutaneous nerve activity and spontaneous ventricular arrhythmias in ambulatory dogs. Heart Rhythm 2015;122:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doytchinova A, Hassel JL, Yuan Y, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm January 2017;14:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Z, Zhao Y, Doytchinova A, et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm February 11 2015;12:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran-Munoz R, Valverde A, Ibancovichi JA, et al. Cardiovascular effects of constant rate infusions of lidocaine, lidocaine and dexmedetomidine, and dexmedetomidine in dogs anesthetized at equipotent doses of sevoflurane. Can Vet J July 2017;58:729–734. [PMC free article] [PubMed] [Google Scholar]
- 12.Chiavaccini L, Claude AK, Meyer RE. Comparison of Morphine, Morphine-Lidocaine, and Morphine-Lidocaine-Ketamine Infusions in Dogs Using an Incision-Induced Pain Model. J Am Anim Hosp Assoc Mar-Apr 2017;53:65–72. [DOI] [PubMed] [Google Scholar]
- 13.Biello P, Bateman SW, Kerr CL. Comparison of fentanyl and hydromorphone constant rate infusions for pain management in dogs in an intensive care unit. Vet Anaesth Analg September 2018;45:673–683. [DOI] [PubMed] [Google Scholar]
- 14.Minghella E, Auckburally A, Pawson P, Scott ME, Flaherty D . Clinical effects of midazolam or lidocaine co-induction with a propofol target-controlled infusion (TCI) in dogs. Vet Anaesth Analg September 2016;43:472–481. [DOI] [PubMed] [Google Scholar]
- 15.Defresne A, Harrison M, Clement F, Barvais L, Bonhomme V. Two different methods to assess sympathetic tone during general anesthesia lead to different findings. J Clin Monit Comput June 25 2019;33:463–469. [DOI] [PubMed] [Google Scholar]
- 16.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) January 2001;14:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontak AC, Victor RG, Vongpatanasin W. Dexmedetomidine as a novel countermeasure for cocaine-induced central sympathoexcitation in cocaine-addicted humans. Hypertension February 2013;61:388–394. [DOI] [PubMed] [Google Scholar]
- 18.Tuncel M, Wang Z, Arbique D, Fadel PJ, Victor RG, Vongpatanasin W. Mechanism of the blood pressure--raising effect of cocaine in humans. Circulation March 5 2002;105:1054–1059. [DOI] [PubMed] [Google Scholar]
- 19.Grassi G, Seravalle G, Buzzi S, et al. Muscle and skin sympathetic nerve traffic during physician and nurse blood pressure measurement. Journal of hypertension June 2013;31:1131–1135. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi T, Morimoto M, Taniguchi Y, Takasaki M, Totoki T. Cutaneous distribution of sympathetic postganglionic fibers from stellate ganglion: A retrograde axonal tracing study using wheat germ agglutinin conjugated with horseradish peroxidase. J Anesth 1994;8:441–449. [DOI] [PubMed] [Google Scholar]
- 21.Taneyama C, Goto H, Kohno N, Benson KT, Sasao J, Arakawa K. Effects of fentanyl, diazepam, and the combination of both on arterial baroreflex and sympathetic nerve activity in intact and baro-denervated dogs. Anesth Analg July 1993;77:44–48. [DOI] [PubMed] [Google Scholar]
- 22.Rosow CE, Moss J, Philbin DM, Savarese JJ. Histamine release during morphine and fentanyl anesthesia. Anesthesiology February 1982;56:93–96. [DOI] [PubMed] [Google Scholar]
- 23.Pacentine GG, Muzi M, Ebert TJ. Effects of fentanyl on sympathetic activation associated with the administration of desflurane. Anesthesiology April 1995;82:823–831. [DOI] [PubMed] [Google Scholar]
- 24.Kienbaum P, Heuter T, Scherbaum N, Gastpar M, Peters J. Chronic mu-opioid receptor stimulation alters cardiovascular regulation in humans: differential effects on muscle sympathetic and heart rate responses to arterial hypotension. J Cardiovasc Pharmacol September 2002;40:363–369. [DOI] [PubMed] [Google Scholar]
- 25.Bechir M, Schwegler K, Chenevard R, et al. Anxiolytic therapy with alprazolam increases muscle sympathetic activity in patients with panic disorders. Auton Neurosci July 31 2007;134:69–73. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe Y, Higuchi H, Ishii-Maruhama M, et al. Effect of a low dose of midazolam on high blood pressure in dental patients: a randomised, double-blind, placebo-controlled, two-centre study. Br J Oral Maxillofac Surg May 2016;54:443–448. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Kong AL, Chen R, et al. Propofol and arrhythmias: two sides of the coin. Acta pharmacologica Sinica June 2011;32:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wutzler A, De Asmundis C, Matsuda H, et al. Effects of propofol on ventricular repolarization and incidence of malignant arrhythmias in adults. J Electrocardiol Mar-Apr 2018;51:170–174. [DOI] [PubMed] [Google Scholar]
- 29.Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology May 1992;76:725–733. [DOI] [PubMed] [Google Scholar]
- 30.Sellgren J, Ponten J, Wallin BG. Characteristics of muscle nerve sympathetic activity during general anaesthesia in humans. Acta Anaesthesiol Scand May 1992;36:336–345. [DOI] [PubMed] [Google Scholar]
- 31.Do DH, Bradfield J, Ajijola OA, et al. Thoracic epidural anesthesia can be effective for the short-term management of ventricular tachycardia storm. Journal of the American Heart Association October 27 2017;6:e007080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maslov LN, Khaliulin I, Oeltgen PR, et al. Prospects for Creation of Cardioprotective and Antiarrhythmic Drugs Based on Opioid Receptor Agonists. Med Res Rev September 2016;36:871–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling X, Zhou H, Ni Y, Wu C, Zhang C, Zhu Z. Does dexmedetomidine have an antiarrhythmic effect on cardiac patients? A meta-analysis of randomized controlled trials. PLoS One 2018;13:e0193303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokriski BK, Nagle SE, Papuchis GC, Cohen SM, Waxman GJ. Electroconvulsive therapy-induced cardiac arrhythmias during anesthesia with methohexital, thiamylal, or thiopental sodium. J Clin Anesth May-Jun 1992;4:208–212. [DOI] [PubMed] [Google Scholar]
- 35.Vladinov G, Fermin L, Longini R, Ramos Y, Maratea E, Jr. Choosing the anesthetic and sedative drugs for supraventricular tachycardia ablations: A focused review. Pacing Clin Electrophysiol November 2018;41:1555–1563. [DOI] [PubMed] [Google Scholar]