Abstract
MicroRNA-150-5p (miR-150-5p) plays a complex role in normal early hematopoietic development and is also implicated in the development of various different leukemias. We have reported previously that, in myeloid and lymphoid malignancies associated with dysregulated fibroblast growth factor receptor 1 (FGFR1) activities, miR-150-5p is down-regulated compared with healthy cells. Here, using murine cells, we found that this down-regulation is accompanied by CpG methylation of the miR-150-5p promoter region. Of note, analysis of human acute lymphoblastic leukemia (ALL) cohorts also revealed an inverse relationship between miR-150-5p expression and disease progression. We also found that the DNA methyltransferase 1 (DNMT1) enzyme is highly up-regulated in FGFR1-driven leukemias and lymphomas and that FGFR1 inhibition reduces DNMT1 expression. DNMT1 knockdown in stem cell leukemia/lymphoma (SCLL) cells increased miR-150-5p levels and reduced levels of the MYB proto-oncogene transcription factor, a key regulator of leukemogenesis. FGFR1 directly activates the MYC proto-oncogene basic helix-loop-helix transcription factor, which, as we show here, binds and activates the DNMT1 promoter. MYC knockdown decreased DNMT1 expression, which, in turn, increased miR-150-5p expression. One of the known targets of miR-150-5p is MYB, and treatment of leukemic cells with the MYB inhibitor mebendazole dose-dependently increased apoptosis and reduced cell viability. Moreover, mebendazole treatment of murine xenografts models of FGFR1-driven leukemias enhanced survival. These findings provide evidence that MYC activates MYB by up-regulating DNMT1, which silences miR-150-5p and promotes SCLL progression. We propose that inclusion of mebendazole in a combination therapy with FGFR1 inhibitors may be a valuable option to manage SCLL.
Keywords: leukemia, microRNA (miRNA), Myc (c-Myc), fibroblast growth factor receptor (FGFR), epigenetics, DNMT1, MYB
Introduction
Malignant neoplasms associated with rearrangements of FGFR1,2 also referred to as stem cell leukemia/lymphoma (SCLL) syndrome, present as a myeloproliferative disorder that progresses to AML, and these patients may also develop B or T cell lymphomas (1). In this disease, FGFR1 is constitutively activated as a result of chromosome translocations that juxtapose a dimerization motif N-terminal to the FGFR1 kinase domain (2), leading to constitutive and ligand-independent activation. Inactivation of the kinase domain abrogates the oncogenic potential of these chimeric oncogenes (3), and pharmacological suppression of FGFR1 activity also suppresses progression of the disease (4–5). FGFR1 activation leads to profound changes in gene expression profiles, with activation of genes that promote survival, proliferation, and the stem cell phenotype and suppression of genes that lead to differentiated phenotypes (6–7). In addition to gene expression changes, the microRNA profile of these cells is also altered as a result of FGFR1 activation (8), which has downstream effects on gene expression and protein production. We recently described up-regulation of several miRNAs that promote SCLL progression, with members of the miR17/92 cluster promoting cell survival and proliferation (8). In addition, miR339 has been shown to regulate the BCL2L11 and BAX apoptosis-related genes, influencing cell survival (9). FGFR1 has been shown to promote expression of these miRNAs, but, in this screen for FGFR1-regulated miRNAs, we also identified a series that was down-regulated in SCLL cells, including miR-150-5p (8).
miR-150-5p appears to be differentially expressed during normal hematopoiesis and is frequently deregulated in various types of hematological malignancies (10). Down-regulation of miR-150-5p has also been reported in chronic myeloid leukemia (CML), acute myeloid leukemia (AML), and lymphoma (10), and up-regulation has been reported in myelodysplastic syndrome and chronic lymphocytic leukemia (10). miR-150-5p is thought to control development of the myeloid lineage by fine-tuning the transcription factor Myb and its downstream products (11), promoting differentiation of hematopoietic stem cells (HSCs). Here we demonstrate that miR-150-5p expression is silenced by promoter methylation by Dnmt1, which is highly up-regulated in SCLL cells as a result of FGFR1-driven up-regulation of Myc. Constitutive FGFR1 expression in SCLL, therefore, appears to drive up-regulation of Myb through a cascade of intermediate steps, and pharmacological inhibition of Myb in these cells suppresses cell growth in vitro and leukemia progression in vivo.
Results
miR-150-5p is down-regulated by FGFR1
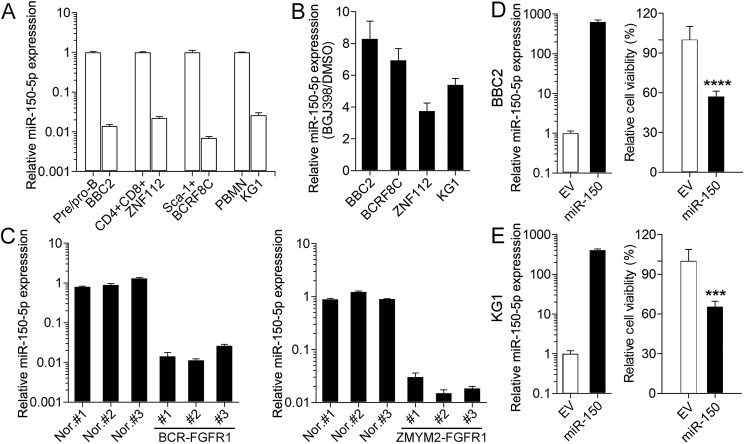
As part of our analysis of miRNAs regulated by FGFR1 in cells derived from BCR-FGFR1 transformed mouse hematopoietic stem cells (BBC2), we identified an association between the presence of activated FGFR1 kinase and down-regulation of miR-150-5p (8). To expand this analysis, we compared other SCLL cell lines with their normal cell counterparts isolated from the spleens of normal mice. BBC2 cells are arrested in a pre/pro-B cell stage (14) and were compared with B220+/IgM− cells isolated from normal spleen. ZNF112 cells, derived from stem cells transformed with ZMYM2-FGFR1 (15), exhibit a CD4+/CD8+ immunophenotype and were compared with double-positive T cells from normal spleen. BCRF8C was derived from NSG hematopoietic stem cells and shows a Sca1+ immunophenotype (7). Normal Sca1+ cells were isolated from normal mouse bone marrow. KG1 cells (16) were derived from a human AML patient and express the FGFR1OP2-FGFR1 chimeric kinase. As controls for this human cell line, we used human-derived mononuclear cells. As shown in Fig. 1A, the expression levels of miR-150-5p in these four cells lines was reduced significantly (>95%) compared with their normal cell counterparts. When the same cell lines were treated with the BGJ389 FGFR1 inhibitor (17), miR-150-5p expression levels were highly up-regulated in all cases (Fig. 1B). The miRNA levels in primary SCLL cells developed in syngeneic hosts using the transduction and transplantation approach described previously (15) also showed that miR-150-5p levels were significantly reduced in spleen-derived cells from diseased mice (n = 3) from both BCR-FGFR1 and ZMYM2-FGFR1 (Fig. 1C) transformed stem cells compared with normal spleen cells (n = 3). It appears, therefore, that the high-level expression of FGFR1 in SCLL cells correlates with low-level expression of miR-150-5p both in vitro and in vivo and that pharmacological suppression of FGFR1 function leads directly to increased miR-150-5p expression levels. In contrast, a housekeeping miRNA, Let-7a, shows no difference in levels in these comparisons (Fig. S1), supporting the specificity of the observations for miR-150-5p.
Figure 1.
miR-150-5p is down-regulated in SCLL. A, relative expression levels (on a log scale) in BBC2 cells relative to normal pre/pro-B cells, ZNF112 relative to normal CD4+CD8+ cells, BCRF8C relative to normal Sca1+ cells, and KG1 cells relative to peripheral blood mononuclear cells all show highly significant down-regulation of miR-150-5p in SCLL cell lines. B, when the same cell lines are treated with the BGJ398 FGFR1 inhibitor, miR-150-5p levels are increased compared with DMSO-treated controls in all cases. C, analysis of miR-150-5p levels in spleen cells from mice transplanted with either BCR-FGFR1– or ZMYM2-FGFR1–transformed hematopoietic cells show significant down-regulation of miR-150-5p (on a log scale). Nor., normal control. D, when miR-150-5p is overexpressed in BBC2 cells, there is a significant reduction in cell viability. D and E, overexpression of miR-150-5p in human KG1 cells (E) shows a similar reduction in cell viability (D). ***, p < 0.001; ****, p < 0.0001. EV, empty vector.
miR-150-5p overexpression induces apoptosis
To investigate the effect of miR-150-5p on cell viability, we overexpressed miR-150-5p in mouse BBC2 and human KG1 cells (Fig. 1D). When miR-150-5p expression levels were analyzed in BBC2 cells, there was a >600-fold increase compared with parental cells transduced with the empty vector, which led to reduced (∼35%) viability (Fig. 1, D and E), a reduced percentage of cells in G2/M, and an increase in Annexin V–positive cells in overexpressing cells (Fig. S2). The same effects were seen in KG1 cells overexpressing miR-150-5p (Fig. 1E and Fig. S2). Thus, it appears that increased apoptosis is one of the consequences of high-level miR-150-5p expression.
miR-150-5p expression is associated with down-regulation of Myb
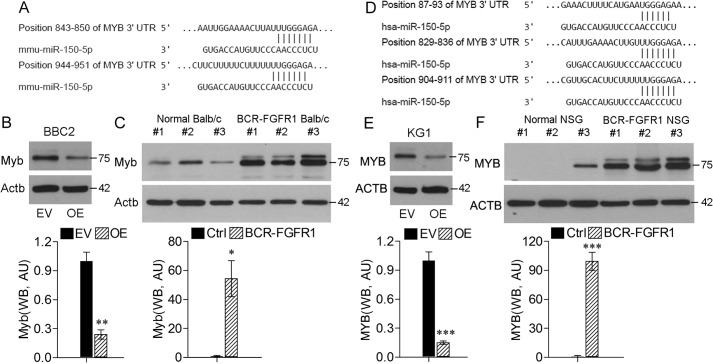
The Myb gene is one of the known targets of miR-150-5p in both humans and mice (11, 18). As shown in Fig. 2A and Fig. S3, there are highly conserved target sites in the murine Myb 3′ UTR with homology to miR-150-5p. Overexpression of miR-150-5p in BBC2 cells shows a significant down-regulation of Myb (Fig. 2B), supporting the idea that miR-150-5p may affect SCLL progression through regulation of Myb expression. Analysis of spleen cells from syngeneic mice transplanted with BCR-FGFR1–transduced hematopoietic stem cells (Fig. 2C) confirms up-regulation of Myb in in vivo models. Similarly, there are conserved target sites in the human MYB gene (Fig. 2D), and overexpression of miR-150-5p in human KG1 SCLL cells (Fig. 2E) shows significantly reduced MYB levels. In human cell SCLL models in NSG mice transplanted with BCR-FGFR1–transduced human CD34+ cells, MYB is also up-regulated (Fig. 2F). Thus, it appears that expression of the FGFR1 chimeric kinases leads to Myb activation in SCLL cells by down-regulating miR-150-5p in vivo as well as in vitro.
Figure 2.
Myb is down-regulated in SCLL cells. A, location of the two miR-150-5p target sites in the 3′ UTR of murine Myb. B, when miR-150-5p is overexpressed (OE) in BBC2 cells (top panel), there is a significant reduction in Myb protein levels compared with empty vector (EV)–transformed cells (bottom panel, showing quantified levels in arbitrary units (AU)). C, analysis of spleen cells from BALB/c mice transplanted with mouse hematopoietic stem cells (n = 3) transduced with BCR-FGFR1 shows increased levels of Myb expression compared with normal BALB/c spleen cells. D, location of the 3 miR-150-5p target sites in the 3 UTR of human MYB. E, human KG1 SCLL cells also show reduced levels of MYB when miR-150-5p is overexpressed. F, human CD34+ cord blood cells transformed with BCR-FGFR1 and transplanted into immunocompromised NSG mice show the same increased MYB expression levels in isolated spleen cells compared with spleen cells from normal NSG mice. *, p < 0.01; **, p < 0.001; ***, p < 0.0001. Ctrl, control; WB, western blot.
miR-150-5p is suppressed in SCLL cells through promoter methylation
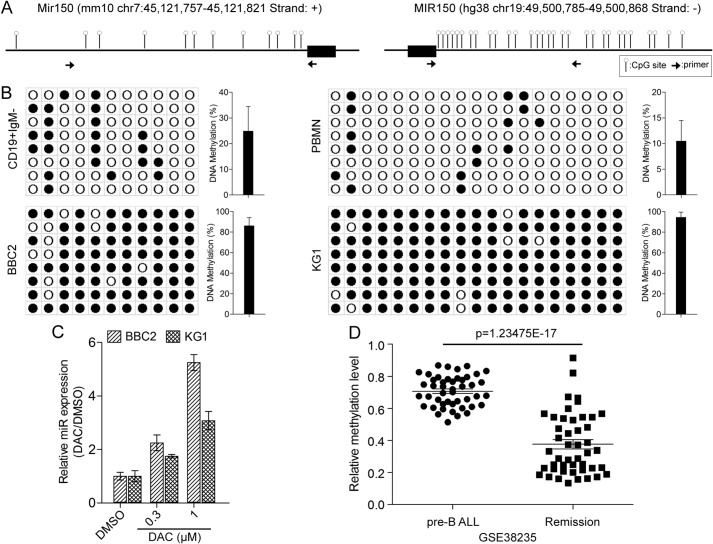
To investigate the mechanism underlying the down-regulation of miR-150-5p in SCLL cells, we analyzed the promoter region in BBC2 and KG1 cells (Fig. 3A). There are fewer CpG dinucleotides in the murine promoter region compared with the human promoter. We designed primers to investigate the methylation status of promoter regions with the highest density of CpGs, as shown in Fig. 3A, and compared mouse normal CD19+IgM− flow-sorted cells from the spleen with BBC2 cells. Human peripheral mononuclear cells were used as the normal counterpart for human KG1 AML cells. Following bisulfite conversion of DNA from both cell lines and controls, the miR-150-5p promoter regions were PCR-amplified and cloned into the pGEM-T Easy vector. Sanger sequencing of randomly selected individual clones identified methylated CpG sites. As seen in Fig. 3B, there was limited methylation in either promoter region from the normal cells. In contrast, extensive methylation was seen throughout the promoter region in both cell lines, suggesting a mechanism for miR-150-5p silencing. When BBC2 and KG1 cells were treated with the 5′-azacytidine methylation inhibitor, compared with DMSO-treated cells, there is a dose-dependent increase in miR-150-5p expression levels in both cell lines, demonstrating the functional relationship between methylation in the miR-150-5p promoter and its expression levels (Fig. 3C). To investigate the relationship between methylation in the miR-150-5p promoter and clinical parameters in leukemic samples, we retrospectively analyzed the GSE 38235 dataset from the GEO (19), which compared the methylome and transcriptome between matched pre-B-ALL tumor samples containing high levels of leukemic blasts and blast-free remission samples from 46 patients. The methylation data for the miR-150-5p promoter showed a highly significant reduction in methylation levels in the remission samples compared with their tumor counterparts (Fig. 3D), supporting the correlation between inactivation of miR-150-5p and increased aggressiveness of this leukemia.
Figure 3.
The miR-150-5p promoter is highly methylated in SCLL cells. A, distribution of CpG dinucleotides (lollipops) within the murine (left panel) and human (right panel) miR150 promoters shows higher CpG numbers in human cells. The miR-150-5p gene in each case is indicated by the black bars, and the position of the primer pairs used to amplify the promoters is indicated by the opposing arrows. B, DNA sequence analysis of the miRNA promoter in individual clones from bisulfite-treated DNA from BBC2 cells (left panel) or KG1 cells (right panel) shows extensive methylation at CpG dinucleotides (closed circles) throughout the region compared with normal CD19+IgM− B cells or PBMN cells. C, treatment of SCLL cells with the methylation inhibitor 5′-azacytidine (DAC) shows a dose-dependent increase in miR-150-5p expression levels. D, analysis of matched bone marrow samples from pre-B-ALL patients with disease or in remission shows a highly significant reduction in methylation levels in the remission phase.
miR-150-5p promoter methylation is facilitated by Dnmt1
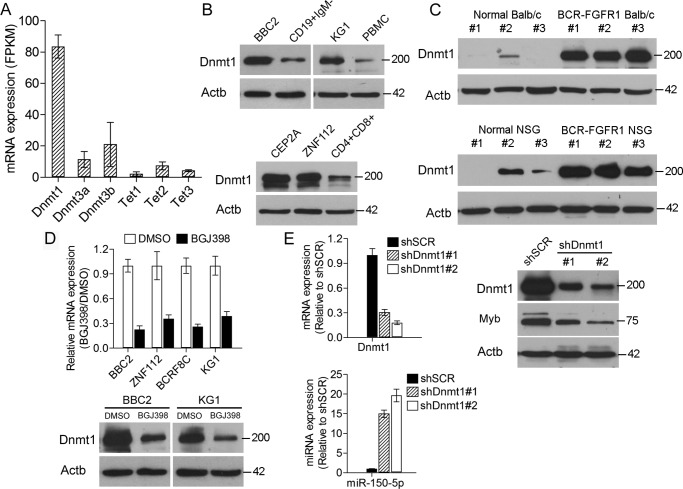
Because methylation status is determined by the action of proteins such as those in the DNMT and ten-eleven translocation families, we analyzed RNA-Seq data generated from the SCLL cell lines BBC2, ZNF112, BCRF8C, and KG1. In each case, there was consistent high-level expression of Dnmt1. When the data for these four cell lines were combined (Fig. 4A), the selective up-regulation of Dnmt1 was confirmed. In a comparison of BBC2 with normal CD19+IgM− pre/pro-B cells, KG1 with PBMCs, CEP2A and ZNF112 cells with normal CD4+CD8+ cells, increased Dnmt1 levels were seen in all cases of SCLL (Fig. 4B). Similarly, analysis of splenic cells derived from mouse stem cells transformed with BCR-FGFR1 showed increased levels of Dnmt1 compared with normal spleen cells (Fig. 4C). Consistently, analysis of leukemic cells derived from BCR-FGFR1–transformed human CD34+ cord blood cells transplanted into immunocompromised mice (NSG) also showed increased Dnmt1 protein levels. Analysis of expression and protein levels in four different SCLL cell lines after treatment with the BGJ398, FGFR1 inhibitor showed a significant reduction in Dnmt1 expression levels (Fig. 4D). Down-regulation of Dnmt1 using shRNA (Fig. 4E) showed a proportional increase in miR-150-5p expression levels and increased Myb levels. These data suggest that methylation of the miR-150-5p promoter and, hence, silencing of its expression are mediated by Dnmt1, which is a consequence of FGFR1 activation.
Figure 4.
Dnmt1 expression correlates with miR-150-5p levels in SCLL cells. A, RNA-Seq–derived mRNA levels, expressed as fragments per kilobase of transcripts per one million mapped reads (FPKM) for members of the Dnmt and Tet families of genes in four SCLL cell lines (BBC2, ZNF112, BCRF8C, and KG1), show highly elevated levels specifically for Dnmt1. B, analysis of protein levels in BBC2 and KG1 cells (top panel) compared with either normal CD19+IgM− or peripheral blood mononuclear cells, respectively, shows the same increase in Dnmt1 levels in mouse and human cell lines. Analysis of Dnmt1 levels in mouse SCLL T-lymphoma cell lines (CEP2A and ZNF112) shows the same increase compared with normal CD4+CD8+ cells (bottom panel). C, analysis of spleen cells from normal mice (BALB/c) and primary transformed hematopoietic stem cells in vivo following transduction with BCR-FGFR1 (top panel) also shows increased Dnmt1 levels in the transformed cells. Similarly, analysis of spleen cells from primary human CD34+ cord blood stem cells transformed with BCR-FGFR1 shows increased levels of Dnmt1 (bottom panel). D, when four different SCLL cell lines are treated with the BGJ398 FGFR1 inhibitor, there is a significant reduction in Dnmt1 mRNA levels (top panel). Analysis of Dnmt1 protein levels in BBC2 and KG1 cells following BGJ398 treatment (bottom panel) also shows reduced levels compared with DMSO treated cells. E, knockdown of Dnmt1 using two different shRNAs (left panel, top) leads to proportional increased expression levels of miR-150-5p-5p (left panel, bottom). Western blot analysis (right panel) shows that knockdown of Dnmt1 leads to up-regulation of Myb protein levels.
Myc regulates Dnmt1 expression
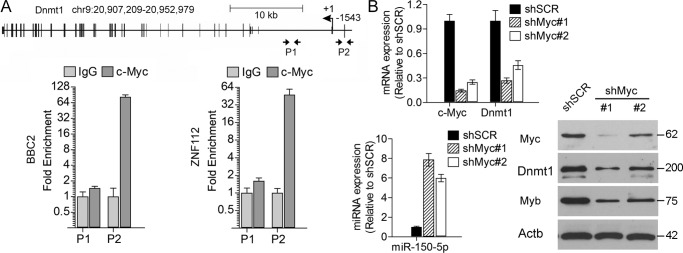
It has been shown that MYC is one of the transcription factors that regulates DNMT1 expression (20), and we have shown previously that FGFR1 up-regulates Myc in SCLL cells (13). In a ChIP-qPCR analysis of the Dnmt1 promoter regions in mouse BBC2 and ZNF112 cells as well as human KG1 cells, a significantly increased level of occupancy was seen in reported Myc-binding regions (20) (Fig. 5A and Fig. S4). Knockdown of Myc in BBC2 cells (Fig. 5B) using shRNAs showed a concomitant reduction in Dnmt1 expression levels. Analysis of miR-150-5p expression in these cells showed a consequential proportional increase in expression levels, supporting the suggestion that Myc, which is up-regulated by FGFR1, in turn leads to activation of Dnmt1 and epigenetic silencing of miR-150-5p. Down-regulation of Myc also leads to reduced Myb expression levels, demonstrating that Myc cooperates with Myb to promote SCLL syndrome via Dnmt1-mediated epigenetic silencing of miR-150-5p.
Figure 5.
Dnmt1 expression is regulated by Myc in SCLL cells. A, organization of the Dnmt1 locus in mice. The positions of the primer pairs within the promoter (P2) and intron 1 (P1), regions used for ChIP, are indicated by the opposing arrows. Compared with the IgG control, there is significant enrichment of Myc binding in the Dnmt1 promoter region (P2) in both BBC2 and ZNF112 cells. B, knockdown of Myc using two different shRNAs shows a concomitant reduction in Dnmt1 expression levels. As a result of Myc knockdown, levels of miR-150-5p show a proportional increase compared with cells expressing a scrambled control (left panel, bottom). Western blot analysis (right panel) shows the relative knockdown levels of Myc using the two shRNAs and the proportional reduction in Dnmt1 and Myb levels.
Targeting c-Myb suppresses SCLL development
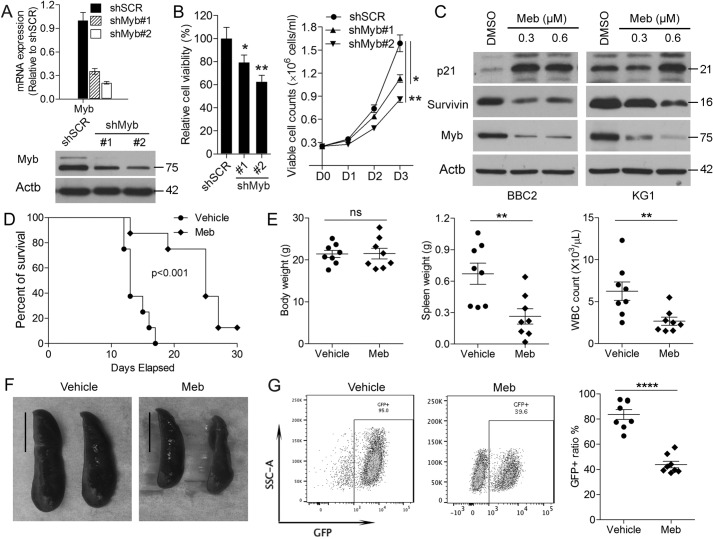
Myc has been shown to activate Myb by inducing epigenetic silencing of miR-150-5p, which normally suppresses Myb expression. It appears, therefore, that Myb up-regulation is important in SCLL development and that its expression is a direct consequence of FGFR1 activation. To investigate the role of Myb in SCLL cell survival, we treated SCLL cells with mebendazole, which has been shown to lead to Myb degradation (21). When BBC2, ZNF112, and KG1 cells were treated with mebendazole (Fig. S5), there was a dose-dependent reduction in cell viability in all three cell lines, supporting an active role of Myb in SCLL development. Consistently, when Myb was inactivated in BBC2 cells using shRNAs (Fig. 6A), there was a significant reduction in cell viability and proliferation compared with treatment with a scrambled shRNA (Fig. 6B). Mebendazole inactivation of Myb inactivation in BBC2 and KG1 cells (Fig. 6C) led to up-regulation of p21, demonstrating withdrawal from the cell cycle and a reduction in the levels of survivin, which normally reduces apoptosis. Consistent with protein expression changes, flow cytometric analysis demonstrated that mebendazole treatment of SCLL cells reduced progression through the cell cycle and led to significantly increased ratios of Annexin V+ cells among both BBC2 and KG1 (Fig. 5) cells compared with vehicle-only-treated cells, demonstrating increased apoptosis. To evaluate the role of Myb on SCLL progression in vivo, we treated mice that had been transplanted with BCR-FGFR1–transformed HSCs with an oral suspension of mebendazole using 1 mg/mouse/day for 2 weeks (22). These cells coexpress GFP from the transduction vector (14), and flow cytometry can monitor the disease load in these animals during disease development and at autopsy. As shown by the Kaplan-Meier analysis (Fig. 6D), mice treated with mebendazole showed a significant improvement in survival compared with vehicle-treated mice (n = 5). Although there were no effects on body weight, both spleen weight and white blood cell count in peripheral blood were reduced in drug-treated animals (Fig. 6E). This suppression of leukemogenesis was evident from the reduced spleen sizes in treated animals (Fig. 6F) as well as the presence of reduced GFP-positive cells in spleen samples (Fig. 6G).
Figure 6.
Suppression of Myb leads to increased survival in SCLL models in vivo. A and B, knockdown of Myb in BBC2 cells using two different shRNAs (#1 and #2) leads to reduced mRNA and protein levels (A) and a proportional reduction in cell viability over a 3-day observation period (B). C, Western blot analysis of the mouse BBC2 and human KG1 cell lines treated with mebendazole (Meb) shows increased levels of p21 and decreased levels of survivin proportional to the reduction in Myb levels. D, In vivo, BBC2 xenografts (n = 8) show increased survival in mebendazole-treated animals. Although there was no change in overall body weight, there was significantly reduced spleen weight and white cell count in the peripheral blood in treated animals. F, the decreased spleen size is clearly shown in mebendazole-treated animals, and flow cytometry analysis of the relative number of GFP+ leukemia cells (coexpressed from the pMIG vector with BCR-FGFR1) shows decreased levels in spleens from treated animals compared with vehicle-treated animals. Scale bar = 1 cm. ns, not significant. *, p < 0.01; **, p < 0.001; ****, p < 0.00001.
Discussion
Development of the hematopoietic system is regulated by a dynamic interplay between various transcription factors in response to environmental cues. One way that rapid responses in levels of transcription factors can be regulated is through microRNA-mediated suppression of expression. MicroRNAs have been extensively implicated in normal and abnormal development of hematopoiesis, and miR-150-5p has been shown to be selectively expressed in mature and resting B and T cells but not in their progenitors. The same is true in the stem cell leukemias seen in the SCLL syndrome we describe here, where miR-150-5p is down-regulated in immature T, B, and myeloid leukemic cells compared with high levels in normal cell counterparts. The expression levels of miR-150-5p are also markedly decreased in ALL, AML, and CML compared with healthy controls (10). Recently, an essential role of miR-150-5p was identified in the pathogenesis of MLL-rearranged AML. Repression of miR-150-5p maturation by MLL fusion genes accelerated leukemogenesis in an MLL/AF9 murine model, and miR-150-5p expression in this model inhibited leukemia cell growth by targeting MYB and FLT3 (23).
miR-150-5p is thought to control development of the myeloid lineage by fine-tuning the Myb transcription factor and its downstream products, promoting differentiation of HSCs toward megakaryocytes rather than erythrocytes. Myb is highly expressed in lymphocyte progenitors, down-regulated upon maturation, and again up-regulated after activation of the mature cells. Myb has also been shown to play a critical role in normal development of hematopoietic cells through control of cell cycle progression and differentiation in stem and progenitor cells (10). Loss of Myb expression in mice results in significantly abnormal development of the hematopoietic system and has also been frequently associated with hematopoietic malignancies (24). In FGFR1-driven leukemogenesis, we show that Myb is highly expressed in cells from this stem cell leukemia syndrome and that degradation of Myb by either forced expression of miR-150-5p or treatment with mebendazole leads to reduced cell proliferation and increased cell apoptosis, suggesting a tumor promotion role of Myb in this context.
The Myc oncogene is frequently overexpressed in leukemias, as we have shown in various SCLL cell models (13). Inactivation of Myc using the 10058-F4 inhibitor in these cells results in suppression of leukemogenesis in vivo and, in this study, we demonstrate that Myc activation leads to epigenetic silencing of miR-150-5p as a result of direct regulation of the Dnmt1 promoter. Dnmt1 is responsible for maintenance of methylation in the genome through cell division, whereas other members of the family establish methylation marks de novo (25). Similarly, the ten-eleven translocation family of enzymes is involved in removing epigenetic marks, and, although they are frequently mutated in hematopoietic malignancies (26), they do not appear to be affected by overexpression of FGFR1. Tumor development is usually the consequence of dysregulation of multiple oncogenes. Here we demonstrate a paradigm that the Myc oncogene activates Myb through epigenetic silencing of miR-150-5p by direct promoter binding and activation of Dnmt1 to promote leukemogenesis in SCLL.
There are a number of parallels between chimeric FGFR1-driven leukemias and BCR-ABL1 in CML, which develops as a chronic myeloproliferative disease, that extend to the studies presented here. Transformation of CD34+ cells by various fusion kinases leads to up-regulation of Myc and down-regulation of miR-150-5p, suggesting, in a large correlative study, transformation of CD34+ cells by BCR-ABL1 fusion kinases leads to up-regulation of MYC and MYB, and down-regulation and miR-150-5p, suggesting that MYC and miR-150-5p are associated with MYB expression in CML (27). In that study, MYC reportedly occupied the −11.7 kb and −0.35 kb regulatory regions of the miR-150 promoter, which is in contrast to our observations in SCLL, where we demonstrate that miR-150-5p silencing is mediated by methylation of its promoter by Dnmt1 as a result of Myc activation. This conclusion regarding our mouse SCLL models is supported following our analysis of a human pre-B-ALL patient cohort, comparing samples with disease and in remission, revealing that differential methylation of the miR-150-5p promoter is associated with remission samples.
Previous studies showed that targeting SCLL cells with FGFR1 inhibitors, most notably BGJ398, can suppress leukemogenesis in mouse models in vivo and affects cell proliferation in SCLL cell lines in vitro (17). Through a better understanding of the molecular etiology of FGFR1-driven neoplasms of lymphoid and myeloid origin, we demonstrated that targeting these downstream effects also suppress leukemogenesis to a greater or lesser degree. The identification of miR-150-5p as a silenced tumor suppressor in SCLL provides an additional treatment option to target this pathway. As a proof of concept, there have been attempts to restore miR-150-5p expression not only in solid tumors but also in hematological malignancies (23, 28). Notably, treatment of FLT3-overexpressing AML with FLT3L-guided miR-150-5p–based nanoparticles shows high selectivity and efficiency and significant inhibition of AML progression (23). Considering the complexity of preparing the miRNA nanoparticles and limitations of delivery methods, pharmaceutical targeting of this pathway may prove to be the preferred approach. The demonstration that mebendazole, which interferes with proteasome degradation of Myb (21), also suppresses SCLL development in vivo and reduces cell viability in vitro suggests that mebendazole may be an important way of treating SCLL, especially in combination with FGFR1 inhibitors.
Experimental procedures
Cell culture and drug treatment
Cells were cultured in RPMI 1640 medium containing 10% FBS. Cell viability was measured using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). To evaluate the effect of methylation on miR-150-5p expression, BBC2 and KG1 cells were treated with 0.3 and 1 μm 2′-deoxy-5-azacytidine for 72 h. For FGFR and Myb inhibition assays, cells were treated with the indicated concentrations of BGJ398 and mebendazole (Selleckchem, Houston, TX) for 24 h, and then cells were harvested for further cellular and molecular analysis.
Cell cycle and apoptosis analysis
106 cells were labeled with Hoechst 33342 (Thermo Fisher Scientific, Waltham, MA) for 1 h at 37 °C, and cell cycle profiles were determined using the BD LSR II flow cytometer (BD Biosciences). To measure apoptosis, 106 cells were stained with APC Annexin V and the DNA-binding dye 7-amino-actinomycin (Biolegend, San Diego, CA) according to the manufacturer's protocol and analyzed by BD FACSCanto II flow cytometry (BD Biosciences).
Transduction of target cells
For overexpression of miR-150-5p, the ∼500-bp fragment including miR-150-5p and flanking sequences was cloned into the pMSCV-PIG vector, and a retrovirus was prepared using the amphotropic packing system as described previously (9). For shRNA knockdown, BBC2 cells were transduced with lentiviral particles prepared from clones TRCN225698 and TRCN225699 for shDnmt1, TRCN42513 and TRCN42517 for shMyc, and TRCN42499 and TRCN426969 for shMyb (Sigma) and then selected with 1 μg/ml puromycin to generate stable cell lines.
Molecular analyses
Western blotting, DNA preparation/cloning, RNA extraction, and reverse transcription of mRNA are standard procedures that have been described previously in detail. For miRNA expression quantification, total RNA was reverse-transcribed with the miRNA cDNA Synthesis Kit (Applied Biological Materials Inc., Richmond, BC, Canada), and then standard real-time PCR reactions were performed.
Bisulfite Sanger sequencing
For bisulfite Sanger sequencing, bisulfite conversion was performed as described previously (12) using the EZ DNA Methylation-Gold Kit (Zymo Research Corp., Irvine, CA) according to the vendor's protocol. Briefly, 0.5 μg of DNA isolated from target cells was heat-denatured, treated with bisulfite (98 °C for 10 min and then 55 °C for 4 h) to convert unmethylated cytosine to uracil, followed by nested PCR with appropriate PCR primers. The PCR products were cloned into the TA vector, and single colonies were selected for Sanger sequencing (MCLAB, San Francisco, CA).
RNA-Seq data analysis
The RNA-Seq data for BGJ398- and DMSO-treated SCLL lines have been described previously (7) and deposited into the GEO database (accession number GSE110457). The normalized fragments per kilobase of transcripts per one million mapped reads data were used to evaluate the expression level of DNA methylation–related genes.
ChIP-qPCR
ChIP assays were performed using a ChIP assay kit (Millipore) as described previously (13). In brief, chromatin was cross-linked with 1% formaldehyde for 10 min at room temperature, sheared to an average size of ∼500 bp, and then immunoprecipitated with an anti-Myc antibody (Abcam). The ChIP-qPCR primers were designed to amplify a proximal promoter region containing putative Myc binding sites (5′-ACCACATGGT-3′ for the mouse locus and 5′-ACCACGTGGC-3′ for the human locus) in the DNMT1 promoter. An intronic region was selected as a negative control. Each immunoprecipitated DNA sample was quantified using qPCR, and all ChIP-qPCR signals were normalized to an IgG control to calculate the relative -fold of enrichment.
In vivo engraftment
Animal protocols followed guidelines and procedures approved by the Institutional Animal Care and Use Committee of Augusta University. Approximately 1 × 106 cells were injected into the tail veins of 6- to 8-week-old, female, sublethally (600 rad) irradiated BALB/c mice, and after 1 week of expansion growth, randomly grouped mice were treated with either inhibitor or vehicle control as described previously (4).
Author contributions
T. H. and J. K. C. conceptualization; T. H., B. C., Y. L., and S. L. data curation; T. H. and Y. C. formal analysis; T. H. and J. K. C. supervision; T. H. and Y. C. validation; T. H., Y. C., B. C., Y. L., and S. L. investigation; T. H., Y. C., B. C., Y. L., and S. L. methodology; J. K. C. funding acquisition; J. K. C. visualization; J. K. C. writing-original draft; J. K. C. project administration.
Supplementary Material
Acknowledgment
We thank Dr. Jan van Riggelen for helpful discussions and providing the DNMT1 antibody.
This work was supported by National Cancer Institute (NCI) Grant CA076167. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S5.
- FGFR
- fibroblast growth factor receptor
- SCLL
- stem cell leukemia/lymphoma
- AML
- acute myeloid leukemia
- miRNA
- microRNA
- CML
- chronic myeloid leukemia
- HSC
- hematopoietic stem cell
- DNMT
- DNA methyltransferase
- qPCR
- quantitative PCR
- ALL
- acute lymphoblastic leukemia.
References
- 1. Jackson C. C., Medeiros L. J., and Miranda R. N. (2010) 8p11 myeloproliferative syndrome: a review. Hum. Pathol. 41, 461–476 10.1016/j.humpath.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Baumann H., Kunapuli P., Tracy E., and Cowell J. K. (2003) The oncogenic fusion protein tyrosine kinase ZNF198/fibroblast growth factor receptor-1 has signaling function comparable with interleukin-6 cytokine receptors. J. Biol. Chem. 278, 16198–16208 10.1074/jbc.M300018200 [DOI] [PubMed] [Google Scholar]
- 3. Roumiantsev S., Krause D. S., Neumann C. A., Dimitri C. A., Asiedu F., Cross N. C., Van Etten R. A. (2004). Distinct stem cell myeloproliferative/T lymphoma syndromes induced by ZNF198-FGFR1 and BCR-FGFR1 fusion genes from 8p11 translocations. Cancer Cell 5, 287–298 10.1016/S1535-6108(04)00053-4 [DOI] [PubMed] [Google Scholar]
- 4. Ren M., Qin H., Ren R., and Cowell J. K. (2013) Ponatinib suppresses the development of myeloid and lymphoid malignancies associated with FGFR1 abnormalities. Leukemia 27, 32–40 10.1038/leu.2012.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khodadoust M. S., Luo B., Medeiros B. C., Johnson R. C., Ewalt M. D., Schalkwyk A. S., Bangs C. D., Cherry A. M., Arai S., Arber D. A., Zehnder J. L., and Gotlib J. (2016) Clinical activity of ponatinib in a patient with FGFR1-rearranged mixed-phenotype acute leukemia. Leukemia 30, 947–950 10.1038/leu.2015.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ren M., Qin H., Kitamura E., and Cowell J. K. (2013) Dysregulated signaling pathways in the development of CNTRL-FGFR1-induced myeloid and lymphoid malignancies associated with FGFR1 in human and mouse models. Blood 122, 1007–1016 10.1182/blood-2013-03-489823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silva J., Chang C.-S., Hu T., Qin H., Kitamura E., Hawthorn L., Ren M., and Cowell J. K. (2018) Distinct signaling programs associated with progression of FGFR1 driven leukemia in a mouse model of stem cell leukemia lymphoma syndrome. Genomics 10.1016/j.ygeno.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 8. Hu T., Chong Y., Qin H., Kitamura E., Chang C.-S., Silva J., Ren M., and Cowell J. K. (2018) The miR-17/92 cluster is involved in the molecular etiology of the SCLL syndrome driven by the BCR-FGFR1 chimeric kinase. Oncogene 37, 1926–1938 10.1038/s41388-017-0091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu T., Chong Y., Lu S., Wang R., Qin H., Silva J., Kitamura E., Chang C.-S., Hawthorn L., and Cowell J. K. (2018) MicroRNA 339 promotes development of stem cell leukemia/lymphoma syndrome through downregulation of the BCL2L11 and BAX pro-apoptotic genes. Cancer Res. 78, 3522–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He Y., Jiang X., and Chen J. (2014) The role of miR150 in normal and malignant hematopoiesis. Oncogene 33, 3887–3893 10.1038/onc.2013.346 [DOI] [PubMed] [Google Scholar]
- 11. Xiao C., Calado D. P., Galler G., Thai T. H., Patterson H. C., Wang J., Rajewsky N., Bender T. P., and Rajewsky K. (2007) MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 131, 146–159 10.1016/j.cell.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 12. Hu T., Zhu X., Pi W., Yu M., Shi H., and Tuan D. (2017) Hypermethylated LTR retrotransposon exhibits enhancer activity. Epigenetics 12, 226–237 10.1080/15592294.2017.1289300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu T., Wu Q., Chong Y., Qin H., Poole C. J., van Riggelen J., Ren M., and Cowell J. K. (2018) FGFR1 fusion kinase regulation of MYC expression drives development of stem cell leukemia/lymphoma syndrome. Leukemia 32, 2363–2373 10.1038/s41375-018-0124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren M., Tidwell J. A., Sharma S., and Cowell J. K. (2012) Acute progression of BCR-FGFR1 induced murine B-lympho/myeloproliferative disorder suggests involvement of lineages at the pro-B Cell Stage. PLoS ONE 7, e38265 10.1371/journal.pone.0038265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ren M., Li X., and Cowell J. K. (2009) Genetic fingerprinting of the development and progression of T-cell lymphoma in a murine model of atypical myeloproliferative disorder initiated by the ZNF198-FGFR1 chimeric tyrosine kinase. Blood 114, 1576–1584 10.1182/blood-2009-03-212704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furley A. J., Reeves B. R., Mizutani S., Altass L. J., Watt S. M., Jacob M.C., van den Elsen P., Terhorst C., and Greaves M. F. (1986) Divergent molecular phenotypes of KG1 and KG1a myeloid cell lines. Blood 68, 1101–1107 10.1182/blood.V68.5.1101.1101 [DOI] [PubMed] [Google Scholar]
- 17. Wu Q., Bhole A., Qin H., Karp J., Malek S., Cowell J. K., and Ren M. (2016) Targeting FGFR1 to suppress leukemogenesis in syndromic and de novo AML in murine models. Oncotarget 7, 49733–49742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin Y. C., Kuo M. W., Yu J., Kuo H. H., Lin R. J., Lo W. L., and Yu A. L. (2008) c-Myb is an evolutionary conserved miR-150 target and miR-150/c-Myb interaction is important for embryonic development. Mol. Biol. Evol. 25, 2189–2198 10.1093/molbev/msn165 [DOI] [PubMed] [Google Scholar]
- 19. Busche S., Ge B., Vidal R., Spinella J. F., Saillour V., Richer C., Healy J., Chen S.-H., Droit A., Sinnett D., and Pastinen T. (2013) Integration of high-resolution methylome and transcriptome analyses to dissect epigenomic changes in childhood acute lymphoblastic leukemia. Cancer Res. 73, 4323–4336 10.1158/0008-5472.CAN-12-4367 [DOI] [PubMed] [Google Scholar]
- 20. Poole C. J., Zheng W., Lodh A., Yevtodiyenko A., Liefwalker D., Li H., Felsher D. W., and van Riggelen J. (2017) DNMT3B overexpression contributes to aberrant DNA methylation and MYC-driven tumor maintenance in T-ALL and Burkitt's lymphoma. Oncotarget 8, 76898–76920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walf-Vorderwülbecke V., Pearce K., Brooks T., Hubank M., van den Heuvel-Eibrink M. M., Zwaan C. M., Adams S., Edwards D., Bartram J., Samarasinghe S., Ancliff P., Khwaja A., Goulden N., Williams G., de Boer J., and Williams O. (2018) Targeting acute myeloid leukemia by drug-induced c-MYB degradation. Leukemia 32, 882–889 10.1038/leu.2017.317 [DOI] [PubMed] [Google Scholar]
- 22. Mukhopadhyay T., Sasaki J., Ramesh R., and Roth J. A. (2002) Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin. Cancer Res. 8, 2963–2969 [PubMed] [Google Scholar]
- 23. Jiang X., Bugno J., Hu C., Yang Y., Herold T., Qi J., Chen P., Gurbuxani S., Arnovitz S., Strong J., Ferchen K., Ulrich B., Weng H., Wang Y., Huang H., et al. (2016) Eradication of acute myeloid leukemia with FLT3 ligand-targeted miR-150-5p nanoparticles. Cancer Res. 76, 4470–4480 10.1158/0008-5472.CAN-15-2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pattabiraman D. R., and Gonda T. J. (2013) Role and potential for therapeutic targeting of MYB in leukemia. Leukemia 27, 269–277 10.1038/leu.2012.225 [DOI] [PubMed] [Google Scholar]
- 25. Gujar H., Weisenberger D. J., and Liang G. (2019) The roles of human DNA methyltransferases and their isoforms in shaping the epigenome. Genes 10, E172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Solary E., Bernard O. A., Tefferi A., Fuks F., and Vainchenker W. (2014) The ten-eleven translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases. Leukemia 28, 485–496 10.1038/leu.2013.337 [DOI] [PubMed] [Google Scholar]
- 27. Srutova K., Curik N., Burda P., Savvulidi F., Silvestri G., Trotta R., Klamova H., Pecherkova P., Sovova Z., Koblihova J., Stopka T., Perrotti D., and Polakova K. M. (2018) BCR-ABL1 mediated miR-150-5p downregulation through MYC contributed to myeloid differentiation block and drug resistance in chronic myeloid leukemia. Haematologica 103, 2016–2025 10.3324/haematol.2018.193086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoareau-Aveilla C., Valentin T., Daugrois C., Quelen C., Mitou G., Quentin S., Jia J., Spicuglia S., Ferrier P., Ceccon M., Giuriato S., Gambacorti-Passerini C., Brousset P., Lamant L., and Meggetto F. (2015) Reversal of microRNA-150 silencing disadvantages crizotinib-resistant NPM-ALK+ cell growth. J. Clin. Invest. 125, 3505–3518 10.1172/JCI78488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.