Abstract
Healthcare-Associated Infections (HAIs) represent a frequent complication for hospitalized patients and more rarely for workers. In recent years, substantial scientific evidence has been reached regarding the role played by the inanimate surfaces, especially those touched in patient-care areas, in the transmission of nosocomial pathogens. Therefore, it is essential to find new collective protective measures to minimize microbial contamination in healthcare facilities, thereby preventing the spread of multi-drug resistant bacteria. We present an overview of the major nano-enabled AntiMicrobial Coatings (AMCs) which may be used as collective protective measures in healthcare setting, discussing also some aspects related to their effectiveness and safety. AMCs may be classified within three groups on base of their mechanism of action: surfaces releasing active compound, contact-killing surfaces and anti-adhesive surfaces. To date, little information is available on the effectiveness of AMCs to reduce the risk of HAIs since the most of studies do not reach conclusive results on their beneficial effects. Moreover, the lack of standard protocols for assessing antimicrobial efficacy and poor data about the interaction between AMCs and disinfectants prevent their placing on the market. Further studies are needed for assessing risks and benefits of AMCs as collective protective measures in healthcare setting.
Keywords: Nanomaterials, Antimicrobial coatings, Healthcare-associated infections, Biological risk, Collective protective measures
Introduction
Nano-enabled materials are widespread produced and used in different sectors: from energy to medicine, from automotive to construction, from agriculture and food to personal care1). Major occupational concerns are related to their potential adverse health effects2) and the related strategies for preventing workers’ exposure3).
The novel properties of nanoscale materials may offer also new opportunities to improve the protection of workers exposed to traditional risks through the development of new devices and technologies. As an example, nanotechnologies had several applications in protective clothing and smart textiles for the improvement of personal protective equipment due to their increased thermal, electrical, mechanical and antimicrobial properties4,5,6,7); engineered dressings may reduce the risk of microbial infection8); nanocellulose sheets can be used as wound dressing and healing materials in medicine9) with promising application for the development of sterile coating for medical applications in surgeries10).
In this framework and in accordance with European strategies11), the development of nanotechnology has opened up new horizons in the research of “non-traditional” antimicrobial compounds that have led to the production of AntiMicrobial Coatings (AMCs).
As known, Healthcare-Associated Infections (HAIs) are the most common complication in hospitalized patients with a significant clinical and economic impact. People who may be at risk of contracting these infections are first of all patients and, with less frequency, healthcare personnel, volunteer assistants, trainees and students12).
A recent survey on all types of health-associated infections carried out by the European Center for Disease Prevention and Control (ECDC) shows that in Europe 3.2 million patients are affected by at least one HAIs and 37,000 die as direct consequence of these13). In particular in Italy, each year about 450–700,000 new cases of HAIs are registered (primarily urinary infections followed by pneumonia and sepsis) almost half of which are preventable14). Healthcare-associated infections are closely connected with the problem of the antibiotic resistance, increasing global phenomenon. According to the latest report of the Organization for Economic Cooperation and Development (OECD), Italy is the third country with the highest percentage of antibiotic resistant bacteria (33–34% in 2014, doubled compared to 2005 when it was 16–17%), behind only Turkey and Greece15).
Although, in 20–40% of cases, the source of HAIs is the endogenous flora of the patient, environmental matrices (air, water) and surfaces (patient rooms, medical equipment, etc.) are also involved in the transmission of the microorganisms16). Substantial scientific evidence has been reached in recent years regarding the role played by the inanimate surfaces in the transmission of nosocomial pathogens, including multidrug-resistant bacteria17, 18).
For this reason it is of the utmost importance to clean and disinfect regularly environmental surfaces with particular attention to those more frequently touched by patient and healthcare personnel (High Touch Surfaces, HTSs) such as bedside tables, switches, push-buttons, computer keyboards, electro-medical devices, blood pressure devices, etc19). Some studies20,21,22) have however documented the lack of compliance with established guidelines for disinfection and sterilization in healthcare setting.
In this context, is essential to find new collective protective measures to minimize microbiological contamination on frequently touched surfaces in healthcare facilities, thereby preventing the growth and spread of microorganisms, especially multidrug-resistant bacteria. In this paper, we intend to provide a representative overview of the major AMCs that can be used as collective protective measures to safeguard the health of patients and workers. We also discuss aspects related to the efficacy, long-term stability and safety of antimicrobial coatings in healthcare facilities.
Methods
Search strategy
We reviewed the international literature including peer-reviewed journal articles and studies extrapolated from technical reviews, books and reports. In order to identify relevant publications, a literature search was performed in PubMed and Scopus databases using a search strategy adapted to each database structure. We used the following search terms ‘antimicrobial’, ‘antibacterial’, ‘microorganism’, healthcare-associated infections’, ‘biological risk’ coupled with the keywords ‘coating’ OR ‘surfaces’ AND ‘nano’. Papers and documents in English language published in the last 15 yr have been examined in this study.
Results
Antimicrobial coatings classification
Different antimicrobial surfaces are described in literature, but these can be classified in three main groups according to their mechanism of action: ‘surfaces releasing the active compound’, ‘contact-killing surfaces’ and ‘anti-adhesive surfaces23).
Surfaces releasing the active compound
Currently, the majority of AMCs releases the active compound. They are commonly produced by combining of a porous substrate with the antimicrobial agent; the latter can be either deposited directly on the surface or inside polymeric matrix24). Self-disinfecting surfaces containing silver, copper or zinc nanoparticles or titanium dioxide are widely documented in literature. Their antimicrobial action takes place through various mechanisms of action; among these, the most common are oxidative stress, metal ion release and non-oxidative mechanisms25, 26). As is well known, different nanoparticles (NPs) may generate distinctive reactive oxygen species (ROS), such as superoxide (O2−) or hydroxyl radical (OH), hydrogen peroxide (H2O2) which are able to pass through the bacterial membrane and cause cell death27). However, as regards the metal ion release, it has been shown that copper oxide (CuO) NPs can interact with functional groups of proteins altering the normal physiologicalprocesses in the bacterial cell. About non-oxidative mechanisms, these involve interaction of the NPs with bacterial membrane or cell wall. NPs present some features which make them better suited to combat infectious agents, such as their functionalization with different (bio) molecules (Ag, Au, Al, Cu, Zn, etc.), controlled time-release and especially mechanisms of action different from those of the antibiotics28).
Silver-based nanoparticles (AgNPs), widely used for decades, show broad-spectrum antimicrobial activities. However, they have limits related to high costs and low durability, as they tend to oxidize and lose their effectiveness in releasing silver ions. In addition, the active compound may gradually become inactive and therefore it may induce the formation of resistant bacterial strains29). Not only AgNPs are considered to be very effective against bacteria but also other metallic nanoparticles (CuONPs, TiONPs, AuNPs, and Fe3O2NPs) have shown bactericidal effects30,31,32) because of their interaction with functional groups of proteins and nucleic acids, amino and carboxyl groups27). The most important limit of the surfaces releasing active compounds is their potential toxicity; the active compound can be released from coatings into the environment where it may have adverse effects against eukaryotic cells, especially against aquatic organisms33, 34). In a recent study35), the synergic bactericidal effects of reduced graphene oxide (rGO) and AgNPs were responsible for the increase in antibacterial activity of rGO-nAg nanocomposite with very promising results against several clinically relevant pathogens.
Contact-killing surfaces
In these surfaces, the active compound is covalently anchored to the coating and interacts with bacterial cell through direct contact. The biocide (e.g. quaternary ammonium polymers or peptides) may become active upon contact with bacterial cell or after activation by light as in the case of titanium dioxide (TiO2) or photosensitizers. Several biocides are known, such as quaternary phosphoniums compounds (QPCs), carbon nanotubes, antibacterial peptides, quaternary ammonium compounds (QACs) and N-chloramines, but the last two are the most studied36).
In the case of antibacterial surfaces containing ammonium salts or quaternary ammonium compounds (QACs), the positively charged nitrogen in the ammonium group interacts with the negatively charged of the phospholipids in the bacterial membrane, causing the disruption of Gram-positive and Gram-negative cells37, 38). Otherwise, N-chloramines are formed by chlorination of amine, amide or imide groups and contain one or multidrug-resistant N-Cl bonds in which Cl is partially positively charged. The mode of action of surface bound N-chloramines has been hypothesised to be based on active chlorine transfer from surface N-chloramines to the external protein matrix of bacteria39). Other cationic agents, such as polymers and polysaccharide chitosan act by damaging the cell membrane and cause cell death. In addition, antimicrobial peptides (AMPs) have been successfully used for their broad-spectrum antimicrobial activitie40). Their mechanisms of action have been widely studied, including ‘polymeric spacer effect’, ‘ion-exchange mechanism’ and ‘phospholipid sponge effect’; anyway, the surface positive charge density seems to be a key parameter to define antibacterial efficacy41).
Anti-adhesive surfaces
Anti-adhesive surfaces aim to repel microbes or decrease their surface attachment. For this purpose, chemical composition, hydrophilicity, hydrophobicity and topography are modified in order to reduce bacterial adhesion during the initial stage of the biofilm formation process38, 42). As it is known, biofilm is a thick layer of bacteria aggregated to each other on surfaces within the extracellular matrix produced by themselves. Biofilm protects them from adverse environmental conditions, also inhibiting the penetration of antibiotics thus promoting the antibiotic resistance43, 44).
Recent studies have shown45,46,47,48,49) that some nanoparticles (AuNPs, ZnONPs, CuNPs, GONPs) are able to hinder biofilm formation by interacting with the extracellular matrix and the bacterial communication—quorum sensing (QS). This latter plays an important role on the bacterial communication through the production of signal molecules able to synchronize the expression of genes which bacteria use to respond to changes in the environment50).
Safe By Design (SbD) approach
Whatever technology is used for the production of antimicrobial surfaces, it must however takes into account the potential health and safety risks associated with the final product. For this purpose, some European research teams have recently proposed a Safe-By-Design (SbD) approach in Antimicrobial Coatings development aimed to obtain safe products and compliant with all European regulations23). To develop innovative but at the same time safe antimicrobial coatings, during the early design phase, it is necessary to consider various aspects including their efficacy and long-term stability, but also the potential release of the active compound from the coating into the environment and its toxicity.
In this regard, some European research programs (NanoFase, SafeNano, ProSafe, NaNo-Reg, NaNo-Reg2, Euro-NanoTox) have examined different issues related to the toxicity and fate of nanomaterials into environment in order to set toxicological measurements and establish international standards24). Silver-resistant bacterial strains have been found in hospital sewage systems51), while in the study of Pal et al.52), resistance genes to metals and biocides have been found in different environments, including those not influenced by large-scale human use of antimicrobials53). As these genes were found together with antibiotic resistance genes on mobile genetic elements such as plasmids or transposable elements54), it is clear that these resistance mechanisms are aimed to protect bacteria both from toxic effects of antibiotics and antibacterial compound, contributing to the maintenance and spread of multi-drug resistance strains in the environment. In this regard, particular attention should be focused on cleaning procedures in healthcare facilities, because during these operations, small amounts of biocides from AMCs could be likely removed. These released into the environment may be very harmful to human or animal health. Hence, is needed that hospital wastewater and cleaning effluent containing potential biocides or multidrug-resistant bacteria are properly treated55).
AMCs effectiveness and long-term stability
In literature, little information is available on the effectiveness of AMCs in healthcare setting since the most of studies do not reach conclusive results on beneficial effects and furthermore poor data are available on the long-term duration of antimicrobial effects. In a recent paper, Muller et al.56) have carried out a systematic review on the use of self-disinfecting surfaces in patient rooms in order to assess whether these were able to reduce the degree of microbial contamination when compared with standard surfaces. The results show that only 11 out of a total 6,011 studies were eligible under criteria fixed since the most studies was not randomized or, in other cases, confounding factors had not been taken in account. Eleven studies that passed the evaluation criteria concerned mostly copper (n=7), while few papers were found on the impact of non-copper antimicrobial surfaces on microbial contamination in healthcare setting: silver (n=1), metal-alloy (n=1) and organo-silane (n=1). This is partly due to the lack of standardized methods, universally recognized by the scientific community, essential for evaluating antimicrobial efficacy against tested microorganisms and the long-term stability of AMCs. Some industrial standard tests are usually used for assessing the antimicrobial efficacy on surfaces but they are not suitable for testing products to use in healthcare facilities since they do not provide data regarding the toxicity or the potential release of the active compound in the environment23) and thus they do not meet SbD criteria. Moreover, many industrial standard tests have been modified over the past years adapting them to specific context, since AMCs based on diverse mechanisms of action (active compound releasing, contact-killing and antiadhesive surfaces) require different in vitro tests.
For example, ASTM E2149 is the most commonly used method for evaluating biocide-releasing surfaces57) and less suited for non-leaching (immobilized and not water-soluble) antimicrobial products. Adhesion-based methods, on the other hand, are suited for evaluating effectiveness of contact-killing surfaces58). In a recent study, van de Lagemaat et al.59) found that ‘Petrifilm system’ and ‘Japanese Industrial Standard (JIS Z 2801)’ were the best methods to assess the antimicrobial activity of contact-killing surfaces, if they were used with a complementary assay (zone of inhibition on agar) to exclude bacterial death due to the release of active compound in the medium. Although the JIS Z 2801 has also been adopted as an International Organization for Standardization procedure (ISO 22196:2011), it is a questionable method, in certain respects. Under experimental conditions, indeed, the microbial inoculum is spread over a wide surface covered with a thin sterile film to ensure close contact with the antimicrobial surface at an incubation temperature of 35°C and in humid chamber, for a period of 24 h. In indoor environments, on the contrary, the microbial contaminants dry quickly onto surfaces where they usually form cell aggregates loosely grouped together, not in direct contact with the surface. Consequently, it is very likely that under these latter conditions, antimicrobial efficacy is lower other than obtained under optimal experimental conditions.
Conclusions
Despite the efforts to prevent and control HAIs, these remain a frequent complication for hospitalized patients and a big challenge for the health system. Nano-enabled antimicrobial coatings on inanimate surfaces, work equipment, personal protective equipment (gloves, face mask, etc.) might make an important contribution to the fight against nosocomial infections, also preventing the spread of antibiotic-resistant bacteria, concomitantly with the adoption of additional prevention and protection measures.
However, as regards the self-disinfecting surfaces, their potential use as measures of collective protection against the infectious risk is still very limited because, to date, few studies have shown the effectiveness of antimicrobial coatings, under conditions of common use in patients’ rooms, in reducing the risk of healthcare-associated infections.
Another issue that needs to consider is that, in the most of cases, the data available in literature on the effectiveness of new AMCs are not sufficiently complete in respect of certain aspects concerning the safety such as the potential induction of antimicrobial resistance and/or eco-toxicological effects. Our findings confirm the need to develop innovative but at the same time safe antimicrobial coatings, therefore the development and implementation of SbD approach is a key point for the design of self-disinfecting surfaces for healthcare environments.
New standardized protocols have also to be developed and widely accepted by the scientific community because those currently in use are mostly inappropriate, as they do not describe the real performance of coatings under microclimatic conditions as those commonly present in indoor environments.
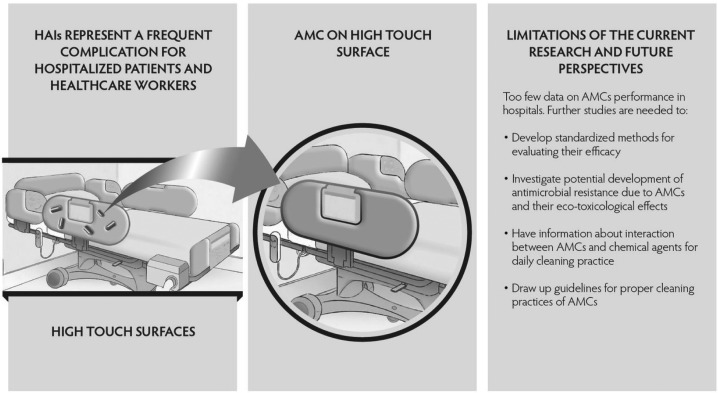
In addition, the lack of information about interaction between chemical agents for daily cleaning practice and AMCs is one of the main impediments to their commercialization and placing in hospitals in which, various studies20, 21) have documented the lack of compliance with established rules for the disinfection. Therefore, the development of guidelines for proper cleaning practices of AMCs represents a great challenge for all scientific community. At the same time, however, it is important to search innovative methods for surface disinfection in addition to those currently available, because the healthcare workers are exposed to complex mixtures of disinfectants with alarming acute and chronic effects (skin irritation, chronic bronchitis and asthma, etc.). In Fig. 1 the main topics discussed in this review are summarized.
Fig. 1.
Nano-enabled antimicrobial coatings (AMCs) as collective protection measures against healthcare-associated infections (HAIs).
In conclusion, further studies are needed for assessing risks and benefits deriving from the use of nano-enabled AMCs as collective protective measures and/or personal protective equipment in order to safeguard patients and workers from biological risk and verifying their effectiveness directly in workplace environments, such as hospital wards, surgeries and patients’ rooms.
Acknowledgments
This work was supported by Italian Workers’ Compensation Authority (INAIL), Department of Occupational and Environmental Medicine, Epidemiology and Hygiene, Monte Porzio Catone, Rome, Italy (INAIL Research Programme 2016-2018, Project No. P8-O3).
References
- 1.Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF, Jr, Rejeski D, Hull MS. (2015) Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6, 1769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietroiusti A, Magrini A. (2014) Engineered nanoparticles at the workplace: current knowledge about workers’ risk. Occup Med (Lond) 64, 319–30. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2017) WHO guidelines on protecting workers from potential risks of manufactured nanomaterials. WHO, Geneva, Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 4.Thilagavathi G, Raja ASM, Kannaian T. (2008) Nanotechnology and protective clothing for defencepersonnel. Def Sci J 58, 451–9. [Google Scholar]
- 5.Dolez PI, Vu-Khanh T. (2009) Recent developments and needs in materials used for personal protective equipment and their testing. Int J Occup Saf Ergon 15, 347–62. [DOI] [PubMed] [Google Scholar]
- 6.Syduzzaman M, Patwary SU, Farhana K, Ahmed S. (2015) Smart textiles and nano-technology: a general overview. J Text Sci Eng 5, 1. [Google Scholar]
- 7.Song G, Mandal S, Rossi RM .(2017) Thermal Protective Clothing for Firefighters, In: Woodhead Publishing Series in Textiles, Woodhead Publishing, Sawston. [Google Scholar]
- 8.Hajiali H, Summa M, Russo D, Armirotti A, Brunetti V, Bertorelli R, Athanassiou A, Mele E. (2016) Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J Mater Chem B Mater Biol Med 4, 1686. [DOI] [PubMed] [Google Scholar]
- 9.Bayer IS, Fragouli D, Attanasio A, Sorce B, Bertoni G, Brescia R, Di Corato R, Pellegrino T, Kalyva M, Sabella S, Pompa PP, Cingolani R, Athanassiou A. (2011) Water-repellent cellulose fiber networks with multifunctional properties. ACS Appl Mater Interfaces 3, 4024–31. [DOI] [PubMed] [Google Scholar]
- 10.Bideau B, Bras J, Saini S, Daneault C, Loranger E. (2016) Mechanical and antibacterial properties of a nanocellulose-polypyrrole multilayer composite. Mater Sci Eng C 69, 977–84. [DOI] [PubMed] [Google Scholar]
- 11.European Commission (2018) CORDIS Community Research and Development Information Service. Projects and Results. https://cordis.europa.eu/projects/result_en. Accessed September 13, 2018.
- 12.World Health Organization (2011) Report on the burden of endemic health care-associated infection worldwide. World Health Organization (WHO). http://www.who.int/iris/handle/10665/80135. Accessed September 24, 2018.
- 13.European Centre for Disease Prevention and Control (2013) Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011e 2012. ECDC, Stockholm. [DOI] [PubMed] [Google Scholar]
- 14.Italian Ministry of Health (2012) Manuale di formazione per il governo clinico: la sicurezza dei pazienti e degli operatori 2012. http://www.area-c54.it/public/manuale%20appropriatezza.pdf.
- 15.Organization for Economic Cooperation and Development (2016) Antimicrobial resistance. www.oecd.org/health/antimicrobial-resistance.htm. Accessed September 24, 2018.
- 16.Dancer SJ. (2009) The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 73, 378–85. [DOI] [PubMed] [Google Scholar]
- 17.Weber DJ, Anderson D, Rutala WA. (2013) The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis 26, 338–44. [DOI] [PubMed] [Google Scholar]
- 18.Sattar SA, Maillard JY. (2013) The crucial role of wiping in decontamination of high-touch environmental surfaces: review of current status and directions for the future. Am J Infect Control 41 Suppl, S97–104. [DOI] [PubMed] [Google Scholar]
- 19.Cobrado L, Silva-Dias A, Azevedo MM, Rodrigues AG. (2017) High-touch surfaces: microbial neighbours at hand. Eur J Clin Microbiol Infect Dis 36, 2053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutala WA, Weber DJ. (2004) Disinfection and sterilization in health care facilities: what clinicians need to know. Clin Infect Dis 39, 702–9. [DOI] [PubMed] [Google Scholar]
- 21.Rutala WA, Weber DJ. (2007) How to assess risk of disease transmission to patients when there is a failure to follow recommended disinfection and sterilization guidelines. Infect Control Hosp Epidemiol 28, 146–55. [DOI] [PubMed] [Google Scholar]
- 22.Hayden MK, Bonten MJM, Blom DW, Lyle EA, van de Vijver DAMC, Weinstein RA. (2006) Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 42, 1552–60. [DOI] [PubMed] [Google Scholar]
- 23.Ahonen M, Kahru A, Ivask A, Kasemets K, Kõljalg S, Mantecca P, Vinković Vrček I, Keinänen-Toivola MM, Crijns F. (2017) Proactive approach for safe use of antimicrobial coatings in healthcare settings: opinion of the COST action network AMiCI. Int J Environ Res Public Health 14, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adlhart C, Verran J, Azevedo NF, Olmez H, Keinänen-Toivola MM, Gouveia I, Melo LF, Crijns F. (2018) Surface modifications for antimicrobial effects in the healthcare setting: a critical overview. J Hosp Infect 99, 239–49. [DOI] [PubMed] [Google Scholar]
- 25.Rojas-Andrade MD, Chata G, Rouholiman D, Liu J, Saltikov C, Chen S. (2017) Antibacterial mechanisms of graphene-based composite nanomaterials. Nanoscale 9, 994–1006. [DOI] [PubMed] [Google Scholar]
- 26.Zaidi S, Misba L, Khan AU. (2017) Nano-therapeutics: a revolution in infection control in post antibiotic era. Nanomedicine (Lond) 13, 2281–301. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Hu C, Shao L. (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine 12, 1227–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, Fernandes AR. (2018) Nano-strategies to fight Multidrug resistant bacteria—“A Battle of the Titans”. Front Microbiol 9, 1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunne CP, Keinänen-Toivola MM, Kahru A, Teunissen B, Olmez H, Gouveia I, Melo L, Murzyn K, Modic M, Ahonen M, Askew P, Papadopoulos T, Adlhart C, Crijns FRL. (2017) Anti-microbial coating innovations to prevent infectious diseases (AMiCI): cost action ca15114. Bioengineered 8, 679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dakal TC, Kumar A, Majumdar RS, Yadav V. (2016) Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol 7, 1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemeg HA. (2017) Nanomaterials for alternative antibacterial therapy. Int J Nanomedicine 12, 8211–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slavin YN, Asnis J, Häfeli UO, Bach H. (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnology 15, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivask A, Juganson K, Bondarenko O, Mortimer M, Aruoja V, Kasemets K, Blinova I, Heinlaan M, Slaveykova V, Kahru A. (2014) Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology 8 Suppl 1, 57–71. [DOI] [PubMed] [Google Scholar]
- 34.Käkinen A, Bondarenko O, Ivask A, Kahru A. (2011) The effect of composition of different ecotoxicological test media on free and bioavailable copper from CuSO4 and CuO nanoparticles: comparative evidence from a Cu-selective electrode and a Cu-biosensor. Sensors (Basel) 11, 10502–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prasad K, Lekshmi GS, Ostrikov K, Lussini V, Blinco J, Mohandas M, Vasilev K, Bottle S, Bazaka K, Ostrikov K. (2017) Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci Rep 7, 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiller JC, Liao CJ, Lewis K, Klibanov AM. (2001) Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci USA 98, 5981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buffet-Bataillon S, Tattevin P, Bonnaure-Mallet M, Jolivet-Gougeon A. (2012) Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds—a critical review. Int J Antimicrob Agents 39, 381–9. [DOI] [PubMed] [Google Scholar]
- 38.Hasan J, Crawford RJ, Ivanova EP. (2013) Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol 31, 295–304. [DOI] [PubMed] [Google Scholar]
- 39.Kaur R, Liu S. (2016) Antibacterial surface design—contact kill. Prog Surf Sci 91, 136–53. [Google Scholar]
- 40.Ren X, Kocer HB, Kou L, Worley SD, Broughton RM, Tzou YM, Huang TS. (2008) Antimicrobial polyester. J Appl Polym Sci 109, 2756–61. [DOI] [PubMed] [Google Scholar]
- 41.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. (2007) Permanent, non-leaching antibacterial surface—2: how high density cationic surfaces kill bacterial cells. Biomaterials 28, 4870–9. [DOI] [PubMed] [Google Scholar]
- 42.Pavithra D, Doble M. (2008) Biofilm formation, bacterial adhesion and host response on polymeric implants—issues and prevention. Biomed Mater 3, 034003. [DOI] [PubMed] [Google Scholar]
- 43.Lebeaux D, Ghigo JM, Beloin C. (2014) Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 78, 510–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khameneh B, Diab R, Ghazvini K, Fazly Bazzaz BS. (2016) Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog 95, 32–42. [DOI] [PubMed] [Google Scholar]
- 45.Slomberg DL, Lu Y, Broadnax AD, Hunter RA, Carpenter AW, Schoenfisch MH. (2013) Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl Mater Interfaces 5, 9322–9. [DOI] [PubMed] [Google Scholar]
- 46.Chen CW, Hsu CY, Lai SM, Syu WJ, Wang TY, Lai PS. (2014) Metal nanobullets for multidrug resistant bacteria and biofilms. Adv Drug Deliv Rev 78, 88–104. [DOI] [PubMed] [Google Scholar]
- 47.Miao L, Wang C, Hou J, Wang P, Ao Y, Li Y, Geng N, Yao Y, Lv B, Yang Y, You G, Xu Y. (2016) Aggregation and removal of copper oxide (CuO) nanoparticles in wastewater environment and their effects on the microbial activities of wastewater biofilms. Bioresour Technol 216, 537–44. [DOI] [PubMed] [Google Scholar]
- 48.Yu Q, Li J, Zhang Y, Wang Y, Liu L, Li M. (2016) Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci Rep 6, 26667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulshrestha S, Qayyum S, Khan AU. (2017) Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: a comparative study on inhibition of gram-positive and gram-negative biofilms. Microb Pathog 103, 167–77. [DOI] [PubMed] [Google Scholar]
- 50.Papenfort K, Bassler BL. (2016) Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol 14, 576–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lima de Silva AA, de Carvalho MA, de Souza SA, Dias PM, da Silva Filho RG, de Meirelles Saramago CS, de Melo Bento CA, Hofer E. (2012) Heavy metal tolerance (Cr, Ag AND Hg) in bacteria isolated from sewage. Braz J Microbiol 43, 1620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pal C, Bengtsson-Palme J, Kristiansson E, Larsson DG. (2015) Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genomics 16, 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wardwell LH, Jude BA, Moody JP, Olcerst AI, Gyure RA, Nelson RE, Fekete FA. (2009) Co-selection of mercury and antibiotic resistance in sphagnum core samples dating back 2000 years. Geomicrobiol J 26, 238–47. [Google Scholar]
- 54.Pal C, Asiani K, Arya S, Rensing C, Stekel DJ, Larsson DGJ, Hobman JL. (2017) Metal resistance and its association with antibiotic resistance. Adv Microb Physiol 70, 261–313. [DOI] [PubMed] [Google Scholar]
- 55.Dunne SS, Ahonen M, Modic M, Crijns FRL, Keinänen-Toivola MM, Meinke R, Keevil CW, Gray J, O’Connell NH, Dunne CP. (2018) Specialized cleaning associated with antimicrobial coatings for reduction of hospital-acquired infection: opinion of the COST Action Network AMiCI (CA15114). J Hosp Infect 99, 250–5. [DOI] [PubMed] [Google Scholar]
- 56.Muller MP, MacDougall C, Lim M, Armstrong I, Bialachowski A, Callery S, Ciccotelli W, Cividino M, Dennis J, Hota S, Garber G, Johnstone J, Katz K, McGeer A, Nankoosingh V, Richard C, Vearncombe M, Ontario Agency for Health Protection and Promotion Public Health OntarioProvincial Infectious Diseases Advisory Committee on Infection Prevention and ControlProvincial Infectious Diseases Advisory Committee on Infection Prevention and Control (2016) Antimicrobial surfaces to prevent healthcare-associated infections: a systematic review. J Hosp Infect 92, 7–13. [DOI] [PubMed] [Google Scholar]
- 57.Sjollema J, Zaat SAJ, Fontaine V, Ramstedt M, Luginbuehl R, Thevissen K, Li J, van der Mei HC, Busscher HJ. (2018) In vitro methods for the evaluation of antimicrobial surface designs. Acta Biomater 70, 12–24. [DOI] [PubMed] [Google Scholar]
- 58.Feng G, Cheng Y, Wang SY, Borca-Tasciuc DA, Worobo RW, Moraru CI. (2015) Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: how small is small enough? NPJ Biofilms Microbiomes 1, 15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van de Lagemaat M, Grotenhuis A, van de Belt-Gritter B, Roest S, Loontjens TJA, Busscher HJ, van der Mei HC, Ren Y. (2017) Comparison of methods to evaluate bacterial contact-killing materials. Acta Biomater 59, 139–47. [DOI] [PubMed] [Google Scholar]