Abstract
Tissue colonization (homing) by blood-borne cells critically hinges on the ability of the cells to adhere to vascular endothelium with sufficient strength to overcome prevailing hemodynamic shear stress. These adhesive interactions are most effectively engendered via binding of the endothelial lectin E-selectin (CD62E) to its cognate ligand, sialyl Lewis-X (sLeX), displayed on circulating cells. Although chimeric antigen receptor (CAR) T-cell immunotherapy holds promise for treatment of various hematologic and non-hematologic malignancies, there is essentially no information regarding the efficiency of CAR T-cell homing. Accordingly, we performed integrated biochemical studies and adhesion assays to examine the capacity of human CAR T-cells to engage E-selectin. Our data indicate that CAR T-cells do not express sLeX and do not bind E-selectin. However, enforced sLeX display can be achieved on human CAR T-cells by surface fucosylation, with resultant robust E-selectin binding under hemodynamic shear. Importantly, following intravascular administration into mice, fucosylated human CAR-T cells infiltrate marrow with 10-fold higher efficiency than do unfucosylated cells. Collectively, these findings indicate that custom installation of sLeX programs tissue colonization of vascularly administered human CAR T-cells, offering a readily translatable strategy to augment tissue delivery, thereby lowering the pertinent cell dosing and attendant cell production burden, for CAR T-cell immunotherapy applications.
Keywords: immunotherapy, cancer therapy, glycosylation, glycobiology, fucosyltransferase, CAR T cell, E-selectin ligand, sialyl Lewis X, sLeX, translational glycobiology
Introduction
Immunotherapy is an emerging approach for cancer treatment that offers the opportunity to specifically target tumor cells and thereby avoid systemic toxicities of conventional radiochemotherapy. One major advance in this approach is chimeric antigen receptor (CAR)3 T-cell therapy, which has shown curative potential against B-cell malignancies and holds great promise for treating other hematologic malignancies and solid tumors. However, general applicability of this therapeutic approach is limited by production costs related to the culture-expansion of requisite numbers of CAR T-cells to achieve therapeutic response(s). Furthermore, studies indicate that systemic toxicities result from dose-related off-target dissemination of these cytotoxic lymphocytes (1–3). Thus, to optimize the applicability of CAR T-cell therapeutics, it is critical to devise strategies to improve lesion-specific colonization of this “live drug,” thereby improving efficacy and dropping production costs and incidence of adverse events.
It is well known that tumor regression by intravascularly administered CAR T-cells depends on the ability of the cells to infiltrate affected site(s), whether within bone marrow parenchyma for hematologic malignancies, or within primary solid tumor beds, or within metastatic tumor foci (4, 5). As opposed to non-specific entrapment, which results in stochastic cellular accumulation in both unaffected and lesional tissue, the ability of circulating cells to engage vascular endothelium allows for “homing” in which immunotherapeutic cells selectively colonize target sites. Homing is initiated by tethering and rolling of blood-borne cells onto target endothelium (6), a process most efficiently mediated by E-selectin receptor–ligand interactions. E-selectin is an endothelial lectin that is constitutively expressed by bone marrow and skin microvessels (7), is potently induced within essentially all microvascular endothelial beds by inflammatory cytokines (e.g. TNFα and interleukin-1β) (8), and is also characteristically expressed by cancer microvessels (9). Notably, bone is a common metastastic site for a variety of solid malignancies, and a recent study reported that marrow microvessel expression of E-selectin promotes bone metastasis of cancer cells (10). Therefore, the ability of CAR T-cells to home to E-selectin–bearing sites such as marrow is critical for precise targeting of osteotropic metastatic cancers such as prostate, breast, and lung adenocarcinomas, as well as for hematologic malignancies such as acute leukemias and multiple myeloma.
The tetrasaccharide glycan known as “sialyl Lewis X” (sLeX, CD15s) is the canonical binding determinant of E-selectin (6). sLeX is a sialo-fucosylated lactosaminyl glycan, displayed at the termini of specialized membrane glycoproteins (11) and glycolipids (12) of leukocytes. Although sLeX expression on circulating native human T-cells is well-characterized, no prior study has evaluated the expression of sLeX by human CAR T-cells. Indeed, to date, there is no information regarding the ability of CAR T-cells to engage endothelial cells under hemodynamic flow conditions. Here, using CAR T-cells expressing antibody specificity for human epidermal growth factor receptor (EGFR), a clinically targetable cell membrane protein highly amplified in many types of cancer (13, 14), we report that typical in vitro conditions used for CAR T-cell propagation/expansion deaden cell surface sLeX display, leading to a commensurate reduction in E-selectin–mediated tethering and rolling on endothelial cells under shear stress conditions. However, culture-expanded CAR T-cells display uniformly high levels of type 2 sialyllactosamines (sialylLacNAc) that can be converted to sLeX via enzyme-based cell surface fucosylation (exofucosylation) (6). This enforced sLeX expression yields significantly higher CAR T-cell tethering and rolling adhesive interactions on endothelial cells expressing E-selectin, and, upon intravascular injection into mice, these cells infiltrate bone marrow with ∼10-fold higher efficiency than unfucosylated CAR T-cells. Collectively, these findings indicate that deficits in CAR T-cell homing can be remedied by cell surface glycoengineering, providing a readily translatable strategy for improving colonization of CAR T-cells within marrow and other tissues whose endothelial beds express E-selectin.
Results and discussion
Human CAR T-cells directed against human EGFR, which is highly amplified in various cancers (13, 14), were manufactured by lentiviral transduction of purified human T-cells with the huEGFR-BBZ CAR construct co-expressing mCherry to report transduction. T-cells were stimulated with anti-CD3/CD28 microbeads before transduction with CAR construct and culture-expanded for 10 days in growth medium supplemented with either FBS or human AB serum (HS) and IL-2. The 10-day expanded human CAR T-cells were then co-cultured with U87 cells (an EGFR-expressing human cell line) for 7 additional days to allow antigen-specific expansion (15).
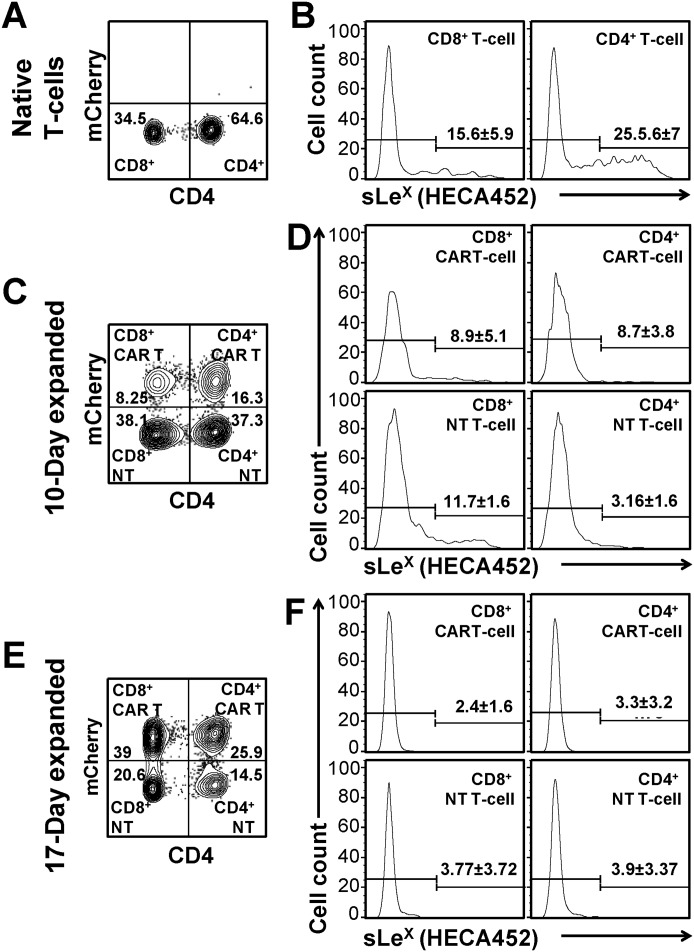
We first sought to assess whether culture expansion modifies sLeX display on CAR T-cells (Fig. 1). To this end, we measured binding of the mAb HECA452 (which recognizes sLeX) to CAR T-cells manufactured as described above. We observed that native (i.e. freshly obtained from normal blood) human T-cells exhibit heterogeneity in sLeX expression, with an average of ∼25% of CD4+ and ∼15% of CD8+ T-cells characteristically expressing sLeX (Fig. 1B) (11). However, culture-expansion in medium containing either FBS (Fig. 1, C–F) or HS (Fig. 2B, right panel) leads to a significant reduction in CAR T-cell sLeX expression compared to that of native T cells. To determine whether transduction by CAR construct itself alters sLeX display, and to evaluate whether culture-expansion differentially affects CD4+ or CD8+ T-cells, we divided the CAR-transduced and expanded T-cells into the following subpopulations based on mCherry expression and CD4 staining (Fig. 1C): CD4+CAR T-cells (CD4+mCherry+), CD4+nontransduced (NT) T-cells (CD4+mCherry−), CD8+CAR T-cells (CD8+mCherry+), and CD8+ NT T-cells (CD8+mCherry−). After 10 days in culture, ∼3–8% of CD4+ and ∼8%–11% of the CD8+ T-cells (both CAR and NT T-cells) express sLeX (Fig. 1D). At this time, the surface density of sLeX on the minor population of sLeX+ cells was also low. Co-culturing these cells for 7 additional days with U87 cell line further reduces sLeX expression (Fig. 1F); after 17 days, on an average, only ∼3% of cells express sLeX in both CD4+ and CD8+ T-cell compartments (CAR or NT T-cells), and these cells display very low sLeX surface density. Notably, upon expansion, both CAR and NT T-cells drop sLeX levels by similar proportions. Together, our results indicate that culture-expansion progressively deadens expression of the tetrasaccharide sLeX within both CD4+ and CD8+ T-cell compartments, and, importantly, transduction by lentivirus encoding CAR construct in itself has no effect on sLeX display. In other words, culture-expansion itself, in either FBS or HS, markedly dampens sLeX display.
Figure 1.
Culture expansion progressively depletes sLeX expression on CAR T-cells, whereas transduction with the CAR construct has no effect on sLeX display. A–F, sLeX expression (measured by mAb HECA452 binding) on the surface of native human T-cells (A and B), 10-day expanded (C and D), and 17-day expanded (E and F) CAR-transduced T-cells. A, C, and E: representative flow cytometry contour plots presenting mCherry expression and CD4 staining of native (A), 10-day (C), and 17-day (E) expanded CAR-transduced T-cells. mCherry+ cells indicate CAR T-cells and mCherry− cells indicate NT T-cells. Numbers within each gate represent percentage of positive cells. B, D, and F: representative flow cytometry histograms presenting sLeX expression on native CD8+ (B, left) and CD4+ (B, right) T-cells; 10-day CD8+ CAR T-cells (D, top left) and CD4+ CAR T-cells (D, top right); CD8+ NT T-cells (D, bottom left) and CD4+ NT T-cells (D, bottom right); 17-day CD8+ CAR T-cells (F, top left) and CD4+ CAR T-cells (F, top right); and CD8+ NT T-cells (F, bottom left) and CD4+ NT T-cells (F, bottom right). Numbers above each gate represent the percentage of positive cells. Mean ± S.D. of three to four experiments.
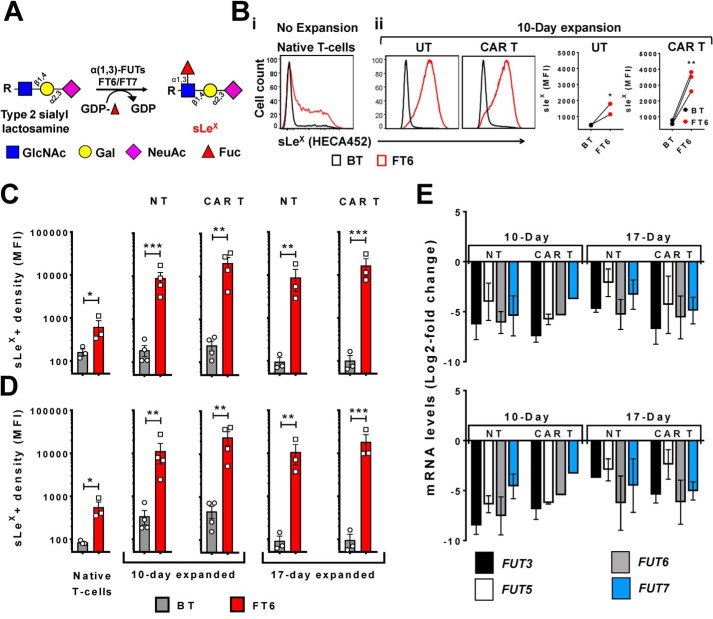
Figure 2.
Exofucosylation custom-installs sLeX display on culture-expanded CAR T-cells and augments CAR T-cell E-selectin binding function. A, schematic of the glycosyltransferase-programmed stereosubstitution (exofucosylation) reaction, in which type 2 sialylLacNAc acceptors present on the cell surface are converted to sLeX using purified α(1,3)-fucosyltransferases (FT6 or FT7) and GDP-fucose. B i, sLeX expression (measured by HECA452 binding) on native human T-cells that were either treated with buffer alone (BT, black line) or exofucosylated with purified FT6 enzyme (FT6, red line). B ii, sLeX expression on UT or CAR T-cells expanded using medium containing 10% HS and IL-2 for10 days: Left, representative histograms presenting sLeX expression as measured by HECA452 binding (Black lines represent BT, and red lines represent FT6); Right, aggregate data presenting the mean fluorescence intensity (MFI) of HECA452 binding to 10-day UT and CAR T-cells with or without exofucosylation by FT6. n = 3 (each data point represents an independent T-cell donor). Ratio paired t test comparing BT and FT6 treatment groups: *, p < 0.05; **, p < 0.01. C and D: Culture-expanded T-cells that had undergone transduction were either treated with buffer alone (BT) or exofucosylated with FT6 (FT6), followed by staining with mAbs against CD4 and sLeX (HECA452). mCherry+ indicates CAR T-cells that were successfully transduced and mCherry− indicates NT T-cells. The bar plots represent mean fluorescence intensity (MFI) of HECA45 binding. C, sLeX expression by BT (gray columns) and FT6-treated (red columns) CD4+ T-cell populations; Left to right: native, 10-day NT, 10-day CAR T, 17-day NT, and 17-day CAR T-cells. n = 3–4. Error bars represent S.E.; Ratio paired t test: *, p < 0.05; **, p < 0.01; ***, p < 0.001. D, sLeX expression on BT (gray columns) and FT6-treated (red columns) CD8+ T-cell populations; Left to right: native, 10-day NT, 10-day CAR, 17-day NT, and 17-day CAR T-cells. n = 3–4. Error bars indicate S.E.; Ratio paired t test: *, p < 0.05; **, p < 0.01; ***, p < 0.001. E, quantitative RT-PCR analysis of the human α(1,3)-fucosyltransferase enzyme transcripts FUT3, FUT5, FUT6, and FUT7 in 10- and 17-day-expanded CD4+ (top panel) and CD8+ (bottom panel) T-cell populations. Gene expression values are presented as log2 -fold change compared with expression of each gene in native (freshly isolated) human CD4+ or CD8+ T-cells. n = 2 independent donors, triplicates. Data are presented as mean ± S.E.
The tetrasaccharide sLeX (Neu5Ac-α(2,3)-Gal-β(1,4)-[Fuc-α(1,3)-]GlcNAcβ1-R) is composed of a type 2 lactosamine (type 2 LacNAc) disaccharide core in which a galactose (Gal) is β(1,4)-linked to N-acetylglucosamine (GlcNAc) (i.e. Gal-β(1,4)–GlcNAc). When this LacNAc is modified by a sialic acid (N-acetylneuraminic acid, Neu5Ac) residue in α(2,3)-linkage to Gal, this structure is called a “type 2 sialylLacNAc” (Neu5Ac-α(2,3)-Gal-β(1,4)-GlcNAcβ1-R). Addition of a fucose (Fuc) residue in α(1,3)-linkage to the GlcNAc within type 2 sialylLacNAc creates sLeX. All of these reactions are dictated by glycosyltransferases, which function in an assembly line–like sequence. As such, type 2 sialylLacNAc is the precursor of sLeX, and, when present on the cell surface, this trisaccharide acceptor can be converted to sLeX by glycosyltransferase-programmed stereosubstitution, wherein fucose is installed at α(1,3)-linkage to the GlcNAc in vitro using purified α(1,3)-fucosyltransferase enzyme (fucosyltransferase 6 (FT6)) and the nucleotide sugar donor GDP-fucose (Fig. 2A) (6). Accordingly, we sought to assess whether CAR T-cells culture-expanded according to conventional methods express type 2 sialylLacNAc that can be converted to sLeX by exofucosylation. We observed that exofucosylation enforces sLeX display on T-cells expanded in medium containing HS (Fig. 2B, ii) or FBS (Fig. 2, C and D). Notably, even after exofucosylation, only a fraction of the native (i.e. unexpanded) T-cells display sLeX (45% of CD4+ and 35% of CD8+ T-cells) (Fig. 2B, i, and Fig. S1A), indicating that a significant proportion of native T-cells do not innately express type 2 sialylLacNAc. Strikingly, all four aforementioned culture-expanded T-cell subsets, as identified based on mCherry expression and CD4 staining (Fig. 1C) (i.e. CD4+ or CD8+ CAR or NT T-cells), display uniformly high levels of sLeX after exofucosylation (Fig. 2, C and D, and Fig. S1, B and C). Furthermore, untransduced (UT) T-cells (never transduced but expanded under similar conditions as CAR-transduced cells) exhibit similar boost in sLeX display upon exofucosylation, as do NT or CAR T-cells (Fig. 2B, ii). Importantly, post-exofucosylation, similar sLeX levels were observed on CAR and NT T-cells, indicating that procedures/techniques associated with CAR transduction do not alter the expression of type 2 sialylLacNAc acceptors.
Our finding that exofucosylation is able to complete sLeX tetrasaccharide structure on the surface of CAR T-cells, and is able to restore E-selectin binding function of CAR T-cells, indicates that there are sialylated acceptors present on the right protein backbones on CAR T-cell surface, but the innate fucosylation machinery is down-regulated in these cells. We tested this hypothesis by performing quantitative RT-PCR of native (freshly isolated) human T-cells, and, NT and CAR T-cells cultured for 10 or 17 days to assess expression of α(1,3)-fucosyltransferase (FUT) enzymes that add fucose to the type 2 sialylLacNAc acceptors (FUT3, FUT5, FUT6, and FUT7). We found that each of the α(1,3)-FUT enzymes is significantly down-regulated in CD4+ and CD8+ NT or CAR T-cells (Fig. 2E).
To evaluate the E-selectin–reactive glycoproteins engendered by exofucosylation of native and culture-expanded T-cells, we performed Western blot analysis using E-selectin–Ig chimera as a probe. Native T-cells show relatively modest E-selectin–Ig reactivity at a band of 150-kDa molecular mass, which comprises two glycoprotein E-selectin ligands: The PSGL1 glycoform known as cutaneous lymphocyte antigen (CLA) and the CD43 glycoform that binds E-selectin (CD43E) (11). A higher-molecular-mass-band (>250 kDa) is also found, representing the homodimer of PSGL1. Upon exofucosylation of native T cells, the E-selectin binding capacity of each of these glycoproteins increases dramatically (Fig. 3A). In contrast, culture-expanded T-cells, both CAR and NT T-cells, do not exhibit any appreciable E-selectin binding by Western blot analysis. However, upon exofucosylation, markedly increased E-selectin binding was evident on CAR T-cells culture-expanded for 10 days (Fig. 3A) and for 17 days (Fig. S1E). Immunoprecipitation by E-selectin–Ig followed by Western blotting indicates that exofucosylation profoundly increases both cutaneous lymphocyte antigen and CD43E expression on CAR T-cells, and also enforces expression of the E-selectin–binding glycoform of CD44 known as Hematopoietic Cell E-/L-selectin Ligand (HCELL) (Fig. 3B) (16). Together, these results indicate that exofucosylation can install high-level sLeX display on the membrane glycoproteins PSGL-1, CD43, and CD44 of culture-expanded CAR T-cells.
Figure 3.
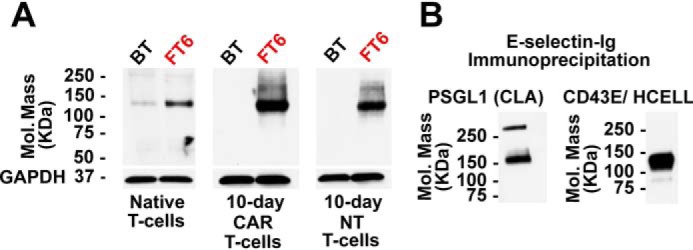
Identification of the proteins carrying sLeX in exofucosylated CAR T-cells. A, Western blot using E-selectin–Ig chimera as a probe of BT- or FT6-treated native T-cells, 10-day CAR T-cells, and 10-day NT T-cells. GAPDH staining serves as a loading control. B, Western blot analysis of E-selectin–reactive glycoproteins of exofucosylated CAR T-cells. E-selectin–Ig immunoprecipitated glycoproteins from FT6-exofucosylated CAR T-cells were stained with antibodies against PSGL1 (left), and CD43 and CD44 (right). The numbers on the left indicate molecular mass in kilodaltons.
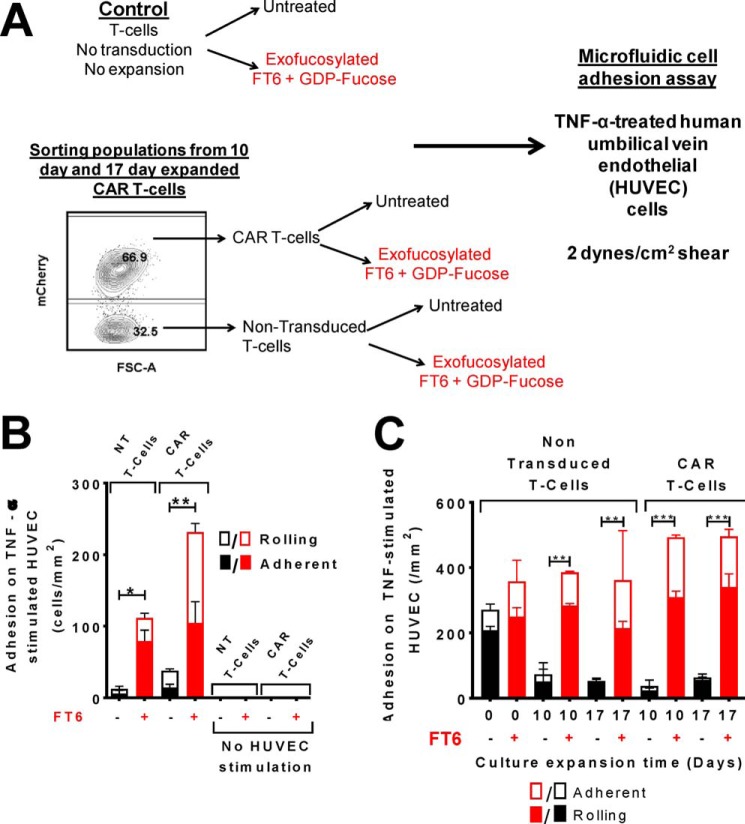
To investigate whether installation of sLeX on CAR T-cells by exofucosylation translates to functional E-selectin–mediated tethering and rolling activity, we performed in vitro microfluidic flow chamber assays to evaluate T-cell–adhesive interactions on E-selectin–expressing endothelial beds under hemodynamic shear conditions. To this end, we perfused untreated or exofucosylated T-cells over TNFα-stimulated HUVEC monolayers at 2 dynes/cm2 shear stress (Fig. 4A). These conditions closely mimic physiological environment prevailing within microvasculature (17). As expected, based on a prior study (11), native T-cells display robust rolling on E-selectin–expressing HUVEC monolayers; however, we observed only a moderate increase in the rolling flux of native T-cells after exofucosylation (Fig. 4C). Notably, consistent with diminished cell surface sLeX levels, both 10- and 17-day–expanded T-cells (CAR and NT) display profoundly impaired tethering and rolling. Importantly, exofucosylation leads to strikingly higher rolling and adhesion of culture-expanded T-cells (both CAR and NT) on E-selectin–bearing HUVEC monolayers (Fig. 4, B and C). These findings indicate that conventional culture techniques markedly induce expression of type 2 sialylLacNAc glycans on the T-cell surface. Notably, transduction with CAR construct does not itself modify expression of sialylLacNAc structures, as NT and CAR T-cells each exhibit similar rolling and adhesion flux upon exofucosylation.
Figure 4.
Exofucosylation augments tethering and rolling function of culture-expanded CAR T-cells on TNFα-stimulated HUVECs. A, schematic of the experiment evaluating ex vivo tethering and rolling function of CAR-transduced and culture-expanded T-cells. T-cells were either treated with buffer alone (BT) or exofucosylated with FT6. The 10- and 17-day-expanded CAR-transduced T-cells were then flow-sorted based on mCherry expression to isolate NT (i.e. mCherry−) and CAR T-cells (i.e. mCherry+ cells). These cells were then perfused over a monolayer of TNFα-stimulated HUVECs at 2 dynes/cm2 shear stress at 2 × 106/ml cell concentration. Rolling and adherent cells were counted and normalized to cell count per unit area. B, quantitation of E-selectin–mediated recruitment of BT (black columns) and FT6-treated (red columns) T-cell populations on TNFα-stimulated HUVECs. The plots present normalized counts of rolling (solid columns) and firmly adherent (open columns) T-cells. No T-cell rolling was observed in the absence of HUVEC stimulation. n = 2–3; ordinary one-way ANOVA (p = 0.01) with Sidak's multiple comparison test comparing BT with FT6-treated cells for each T-cell population: *, p < 0.05; **, p < 0.01. C, Column plot presenting normalized counts of rolling (solid columns) and firmly adherent (open columns) cells on HUVEC monolayers for native T-cells (nontransduced and nonexpanded, freshly isolated), NT T-cells (nontransduced and expanded), and CAR T-cells expanded for 0, 10, or 17 days in culture. Black columns represent BT T-cells, and red columns represent FT6-treated T-cells. Data represent mean ± S.E. of two independent experiments, triplicates. Ordinary one-way ANOVA (p = 0.0001) with Bonferroni's multiple comparison test comparing BT with FT6 categories for each T-cell population: **, p < 0.01; ***, p < 0.001.
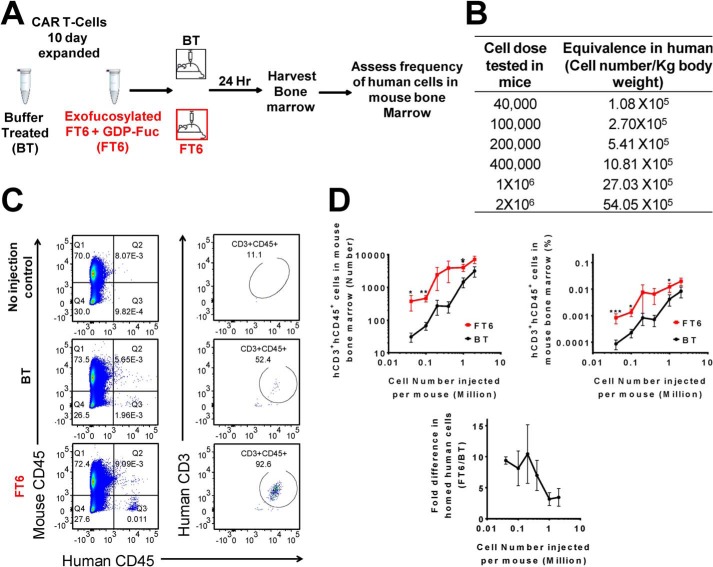
Given that enforcing sLeX display on CAR T-cells enhances their adhesive interactions on E-selectin–bearing endothelial cells in vitro, we reasoned that exofucosylation may also improve the ability of CAR T-cells to home to tissue sites where microvascular endothelial cells express E-selectin. Since bone marrow microvessels constitutively express E-selectin (7), we used homing to bone marrow as a model to test in vivo tissue-specific homing of exofucosylated CAR T-cells. Importantly, many solid malignancies that amplify membrane EGFR expression are osteotropic, providing key rationale for analyzing the capacity of EGFR-directed CAR T-cells to colonize bone marrow. Previous studies from our laboratory that have directly assessed (via real-time intravital microscopy) the capacity of systemically administered human cells to extravasate (including hematopoietic stem cells (18), mesenchymal stem cells (16, 19–21), iPSC-derived hematopoietic stem cells (22), and leukocytes (18, 23)), have consistently revealed that enforced expression of E-selectin ligands uniformly leads to transendothelial migration at endothelial beds that express E-selectin. Thus, an increased human cell count in the marrow is a definite measure of marrow parenchymal infiltration. Accordingly, we intravenously injected varying numbers of buffer-treated (BT) and FT6-exofucosylated (FT6) CAR T-cells into immunocompromised mice and collected flushed marrow cells to quantify parenchymal infiltrates of human T-cells (Fig. 5). We administered five cell doses for each treatment (BT or FT6) and collected marrow cells 24 h post-injection (Fig. 5B).
Figure 5.
Exofucosylation significantly improves CAR T-cell homing to bone marrow. A, schematic of CAR T-cell homing experiment. 10- or 17-day CAR T-cells were either treated with buffer alone (BT) or exofucosylated using FT6 (FT6). These cells were injected through the retro-orbital plexus of NSG mice at varying cell doses. 24 h after injection, mouse bone marrow (BM) was harvested and interrogated by flow cytometry. B, Number of cells in each category (BT or FT6) injected in individual mice, translated to number of cells per kilogram of body weight according to the following formula: human cell dose (cell number per kilogram) = mouse cell dose (cell number per kilogram) × (mouse Km/human Km) (24). C, representative dot plots showing flow cytometry analysis of mouse bone marrow. Bone marrow cells were co-stained with antibodies against mouse CD45 (mCD45), human CD45 (hCD45), and human CD3 (hCD3). mCD45− and hCD45+ cells (left) were subgated based on hCD3 expression (right) to identify hCD3 and hCD45 double-positive cells. Shown are bone marrow cells of mice receiving no human cell injection (top), 1 × 106 BT CAR T-cells (center), and 1 × 106 FT6-treated CAR T cells (bottom). Counting beads were used to calculate the absolute number of human cells in the bone marrow. D, quantitation of CAR T-cell marrow homing, presented as a function of injected cell number. Total number of bone marrow–infiltrating T-cells (top left), percentage of human cells in mouse bone marrow (top right), and -fold difference in homed human cells in mouse bone marrow (bottom), calculated as follows: -fold difference = percentage of hCD3+ hCD45+ FT6 cells in mouse BM/percentage of hCD3+ hCD45+ BT cells in mouse BM. Data are presented as mean ± S.E. of three independent experiments. Each data point represents three to four mice. Ratio-paired t test comparing BT and FT6 conditions for each cell dose: *, p < 0.05; **, p < 0.01.
Our data indicate that exofucosylation significantly improves marrow homing of CAR T-cells, as, at each cell dose, we found higher numbers and higher proportions of FT6 CAR T-cells in marrow compared with injected BT cells (Fig. 5D, top panels). Notably, the biggest difference in marrow infiltration between FT6 and BT CAR T-cells was observed at cell doses much lower than those routinely used in clinical applications (i.e. injection of 40,000–100,000 cells/mouse (weight, 30 g) is reflective of doses of 1.08–2.7 × 105/kg of body weight in a human (24)) (Fig. 5D, bottom panel). At these doses, we observed ∼10-fold higher infiltration of exofucosylated cells compared to unfucosylated cells. In other words, we found that injection of 40,000 exofucosylated human CAR T-cells delivers the same number of CAR T-cells into bone marrow as injection of 400,000 buffer-treated cells. Importantly, the difference in marrow infiltration between FT6 and BT cells is inversely proportional to cell dose, as, at higher doses (1–2 × 106 cells/mouse, i.e. 2.7–5.4 × 106/kg of body weight human dose), only a 3.5 ± 2.6–fold higher frequency of exofucosylated cells was observed in marrow compared to unfucosylated cells. Together, these findings support the notion that intravenous infusion of high cell doses leads to saturation of tissue beds in a stochastic fashion, i.e. regardless of molecular effectors of cell migration, leading to nonspecific accumulation of administered cells (25). In contrast, enforcing sLeX display by exofucosylation pilots CAR T-cell colonization preferentially into tissues whose endothelial beds express E-selectin. Thus, exofucosylation can potentially reduce CAR T-cell dosing by 10-fold. Notably, we did not observe any difference in spleen infiltration between unfucosylated and exofucosylated CAR T-cells (Fig. S2), indicating that exofucosylation specifically increases CAR T-cell infiltration into E-selectin–bearing tissues. In particular, the results herein prompt future investigations using exofucosylated EGFR-directed CAR T-cells to assess their efficacy in eradication of osteotropic tumors that characteristically display high levels of EGFR, such as in breast, lung, and prostate cancers; notably, heightened EGFR expression is a driver of bone metastasis of prostate cancer (26).
To determine whether exofusylation alters CAR T-cell cytotoxicity, we investigated cytolysis by these cells in vitro (Fig. S3). We observed that CAR-T cells produce highly specific cytolysis of target U87 cells, which remains unaltered after exofucosylation. Thus, the anti-tumor cytotoxicity of CAR T-cells is not modified by cell surface glycoengineering.
In this study, we investigated the first step of CAR T-cell homing, i.e. E-selectin–mediated recruitment of CAR T-cells under hemodynamic shear conditions, an important but hitherto unexplored prerequisite for target-specific colonization of CAR T-cells. Our study provides first evidence that CAR T-cells display an inability to engage E-selectin by virtue of the fact that these cells are deficient in cell surface sLeX display. Our results indicate that the absence of sLeX display on CAR T-cells is not due to transduction of the CAR construct but a consequence of down-regulation of α(1,3)-FUTs during cell expansion, regardless of whether cells are cultured in FBS or HS. These results are in line with the findings of a prior study that reported suppression of the α(1,3)-FUT enzyme FUT7 upon culturing human T-cells using conventional culture methods (27). To enforce E-selectin binding activity, we employed exofucosylation to custom-install sLeX on CAR T-cell surface, with resultant marked improvement in CAR T-cell homing to bone marrow, an E-selectin–bearing target tissue. Notably, sLeX installed via exofucosylation persists on the cell surface for ∼48 h, after which the sLeX levels dissipate because of natural turnover of cell surface glycoconjugates. Our central hypothesis is that installation of sLeX on CAR T-cells via exofucosylation will provide an initial boost in CAR T-cell targeting to E-selectin–bearing endothelial beds (as characteristic of native marrow endothelial beds and tumor endothelial beds) immediately after injection, and, this higher initial seeding of CAR T-cells within the tumor site will accentuate tumor elimination by CAR T-cells. Once CAR T-cells have infiltrated the tumor parenchyma and come in contact with relevant lesional cells, they will undergo intensive antigen-specific proliferation. Indeed, beyond induction of proliferation, the high affinity of the CAR for its cognate antigen will anchor the cells within the tumor parenchyma. However, alternate genetic approaches may also be utilized in order to achieve more stable sLeX display on the cell surface; e.g. using transfection of modified mRNA encoding FUT6 transcript (28), which allows for a non-permanent and non-genome integrative gene expression lasting about 4–5 days post-transfection (19), one can achieve custom modification of cell surface sLeX display for a longer duration. Furthermore, co-expression of FT6 enzyme together with the CAR construct, using a lentiviral delivery system, can create CAR T-cells with permanent display of cell surface sLeX.
Collectively, our findings reveal that cell surface glycoengineering can result in efficient and specific piloting of CAR T-cells into tissue sites that contain E-selectin–expressing endothelial beds. Importantly, apart from its constitutive expression on marrow microvessels, E-selectin is also characteristically expressed by the microvascular endothelial cells of cancers, and, thus, is strategically placed to recruit CAR T-cells. Notably, bone is a common metastastic site for a variety of solid malignancies, and marrow microvessel expression of E-selectin is known to promote bone metastasis of cancer cells. Therefore, the ability of CAR T-cells to home to E-selectin–bearing sites such as marrow is critical for precise targeting of osteotropic metastatic cancers such as prostate, breast, and lung adenocarcinomas as well as for hematologic malignancies such as acute leukemias and multiple myeloma. This improved homing may permit more efficient localization of these immunotherapeutic cells at lesional tumor sites, achieving requisite tissue infiltration with administration of fewer CAR T-cells. Thus, glycoengineering of the CAR T-cell surface could result in a significantly reduced burden of cell expansion, commensurately yielding less costly CAR T-cell therapeutics with higher clinical efficacy.
Materials and methods
Study approval
Collection and use of human T-cells were in accordance with procedures approved by institutional review board of the Dana Farber/Harvard Cancer Center (protocol 01-206) and Massachusetts General Hospital. The procedures included in the study abide by the Declaration of Helsinki principles. All animal studies were in accordance with the National Institutes of Health guidelines for the care and use of animals under approval of the institutional animal care and use committees of Brigham and Women's Hospital.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were cultured in EBM2 medium (Lonza, Walkersville, MD) with 5% FBS.
Production and culture of huEGFR CAR T-cells
The huEGFRscFv-BBz chimeric antigen receptor was designed based on the heavy and light chains of cetuximab to form a single-chain variable fragment (29, 30) that was fused to a portion of the extracellular and transmembrane domains of human CD8α, followed by the intracellular domains of 4-1BB and CD3ζ. The bicistronic vector also encoded truncated mCherry as a selectable marker and was placed following a T2A ribosomal skip sequence. The plasmid encoding huEGFRscFv-BBz-CAR was synthesized, and a third-generation lentivirus was produced. T-cells were isolated from leukapheresis products obtained from deidentified healthy donors and were stimulated in culture with Dynabeads Human T Activator CD3/CD28 (Life Technologies) at a bead-to-cell ratio of 3:1. T-cells were cultured in RPMI 1640 medium supplemented with either 10% fetal bovine serum or with human AB serum, Hepes buffer (20 mm), penicillin (100 IU/ml), streptomycin (100 μg/ml), and IL-2 (20 IU/ml). One day following bead stimulation, T cells were either transduced with CAR plasmid (CAR T) or left untransduced (UT) and then expanded for 10 days. After 10 days of culture, a portion of the T-cells was debeaded and cryopreserved; the remaining 10-day-expanded T cells were stimulated with irradiated U87 cells (a human glioma cell line that expresses EGFR), culture-expanded for an additional 7 days, and then cryopreserved until further use. Surface expression of the CAR was confirmed and quantified by flow cytometry based on expression of mCherry; mCherry expression served as the reporter for identifying the extent of successful CAR transduction in cells that had undergone transduction.
Antibodies
Antibodies used for flow cytometry were as follows: HECA452-FITC (catalog no. 321308, Biolegend), HECA452-Pacific Blue (catalog no. 321307, Biolegend) hCD45-FITC (clone HI130, catalog no. 304006, Biolegend), hCD3-PE-Cy7 (clone OKT3, catalog no. 317334, Biolegend), mCD45-APC (30-F11, catalog no. 103112, Biolegend), hCD4 (clone OKT4, catalog no. 317416, Biolegend), mouse E-selectin–Ig chimera (catalog no. 575-ES, R&D Systems), anti-PSGL1 (clone KPL1, catalog no. 556053, BD Pharmingen), anti-CD43 (clone L60, catalog no. 551457, BD Pharmingen), rat anti-mouse E-selectin (catalog no. MAB5751-500, R&D Systems), goat anti-rat IgG-HRP (catalog no. 3030-05, Southern Biotech), and goat anti-mouse-Ig-HRP (catalog no. 554002, BD Biosciences).
Flow cytometry and cell sorting
For flow cytometry analysis, cryopreserved T-cells were first revived and washed with PBS with 2% FBS. Cells were then incubated with human Fc receptor blocking reagent TruStain (catalog no. 422302, Biolegend). 5 × 106 cells were then stained with 100 μl of appropriate antibody mixture prepared in PBS and 2% FBS. Staining was performed at 4 °C for 30 min. Stained cells were then washed three times in PBS with 2% FBS and resuspended in 200 μl of PBS before analysis. Flow cytometry was performed using BD FACS instruments (BD Biosciences). For multicolor experiments, compensation controls were set up using UltraComp eBeads (Invitrogen), and spectral overlap was calculated using the automatic module of BD FACSDIVA. Flow cytometry data thus obtained were analyzed using FlowJo software (Tree Star, Ashland, OR). CAR T-cells and NT T-cells were isolated using a BD flow-assisted cell sorter based on expression of the mCherry reporter.
Exofucosylation
Native T-cells, and, 10-day-expanded or 17-day-expanded CAR T-cells were divided into two treatment groups. One group was exofucosylated with FT6, and the other group was treated with reaction buffer alone (BT). For exofucosylation, 5 × 106 cells were incubated with 60 μl of reaction mixture containing 1 mm GDP-fucose and 60 μg/ml purified FT6 enzyme (Warrior Therapeutics LLC, Sudbury, MA) in reaction buffer at 37 °C for 1 h (19). For buffer treatment, cells were incubated with reaction buffer alone (without enzyme or GDP fucose) at 37 °C for 1 h. Both groups were then washed with 1× PBS prior to downstream analysis.
Preparation of cell lysates
Cell lysis was performed using a buffer containing 150 mm NaCl, 50 mm Tris-HCI (pH 7.4), 0.02% NaN3, 20 mg/ml PMSF, 2% Nonidet P-40, and protease inhibitor mixture (Roche). After suspending the cell pellet in the aforementioned buffer, the resulting suspension was incubated on ice for 30 min and then sonicated. The samples were then centrifuged at 12,000 × g for 5 min to remove particulate matter. The supernatant was then either boiled in reducing Laemmli loading buffer (Boston Bioproducts) to prepare for Western blotting or used for immunoprecipitation.
Immunoprecipitation of E-selectin ligands
Cell lysates were precleared using protein G–agarose beads (Invitrogen) and then incubated with the murine E-selectin–human Fc chimera (E-Ig, Bio-Techne) in the presence of 2 mm CaCl2. Immunoprecipitated glycoproteins were collected by boiling the beads in the presence of 2-mercaptoethanol in Laemmli loading buffer.
Western blotting
Reduced cell lysate protein samples or immunoprecipitation products were resolved on a 4%–20% SDS-PAGE gel (Bio-Rad) and then transferred onto a PVDF membrane (Bio-Rad). The membrane was then blocked with 10% milk in TBS-T (TBS and 0.1% Tween 20) buffer. For detection of E-selectin ligands, membranes were first incubated with E-Ig in TBS-T buffer containing 2 mm CaCl2 followed by incubation with rat anti-mouse E-selectin antibody (Bio-Techne) and then probed with HRP-conjugated goat anti-rat IgG antibody (Southern Biotech). In other cases, membranes were incubated with monoclonal antibodies against either PSGL1 (clone KPL1, Biolegend), CD43 (clone IG10, BD Biosciences), or CD44 (clone 156-3C11, Cell Signaling), followed by incubation with HRP-conjugated goat anti-mouse IgG1 antibody (BD Biosciences). Protein bands were detected by chemiluminescence using Lumi-Light substrate (Roche).
Quantitative RT-PCR
Total cellular RNA was reverse-transcribed using SuperScript VILO cDNA Conversion Kit (Invitrogen) according to the following conditions: 25 °C for 10 min, 42 °C for 60 min, and 85 °C for 5 min. Quantitative RT-PCR was performed with specific primers to amplify target genes (listed in Table S1) using SYBR Select Master Mix (Applied Biosystems, Foster City, CA) and the StepOne Plus PCR Detection System (Applied Biosystems) according to the following thermal cycling sequence: initial activation at 95 °C for 20 s, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Expression of a particular gene in test cells (10-day or 17-day CAR T-cells) is presented as log2 -fold change compared to expression of the said gene in native human T-cells (untransduced and nonexpanded T-cells).
Microfluidic flow chamber experiment to evaluate tethering and rolling function of CAR T-cells ex vivo
Microfluidic cell adhesion assay was performed using a microfluidic flow chamber as described previously (31). For this assay, HUVECs were cultured in 100-mm tissue culture dishes to obtain a uniform monolayer. HUVEC monolayers were either left unstimulated or stimulated to express E-selectin by incubating the cells with 40 ng/ml TNFα for 4 h at 37 °C. The microfluidic flow chamber was vacuum-sealed on the stimulated or unstimulated HUVEC monolayers and mounted over a microscope stage. Native T-cells and culture-expanded NT or transduced T-cells with or without exofucosylation were resuspended at 2 × 106/ml cell concentration in HEPES buffer with 2 mm CaCl2 and perfused into the microfluidic flow chamber using a syringe pump at 2 dynes/cm2 wall shear stress. Cellular interactions within the microfluidic chamber were recorded using Moticam (Motic, Richmond, BC, Canada) camera and μManager image acquisition software. Total rolling and adherent cells were enumerated using ImageJ analysis software.
Murine model to evaluate CAR T-cell homing
Age-matched NCG mice (NOD-Prkdcem26Cd52Il2rgem26Cd22/NjuCr) were purchased from Charles River (Wilmington MA). Buffer-treated or exofucosylated CAR T-cells were resuspended in 1× PBS. The cells were then divided into seven different cell doses as indicated in Fig. 3B, with each cell dose resuspended in a 100-μl injection volume. The cells were then injected into the retro-orbital plexus of individual mice using a 29-gauge hypodermic needle. 24 h after injection, mice were sacrificed, and bone marrow was harvested from two femora and two tibiae of each mouse. The bone marrow cells were subjected to red blood cell lysis, washed, and then stained with antibodies against mouse CD45, human CD45, and human CD3. Bone marrow cells were then analyzed using flow cytometry to determine human cell infiltration within mouse bone marrow.
Cytotoxicity assay
Human EGFR CAR T-cells were co-cultured with click beetle green luciferase–expressing U87 cells as targets at the indicated ratios (Fig. S3) for 16 h. Luciferase activity was measured with a Synergy Neo2 luminescence microplate reader (Biotek), and the percentage of specific lysis was calculated utilizing the following equation: percent specific lysis = (total relative light units/target cell specific relative light units) ×100.
Statistics
Data are reported as mean ± S.E. The p values were calculated using a ratio paired t test or ordinary one-way ANOVA with Sidak's multiple comparison test, as indicated in the figure legends. p < 0.05 is considered significant.
Author contributions
R. S. conceptualization; N. M., M. S., and A. P. C. data curation; N. M. and M. S. formal analysis; N. M. and R. S. investigation; N. M. visualization; N. M., A. P. C., and M. V. M. methodology; N. M. and R.S. writing-original draft; N. M., A. P. C., and R. S. writing-review and editing; M. V. M. and R. S. resources; R. S. funding acquisition; R. S. validation.
Supplementary Material
Acknowledgment
We thank Kyle C. Martin for assistance with mouse studies.
This work was supported by National Institutes of Health Grant PO1-HL107146 and the Team Jobie Fund (to R. S.). According to National Institutes of Health policies and procedures, the Brigham and Women's Hospital has assigned intellectual property rights regarding cell surface glycan engineering to R. S., and R. S. has licensed portions of this technology to an entity he has founded (Warrior Therapeutics, LLC), to BioTechne, Inc., and to Mesoblast Ltd. R. S.'s ownership interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policy.
This article contains Figs. S1–S3 and Table S1.
- CAR
- chimeric antigen receptor
- TNF
- tumor necrosis factor
- EGFR
- epidermal growth factor receptor
- sLeX
- sialyl Lewis-X
- HS
- human AB serum
- NT
- nontransduced
- Gal
- galactose
- Neu5Ac
- N-acetylneuraminic acid
- Fuc
- fucose
- HUVEC
- human umbilical vein endothelial cell
- BT
- buffer-treated
- TBS
- Tris-buffered saline
- ANOVA
- analysis of variance
- BM
- bone marrow
- LacNAc
- lactosamine
- GlcNAc
- N-acetylglucosamine
- IL-2
- Interleukin-2.
References
- 1. Hay K. A., Hanafi L. A., Li D., Gust J., Liles W. C., Wurfel M. M., López J. A., Chen J., Chung D., Harju-Baker S., Cherian S., Chen X., Riddell S. R., Maloney D. G., and Turtle C. J. (2017) Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 130, 2295–2306 10.1182/blood-2017-06-793141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill J. A., Li D., Hay K. A., Green M. L., Cherian S., Chen X., Riddell S. R., Maloney D. G., Boeckh M., and Turtle C. J. (2018) Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 131, 121–130 10.1182/blood-2017-07-793760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morgan R. A., Yang J. C., Kitano M., Dudley M. E., Laurencot C. M., and Rosenberg S. A. (2010) Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing erbb2. Mol. Ther. 18, 843–851 10.1038/mt.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mukai S., Kjaergaard J., Shu S., and Plautz G. E. (1999) Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer Res. 59, 5245–5249 [PubMed] [Google Scholar]
- 5. Sackstein R., Schatton T., and Barthel S. R. (2017) T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Invest. 97, 669–697 10.1038/labinvest.2017.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sackstein R. (2009) Glycosyltransferase-programmed stereosubstitution (GPS) to create HCELL: engineering a roadmap for cell migration. Immunol. Rev. 230, 51–74 10.1111/j.1600-065X.2009.00792.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schweitzer K. M., Dräger A. M., van der Valk P., Thijsen S. F., Zevenbergen A., Theijsmeijer A. P., van der Schoot C. E., and Langenhuijsen M. M. (1996) Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am. J. Pathol. 148, 165–175 [PMC free article] [PubMed] [Google Scholar]
- 8. Tamaru M., Tomura K., Sakamoto S., Tezuka K., Tamatani T., and Narumi S. (1998) Interleukin-1β induces tissue- and cell type-specific expression of adhesion molecules in vivo. Arterioscler. Thromb. Vasc. Biol. 18, 1292–1303 10.1161/01.ATV.18.8.1292 [DOI] [PubMed] [Google Scholar]
- 9. Kannagi R., Izawa M., Koike T., Miyazaki K., and Kimura N. (2004) Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Science 95, 377–384 10.1111/j.1349-7006.2004.tb03219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Esposito M., Mondal N., Greco T. M., Wei Y., Spadazzi C., Lin S. C., Zheng H., Cheung C., Magnani J. L., Lin S. H., Cristea I. M., Sackstein R., and Kang Y. (2019) Bone vascular niche e-selectin induces mesenchymal-epithelial transition and WNT activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 21, 627–639 10.1038/s41556-019-0309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silva M., Fung R. K. F., Donnelly C. B., Videira P. A., and Sackstein R. (2017) Cell-specific variation in E-selectin ligand expression among human peripheral blood mononuclear cells: implications for immunosurveillance and pathobiology. J. Immunol. 198, 3576–3587 10.4049/jimmunol.1601636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mondal N., Stolfa G., Antonopoulos A., Zhu Y., Wang S. S., Buffone A. Jr., Atilla-Gokcumen G. E., Haslam S. M., Dell A., and Neelamegham S. (2016) Glycosphingolipids on human myeloid cells stabilize E-selectin-dependent rolling in the multistep leukocyte adhesion cascade. Arterioscler. Thromb. Vasc. Biol. 36, 718–727 10.1161/ATVBAHA.115.306748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seshacharyulu P., Ponnusamy M. P., Haridas D., Jain M., Ganti A. K., and Batra S. K. (2012) Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 16, 15–31 10.1517/14728222.2011.648617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mellinghoff I. K., Wang M. Y., Vivanco I., Haas-Kogan D. A., Zhu S., Dia E. Q., Lu K. V., Yoshimoto K., Huang J. H., Chute D. J., Riggs B. L., Horvath S., Liau L. M., Cavenee W. K., Rao P. N., et al. (2005) Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 10.1056/NEJMoa051918 [DOI] [PubMed] [Google Scholar]
- 15. Johnson L. A., Scholler J., Ohkuri T., Kosaka A., Patel P. R., McGettigan S. E., Nace A. K., Dentchev T., Thekkat P., Loew A., Boesteanu A. C., Cogdill A. P., Chen T., Fraietta J. A., Kloss C. C., et al. (2015) Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci. Transl. Med. 7, 275ra22 10.1126/scitranslmed.aaa4963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sackstein R., Merzaban J. S., Cain D. W., Dagia N. M., Spencer J. A., Lin C. P., and Wohlgemuth R. (2008) Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 14, 181–187 10.1038/nm1703 [DOI] [PubMed] [Google Scholar]
- 17. Ballermann B. J., Dardik A., Eng E., and Liu A. (1998) Shear stress and the endothelium. Kidney Int. Suppl. 67, S100–S108 [DOI] [PubMed] [Google Scholar]
- 18. Dagia N. M., Gadhoum S. Z., Knoblauch C. A., Spencer J. A., Zamiri P., Lin C. P., and Sackstein R. (2006) G-CSF induces E-selectin ligand expression on human myeloid cells. Nat. Med. 12, 1185–1190 10.1038/nm1470 [DOI] [PubMed] [Google Scholar]
- 19. Dykstra B., Lee J., Mortensen L. J., Yu H., Wu Z. L., Lin C. P., Rossi D. J., and Sackstein R. (2016) Glycoengineering of E-selectin ligands by intracellular versus extracellular fucosylation differentially affects osteotropism of human mesenchymal stem cells. Stem Cells 34, 2501–2511 10.1002/stem.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thankamony S. P., and Sackstein R. (2011) Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc. Natl. Acad. Sci. U.S.A. 108, 2258–2263 10.1073/pnas.1018064108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdi R., Moore R., Sakai S., Donnelly C. B., Mounayar M., and Sackstein R. (2015) HCELL expression on murine MSC licenses pancreatotropism and confers durable reversal of autoimmune diabetes in NOD mice. Stem Cells 33, 1523–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee J., Dykstra B., Spencer J. A., Kenney L. L., Greiner D. L., Shultz L. D., Brehm M. A., Lin C. P., Sackstein R., and Rossi D. J. (2017) mRNA-mediated glycoengineering ameliorates deficient homing of human stem cell-derived hematopoietic progenitors. J. Clin. Invest. 127, 2433–2437 10.1172/JCI92030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Videira P. A., Silva M., Martin K. C., and Sackstein R. (2018) Ligation of the CD44 glycoform HCELL on culture-expanded human monocyte-derived dendritic cells programs transendothelial migration. J. Immunol. 201, 1030–1043 10.4049/jimmunol.1800188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nair A. B., and Jacob S. (2016) A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 10.4103/0976-0105.177703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sackstein R. (2018) The first step in adoptive cell immunotherapeutics: assuring cell delivery via glycoengineering. Front. Immunol. 9, 3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Day K. C., Lorenzatti Hiles G., Kozminsky M., Dawsey S. J., Paul A., Broses L. J., Shah R., Kunja L. P., Hall C., Palanisamy N., Daignault-Newton S., El-Sawy L., Wilson S. J., Chou A., Ignatoski K. W., et al. (2017) HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 77, 74–85 10.1158/0008-5472.CAN-16-1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knibbs R. N., Craig R. A., Natsuka S., Chang A., Cameron M., Lowe J. B., and Stoolman L. M. (1996) The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J. Cell Biol. 133, 911–920 10.1083/jcb.133.4.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mondal N., Dykstra B., Lee J., Ashline D. J., Reinhold V. N., Rossi D. J., and Sackstein R. (2018) Distinct human α(1,3)-fucosyltransferases drive Lewis-X/sialyl Lewis-X assembly in human cells. J. Biol. Chem. 293, 7300–7314 10.1074/jbc.RA117.000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caruso H. G., Torikai H., Zhang L., Maiti S., Dai J., Do K. A., Singh H., Huls H., Lee D. A., Champlin R. E., Heimberger A. B., and Cooper L. J. (2016) Redirecting T-cell specificity to EGFR using mRNA to self-limit expression of chimeric antigen receptor. J. Immunother. 39, 205–217 10.1097/CJI.0000000000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Banisadr A., Safdari Y., Kianmehr A., and Pourafshar M. (2018) Production of a germline-humanized cetuximab scFv and evaluation of its activity in recognizing EGFR-overexpressing cancer cells. Hum. Vaccin. Immunother. 14, 856–863 10.1080/21645515.2017.1407482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mondal N., Buffone A. Jr., Stolfa G., Antonopoulos A., Lau J. T., Haslam S. M., Dell A., and Neelamegham S. (2015) St3gal-4 is the primary sialyltransferase regulating the synthesis of E-, P-, and L-selectin ligands on human myeloid leukocytes. Blood 125, 687–696 10.1182/blood-2014-07-588590 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.