Abstract
Many retinal diseases are associated with pathological cell swelling, but the underlying etiology remains to be established. A key component of the volume-sensitive machinery, the transient receptor potential vanilloid 4 (TRPV4) ion channel, may represent a sensor and transducer of cell swelling, but the molecular link between the swelling and TRPV4 activation is unresolved. Here, our results from experiments using electrophysiology, cell volumetric measurements, and fluorescence imaging conducted in murine retinal cells and Xenopus oocytes indicated that cell swelling in the physiological range activated TRPV4 in Müller glia and Xenopus oocytes, but required phospholipase A2 (PLA2) activity exclusively in Müller cells. Volume-dependent TRPV4 gating was independent of cytoskeletal rearrangements and phosphorylation. Our findings also revealed that TRPV4-mediated transduction of volume changes is dependent by its N terminus, more specifically by its distal-most part. We conclude that the volume sensitivity and function of TRPV4 in situ depend critically on its functional and cell type–specific interactions.
Keywords: transient receptor potential channels (TRP channels); aquaporin 4 (AQP4); polyunsaturated fatty acid (PUFA); lipid; retina; osmo-sensing; osmo-sensor; transient receptor potential vanilloid 4 (TRPV4); volume sensing; volume sensor; cell swelling; phospholipase A2 (PLA2), 5′,6′-epoxy-8Z,11Z,14Z-eicosatrienoic acid (5′,6′-EET); glaucoma; Xenopus oocytes; Müller cells; retinal ganglion cells
Introduction
The transient receptor potential vanilloid 4 (TRPV4)3 channel is a Ca2+-permeable cation channel expressed in a variety of tissues, including the retina, where the channel is localized to Müller cells, microvascular endothelial cells, retinal pigment epithelium, and retinal ganglion cells (1–3). As an indication of TRPV4 being a key component of the response mechanism during pathological events, excessive TRPV4 influx may drive reactive gliosis and glial cytokine release (1, 4), modulate the integrity of the blood-retina barrier (5, 6), and predispose retinal ganglion cells to activation of Ca2+-dependent pro-apoptotic signaling pathways (7). TRPV4 channels display remarkable gating promiscuity and are activated by a palette of physical stimuli, such as mechanical forces, post-translational modifications, and chemical stimuli, such as synthetic lipid ligands (1, 8–12) (but see Ref. 13), in a manner that mimics the volume-dependent activation of the channel (14). TRPV4 responds to isosmolar cell swelling as well as to osmolarity-induced cell swelling, translated via aquaporin water channels, and thus functions as a sensor of abrupt volume changes irrespective of the origin of the cell swelling (14–16). However, a key unresolved question pertains to the structural determinants that mediate the molecular coupling between cell swelling and channel activation. Müller glia, endothelial cells, and HEK293 cells may require an intermediate step involving phospholipase A2 (PLA2) activation and cytochrome P450 (CYP450)-dependent arachidonic acid (AA) metabolites for swelling-dependent activation of TRPV4 (1, 8, 17), whereas cell swelling has led to TRPV4 activation independently of PLA2 and AA metabolites in retinal ganglion cells, sensory neurons, yeast, renal vascular cells, and Xenopus oocytes (1, 13, 18). It thus remains unresolved whether swelling-induced activation of TRPV4 can occur directly or whether an intracellular signaling cascade is required to couple cell swelling to TRPV4 activation.
TRPV4 belongs to a family of channels, of which several members display volume sensitivity (19, 20) and activate either in response to cell swelling as TRPV4 (14, 15) or to cell shrinkage as the TRPV1 splice variant, VR.5′sv (21–24). TRPV4 possesses an extensive cytoplasmic N terminus, which contains ankyrin repeats (25, 26) that are recognized as potential binding hubs and thus could represent an important structural element of volume-dependent channel gating. The reports of volume-dependence of TRPV4 were based on introduction of large osmotic gradients of 100–200 mosm (1, 14, 27, 28), which in most cell types will induce cell swelling of a nonphysiological caliber (29). The extent of TRPV4-mediated activation and gating upon small physiologically relevant volume changes remains unexplored. Here, we investigated swelling-induced TRPV4 activation with physiologically relevant volume changes in murine retinal cells and upon heterologous expression in Xenopus oocytes to reveal the molecular coupling between cell swelling and TRPV4 activation.
Results
Swelling-induced activation of heterologously expressed TRPV4 occurs independently of PLA2 activity
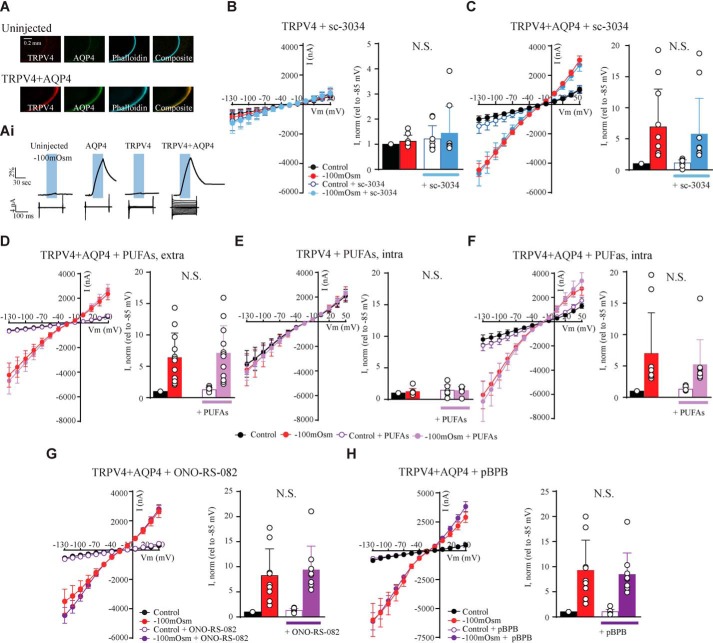
Whereas initial studies suggested that PLA2 activation is required for swelling-induced TRPV4 activation (8, 9, 30), at least two studies reported that canonical PLA2 signaling may not be obligatory in neurons (1, 31). We therefore employed the Xenopus laevis oocyte heterologous expression system based on TRPV4 expression in Xenopus laevis oocytes that were exposed to hyposmotic stimuli in the presence of PLA2 activators and blockers. As an additional control, we co-expressed AQP4 in a subset of oocytes, which we previously showed facilitates TRPV4 activation through a robust increase in water permeability and rate of swelling (32). TRPV4 and AQP4 expression in the plasma membrane was verified in immunofluorescent micrographs after microinjection of cRNA encoding the two proteins, whereas no expression was detected in control (uninjected) oocytes (Fig. 1A). Oocytes were challenged with a hyposmotic gradient of −100 mosm (obtained by removal of 100 mm mannitol from the control solution to decrease the osmolarity while keeping the ionic strength constant, leading to 5–10% cell swelling in the tested time frame) with continuous monitoring of their volume and current activity with conventional two-electrode voltage clamp (see representative volume and current traces in Fig. 1Ai). This osmotic challenge led to robust cell swelling of oocytes co-expressing AQP4 and TRPV4, which was virtually absent in TRPV4-expressing oocytes or uninjected oocytes (Fig. 1Ai).
Figure 1.
TRPV4 is activated by increased cell volume independently of PLA2 activity. A, representative confocal laser-scanning micrographs of an uninjected oocyte (top) and an oocyte expressing TRPV4 + AQP4 (bottom) after immunolabeling with phalloidin and anti-AQP4 and anti-TRPV4 antibodies confirmed the plasma membrane expression. Ai, representative volume and current traces obtained from oocytes voltage-clamped at Vm = −20 mV and challenged with a hyposmotic gradient (−100 mosm, indicated by a blue bar). Current traces were recorded with a 200-ms step protocol from an uninjected oocyte (far left) and oocytes expressing either AQP4 (middle left), TRPV4 (middle right), or TRPV4 + AQP4 (far right). B–H, summarized I/V curves from TRPV4– and TRPV4 + AQP4–expressing oocytes in control solution (black) or during application of a hyposmotic solution (red) without drug or in control solution and hyposmotic solution with sc-3034 (PLA2 activator) (B and C; control solution in white, hyposmotic solution in blue), externally added PUFAs (D; control solution in white, hyposmotic solution in light purple), microinjected PUFAs (E and F; control solution in white, hyposmotic solution in light purple), ONO-RS-082 (PLA2 inhibitor) (G; control solution in white, hyposmotic solution in dark purple), or pBPB (PLA2 inhibitor) (H; control solution in white, hyposmotic solution in dark purple). Insets, TRPV4-mediated current activity at −85 mV obtained after exposure to −100 mosm (red), in control solution with drug (white), and after exposure to −100 mosm with drug (blue, light, or dark purple) was normalized to that obtained in control condition without drug. N.S., not significant (p > 0.05); one-way ANOVA, n = 9–10 oocytes. Error bars, S.D. (bar graphs) or S.E. (I/V curves).
Activation of PLA2 by sc-3034 (3 μm) added to the test solution failed to affect TRPV4-mediated current activity, whether or not the oocyte was exposed to an osmotic challenge during the exposure (n = 10, Fig. 1 B and C). Despite earlier reports of PLA2 activity in oocytes (33–36), we excluded the possibility that lack of PLA2-mediated TRPV4 activation could originate from PLA2 absence in the oocytes by circumventing PLA2 enzyme activity. The TRPV4-expressing oocytes were exposed to the downstream metabolites of the PLA2 signaling pathway, a mixture of the polyunsaturated fatty acids (PUFAs) AA (10 μm), oleic acid (10 μm), anandamide (10 μm), and 5′,6′-epoxy-eicosatrienoic acids (EETs) (5 μm) to the extracellular side by inclusion in the test solution (n = 10; Fig. 1D). As these metabolites are released intracellularly upon PLA2 activity, we, in addition, microinjected the PUFAs directly into the oocyte during the electrophysiological recordings (n = 10; Fig. 1, E and F). Exposure of this mixture of PUFAs did not affect the TRPV4-mediated current, whether AQP4 was co-expressed or not and whether or not the cells were exposed to a hyposmotic challenge. Combined, these data illustrate that the canonical PLA2 signaling pathway and/or its associated downstream metabolites do not lead to activation of TRPV4 expressed in Xenopus oocytes.
To determine whether PLA2 was required for the volume-induced TRPV4 activation, two different PLA2 inhibitors (ONO-RS-82 (1 μm) or pBPB (1 μm)) were applied prior to introduction of the osmotic challenge; PLA2 inhibition did not affect the TRPV4-mediated current activity or prevent swelling-induced TRPV4 activation (n = 9, Fig. 1 G and H). None of the tested agonists/antagonists affected the membrane currents of uninjected oocytes or oocytes expressing only AQP4 (data not shown). These results demonstrate that swelling-induced TRPV4 activation can indeed occur independently of PLA2 activation.
Swelling-induced activation of TRPV4 is unaffected by phosphorylation
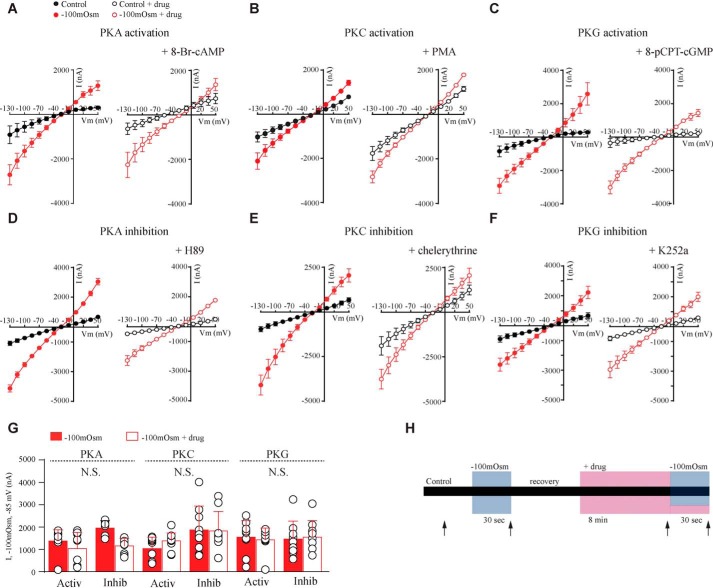
Given that intermediary PLA2 activation is not required for swelling-induced gating of heterologously expressed TRPV4, we determined the involvement of other factors that have previously been implicated in TRPV4 function. To resolve the requirement for protein phosphorylation in swelling-induced activation of TRPV4, TRPV4 + AQP4–expressing oocytes were challenged with a hyposmotic solution (−100 mosm) in the absence and presence of activators or inhibitors of protein kinase G (PKG), A (PKA), or C (PKC) (Fig. 2). These kinase modulators all efficiently target and activate their respective kinases in Xenopus oocytes (37, 38) and do not affect AQP4 expression or activity within the employed time frame (≤10 min) (37, 38). To determine the effect of PKA-, PKC-, or PKG-dependent phosphorylation during swelling-induced activation of TRPV4, 200 nm phorbol 12-myristate 13-acetate (PMA) (PKC activator) or 10 μm chelerythrine (PKC inhibitor), 300 μm 8-Br-cAMP (PKA activator) or 50 μm H89 (PKA inhibitor), or 100 μm 8-pCPT-cGMP (PKG activator) or 1 μm K252a (PKG inhibitor) (n = 9–12, Fig. 2, A–F, right panels) were added to the test solution, and the TRPV4-mediated current activity was measured (see Fig. 2H for a schematic of the experimental paradigm). Summarized data obtained for all six kinase modulators at −85 mV are shown in Fig. 2G (n = 9–12). Inhibition or activation of PKC, PKA, and PKG did not significantly affect the swelling-induced activation of TRPV4.
Figure 2.
No changes in swelling-induced activation of TRPV4 upon phosphorylation. A–F, representative I/V curves of TRPV4-mediated activity in control solution or in hyposmotic solution. Control solution is shown in black and hyposmotic (−100 mosm) in red. A–C, activity with PKA-, PKC-, or PKG activators (right). D–F, activity with PKA, PKC, or PKG inhibitors (right). G, current activity, I, at −85 mV obtained after exposure to −100 mosm is shown as bars. Filled red bars, hypotonic solution–induced TRPV4-mediated activity without drug; open bars, response with drug. H, experimental paradigm. Application of a hyposmotic gradient is indicated by a blue bar, and drug application is shown by a pink bar. Arrows indicate when current activity was recorded. N.S., not significant (p > 0.05), one-way ANOVA, n = 9–12 oocytes. Error bars, S.D. (bar graphs) or S.E. (I/V curves).
Swelling-induced TRPV4 activation occurs independently of cytoskeletal rearrangements
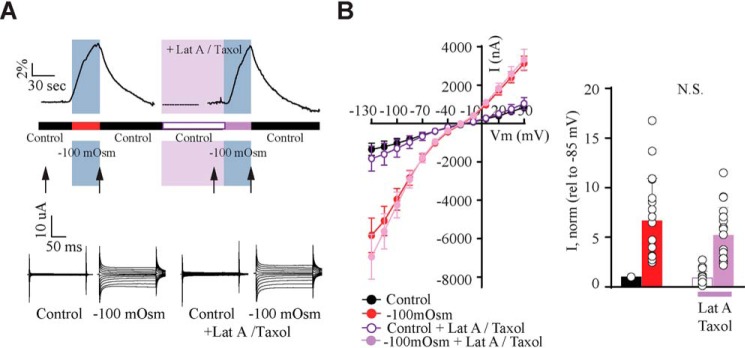
To determine whether swelling-induced TRPV4 activation relies on cytoskeletal dynamics, we monitored swelling-induced activation of TRPV4 in the presence of two inhibitors of cytoskeletal rearrangements, taxol (microtubules) and latrunculin A (actin) (39). Exposure of TRPV4 + AQP4–expressing oocytes to these inhibitors did not affect the TRPV4-mediated current activity or the swelling-induced TRPV4 response (Fig. 3, A and B, n = 12). These results illustrate that swelling-induced TRPV4 activation occurs independently of cytoskeletal rearrangements.
Figure 3.
Cytoskeletal rearrangements are not required for activation of TRPV4. A, experimental paradigm and representative volume traces (top). Application of a hyposmotic gradient is indicated by a blue bar, and drug application is shown by a pink bar. Representative current traces (bottom) were recorded as indicated by arrows (in control and hyposmotic solutions before drug application, after recovery and after latrunculin A and taxol application). B, summarized I/V curves with control solution (black), hyposmotic solution (red), control solution (white), and hyposmotic solution (light purple) after latrunculin A/taxol application. Insets, TRPV4-mediated current activity at −85 mV obtained after exposure to −100 mosm (red), in control solution with latrunculin A/taxol (white) or in hyposmotic solution with latrunculin A/taxol (light purple) was normalized to that obtained in control condition. N.S., not significant (p > 0.05); one-way ANOVA, n = 12 oocytes. Error bars, S.D.
The N terminus of TRPV4 determines the directionality of the volume sensing
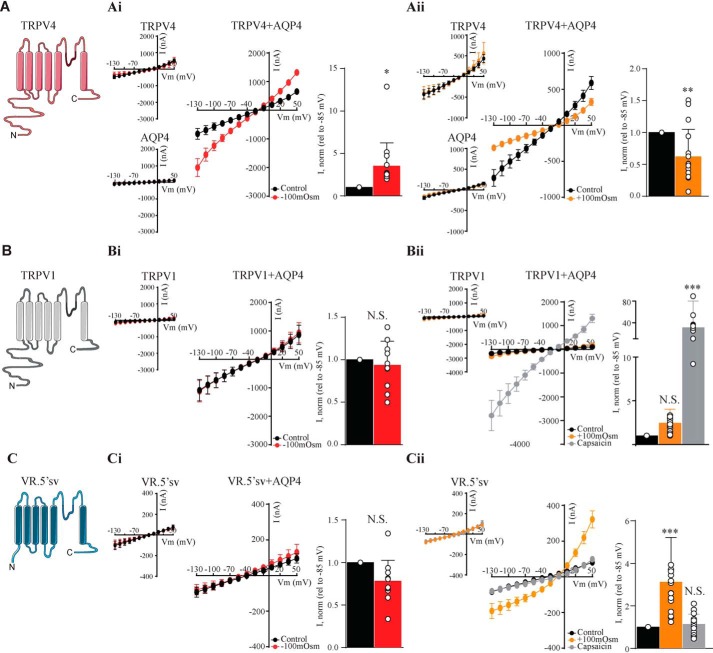
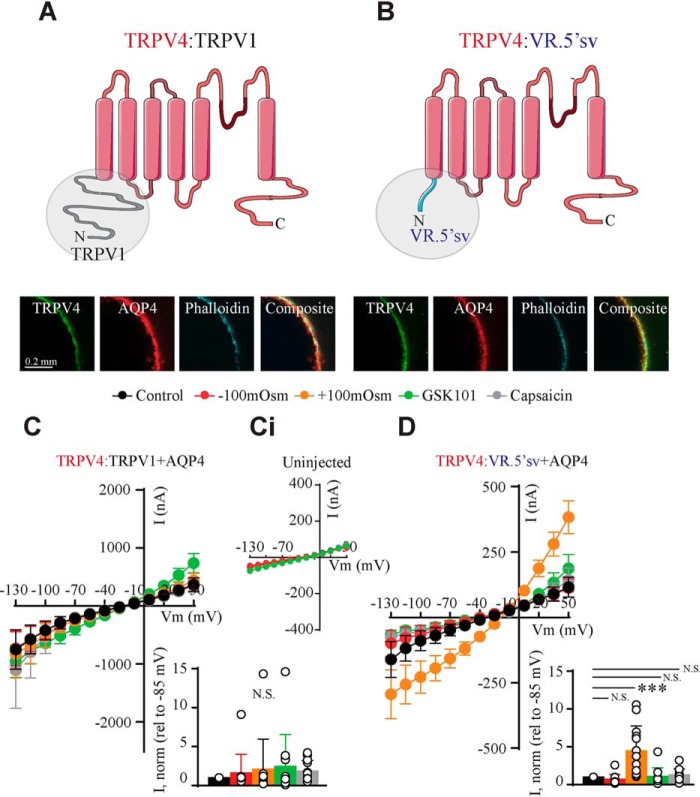
To resolve the structural identity of the TRPV4 volume sensor, we included the phylogenetically related TRPV1 in its full-length version as well as the heavily truncated splice variant thereof (VR.5′sv), the latter of which has been reported to open upon cell shrinkage (21, 40). The experiments were designed for each oocyte to act as its own control, and the effects of osmotic challenges and agonists are presented relative to the basal control current (see histograms in Fig. 4). In this manner, the expression levels of the different constructs need not be identical. Oocytes expressing AQP4 and each of the TRPV constructs (TRPV4 + AQP4 (Fig. 4A), TRPV1 + AQP4 (Fig. 4B), and VR.5′sv + AQP4 (Fig. 4C)) were initially challenged with a hyposmotic (−100 mosm) gradient during current recordings. TRPV4 + AQP4-expressing oocytes presented with increased current activity upon cell swelling (n = 9; Fig. 4Ai), which was in contrast to both TRPV1 and VR.5′sv, neither of which responded to cell swelling (n = 9; Fig. 4, Bi and Ci). Exposure to a hyperosmotic gradient (+100 mosm) led to cell shrinkage-induced current reduction in TRPV4 + AQP4–expressing oocytes (n = 9; Fig. 4Aii) but had no effect on TRPV1 + AQP4–expressing oocytes (which, nonetheless, responded robustly to the TRPV1 agonist capsaicin (10 μm) (n = 9; Fig. 4Bii)). Oocytes expressing the truncated version of TRPV1, VR.5′sv + AQP4, in contrast, displayed robust current activation upon cell shrinkage (n = 9; Fig. 4Cii) but were insensitive to capsaicin (Fig. 4Cii). AQP4-expressing oocytes (Fig. 4 (Ai and Aii)) and oocytes expressing the TRPV constructs in the absence of AQP4 failed to display increased current activity upon cellular volume changes. These related, but differentially gated, ion channels (TRPV1 with its insensitivity to volume changes, VR.5′sv with its shrinkage sensitivity, and TRPV4 with its swelling sensitivity) thus serve as tools to resolve the structural determinant of TRPV4 volume sensitivity.
Figure 4.
Related TRPVs are differentially gated. A–C, schematics of the phylogenetically related TRPV4 (top), TRPV1 (middle), and the V1 splice variant, VR.5′sv (bottom). Shown are summarized I/V curves from TRPV4-expressing (top), TRPV1-expressing (middle), or VR.5′sv-expressing oocytes (bottom) with control solution (black) and hyposmotic solution (red) (Ai–Ci) or with hyperosmotic solution (orange) or capsaicin (gray) (Aii–Cii). Insets, summarized I/V curves from TRPV4-expressing and AQP4-expressing (top), TRPV1-expressing (middle), and VR.5′sv-expressing (bottom) oocytes. Current activity at −85 mV was obtained after exposure to −100 mosm, +100 mosm, and capsaicin was normalized to that obtained in control conditions. N.S., not significant (p > 0.05); *, p < 0.05; **, p < 0.01; ***, p < 0.001; Student's paired t test (Ai–Ci, Aii), one-way ANOVA (Bii–Cii), n = 9 oocytes. Error bars, S.D. (bar graphs) or S.E. (I/V curves).
To identify the N-terminal determination of the volume response, we constructed a set of chimeric TRPV4 channels composed of the N terminus from the phylogenetically related TRPV1 (insensitive to volume changes) and its splice variant, VR.5′sv (shrinkage-activated). Fig. 5 A and B illustrates the schematics and immunofluorescent micrographs demonstrating plasma membrane expression of both chimeras upon microinjection of cRNA encoding TRPV4:TRPV1 and TRPV4:VR.5′sv. Although the basal control current was intact (and employed for normalizing the response to volume changes and agonists within each oocyte), oocytes expressing the TRPV4:TRPV1 chimera failed to respond to both cell swelling and cell shrinkage as well as to GSK101 (TRPV4 agonist) and capsaicin (TRPV1 agonist) (n = 12; Fig. 5C, uninjected oocytes in inset). The chimeric TRPV4:VR.5′ channel, with replacement of the TRPV4 N terminus with the heavily truncated N terminus of VR.5′sv, exhibited significantly elevated current activity in response to cell shrinkage (n = 12; Fig. 5D), whereas swelling-induced activation and GSK101-/capsaicin-elicited activation was absent (Fig. 5D). These results illustrate that the N termini of the related TRPV1 and VR.5′sv can partially substitute for the TRPV4 N terminus and that this domain dictates the volume sensitivity of the channel.
Figure 5.
The N terminus of TRPV4 is essential for the directionality of the volume sensing. A and B, schematics of the constructed chimeras (top) with the N terminus from TRPV1 (left) and the TRPV1 splice variant VR.5′sv (right) and micrographs of the chimeras immunolabeled with phalloidin and anti-AQP4 and anti-TRPV4 antibodies, confirming plasma membrane expression. C and D, summarized I/V curves from TRPV4:TRPV1-expressing (C) or TRPV4:VR.5′sv-expressing (D) oocytes with control solution (black), hyposmotic solution (red), hyperosmotic solution (orange), GSK101 (TRPV4 agonist; green), and capsaicin (TRPV1 agonist; gray). Activity from uninjected oocytes is shown in Ci. Insets, current activity at −85 mV obtained after exposure to −100 mosm, +100 mosm, GSK101, or capsaicin was normalized to that obtained in control conditions. N.S., not significant (p > 0.05); ***, p < 0.001; one-way ANOVA, n = 12 oocytes. Error bars, S.D. (bar graphs) or S.E. (I/V curves).
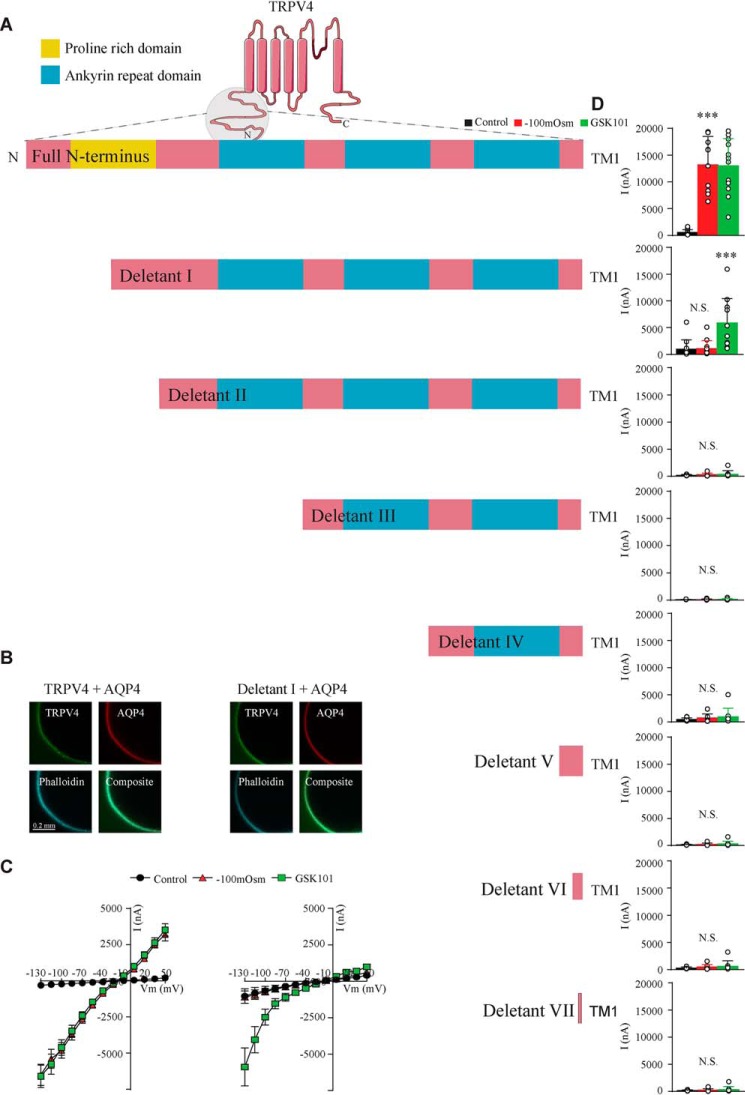
The distant part of the N terminus of TRPV4 is required for volume sensing
To determine which part of the TRPV4 N terminus dictates the volume sensitivity, we compared swelling-induced activation of TRPV4 mutants with modified N termini (schematic in Fig. 6A) with that of the WT TRPV4 (n = 9; Fig. 6A (Full N terminus), B and C (left panels), and D (top)). Oocytes expressing a mutant TRPV4 with a deletion of the most distal part of its N terminus (leaving part of the proline-rich domain intact, Deletant I) failed to respond with increased membrane currents upon cell swelling, while retaining its activation by the TRPV4 agonist GSK101 (Fig. 6A (schematic), B and C (right panels), and D (second panel from the top)). Channels with shorter versions of the N terminus all presented as insensitive to volume changes as well as GSK101 (Fig. 6D) (despite proper membrane targeting; data not shown). The most distant part of the N terminus, including part of the proline-rich domain, is thus required for volume sensing of TRPV4.
Figure 6.
The proline rich distant part of the N terminus of TRPV4 is required for swelling-induced activation. A, schematics of the full-length TRPV4 N terminus and deletants. B, micrographs of TRPV4 and the functioning deletant (Deletant I) immunolabeled with phalloidin and anti-AQP4 and anti-TRPV4 antibodies, confirming plasma membrane expression. C, summarized I/V curves from TRPV4 (left) and Deletant I (right) with control solution (black), hyposmotic solution (red), and GSK101 (green). D, current activity at −130 mV obtained in control solution, after exposure to −100 mosm or GSK101 in the full-length form or deletants shown as bars. N.S., not significant (p > 0.05); ***, p < 0.001; one-way ANOVA, n = 9 oocytes. Error bars, S.D. (bar graphs) or S.E. (I/V curves).
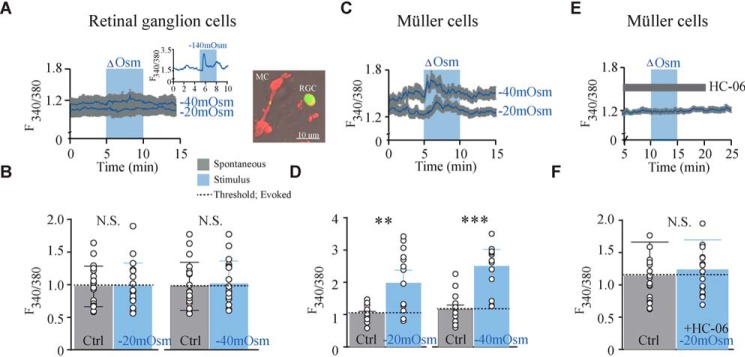
TRPV4 is activated by small, physiologically relevant volume changes in acutely dissociated Müller glia but not in retinal ganglion cells
Studies employing heterologously expressed TRPV4 (Fig. 1) demonstrate that PLA2 is not necessary for TRPV4 activation under the conditions of cell swelling, but it remains unclear (i) whether endogenously expressed TRPV4 ion channels can be activated by physiological relevant osmotic gradients and (ii) whether this process involves PLA2 activation. We took advantage of the retinal preparation that allows simultaneous visualizations of TRPV4-mediated responses in neurons and glial cells (1, 7). Dissociated cells were loaded with the Ca2+ sensor Fura-2AM and exposed to small osmotic gradients of −20 and −40 mosm as [Ca2+]i was monitored in real time. The osmotic gradients were obtained with constant electrolyte concentration by removal of mannitol from the test solution (see “Experimental procedures”) to avoid concomitant changes in ionic driving forces and membrane potentials. Introduction of such osmotic challenges did not translate to altered Ca2+ dynamics in retinal ganglion cells (n = 19 cells; Fig. 7 A and B), although a large osmotic gradient of −140 mosm did provoke a response (Fig. 7A, inset), as observed previously (1, 7). In contrast, exposure to small osmotic gradients consistently elevated cytosolic Ca2+, [Ca2+]i, in Müller glia (∼36% for −20 mosm; ∼48% for −40 mosm, n = 9–13 cells; Fig. 7, C and D). These osmotically induced [Ca2+]i transients in Müller cells were mediated by TRPV4, as indicated by their abrogation in the presence of the TRPV4 inhibitor HC-067047 (HC-06; 1 μm) (n = 19 cells; Fig. 7, E and F). These data demonstrate that TRPV4-dependent transduction of physiologically relevant hyposmotic challenges (and thus volume changes) to Ca2+ signaling is cell type–specific, with glial TRPV4 showing markedly augmented sensitivity to osmotic stressors.
Figure 7.
TRPV4 is activated by small osmotic gradients in Müller cells. A, representative raw trace of responses recorded in Fura-2AM–loaded retinal ganglion cells. Application of osmotic gradients is indicated by the blue bar. Physiologically relevant osmotic gradients (−20 and −40 mosm) and, thus, small volume changes, do not elevate retinal ganglion cell calcium levels above spontaneous calcium spikes. Calcium levels were evoked upon application of a large gradient (−140 mosm) serving as a positive control for responsiveness. Inset, micrograph showing dissociated Müller and retinal ganglion cells. B, summary for results shown in A. Shown are mean values of spontaneous manually detected spikes (peak ± 10 frames pre- and post-peak) before stimulation (Ctrl) and the change in [Ca2+]i measured as spikes detected above a threshold (horizontal line) sat as spontaneous levels during stimulation (−20 or −40 mosm), n = 19 cells. C, representative raw traces of responses recorded in Fura-2AM–loaded Müller cells presented as in A. A summary of results is shown in D, analyzed and processed as in B, n = 10–13 cells. E, representative raw trace of response recorded in Fura-2AM–loaded Müller cells displaying reduced responsiveness in the presence of HC-06 with summary of results in F, n = 19 cells. N.S., not significant (p > 0.05); **, p < 0.01; ***, p < 0.001; two-sample t test. Error bars, S.D.
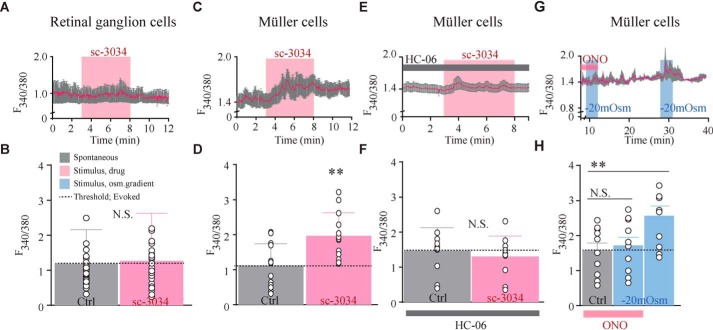
Phospholipase A2 activation is required for swelling-induced TRPV4 activation in Müller cells
We next tested whether the differential volume transduction in neurons and glia correlates with PLA2 activity. Suppression of PLA2 activity has been shown to inhibit TRPV4 (1), but it is not known whether PLA2 activity is sufficient to activate the channel. PLA2 activation was prompted by the PLA2 agonist sc-3034 (3 μm), which had no effect on retinal ganglion cell [Ca2+]i dynamics (n = 29 cells; Fig. 8 A and B) but elicited robust [Ca2+]i activity in Müller cells (∼55%, n = 13 cells; Fig. 8, C and D). sc-3034–induced calcium signals in the glia were abolished by HC-06 (n = 10 cells; Fig. 8, E and F), indicating that PLA2 signaling suffices to activate TRPV4 in Müller cells in the absence of mechanical stressors.
Figure 8.
PLA2 activation is required for swelling-induced TRPV4 activation in Müller cells. A, representative raw trace of response recorded in Fura-2AM–loaded retinal ganglion cells. Activation of PLA2 (sc-3034) is indicated by the pink bar. B, summary for results shown in A. Shown are mean) values before PLA2 activation (Ctrl) and the change in [Ca2+]i during stimulation (sc-3034), n = 29 cells. C, representative raw trace of response recorded in Müller cells with summary of results shown in D, n = 13 cells. E, representative raw trace of responses recorded in Müller cells verifying their TRPV4 origin. The evoked PLA2 activation-induced responses are abolished in the presence of HC-06. F, summary of results in E, n = 10 cells. G, representative raw trace of response recorded in Müller cells after pretreatment with a PLA2 inhibitor (ONO-RS-089, indicated by the pink bar) prior to application of an osmotic gradient (−20 mosm, indicated by the blue bar) and after ONO-RS-089 washout. H, summary of results from G presenting the increase in swelling-induced calcium levels after washout of PLA2 blocker, n = 14 cells. N.S., not significant (p > 0.05); **, p < 0.01; ***, p < 0.001; two-sample t test. Error bars, S.D.
To resolve whether PLA2 activation was required for swelling-induced TRPV4 activation in Müller glia brought about via small gradients (−20 mosm), the cells were pretreated with the PLA2 inhibitor ONO-RS-82 (1 μm) prior to introduction of the osmotic challenges. Notably, wash-in of the PLA2 inhibitor shifted the baseline F340/380 fluorescence ratio to another stable level upon which the osmotic challenge was introduced. The PLA2 inhibitor blocked the swelling-induced [Ca2+]i dynamics in the Müller cells (n = 14 cells; Fig. 8, G and H), demonstrating that volume-dependent activation of TRPV4 requires PLA2 activity. Upon wash-out of the inhibitor, the volume sensitivity was restored to the Müller cells (Fig. 8, G and H). These results show that the molecular properties of TRPV4 volume sensing are cell type–specific, with intermediary PLA2 activation obligatory for TRPV4 activation in Müller cells, whereas heterologously expressed TRPV4 in oocytes and retinal ganglion cells responds to swelling through direct activation of the channel. Thus, sensing of the physico-chemical milieu may require not only the requisite transducer but also the appropriate intracellular context associated with each cell type.
Discussion
We here demonstrate that sensing of volume changes by TRPV4 occurs by distinct pathways in a cell type–specific manner, with or without the requirement of an intermediate PLA2 activation. Our observation that TRPV4 responds to small physiologically relevant levels of cell swelling in Müller cells but not in retinal ganglion cells suggests that the TRPV4-mediated volume response may in fact be physiologically relevant and not exclusively a response to pathological levels of cell swelling. It is generally accepted that TRPV4 is activated by cell swelling, yet whether PLA2 activation is required to mediate the coupling between cell swelling and channel activation has remained a standing question. In tumor cells, pancreatic islets, endothelial cells, epithelial cells, fibroblasts, and trabecular meshwork cells (41–44), swelling has been shown to activate PLA2, but many of these studies have relied on large, unphysiological osmotic gradients (100–200 mosm) that could have introduced additional confounding elements (1, 14, 27, 28). Swelling-induced activation of glial TRPV4 required activity of endogenously expressed PLA2, presumably involving the intermediary PLA2-CYP450-EET axis, in agreement with previous observations based on exposure to large hyposmotic gradients (1). Interestingly, the ganglion cells were insensitive to exposure to small osmotic gradients but demonstrated TRPV4 activation upon exposure to large osmotic gradients, mimicking pathophysiological conditions. It is unclear why TRPV4 is responsive to exposure of a hyposmolar gradient when expressed in Müller cells and not in retinal ganglion cells, given the similarity between the extent of swelling in response to a large osmotic challenge in the cell types (1). If this response is extrapolated to exposure to smaller osmotic gradients, as employed in the present study, it may suggest that PLA2 could function to amplify the response to small levels of swelling. However, such a small osmotic gradient may translate to a slower rate of cell swelling in the ganglion cells compared with the Müller cells, which, in itself, could impose a reduction in the osmotic activation of TRPV4 (14).
PLA2 activation leads to formation of arachidonic acid metabolites, such as EETs. A recent discovery of a conserved EET-binding pocket in TRPV4, involving residues from S4, the S2-S3 linker, and the S4-S5 linker domains (47), supports a direct link between PLA2 activation and TRPV4 gating. To resolve whether PLA2 and its metabolites are required for swelling-induced TRPV4 activation in an isolated setting, we co-expressed TRPV4 in Xenopus oocytes with AQP4 to promote osmotically induced cell swelling (14). We have previously excluded a significant contribution from endogenous Cl− channels (48, 49) and confirmed that currents recorded from TRPV4-expressing oocytes are of TRPV4 origin (by their cationic nature and ruthenium red sensitivity) (14). Activation of PLA2 had no effect on TRPV4-mediated membrane currents, indicating that this canonical signaling pathway did not directly activate TRPV4 in this cell type, as we also observed in retinal ganglion cells. PLA2 activity has previously been demonstrated in Xenopus oocytes (33–36). To rule out the possibility that the lack of effect of PLA2 activation resided in low or absent PLA2 activity in this experimental cell system, we, in addition, exposed TRPV4-expressing oocytes to the downstream products of PLA2, thereby circumventing PLA2. These PLA2 products did not affect the TRPV4-mediated current, whether applied via the surrounding test solution or upon microinjection into the oocytes during the experiment. The volume-dependent activation of TRPV4 in Xenopus oocytes robustly occurred irrespective of PLA2 activity (as tested with two different PLA2 antagonists). This ability to sense volume changes in the absence of PLA2 activity confirms previous studies on both oocytes and yeast (13, 18), as well as on thoracic sensory neurons (31). Accordingly, whereas cell swelling may cause PLA2 activation with subsequent accumulation of arachidonic acid and other metabolites (41, 42, 50), this enzymatic action seems to represent a required intermediary step for swelling-induced TRPV4 gating only in select cell types. Although we currently cannot explain the cell type–specific requirement for PLA2, we hypothesize that the long N terminus of TRPV4 may interact with different cytoskeletal components in the different cell types, which may cause a conformational shift of the channel, mimicking the PLA2-bound protein structure. Alternatively, the retinal ganglion cells may not express the requisite cPLA2 and/or CYP450 enzymes that epoxygenate the arachidonic acid precursor (51) and/or may express a cell type–specific distinct splice variant, as is the case for the volume sensitivity of TRPV1 (40) and piezo2 channels (52). It remains to be seen whether the EET-binding pocket, which mediates TRPV4 activation by EETs (47), is occluded in PLA2-nonresponding oocytes, yeast, and neurons. The complexity of channel gating can, for example, be uncovered by exposing cells to different TRPV4-activating stimuli, which might expose different binding domains. For example, Vriens et al. (9) showed that temperature- and agonist-induced channel gating in HEK293 cells are independent of PLA2 despite the obligatory requirement for swelling-induced activation, potentially due to a different state of Tyr-555 in transmembrane segment 3. Future experiments will determine how mechanical properties and lipid structure (i.e. relative fraction of ω3 versus ω6 polyunsaturated fatty acids) of the membrane influence TRPV4 gating in neurons and glia.
From a functional perspective, augmented volume sensitivity of glia versus neurons appears to be congruent with the central role glia plays in the regulation of a microenvironment in the retina and the brain (45), whereby glia-mediated membrane transport across the parenchyma, subserved by TRPV4, protects the overall homeostasis (15, 16). This bears relevance to neurodegenerative diseases such as glaucoma, in which Müller glia shows substantial early reactive activation that precedes retinal ganglion cell loss (46). It is possible that the PLA2 requirement in Müller cells provides a “safety” toward aberrant volume activation of TRPV4 with each neuronal activity–evoked glia cell swelling presumed to take place in the Müller cells analogously to that of hippocampal astrocytes (53). In support of this hypothesis, we previously demonstrated that TRPV4 activity did not influence the glia cell volume dynamics occurring in the wake of neuronal activity, despite the observed cell swelling (29). In such a scenario, only larger cell swelling may lead to PLA2 activity in the glia cells and thus, in such a setting, prevent TRPV4 activity and associated Ca2+ influx (45).
To reveal a molecular coupling between cell swelling and TRPV4 activation, we tested whether other intermediary regulators could supplant the role of PLA2 in volume-induced TRPV4 gating. A force-sensitive complex tethered to the cytoskeleton (microtubules and F-actin) (54–56) has been proposed to couple membrane stretch to TRPV4 activation (57–59). The kinetics of volume-induced currents were not altered by inhibitors of cytoskeletal rearrangement, leading us to conclude that TRPV4 volume transduction occurs independently of dynamic rearrangement of cytoskeletal components. However, the volume-induced activation may still occur via N-terminal anchoring to cytoskeletal components. The N terminus of TRPV4 displays a site of phosphorylation via an anchored regulatory kinase complex (56). To determine whether swelling-induced activation of TRPV4 required protein phosphorylation, we monitored TRPV4-mediated membrane currents after introduction of membrane-permeable inhibitors and activators of protein kinases A, C, G, all of which have been demonstrated to be present and sensitive to such pharmacological manipulation in the Xenopus oocytes (37, 38). Whereas TRPV4 may certainly be subject to phosphorylation and thus regulated in this fashion in different cell types and experimental settings, our data suggest that activation of protein kinase A, C, or G was not a required molecular link between cell swelling and TRPV4 activation. Therefore, TRPV4 can be activated by volume changes independently of PKA, PKC, or PKG activity. This finding contrasts with previous reports on swelling-induced TRPV4 activation in HEK293 cells, which was enhanced upon PKC (60) and Src family kinase activation (61). However, the amino acid residues involved in these responses appeared to be required for sensitization rather than activation of the channel and thus represent a possible modulatory mechanism (61).
To obtain a molecular handle on the putative TRPV4 volume-sensing domain, we included the phylogenetic relative, TRPV1, and its splice variant VR.5′sv (21). Whereas TRPV1 was unresponsive to hyperosmotically induced volume changes, as earlier reported in situ (22), the splice variant VR.5′sv was activated by cell shrinkage and insensitive to capsaicin, the latter finding supported by earlier reports (21, 23, 40). It has been suggested that truncation of the N terminus, as seen with this splice variant, may affect the trafficking to the cell membrane (62). However, correct membrane targeting in Xenopus oocytes (present study) (63) was evident. This volume-sensing profile is opposite to that of TRPV4, which we here demonstrate was inhibited by cell shrinkage (in addition to its activation by cell swelling). These three distinct volume-sensitivity profiles within one family of proteins provided a unique platform for identification of the volume-sensing domain of TRPV4. TRPV4 possesses an extensive cytoplasmic N terminus containing ankyrin repeats (25, 26) that are recognized as binding hubs for proteins, small ligands (64), or cytoskeletal components (54–56) and could well contain the volume sensor. We confirmed this hypothesis using a chimeric approach, which demonstrated that the volume response of TRPV4 is dictated by the origin of the N terminus; the channel became insensitive to volume changes with an N-terminal replacement with that of the volume-insensitive TRPV1 and a sensor of cell shrinkage with an N-terminal replacement with that of the shrinkage-activated splice variant of TRPV1 (VR.5′sv). The N terminus thus dictates the TRPV4 channel volume sensitivity. Future studies aimed at delineating whether the N terminus is the volume controller among all volume-sensitive TRPV channels would be instrumental for complete understanding of the volume sensitivity of this class of ion channels. Stepwise deletions of the N terminus illustrated that the most distal part of the tail is required for the channel to respond to cellular swelling, as the deletion of the initial segment and part of the proline-rich domain rendered the channel insensitive to volume changes but maintained responsiveness to the agonist GSK101 (although with an altered current/voltage relationship). Deletion of larger sections of the N terminus rendered the channel inactive (unresponsive to volume changes and GSK101), although still correctly targeted to the plasma membrane. Our results suggest that the proline-rich N-terminal domain is necessary for TRPV4-dependent sensing of cellular volume changes. This notion aligns nicely with an earlier report of this very domain interacting with the cytoskeletal protein PACSIN3, which is implemented in the volume-sensing ability of TRPV4 (65). The N terminus of the vanilloid TRP channels thus appears to shape the unique and distinct activation profile that represents the foundation of TRPV4 polymodality, which in turn may bear importance in pathological conditions associated with abnormal nociception, heat transduction, and/or transduction of ambient mechanical stressors (e.g. as observed in gain- or loss-of-function mutations of TRPV4) (57, 66–69).
In conclusion, mechanical gating of TRPV4, which may be involved in its pathological activation in conditions involving cell swelling, appears to occur via cell type–specific pathways, one of which relies on the enzymatic activity of PLA2, as in retinal Müller cells. TRPV4 is, however, a volume sensor in its own right, inasmuch as it can be directly activated by membrane stretch upon cell swelling. This direct swelling-induced activation of TRPV4 occurs in a manner independent of PLA2 activity (and its downstream metabolites), cytoskeletal rearrangements, and kinase activity (PKA, PKC, PKG). The volume sensitivity of TRPV4 is determined by the N terminus, in which the sensor of volume changes appears to reside, at least in part, in the most distal part. These results support the view that sensory transduction mediated by vanilloid TRP channels is a cell type–specific process, with enzymatic activity providing crucial determinants of channel polymodality and dynamic range of activation.
Experimental procedures
Ethical approval
The experiments adhered to the guidelines of the Danish Veterinary and Food Administration (Ministry of Environment and Food), and the experiments conform to the principles and regulations described previously (70) and to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Experiments conducted in X. laevis oocytes were approved by the animal facility at the Faculty of Health and Medical Sciences, University of Copenhagen, whereas experiments conducted in mice (retinas) were approved by the Institutional Animal Care and Use Committee at the University of Utah. The Danish National Committee approved the surgical protocol, by which the oocytes were retrieved, for Animal Studies, Danish Veterinary and Food Administration (Ministry of Environment and Food). Mice were maintained in a 12-h light/dark cycle with free access to food and water.
RNA preparation and heterologous expression in X. laevis oocytes
Rat TRPV1, TRPV4, TRPV4 deletions/mutants, TRPV4 chimeras, VR.5′sv (TRPV1 splice variant), and AQP4 were subcloned into the oocyte expression vector pXOOM. TRPV4 chimeras, VR.5′sv, and TRPV4 deletion mutants were either purchased from GenScript Biotech Corp. (Piscataway, NJ) or generated by use of the QuikChange mutagenesis kit (Stratagene) and sequenced prior to use. All constructs were linearized downstream from the poly(A) segment and in vitro transcribed using T7 mMessage machine according to the manufacturer's instructions (Ambion, Austin, TX). cRNA was extracted with MEGAclear (Ambion, Austin, TX) and microinjected into defolliculated X. laevis oocytes: 4 ng of TRPV RNA/oocyte only or in combination with 10 ng of AQP4 RNA. X. laevis frogs were obtained from Nasco (Fort Atkinson, WI). Oocytes were collected under anesthesia (2 g/liter tricain, 3-aminobenzoic acid ethyl ester; Sigma, A-5040). The preparation of defolliculated oocytes was carried out as described (38), and the oocytes were kept in Kulori medium: 90 mm NaCl, 1 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 5 mm HEPES (pH 7.4) with ruthenium red (100 μm; Sigma Aldrich, R-2751) to suppress TRPV activity (71) for 3–4 days at 19 °C prior to experiments.
Electrophysiology and volume measurements of oocytes
Conventional two-electrode voltage clamp studies were performed with a DAGAN CA-1B high-performance oocyte clamp (DAGAN, Minneapolis, MN) with a Digidata 1440A interface controlled by pCLAMP software, version 10.5 (Molecular Devices, Burlingame, CA). Electrodes were pulled (HEKA, PIP5) from borosilicate glass capillaries to a resistance of 2–4 megaohms when filled with 1 m KCl. The current traces were obtained by stepping the clamp potential from −20 mV to test potentials ranging from +50 to −130 mV (200-ms pulses) in increments of 15 mV. Recordings were low pass–filtered at 500 Hz and sampled at 1 kHz. Oocytes were placed in an experimental chamber and perfused, and volume measurements were performed as described previously (32). In experiments where hyposmotic solutions were used to induce cell swelling, the perfusion solution consisted of 50 mm NaCl, 2 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 10 mm HEPES, 100 mm mannitol (Tris-buffered, pH 7.4, 220 mosm). The hyposmotic solution was made by removal of 100 mm mannitol with resulting osmolarity of 120 mosm, and the hyperosmolar solution was made by the addition of 100 mm mannitol (320 mosm). GSK1016790A and capsaicin (Sigma-Aldrich; 100 nm) were used to elicit TRPV4- and TRPV1-mediated current activity, respectively. Experiments were performed at room temperature, 23 °C. Drugs were locally applied or applied intracellularly by microinjection while recording current activity.
Immunocytochemistry of oocytes
Uninjected oocytes and oocytes expressing TRPV1 or TRPV4 WT, chimeras, or deletion mutants were fixed for 1 h at room temperature in 2% paraformaldehyde, washed, and left in PBS overnight, incubated with primary antibodies (anti-AQP4 (Alomone Laboratories, AQP-004) and anti-TRPV4 (Abcam, ab63079)) and conjugated secondary antibodies (Alexa Fluor® 488 goat anti-sheep, A-11015 (Invitrogen); Alexa Fluor® 546 goat anti-rabbit, A-11010 (Invitrogen); Alexa Fluor® 647 phalloidin (F-actin), A-22287 (Invitrogen)). Oocytes kept in 12-well plates were mounted with ProlongGold, and the wells were sealed with a coverglass and kept from light exposure. Phalloidin was used as a morphological marker. Micrographs were recorded at room temperature using a Zeiss LSM710 point laser (Argon Lasos RMC781272)-scanning confocal microscope with a Zeiss EC Plan-Neofluar ×40/numerical aperture (NA) 1.3 oil objective (Carl Zeiss) and sampled with scan mode as frame with one line step, a frame size of 512 × 512, and a bit depth of 16. No quantification was carried out from the micrographs, as they only served to verify the expression and correct localization of proteins of interest.
Acutely dissociated retina preparation and superfusion of retinal cells
Mice were killed by isoflurane inhalation followed by cervical dislocation, after which the eyes were enucleated and retinas were isolated from the removed eyes by dissection. The retinas were kept in cold Leibovitz 15 (L15) medium (Invitrogen) containing 20 mm d-glucose, 10 mm Na-HEPES, 11 mg/ml L15 powder, 2 mm sodium pyruvate, 0.3 mm sodium ascorbate, and 1 mm GSH. Digestion of the extracellular matrix was carried out with L15 medium containing papain (7 units/ml; Worthington) for 1 h at room temperature. Subsequently, the retinas were rinsed, placed on ice, and cut into pieces. Pieces were mechanically dissociated, and the cells were plated on concanavalin A (1 mg/ml)-coated coverslips. Dissociated cells were loaded with Fura-2 AM (5–10 μm; Invitrogen) for 30–40 min and washed for 10–20 min. Retinal ganglion cells were identified as described previously (7), and Müller glia were identified by their distinctive morphology (72, 73). Retinal cell morphology, stimulus responsiveness, and Ca2+ homeostasis were maintained for several hours under these experimental conditions (1, 7, 74). The perfusion solution used was a Ringer's solution containing 62.5 mm NaCl, 2.5 mm KCl, 1.5 mm NaH2PO4, 1.5 mm MgCl2 (6H2O), 2 mm CaCl2, 10 mm glucose, 10 mm HEPES hemisodium salt, 1 mm pyruvic acid, 1 mm lactic acid, 0.5 mm l-glutamine, 0.5 mm GSH, and 0.3 mm sodium ascorbate (pH 7.4; 300 mosm (adjusted with mannitol)). The hyposmotic solution was made by the removal of mannitol with resulting osmolarity of 280 and 260 mosm.
Optical imaging
Fluorescence imaging of Fura-2AM–loaded superfused plated retinal cells was performed on an inverted Nikon Ti or an upright Nikon E600 FN microscope using ×20 (0.75 NA oil), ×40 (1.3 NA oil and 0.8 NA water), and ×60 (1.0 NA water) objectives. A Lambda DG-4 illumination system (Sutter Instruments) provided the excitation, and micrographs were captured with a 14-bit CoolSNAP HQ2 camera. Fluorescence detection and processing were done blinded using Velocity version 6.3.0 (PerkinElmer) and Excel. Spontaneous activity was quantified by averaging manually detected spikes (peak + 10 frames pre- and post-peak) before stimulation. A threshold that exceeds that mean spontaneous spike value was set to detect evoked responses during stimulation. Results are shown as bars representing averaged 340/380 nm ratios in individual conditions from retinal ganglion cells or Müller cells in a minimum of three animals (N) with cells (n) from each individual animals acutely dissociated onto a minimum of 4 slides.
Chemicals
ONO-RS-082 (PLA2 inhibitor), GSK1016790A, arachidonic acid (all purchased from Sigma), latrunculin A (F-actin polymerization inhibitor agent; ENZO Life Sciences), taxol (tubulin-stabilizing agent), 5′,6′-EET (Cayman Chemicals), and sc-3034 (PLA2 activator; Santa Cruz) were dissolved in DMSO. Oleic acid and anandamide (N-arachidonoylethanolamine) (both purchased from Sigma) were dissolved in ethanol. HC-06 and ruthenium red (Sigma) were dissolved in double-distilled H2O. PMA (PKC activator), chelerythrine (PKC inhibitor), 8-Br-cAMP (PKA activator), H89 (PKA inhibitor), 8-pCPT-cGMP (PKG activator), and K252 (PKG inhibitor) were all purchased from Sigma and dissolved in water, DMSO, or water containing 100 mm Tris base. Vehicles were always included to match solvent concentrations. All drugs were kept at −20 °C until use except from arachidonic acid and ONO-RS-082, which were kept at −80 °C. Working solutions of free fatty acids were used within 4 h after diluting into aqueous solutions.
Data presentation and statistics
GraphPad Prism 7.0 was used for analysis. Data are presented as mean ± S.D. (bar graphs) or mean ± S.E. (I/V curves). Statistical significance was tested with two-sample or paired t test or one-way analysis of variance (ANOVA) with Dunnet's post hoc test as indicated. p values <0.05 were considered statistically significant. The number of experiments performed in oocytes (n) corresponds to independent measurements from at least three different preparations. Data from female and male mice were pooled and number of cells (n) corresponds to measurements of cells from a minimum of 4 slides per animal. A minimum of 3 animals were used. No sex differences were noted.
Author contributions
T. L. T.-B. and N. M. conceptualization; T. L. T.-B., O. Y., S. R., and T. T. P. data curation; T. L. T.-B., O. Y., S. R., and T. T. P. formal analysis; T. L. T.-B. funding acquisition; T. L. T.-B., O. Y., S. R., and T. T. P. investigation; T. L. T.-B., O. Y., S. R., and T. T. P. methodology; T. L. T.-B. writing-original draft; T. L. T.-B. project administration; T. L. T.-B., O. Y., S. R., T. T. P., D. K., and N. M. writing-review and editing; D. K. and N. M. resources; D. K. and N. M. software; D. K. and N. M. supervision.
Acknowledgments
We thank microscopy manager Yasuko Antoku, as well as the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen.
This work was supported by VELUX Foundation Grant 107493 (to T. L. T.-B.); National Institutes of Health Grants R01EY022076, R01EY027920, and P30EY014800; a USTAR Technology Acceleration award; the Willard Eccles Foundation (to D. K.); and unrestricted support from Research to Prevent Blindness (to the Moran Eye Institute at the University of Utah). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TRPV4
- transient receptor potential vanilloid 4
- VR.5′sv
- TRPV1 splice variant
- AQP4
- aquaporin 4
- GSK101
- GSK1016790A
- HC-06
- HC-067047
- PUFA
- polyunsaturated fatty acid
- PLA2
- phospholipase A2
- AA
- arachidonic acid
- 5′6′-EET
- 5,6-epoxy-8Z,11Z,14Z-eicosatrienoic acid
- PKA
- protein kinase A
- PKC
- protein kinase C
- PKG
- protein kinase G
- PMA
- phorbol 12-myristate 13-acetate
- 8-Br-cAMP
- 8-bromoadenosine 3′,5′-cyclic monophosphate
- 8-pCPT-cGMP
- 8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate
- Fura-2AM
- Fura-2-acetoxymethyl ester
- L15
- Leibovitz 15
- NA
- numerical aperture
- ANOVA
- analysis of variance
- CYP450
- cytochrome P450.
References
- 1. Ryskamp D. A., Jo A. O., Frye A. M., Vazquez-Chona F., MacAulay N., Thoreson W. B., and Križaj D. (2014) Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J. Neurosci. 34, 15689–15700 10.1523/JNEUROSCI.2540-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iuso A., and Križaj D. (2016) TRPV4-AQP4 interactions “turbocharge” astroglial sensitivity to small osmotic gradients. Channels 10, 172–174 10.1080/19336950.2016.1140956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Molnár T., Yarishkin O., Iuso A., Barabas P., Jones B., Marc R. E., Phuong T. T., and Križaj D. (2016) Store-operated calcium entry in Müller glia is controlled by synergistic activation of TRPC and Orai channels. J. Neurosci. 36, 3184–3198 10.1523/JNEUROSCI.4069-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsumoto H., Sugio S., Seghers F., Krizaj D., Akiyama H., Ishizaki Y., Gailly P., and Shibasaki K. (2018) Retinal detachment-induced Müller glial cell swelling activates TRPV4 ion channels and triggers photoreceptor death at body temperature. J. Neurosci 38, 8745–8758 10.1523/JNEUROSCI.0897-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arredondo Zamarripa D., Noguez Imm R., Bautista Cortes A. M., Vázquez Ruíz O., Bernardini M., Fiorio Pla A., Gkika D., Prevarskaya N., López-Casillas F., Liedtke W., Clapp C., and Thébault S. (2017) Dual contribution of TRPV4 antagonism in the regulatory effect of vasoinhibins on blood-retinal barrier permeability: diabetic milieu makes a difference. Sci. Rep. 7, 13094 10.1038/s41598-017-13621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phuong T. T. T., Redmon S. N., Yarishkin O., Winter J. M., Li D. Y., and Križaj D. (2017) Calcium influx through TRPV4 channels modulates the adherens contacts between retinal microvascular endothelial cells. J. Physiol. 595, 6869–6885 10.1113/JP275052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ryskamp D. A., Witkovsky P., Barabas P., Huang W., Koehler C., Akimov N. P., Lee S. H., Chauhan S., Xing W., Rentería R. C., Liedtke W., and Krizaj D. (2011) The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J. Neurosci. 31, 7089–7101 10.1523/JNEUROSCI.0359-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., and Nilius B. (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 10.1038/nature01807 [DOI] [PubMed] [Google Scholar]
- 9. Vriens J., Watanabe H., Janssens A., Droogmans G., Voets T., and Nilius B. (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. U.S.A. 101, 396–401 10.1073/pnas.0303329101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liedtke W., Choe Y., Martí-Renom M. A., Bell A. M., Denis C. S., Sali A., Hudspeth A. J., Friedman J. M., and Heller S. (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535 10.1016/S0092-8674(00)00143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandes J., Lorenzo I. M., Andrade Y. N., Garcia-Elias A., Serra S. A., Fernández-Fernández J. M., and Valverde M. A. (2008) IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J. Gen. Physiol. 131, i2 10.1085/JGP1315OIA2 [DOI] [PubMed] [Google Scholar]
- 12. White J. P., Cibelli M., Urban L., Nilius B., McGeown J. G., and Nagy I. (2016) TRPV4: molecular conductor of a diverse orchestra. Physiol. Rev. 96, 911–973 10.1152/physrev.00016.2015 [DOI] [PubMed] [Google Scholar]
- 13. Loukin S. H., Su Z., and Kung C. (2009) Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Lett. 583, 754–758 10.1016/j.febslet.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toft-Bertelsen T. L., Križaj D., and MacAulay N. (2017) When size matters: transient receptor potential vanilloid 4 channel as a volume-sensor rather than an osmo-sensor. J. Physiol. 595, 3287–3302 10.1113/JP274135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jo A. O., Ryskamp D. A., Phuong T. T., Verkman A. S., Yarishkin O., MacAulay N., and Križaj D. (2015) TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal Müller glia. J. Neurosci. 35, 13525–13537 10.1523/JNEUROSCI.1987-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mola M. G., Sparaneo A., Gargano C. D., Spray D. C., Svelto M., Frigeri A., Scemes E., and Nicchia G. P. (2016) The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: a different point of view on the role of aquaporins. Glia 64, 139–154 10.1002/glia.22921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vriens J., Owsianik G., Fisslthaler B., Suzuki M., Janssens A., Voets T., Morisseau C., Hammock B. D., Fleming I., Busse R., and Nilius B. (2005) Modulation of the Ca2-permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ. Res. 97, 908–915 10.1161/01.RES.0000187474.47805.30 [DOI] [PubMed] [Google Scholar]
- 18. Teng J., Loukin S., Zhou X., and Kung C. (2013) Yeast luminometric and Xenopus oocyte electrophysiological examinations of the molecular mechanosensitivity of TRPV4. J. Vis. Exp. 10.3791/50816 10.3791/50816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin M., Berrout J., and O'Neil R. G. (2011) Regulation of TRP channels by osmomechanical stress. in TRP Channels (Zhu M. X., ed) Academic Press, Boca Raton, FL: [PubMed] [Google Scholar]
- 20. Yin J., and Kuebler W. M. (2010) Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem. Biophys. 56, 1–18 10.1007/s12013-009-9067-2 [DOI] [PubMed] [Google Scholar]
- 21. Schumacher M. A., and Eilers H. (2010) TRPV1 splice variants: structure and function. Front. Biosci. (Landmark Ed.) 15, 872–882 10.2741/3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharif Naeini R., Witty M. F., Séguéla P., and Bourque C. W. (2006) An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat. Neurosci. 9, 93–98 10.1038/nn1614 [DOI] [PubMed] [Google Scholar]
- 23. Sudbury J. R., Ciura S., Sharif-Naeini R., and Bourque C. W. (2010) Osmotic and thermal control of magnocellular neurosecretory neurons: role of an N-terminal variant of trpv1. Eur. J. Neurosci. 32, 2022–2030 10.1111/j.1460-9568.2010.07512.x [DOI] [PubMed] [Google Scholar]
- 24. Ciura S., and Bourque C. W. (2006) Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J. Neurosci. 26, 9069–9075 10.1523/JNEUROSCI.0877-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arniges M., Fernández-Fernández J. M., Albrecht N., Schaefer M., and Valverde M. A. (2006) Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J. Biol. Chem. 281, 1580–1586 10.1074/jbc.M511456200 [DOI] [PubMed] [Google Scholar]
- 26. Gaudet R. (2008) A primer on ankyrin repeat function in TRP channels and beyond. Mol. Biosyst. 4, 372–379 10.1039/b801481g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benfenati V., Caprini M., Dovizio M., Mylonakou M. N., Ferroni S., Ottersen O. P., and Amiry-Moghaddam M. (2011) An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl. Acad. Sci. U.S.A. 108, 2563–2568 10.1073/pnas.1012867108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loukin S., Su Z., and Kung C. (2011) Increased basal activity is a key determinant in the severity of human skeletal dysplasia caused by TRPV4 mutations. PLoS One 6, e19533 10.1371/journal.pone.0019533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toft-Bertelsen T. L., Larsen B. R., and MacAulay N. (2018) Sensing and regulation of cell volume—we know so much and yet understand so little: TRPV4 as a sensor of volume changes but possibly without a volume-regulatory role? Channels 12, 100–108 10.1080/19336950.2018.1438009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nilius B., Vriens J., Prenen J., Droogmans G., and Voets T. (2004) TRPV4 calcium entry channel: a paradigm for gating diversity. Am. J. Physiol. Cell Physiol. 286, C195–C205 10.1152/ajpcell.00365.2003 [DOI] [PubMed] [Google Scholar]
- 31. Lechner S. G., Markworth S., Poole K., Smith E. S., Lapatsina L., Frahm S., May M., Pischke S., Suzuki M., Ibañez-Tallon I., Luft F. C., Jordan J., and Lewin G. R. (2011) The molecular and cellular identity of peripheral osmoreceptors. Neuron 69, 332–344 10.1016/j.neuron.2010.12.028 [DOI] [PubMed] [Google Scholar]
- 32. Fenton R. A., Moeller H. B., Zelenina M., Snaebjornsson M. T., Holen T., and MacAulay N. (2010) Differential water permeability and regulation of three aquaporin 4 isoforms. Cell. Mol. Life Sci. 67, 829–840 10.1007/s00018-009-0218-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Almilaji A., Szteyn K., Fein E., Pakladok T., Munoz C., Elvira B., Towhid S. T., Alesutan I., Shumilina E., Bock C. T., Kandolf R., and Lang F. (2013) Down-regulation of Na/K+ ATPase activity by human parvovirus B19 capsid protein VP1. Cell Physiol. Biochem. 31, 638–648 10.1159/000350083 [DOI] [PubMed] [Google Scholar]
- 34. Carnero A., Dolfi F., and Lacal J. C. (1994) ras-p21 activates phospholipase D and A2, but not phospholipase C or PKC, in Xenopus laevis oocytes. J. Cell Biochem. 54, 478–486 10.1002/jcb.240540415 [DOI] [PubMed] [Google Scholar]
- 35. Charpentier G., Béhue N., and Fournier F. (1995) Phospholipase C activates protein kinase C during induction of slow Na current in Xenopus oocytes. Pflugers Arch. 429, 825–831 10.1007/BF00374807 [DOI] [PubMed] [Google Scholar]
- 36. Yamada Y., Stafforini D. M., Imaizumi T., Zimmerman G. A., McIntyre T. M., and Prescott S. M. (1994) Characterization of the platelet-activating factor acetylhydrolase from human plasma by heterologous expression in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. U.S.A. 91, 10320–10324 10.1073/pnas.91.22.10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Assentoft M., Kaptan S., Fenton R. A., Hua S. Z., de Groot B. L., and MacAulay N. (2013) Phosphorylation of rat aquaporin-4 at Ser111 is not required for channel gating. Glia 61, 1101–1112 10.1002/glia.22498 [DOI] [PubMed] [Google Scholar]
- 38. Moeller H. B., Fenton R. A., Zeuthen T., and Macaulay N. (2009) Vasopressin-dependent short-term regulation of aquaporin 4 expressed in Xenopus oocytes. Neuroscience 164, 1674–1684 10.1016/j.neuroscience.2009.09.072 [DOI] [PubMed] [Google Scholar]
- 39. Herman B., Langevin M. A., and Albertini D. F. (1983) The effects of taxol on the organization of the cytoskeleton in cultured ovarian granulosa cells. Eur. J. Cell Biol. 31, 34–45 [PubMed] [Google Scholar]
- 40. Zaelzer C., Hua P., Prager-Khoutorsky M., Ciura S., Voisin D. L., Liedtke W., and Bourque C. W. (2015) ΔN-TRPV1: a molecular co-detector of body temperature and osmotic stress. Cell Rep 13, 23–30 10.1016/j.celrep.2015.08.061 [DOI] [PubMed] [Google Scholar]
- 41. Basavappa S., Pedersen S. F., Jørgensen N. K., Ellory J. C., and Hoffmann E. K. (1998) Swelling-induced arachidonic acid release via the 85-kDa cPLA2 in human neuroblastoma cells. J. Neurophysiol. 79, 1441–1449 10.1152/jn.1998.79.3.1441 [DOI] [PubMed] [Google Scholar]
- 42. Pedersen S., Lambert I. H., Thoroed S. M., and Hoffmann E. K. (2000) Hypotonic cell swelling induces translocation of the α isoform of cytosolic phospholipase A2 but not the γ isoform in Ehrlich ascites tumor cells. Eur. J. Biochem. 267, 5531–5539 10.1046/j.1432-1327.2000.01615.x [DOI] [PubMed] [Google Scholar]
- 43. Jo A. O., Lakk M., Frye A. M., Phuong T. T., Redmon S. N., Roberts R., Berkowitz B. A., Yarishkin O., and Križaj D. (2016) Differential volume regulation and calcium signaling in two ciliary body cell types is subserved by TRPV4 channels. Proc. Natl. Acad. Sci. U.S.A. 113, 3885–3890 10.1073/pnas.1515895113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ryskamp D. A., Frye A. M., Phuong T. T., Yarishkin O., Jo A. O., Xu Y., Lakk M., Iuso A., Redmon S. N., Ambati B., Hageman G., Prestwich G. D., Torrejon K. Y., and Križaj D. (2016) TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci. Rep. 6, 30583 10.1038/srep30583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S. N., Osborne N. N., and Reichenbach A. (2006) Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 25, 397–424 10.1016/j.preteyeres.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 46. Krizaj D. (1995) What is glaucoma? in Webvision: The Organization of the Retina and Visual System (Kolb H., Fernandez E., and Nelson R., eds) University of Utah Health Sciences Center, Salt Lake City, UT: [PubMed] [Google Scholar]
- 47. Berna-Erro A., Izquierdo-Serra M., Sepúlveda R. V., Rubio-Moscardo F., Doñate-Macián P., Serra S. A., Carrillo-Garcia J., Perálvarez-Marín A., González-Nilo F., Fernández-Fernández J. M., and Valverde M. A. (2017) Structural determinants of 5′,6′-epoxyeicosatrienoic acid binding to and activation of TRPV4 channel. Sci. Rep. 7, 10522 10.1038/s41598-017-11274-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ackerman M. J., Wickman K. D., and Clapham D. E. (1994) Hypotonicity activates a native chloride current in Xenopus oocytes. J. Gen. Physiol. 103, 153–179 10.1085/jgp.103.2.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morin X. K., Bond T. D., Loo T. W., Clarke D. M., and Bear C. E. (1995) Failure of P-glycoprotein (MDR1) expressed in Xenopus oocytes to produce swelling-activated chloride channel activity. J. Physiol. 486, 707–714 10.1113/jphysiol.1995.sp020846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thoroed S. M., Lauritzen L., Lambert I. H., Hansen H. S., and Hoffmann E. K. (1997) Cell swelling activates phospholipase A2 in Ehrlich ascites tumor cells. J. Membr. Biol. 160, 47–58 10.1007/s002329900294 [DOI] [PubMed] [Google Scholar]
- 51. Munro A. W., McLean K. J., Grant J. L., and Makris T. M. (2018) Structure and function of the cytochrome P450 peroxygenase enzymes. Biochem. Soc. Trans. 46, 183–196 10.1042/BST20170218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chesler A. T., and Szczot M. (2018) Portraits of a pressure sensor. Elife 7, e34396 10.7554/eLife.34396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Larsen B. R., and MacAulay N. (2014) Kir4.1-mediated spatial buffering of K+: experimental challenges in determination of its temporal and quantitative contribution to K+ clearance in the brain. Channels 8, 544–550 10.4161/19336950.2014.970448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramadass R., Becker D., Jendrach M., and Bereiter-Hahn J. (2007) Spectrally and spatially resolved fluorescence lifetime imaging in living cells: TRPV4-microfilament interactions. Arch. Biochem. Biophys. 463, 27–36 10.1016/j.abb.2007.01.036 [DOI] [PubMed] [Google Scholar]
- 55. Becker D., Bereiter-Hahn J., and Jendrach M. (2009) Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur. J. Cell Biol. 88, 141–152 10.1016/j.ejcb.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 56. Goswami C., Kuhn J., Heppenstall P. A., and Hucho T. (2010) Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS One 5, e11654 10.1371/journal.pone.0011654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suzuki M., Mizuno A., Kodaira K., and Imai M. (2003) Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–22668 10.1074/jbc.M302561200 [DOI] [PubMed] [Google Scholar]
- 58. Matthews B. D., Thodeti C. K., Tytell J. D., Mammoto A., Overby D. R., and Ingber D. E. (2010) Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface β1 integrins. Integr. Biol. (Camb.) 2, 435–442 10.1039/c0ib00034e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soya M., Sato M., Sobhan U., Tsumura M., Ichinohe T., Tazaki M., and Shibukawa Y. (2014) Plasma membrane stretch activates transient receptor potential vanilloid and ankyrin channels in Merkel cells from hamster buccal mucosa. Cell Calcium 55, 208–218 10.1016/j.ceca.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 60. Fan H. C., Zhang X., and McNaughton P. A. (2009) Activation of the TRPV4 ion channel is enhanced by phosphorylation. J. Biol. Chem. 284, 27884–27891 10.1074/jbc.M109.028803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wegierski T., Lewandrowski U., Müller B., Sickmann A., and Walz G. (2009) Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J. Biol. Chem. 284, 2923–2933 10.1074/jbc.M805357200 [DOI] [PubMed] [Google Scholar]
- 62. Hellwig N., Albrecht N., Harteneck C., Schultz G., and Schaefer M. (2005) Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 118, 917–928 10.1242/jcs.01675 [DOI] [PubMed] [Google Scholar]
- 63. Eilers H., Lee S. Y., Hau C. W., Logvinova A., and Schumacher M. A. (2007) The rat vanilloid receptor splice variant VR.5′sv blocks TRPV1 activation. Neuroreport 18, 969–973 10.1097/WNR.0b013e328165d1a2 [DOI] [PubMed] [Google Scholar]
- 64. Garcia-Elias A., Mrkonjic S., Pardo-Pastor C., Inada H., Hellmich U. A., Rubio-Moscardó F., Plata C., Gaudet R., Vicente R., and Valverde M. A. (2013) Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc. Natl. Acad. Sci. U.S.A. 110, 9553–9558 10.1073/pnas.1220231110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. D'hoedt D., Owsianik G., Prenen J., Cuajungco M. P., Grimm C., Heller S., Voets T., and Nilius B. (2008) Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J. Biol. Chem. 283, 6272–6280 10.1074/jbc.M706386200 [DOI] [PubMed] [Google Scholar]
- 66. Liedtke W., and Friedman J. M. (2003) Abnormal osmotic regulation in trpv4−/− mice. Proc. Natl. Acad. Sci. U.S.A. 100, 13698–13703 10.1073/pnas.1735416100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tabuchi K., Suzuki M., Mizuno A., and Hara A. (2005) Hearing impairment in TRPV4 knockout mice. Neurosci. Lett. 382, 304–308 10.1016/j.neulet.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 68. Gevaert T., Vriens J., Segal A., Everaerts W., Roskams T., Talavera K., Owsianik G., Liedtke W., Daelemans D., Dewachter I., Van Leuven F., Voets T., De Ridder D., and Nilius B. (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J. Clin. Invest. 117, 3453–3462 10.1172/JCI31766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mizuno A., Matsumoto N., Imai M., and Suzuki M. (2003) Impaired osmotic sensation in mice lacking TRPV4. Am. J. Physiol. Cell Physiol. 285, C96–C101 10.1152/ajpcell.00559.2002 [DOI] [PubMed] [Google Scholar]
- 70. Grundy D. (2015) Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J. Physiol. 593, 2547–2549 10.1113/JP270818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vincent F., and Duncton M. A. (2011) TRPV4 agonists and antagonists. Curr. Top. Med. Chem. 11, 2216–2226 10.2174/156802611796904861 [DOI] [PubMed] [Google Scholar]
- 72. Dyer M. A., and Cepko C. L. (2000) Control of Müller glial cell proliferation and activation following retinal injury. Nat. Neurosci. 3, 873–880 10.1038/78774 [DOI] [PubMed] [Google Scholar]
- 73. Gaiano N., Nye J. S., and Fishell G. (2000) Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26, 395–404 10.1016/S0896-6273(00)81172-1 [DOI] [PubMed] [Google Scholar]
- 74. Sokabe T., Fukumi-Tominaga T., Yonemura S., Mizuno A., and Tominaga M. (2010) The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J. Biol. Chem. 285, 18749–18758 10.1074/jbc.M110.103606 [DOI] [PMC free article] [PubMed] [Google Scholar]