Abstract
In patients with epilepsy, nonadherence to agreed antiepileptic drug (AED) treatment may result in seizure relapse, and at worst sudden unexpected death. The aim of this study was to examine the extent of both unintentional and intentional nonadherence among Norwegian patients with refractory epilepsy and try to identify possible risk factors. At the National Centre for Epilepsy in Norway, 333 consecutive adult in‐ and outpatients with refractory epilepsy participated in an anonymous survey about adherence to drug treatment. Twenty‐two percentages admitted that they sometimes or often forgot to take their drugs as scheduled, and 19% reported that they, rarely, sometimes or often intentionally did not follow the AED treatment plan agreed upon with their physician. Young age and depression were significantly correlated with unintentional nonadherence. Intentional nonadherence was associated with young age (36 years or younger). We found nonadherence not to be associated with any specific AED. In conclusion, about one‐fifth of patients with refractory epilepsy admitted that they did not adhere to the agreed drug treatment plan, either intentionally or unintentionally. Measures to reduce nonadherence in this patient group may improve seizure control and should be tailored to address both unintentional and intentional lack of adherence.
Keywords: adherence, drug treatment, epilepsy, refractory
1. INTRODUCTION
With a global prevalence of 0.6%‐1.2%, epilepsy is one of the most common neurological disorders.1 About 60%‐70% of patients with epilepsy can become seizure‐free with appropriate treatment with antiepileptic drugs (AEDs).2 The remaining 30%‐40% with drug‐resistant epilepsy often experience psychosocial challenges in addition to recurrent seizures. These patients have an elevated risk of injuries, and at worst sudden unexpected death (SUDEP).
Adherence to treatment is defined as: “the extent to which a person's behaviour – taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider”.3 Consequently, nonadherence to treatment is defined as any deviation from healthcare provider recommendations, both regarding timing or dosage of a prescribed regimen.4 There are many potential consequences of nonadherence to AED treatment plans, including seizure relapse, status epilepticus, hospital admission, and increased healthcare costs. In addition, SUDEP has been related to nonadherence.4, 5, 6, 7, 8, 9
Nonadherence may be due to misunderstandings between the physician and the patient regarding the agreed dosage or medication.10 Chapman et al demonstrated low adherence being related to doubts about AED necessity, concerns about AED treatment, limitations in capability and resources, and perceiving not to be involved in treatment decisions.11 Nonadherence may be unintentional, that is, the patient forgets to take a dose or inadvertently takes an incorrect dosage. However, nonadherence can also be intentional; the patient—for various reasons—chooses not to follow the agreed AED treatment plan.12
We have previously demonstrated nonadherence, intentionally in 30% and unintentionally in 40% of responders in an Internet survey. That cohort was assumed to represent a general epilepsy population.12 Norwegian patients with the most severe epilepsies are referred to the National Centre for Epilepsy. The extent of nonadherence to AED treatment in this subpopulation has not previously been studied. Thus, we were aiming at determining the extent of both unintentional and intentional nonadherence to AED treatment among patients with refractory epilepsy. Additionally, we looked for predisposing factors for nonadherence in this selected patient population.
2. MATERIAL AND METHODS
2.1. Study population and the questionnaire
Consecutive adult patients admitted to the National Centre for Epilepsy in Norway, a tertiary referral center, were invited to complete a questionnaire regarding age, gender, epilepsy and seizure type, seizure frequency, and use of AEDs. In addition, they were asked to respond to a visual analog scale about quality of life (QoL), the Neurological Disorders Depression Inventory for Epilepsy (NDDIE), and the adverse events profile (AEP). Only patients who were considered able to read, understand, and fill out the questionnaire by themselves were recruited for the study. Patients with learning disabilities and patients who did not have sufficient Norwegian language skills were excluded. The time frame for the study was 2015‐2017.
Information on whether the respondents unintentionally or intentionally used AEDs differently than recommended by and agreed upon with their physician was determined from responses to the following two questions: 1) “Do you sometimes inadvertently take your antiepileptic medication differently than agreed upon with your physician?” and 2) “Do you sometimes intentionally (on purpose) take your antiepileptic medication differently than agreed upon with your physician?”
Patients could choose between the following alternative answers for each of these questions based on a four‐point Likert scale: “never,” “rarely,” “sometimes,” or “often.”
During the analysis, we dichotomized the answers into two groups: “never or rarely” vs “sometimes or often” for unintentional nonadherence, and “never” vs “rarely, sometimes, or often” for intentional nonadherence.
The different dichotomization was due to different clinical implications. In unintentional nonadherence, it is of clinical relevance whether this happens rarely or more regularly. Intentional nonadherence on the contrary is a conscious decision to take medication either differently or not at all. In this situation, the willingness of the patients was important, even if would occur seldom.
As the study was anonymous, no ethical approval was required; nevertheless, the study protocol was evaluated by the regional ethics committee (ref. no. :2014/1011A).
2.2. Statistical methods
IBM SPSS Statistics version 25, release 25.0.0.1. (SPSS Inc, Chicago, IL, USA) was used for statistical analyses. All P‐values reported here are based on two‐sided tests, with a significance level of 0.05. To test possible group differences, Pearson's chi‐square tests or independent‐samples t tests were performed. Variables tested in independent‐samples t tests were age, QoL score, NDDIE score, AEP score, and AEP subscores. Independent variables tested for unintentional and intentional nonadherence in Pearson's chi‐square tests were as follows: age (36 years or younger vs 37 years or older), gender, seizure type, seizure frequency (daily or weekly vs less frequently), monotherapy, different AEDs used in monotherapy, polytherapy (3 or more AEDs), NDDIE score > 14, and AEP score > 44.
We applied Hosmer's step‐down procedure, which means that variables that were significant at the 0.25 level were included in the multivariate logistic regression model.13 Odds ratios for factors associated with lack of adherence to the AED treatment plan agreed with the healthcare provider were estimated using bivariate and multivariate logistic regression analysis with 95% confidence intervals.
3. RESULTS
A total of 466 of 513 patients (91%) agreed to participate in the study and completed the questionnaire, either partly or fully. Among these, 333 patients (72%) reported to have experienced seizures during the last 12 months despite the use of AEDs. Further analysis was done with these 333 patients. Demographic and clinical characteristics of the participants are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the study participants (n = 333)
| Characteristics | n (%) | Median (range); Mean (SD) |
|---|---|---|
| Age (y) (N = 331) | 37 (17‐72); 37.7 (13.00) | |
| Female patients (N = 332) | 188 (56.6) | |
| Male patients (N = 332) | 144 (43.4) | |
| Age at first seizure (y) (N = 311) | 15.0 (0‐68); 18.1 (13.53) | |
| Seizure types | ||
| Tonic‐clonic (N = 286) | 198 (69.2) | |
| Seizure with loss of consciousness (N = 289) | 198 (68.5) | |
| Seizure without loss of consciousness (N = 279) | 220 (78.9) | |
| Seizure frequency (N = 333) | ||
| Daily/weekly | 210 (63.1) | |
| Monthly/yearly | 123 (36.9) | |
| Number of AEDs in use (N = 333) | ||
| 1 | 97 (29.1) | |
| 2 | 151 (45.3) | |
| 3 | 69 (20.7) | |
| 4 | 16 (4.8) | |
| NDDIE score (N = 315) | 12 (6‐24); 12.6 (4.11) | |
| AEP score (N = 283) | 44 (19‐71); 42.8 (10.84) | |
| AED used | Polytherapy (N = 333) n (%) | Monotherapy (N = 97) n (%) |
| Lamotrigine | 132 (39.6) | 35 (36.1) |
| Valproate | 107 (32.1) | 15 (15.5) |
| Levetiracetam | 94 (28.2) | 13 (13.4) |
| Oxcarbazepine | 51 (15.3) | 7 (7.2) |
| Lacosamide | 42 (12.6) | 1 (1) |
| Zonisamide | 40 (12.0) | 2 (2.1) |
| Carbamazepine | 33 (9.9) | 4 (4.1) |
| Topiramate | 31 (9.3) | 5 (5.2) |
| Eslicarbazepine | 31 (9.3) | 6 (6.2) |
| Clobazam | 30 (9.0) | 1 (1) |
| Perampanel | 24 (7.2) | 0 |
| Clonazepam | 13 (3.9) | 5 (5.2) |
| Phenobarbital | 7 (2.1) | 1 (1.0) |
| Phenytoin | 6 (1.8) | 1 (1.0) |
| Vigabatrin | 4 (1.2) | 0 |
| Ethosuximide | 3 (0.9) | 1 (1.0) |
| Gabapentin | 2 (0.6) | 0 |
| Pregabalin | 2 (0.6) | 0 |
| Brivaracetam | 1 (0.3) | 0 |
| Diazepam | 1 (0.3) | 0 |
| Sulthiame | 1 (0.3) | 0 |
| Acetazolamide | 1 (0.3) | 0 |
N, Number of responses to each question.
Of the 321 patients who answered the question on unintentional nonadherence, 72 (22%) reported that they sometimes or often forgot to take their drugs as scheduled. Of the 325 patients who answered the question on intentional nonadherence, 61 (19%) reported that on some occasions they intentionally did not follow the AED treatment plan agreed upon with their physician (Figure 1). There were 36 patients who responded positively to both questions.
Figure 1.
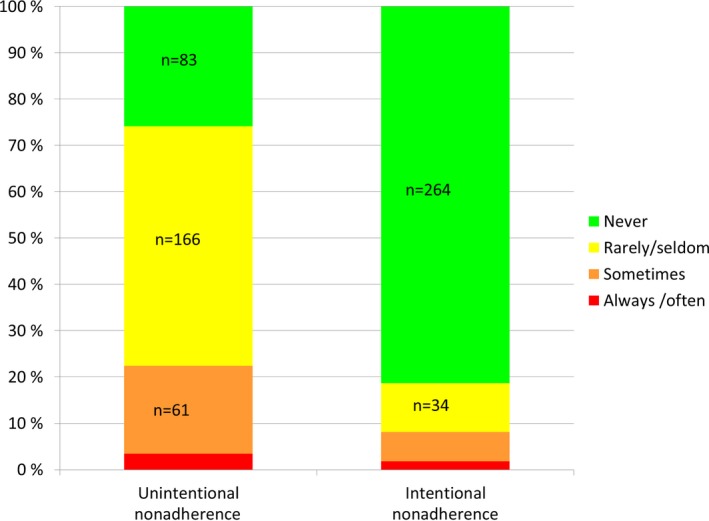
Results (%) of reported unintentional (n = 321) and intentional (n = 325) nonadherence
Of 22 different AEDs, the most commonly used drugs were lamotrigine, valproate, and levetiracetam, used as mono‐ or polytherapy. These three drugs were used by 65% of the patients in monotherapy.
Unintentional nonadherence was significantly correlated with young age (mean 32.7; SD 11.94) (mean difference 6.44; confidence interval [CI] 3.038‐9.832; P < .001) and higher NDDIE score (mean difference 1.41; CI 0.289‐2.530; P = .014). Intentional nonadherence was correlated only with young age (mean 33.7; SD 11.71; mean difference 5.17; CI 1.532‐8.800; P = .005).
The following independent variables were significantly associated with unintentional nonadherence in logistic regression analysis: scoring 15 or higher in the NDDIE (odds ratio [OR] 2.03; CI 1.060‐3.903; P = .033) and being younger than the median age (36 years or younger) (OR 2.309; CI 1.222‐2.309; P = .010).
The only independent factor significantly associated with intentional nonadherence was being younger than the median age (OR 2.46; CI 1.252‐4.808; P = .009). We found no association between both intentional or unintentional nonadherence and the following factors: gender, seizure type, seizure frequency (daily or weekly vs less frequently), monotherapy, different AEDs used in monotherapy, polytherapy (3 or more AEDs), NDDIE score >14, and AEP score >44.
There were no differences between men and women regarding nonadherence.
4. DISCUSSION
The main result from this survey among Norwegian patients with refractory epilepsy was that approximately one in five rarely, sometimes, or often makes the conscious decision not to follow the AED treatment plan as agreed with their neurologist, but rather decides to take their AEDs differently than prescribed. Also, about one‐fifth reported that they sometimes or often forgot to take their AEDs as scheduled.
Unintentional nonadherence was associated with young age and symptoms of depression, while intentional nonadherence was associated with young age.
Most publications on adherence to AED treatment do not differentiate between unintentional and intentional nonadherence. We believe this distinction is important when addressing measures to improve the treatment of this patient group.12
In previous studies, there are considerable variations regarding estimates of poor adherence to treatment in epilepsy populations. A recent review reported nonadherence in 26%‐79% of patients.4 Different study populations, different definitions of adherence, and different methods to measure nonadherence may account for the wide variability.
We found a correlation between nonadherence and young age and symptoms of depression. This is in line with other studies.7, 14, 15 In contrast to other studies, we did not find a high score of adverse events15, 16 to be a risk factor for nonadherence, neither did we find male gender4, 12 to be a risk factor for intentional nonadherence. We did not find a correlation to either monotherapy or polytherapy. It has been shown that the number of AED doses per day twice or more can have a negative correlation with adherence15, 17. But this is not necessarily dependent of the number of AED in use which could explain our findings.
In various studies on nonadherence, the rate of seizure‐freedom in the respective populations either is not included15 or around 30%.11, 16 Studies on nonadherence to treatment in refractory epilepsy are to our knowledge sparse. A study from the United States, defining refractory epilepsy as those currently using three or more AEDs, regardless of seizure rate, revealed a significant higher rate of adherence to treatment in these patients compared to patients using fewer AED.18
The ILAE task force defined drug‐resistant epilepsy in 2010 as “failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules”.19 Unfortunately, we did not have sufficient information on previously tried AEDs among our patients to apply this definition. We chose to define refractory epilepsy as having had seizures the last year despite the use of AEDs.
A previous open online survey of an epilepsy cohort consisting of 40% seizure‐free patients disclosed that 40% reported unintentional and 30% reported intentional nonadherence to treatment, that is, a higher extent of nonadherence than in the present study.12 In contrast to the previous study where 40% of respondents were seizure‐free during the preceding 12 months, none of the patients in this were seizure‐free. 63% had daily or weekly seizures, the rest monthly or seizures at least once during the last year.
The reasons why patients with refractory epilepsy are more adherent to treatment than those with more easy‐to‐treat epilepsy are not known. With earlier epilepsy onset (median debut at 15 vs 21 years old), regular AED intake may have become an established daily routine. Moreover, those with refractory epilepsy are usually followed more closely by an epileptologist and are probably provided with more thorough information on the necessity for carefully following the agreed AED treatment schedule. Also, fear of sudden unexpected death and more regular therapeutic drug monitoring might better adherence.
For clinicians, the results of this study underline the importance of keeping an eye on nonadherence and implement measures to improve adherence.
Clinical implications of this study point to the importance of acknowledging variable adherence as a factor contributing to variability and poor seizure control.
Further studies are needed to clarify in more detail the reasons for intentional nonadherence.
4.1. Limitations of the study
As the majority of patients were using AED polytherapy, possible correlations between individual AEDs and nonadherence were difficult to detect. Another obvious source of error in studies on nonadherence is that patients might be reluctant to admit nonadherence, whether intentional or unintentional. Even if 96% and 98% of patients had answered the questions on adherence, they might have answered according to what they feel physicians would expect. As patients reported intentionally to take their antiepileptic medication differently than agreed upon with their physician, this could result in a lower or higher dosage, leading to a change in daily dosing regimen but not the total daily dosage.
While we did not recruit patients with learning disabilities, we cannot exclude that some of the patients participating could be living in a nursing home or other institution where there is some additional control on the administration of AEDs.
Ideally, we should have used the definition of drug‐resistant epilepsy by the ILAE task force: “…failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules…”.18 For this, we would have needed information on previously used antiseizure medication (“appropriately chosen”) and dosage, serum concentrations, and possible adverse events of actual and earlier used antiseizure medication (“tolerated, appropriately … used”). As the study is anonymous and based on information available from the questionnaires the respondents have filled in this information is not available. All patients included reported to have had at least one seizure during the last 12 months; due to the described lack of information, we do not know whether seizures could be due to nonadherence or inadequately dosing.
5. CONCLUSIONS
The results from this study demonstrate that in a cohort of patients with refractory epilepsy, about one in five patients is nonadherent to their AED treatment regimen, either intentionally (rarely, sometimes, or often) or unintentionally (sometimes or often). Being of young age is the main risk factor. Minimizing nonadherence is important for improving seizure control and thereby reducing the risk of seizure‐related complications in this patient group.
CONFLICT OF INTEREST
Dr Henning has served as a paid consultant for Eisai, UCB, and LivaNova, outside the submitted work. Dr Lossius has served as a paid consultant for ESAI and UCB, outside the submitted work. The remaining authors have no conflicts of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENT
We thank Lucy Robertson for the critical reading and feedback of this paper.
Henning O, Lossius MI, Lima M, et al. Refractory epilepsy and nonadherence to drug treatment. Epilepsia Open. 2019;4:618–623. 10.1002/epi4.12367
REFERENCES
- 1. Helmers SL, Thurman DJ, Durgin TL, Pai AK, Faught E. Descriptive epidemiology of epilepsy in the U.S. population: a different approach. Epilepsia. 2015;56:942–8. [DOI] [PubMed] [Google Scholar]
- 2. Brodie MJ, Barry SJE, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Adherence to Long‐Term Therapies: Evidence for Action, 2003. http://www.who.int/chronic_conditions/en/adherence_report.pdf. (accessed 15 Nov 2018).
- 4. Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135:507–15. [DOI] [PubMed] [Google Scholar]
- 5. Alvarez V, Westover MB, Drislane FW, Dworetzky BA, Curley D, Lee JW, et al. Evaluation of a clinical tool for early etiology identification in status epilepticus. Epilepsia. 2014;55:2059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia. 2008;49:446–54. [DOI] [PubMed] [Google Scholar]
- 7. Samsonsen C, Reimers A, Bråthen G, Helde G, Brodtkorb E. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55:e125–128. [DOI] [PubMed] [Google Scholar]
- 8. Manjunath R, Davis KL, Candrilli SD, Ettinger AB. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14:372–8. [DOI] [PubMed] [Google Scholar]
- 9. Faught E, Duh MS, Weiner JR, Guerin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology. 2008;71:1572–8. [DOI] [PubMed] [Google Scholar]
- 10. Mevaag M, Henning O, Baftiu A, Granas AG, Johannessen SI, Nakken KO, et al. Discrepancies between physicians' prescriptions and patients' use of antiepileptic drugs. Acta Neurol Scand. 2017;135:80–7. [DOI] [PubMed] [Google Scholar]
- 11. Chapman SCE, Horne R, Eade R, Balestrini S, Rush J, Sisodiya SM. Applying a perceptions and practicalities approach to understanding nonadherence to antiepileptic drugs. Epilepsia. 2015;56:1398–407. [DOI] [PubMed] [Google Scholar]
- 12. Henning O, Johannessen Landmark C, Nakken KO, Lossius MI. Nonadherence to treatment regimens in epilepsy from the patient's perspective and predisposing factors: differences between intentional and unintentional lack of adherence. Epilepsia. 2019;60:e58–e62. [DOI] [PubMed] [Google Scholar]
- 13. Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & sons; 2000: pp. 89–150. [Google Scholar]
- 14. Ettinger AB, Good MB, Manjunath R, Edward Faught R, Bancroft T. The relationship of depression to antiepileptic drug adherence and quality of life in epilepsy. Epilepsy Behav. 2014;36:138–43. [DOI] [PubMed] [Google Scholar]
- 15. May TW, Berkenfeld R, Dennig D, Scheid B, Hausfeld H, Walther S, et al. Patients' perspectives on management and barriers of regular antiepileptic drug intake. Epilepsy Behav. 2018;79:162–8. [DOI] [PubMed] [Google Scholar]
- 16. Laville F, Montana M, Roux N, Rathelot P, Giorgi R, Vanelle P. Factors limiting adherence to antiepileptic treatment: a French online patient survey. J Clin Pharm Ther. 2018;43:73–9. [DOI] [PubMed] [Google Scholar]
- 17. Gollwitzer S, Kostev K, Hagge M, Lang J, Graf W, Hamer HM. Nonadherence to antiepileptic drugs in Germany: a retrospective, population‐based study. Neurology. 2016;87:466–72. [DOI] [PubMed] [Google Scholar]
- 18. Gupte‐Singh K, Wilson JP, Barner JC, Richards KM, Rascati KL, Hovinga C. Patterns of antiepileptic drug use in patients with potential refractory epilepsy in Texas Medicaid. Epilepsy Behav. 2018;87:108–16. [DOI] [PubMed] [Google Scholar]
- 19. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77. [DOI] [PubMed] [Google Scholar]


