Abstract
Background
In the elderly, kidney transplantation is associated with increased survival and improved health-related quality of life compared with dialysis treatment. We aimed to study the short-term health economic effects of transplantation in a population of elderly kidney transplant candidates.
Methods
Self-perceived health, quality-adjusted life years (QALYs) and costs were evaluated and compared 1 year before and 1 year after kidney transplantation in patients included in a single-centre prospective study of 289 transplant candidates ≥65 years of age.
Results
Self-perceived health and QALYs both significantly improved after transplantation. At 1 year, the costs per QALY were substantially higher for transplantation (€88 100 versus €76 495), but preliminary analyses suggest a favourable long-term health economic effect.
Conclusions
Kidney transplantation in older kidney transplant recipients is associated with improved health but also with increased costs the first year after engraftment when compared with remaining on the waiting list. Any long-term cost-effectiveness needs to be confirmed in studies with longer observation times.
Keywords: dialysis, elderly, kidney transplantation, QALY, quality of life
INTRODUCTION
In the elderly, kidney transplantation is an expensive treatment with increased morbidity and mortality in the early post-operative phase. However, in patients with end-stage renal disease (ESRD), successful kidney transplantation increases long-term survival and is superior to long-term dialysis [1–3]. It has recently been documented that older kidney transplant recipients report better health-related quality of life (HRQoL) compared with patients on the transplant waiting list [4].
A limiting factor for offering kidney transplantation to all ESRD patients is the scarcity of organs. Consequently, the waiting time for an organ is long in most countries [5, 6]. Several efforts have been made to increase the number of donors [7–11], but the shortage remains. Many centres are therefore reluctant to enlist elderly patients.
In a recent paper from Sweden, the overall costs for dialysis treatment and kidney transplantation were compared over a 10-year period. The authors concluded that 66–79% of the expected health care costs over 10 years were avoided through kidney transplantation [12] and that kidney transplantation is more cost effective compared with long-term dialysis treatment in patients of all ages.
Quality-adjusted life years (QALYs) is a measure of health that includes both the quality and duration of life. In the evaluation of different treatment alternatives, a cost–utility analysis with comparisons of cost per QALY for each alternative should be performed. Lower cost per QALY has been described for adult patients after kidney transplantation when compared with dialysis [13]. However, the mean age of the transplant population in that study was 43.7 ± 12.5 years, indicating that a small number of older patients were included.
The aims of the present study were to evaluate the 1-year health effect of kidney transplantation expressed as self-perceived health and QALYs in a population of older (≥65 years) patients listed for kidney transplantation at the Norwegian national transplant centre and estimate the cost-effectiveness for transplantation compared with dialysis in a short-term perspective.
MATERIALS AND METHODS
Data were retrieved from a study database (QUESTION65) including 289 patients ≥65 years of age who were accepted for kidney transplantation at the Norwegian national transplant centre at Oslo University Hospital. The study was primarily initiated to evaluate HRQoL by the Kidney Disease Quality of Life Short Form questionnaire [14] from patients on the transplant waiting list and until 5 years post-transplant [4, 15]. During the inclusion period, 66% of all Norwegian eligible wait-listed patients ≥65 years of age were included. Apart from the fact that those not included were slightly younger than those who were included (69.8 ± 4.1 versus 71.1 ± 4.1 years), no major differences were observed [4]. The regional ethics committee approved the study and all patients included signed an informed consent form. Data retrieval for the current cost–utility study was performed on 5 February 2018.
For health–utility analyses, 36-item Short Form Health Survey (SF-36) data were converted to the 6-dimension Short Form (SF-6D). Based on the SF-6D, a preference-based single utility index (SF-Index) was derived as a measure of health [16–19]. An SF-Index of 0 is equivalent to death, whereas an SF-Index of 1 indicates perfect health. Pre-transplant SF-Indices were derived based on the SF-6D collected at baseline and at 6 and 12 months after baseline. Post-transplant SF-Indices were derived at 2, 6 and 12 months after transplantation.
QALYs were computed for 1 year on the transplant waiting list and 1 year after kidney transplantation. In the calculation of individual QALYs, any missing SF-Index value was imputed, if possible, as the mean of existing values before and after the missing value. Patients with no valid pre-transplant SF-Index values were excluded from all QALY analyses (n = 4). Patients with no valid post-transplant SF-Index values were excluded from post-transplant QALY analyses (n = 25). Patients who were censored had their last value before censoring imputed for the rest of the interval. Patients who died during the observation period were given an SF-Index value of 0 from the time of death and through the rest of the year. The mean value of the SF-Index at the margins of each time interval was considered a representative for that interval and these mean values were summarized to individual QALYs for 1 year on the waiting list/post-transplantation. The mean of all individual QALYs was then tested statistically for patients on the waiting list versus transplanted patients.
Direct economic costs were not available for the study population and hence values reported from a similar population in Sweden by Jarl et al. [12] were used. The Swedish data did not discriminate between pre-emptive and dialysis patients on the waiting list (J. Jarl, personal communication, 18 October 2018). We have consequently used the same costs for all patients on the waiting list, independent of their dialysis status.
The median survival was imported from the Norwegian Renal Registry for patients ≥65 years of age who were either listed for transplantation between 2012 and 2017 (n = 437) or received a kidney transplant between 2007 and 2017 (n = 689). Survival data were retrieved on 6 December 2018. Survival on the waiting list was counted from the date of wait-listing until death or censoring due to transplantation or date of survival data retrieval. Post-engraftment survival was counted from the time of transplantation until death or censoring due to the date of data retrieval. According to these data, the median survival on the waiting list without transplantation was 4.4 years versus 8.1 years for those who received a transplant (A. Åsberg, personal communication, leader of the Norwegian Renal Registry). No statistical comparison of survival was performed.
Statistics
Normally distributed continuous data were reported as mean ± standard deviation (SD) and compared using an independent t-test. The last SF-Index before transplantation was compared with the SF-Index at 1 year using a paired t-test. Skewed continuous data were reported as median (range) and compared using a Mann–Whitney test. Categorical data were reported as number (%) and compared using Fisher’s exact test. All statistical tests were two-sided. Statistical significance was defined as a P-value <0.05. All statistical analyses were performed using the SPSS for Windows version 25 (IBM, Armonk, NY, USA).
RESULTS
In total, 205 of the 289 patients (71%) included in QUESTION65 received a transplant during the observation period. The median waiting time among those who received a transplant was 14.6 months (range 0.3–71.9); 150 recipients were on dialysis during the waiting time and their median time on dialysis was 24.0 months (range 2.2–95.3).
SF-Index
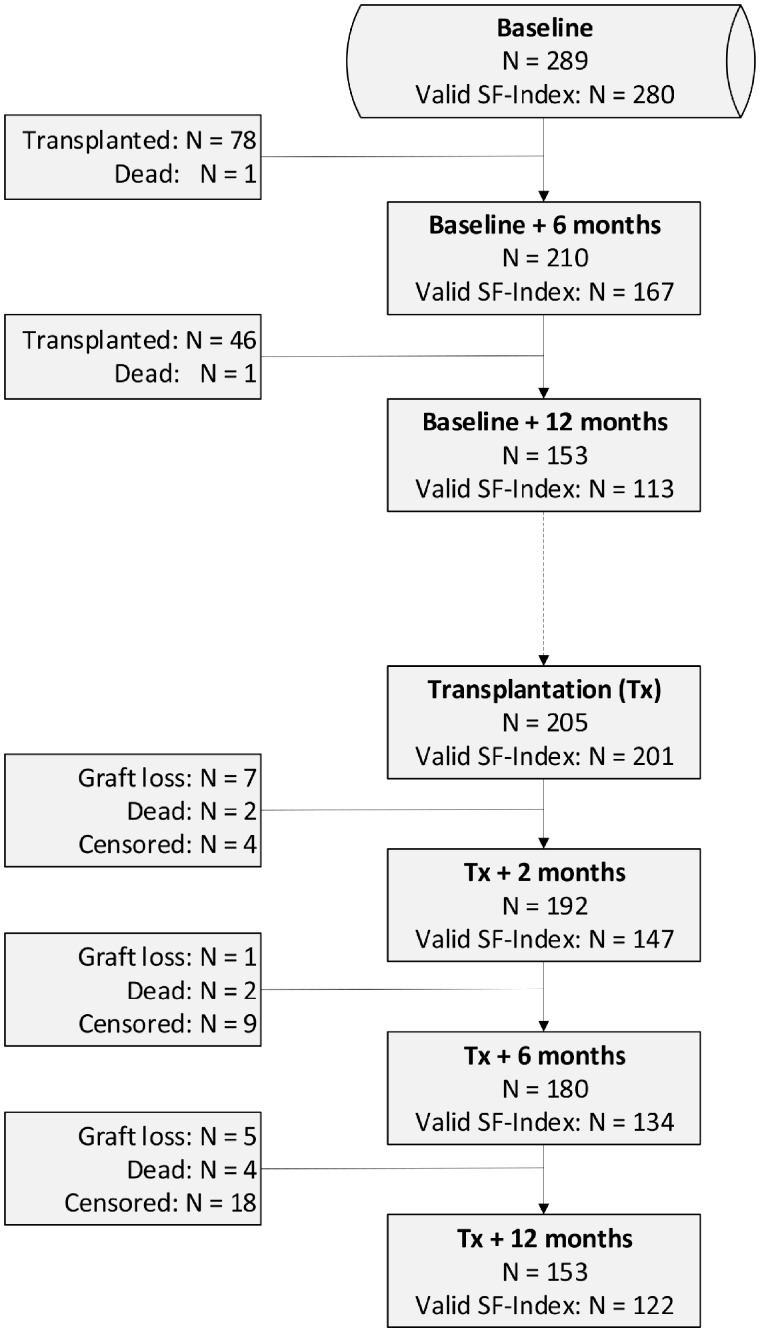
By 5 February 2018, 153 patients had been followed for 1 year post-transplant and 132 patients (86%) had completed the 1-year HRQoL questionnaire. Twelve patients were excluded because of missing values, interfering with the calculation of the SF-Index, leaving 120 patients (78%) for the comparative analyses of SF-Index. Among the 80 patients who were on dialysis at their last visit before transplantation, 60 (75%) received haemodialysis and 20 (25%) received peritoneal dialysis. The median time on dialysis for these recipients was 20.0 months (range 2.2–62.7) for haemodialysis and 20.0 months (range 6.1–44.1) for peritoneal dialysis (P = 0.313). Further demographic and clinical data for the SF-Index comparative cohort (n = 120) are presented in Table 1. Figure 1 shows a flow chart describing the population at different times. The SF-Index at 12 months post-transplantation was significantly higher than the SF-Index at the last visit before transplantation. This was valid both for pre-emptive and dialysis patients (Table 2).
Table 1.
Baseline clinical and demographic data of the comparative study population
| Variable | Total (n = 120) | Pre-emptive (n = 40) | On dialysis (n = 80) | P-value |
|---|---|---|---|---|
| Age (years) | 70.8 ± 4.2 | 70.5 ± 3.9 | 71.0 ± 4.3 | 0.527 |
| Age at transplantation (years) | 71.5 ± 4.2 | 71.2 ± 3.9 | 71.7 ± 4.4 | 0.513 |
| Male, n (%) | 86 (72) | 24 (60) | 62 (78) | 0.055 |
| Liu comorbidity index | 2.6 ± 2.4 | 1.5 ± 1.4 | 3.2 ± 2.6 | <0.001 |
| Waiting time (months) | 13.3 ± 9.1 | 12.6 ± 9.9 | 13.6 ± 8.7 | 0.524 |
| Donor age (years) | 63.9 ± 11.3 | 64.0 ± 12.1 | 63.8 ± 11.0 | 0.923 |
| SF-36 PCS | 54.3 ± 22.7 | 57.3 ± 22.3 | 52.8 ± 22.9 | 0.311 |
| SF-36 MCS | 65.7 ± 19.6 | 65.6 ±18.5 | 65.8 ± 20.3 | 0.974 |
| KDCS | 75.0 ± 11.7 | 79.8 ± 10.1 | 72.7 ±11.9 | 0.001 |
| Living donor, n (%) | 25(21) | 9 (23) | 16 (20) | 0.813 |
| Married, n (%) | 97 (81) | 30 (75) | 67 (84) | 0.325 |
| Divorced, n (%) | 6 (5) | 1 (3) | 5 (6) | 0.662 |
| Widow, n (%) | 13 (11) | 7 (18) | 6 (7.5) | 0.122 |
| Single, n (%) | 4 (3) | 2 (5) | 2 (3) | 0.600 |
| Cause of CKD, n (%) | ||||
| Glomerulonephritis | 34 (28) | 6 (15) | 28 (35) | 0.031 |
| Pyelonephritis | 7 (6) | 2 (5) | 5 (6) | 1.000 |
| Interstitial nephritis | 4 (3) | 2 (5) | 2 (3) | 0.600 |
| APKD | 12 (10) | 7 (18) | 5 (6) | 0.102 |
| Vascular disease | 44 (37) | 17 (43) | 27 (34) | 0.422 |
| Diabetes | 8 (7) | 3 (8) | 5 (6) | 1.000 |
| Other | 11 (9) | 3 (8) | 8 (10) | 0.750 |
| Comorbidity, n (%) | ||||
| Liu score <3 | 85 (71) | 36 (90) | 49 (61) | 0.001 |
| Liu score 4–6 | 27 (23) | 4 (10) | 23 (29) | 0.022 |
| Liu score 7–9 | 7 (6) | 0 | 7 (8) | 0.094 |
| No comorbidity | 29 (24) | 14 (35) | 15 (19) | 0.07 |
| Ischaemic heart disease | 33 (28) | 9 (23) | 24 (30) | 0.516 |
| Heart failure | 7 (6) | 0 | 7 (9) | 0.094 |
| Arrhythmia | 8 (7) | 0 | 8 (10) | 0.051 |
| Other heart disease | 16 (13) | 2 (5) | 14 (18) | 0.086 |
| CVD | 21 (18) | 5 (13) | 16 (20) | 0.445 |
| PVD | 14 (12) | 6 (15) | 8 (10) | 0.547 |
| GI bleeding | 16 (13) | 2 (5) | 14 (18) | 0.086 |
| Diabetes | 28 (23) | 9 (23) | 19 (24) | 1.000 |
| Cancer | 26 (22) | 4 (10) | 22 (28) | 0.034 |
| COPD | 9 (8) | 0 | 9 (11) | 0.028 |
Values are presented as mean ± SD unless stated otherwise. P-values represent statistical comparison between pre-emptive and dialysis patients according to their dialysis status at the last visit before transplantation.
SF-36 PCS: 36-item Short Form Physical Component Score; SF-36 MCS, 36-item Short Form Mental Component Score; KDCS, Kidney Disease Component Score; CKD, chronic kidney disease; APKD, adult polycystic kidney disease; CVD, cerebrovascular disease; PVD, peripheral vascular disease; GI, gastrointestinal; COPD, chronic obstructive pulmonary disease.
FIGURE 1.
Flow chart of patients.
Table 2.
SF-Index comparison between pre-transplant value (mean ± SD) and 2 months, 6 months and 12 months post-transplant
| Pre-emptive |
On dialysis |
Total |
||||
|---|---|---|---|---|---|---|
| n | SF-Index | n | SF-Index | n | SF-Index | |
| Tx0 | 58 | 0.688 ± 0.11 | 143 | 0.684 ± 0.11 | 201 | 0.685 ± 0.11 |
| Tx + 2 | 47 | 0.724 ± 0.12 | 98 | 0.715 ± 0.11 | 145 | 0.718 ± 0.11 |
| P | 0.083 | 0.246 | 0.047 | |||
| Tx + 6 | 43 | 0.739 ± 0.12 | 89 | 0.747 ± 0.13 | 132 | 0.745 ± 0.13 |
| P | 0.005 | <0.001 | <0.001 | |||
| Tx + 12 | 40 | 0.762 ± 0.13 | 80 | 0.746 ± 0.15 | 120 | 0.751 ± 0.14 |
| P | <0.001 | 0.004 | <0.001 | |||
Statistical comparisons were performed using a paired t-test including only pairs with a valid SF-Index at the time points compared.
Tx0, last questionnaire before transplantation; Tx + 2, 2 months post-transplantation; Tx + 6, 6 months post-transplantation; Tx + 12, 12 months post-transplantation; Pre-emptive, patients not on dialysis at Tx0; On dialysis, patients on dialysis at Tx0.
A complementary analysis of the SF-Index including all patients with a valid index at each time of visit was also performed to evaluate any differences between pre-emptive patients and patients on dialysis (Table 3, Figure 2). There were no statistically significant differences between pre-emptive patients and patients on dialysis. Furthermore, SF-Index values decreased on the waiting list but increased post-transplant.
Table 3.
SF-Index computed for all patients with valid values at each point in time
| Pre-emptive |
On dialysis |
Total |
P-value | ||||
|---|---|---|---|---|---|---|---|
| n | SF-Index | n | SF-Index | n | SF-Index | ||
| Baseline | 89 | 0.714 ± 0.11 | 191 | 0.686 ± 0.11 | 280 | 0.695 ± 0.11 | 0.054 |
| Baseline + 6 | 40 | 0.685 ± 0.12 | 127 | 0.691 ± 0.12 | 167 | 0.689 ± 0.12 | 0.800 |
| Baseline + 12 | 29 | 0.686 ± 0.11 | 84 | 0.672 ± 0.13 | 113 | 0.675 ± 0.12 | 0.589 |
| Tx0 | 58 | 0.688 ± 0.11 | 143 | 0.684 ± 0.11 | 129 | 0.692 ± 0.11 | 0.804 |
| Tx + 2 | 48 | 0.727 ± 0.12 | 99 | 0.717 ± 0.11 | 147 | 0.720 ± 0.11 | 0.618 |
| Tx + 6 | 44 | 0.741 ± 0.12 | 90 | 0.749 ± 0.13 | 134 | 0.747 ± 0.13 | 0.706 |
| Tx + 12 | 41 | 0.763 ± 0.13 | 81 | 0.748 ± 0.15 | 122 | 0.753 ± 0.14 | 0.570 |
Statistical comparisons were performed between patients with respect to their dialysis status at the given point in time.
Baseline, time of first questionnaire; Baseline + 6, 6 months after baseline; Baseline + 12, 12 months after baseline; Tx0, last questionnaire before transplantation; Tx + 2, 2 months post-transplantation; Tx + 6, 6 months post-transplantation; Tx + 12, 12 months post-transplantation; Off dialysis, pre-transplant not on dialysis at specified time points, post-transplant not on dialysis at Tx0; On dialysis, pre-transplant on dialysis at specified time points, post-transplant on dialysis at Tx0.
FIGURE 2.
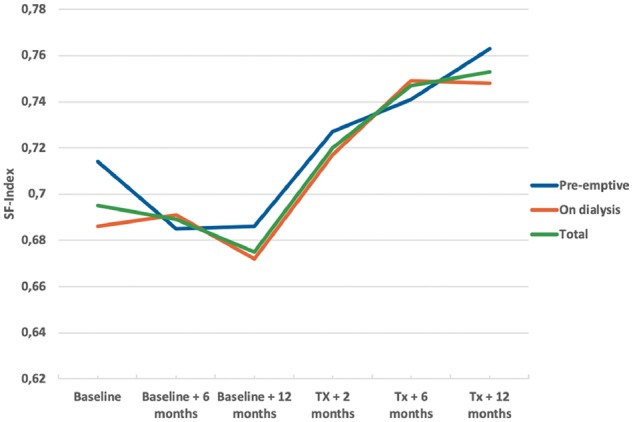
SF-Index from baseline until 1 year after transplantation.
QALYs
Since there was no statistical difference in the SF-Index between pre-emptive and dialysis patients (Table 3), we chose to include all patients in the calculation of QALYs. In total, 4 of 289 patients were excluded from the pre-transplant and 29 of 205 patients were excluded from the post-transplant QALY evaluations because of missing values; consequently 285 and 176 patients were included in the pre- and post-transplant QALY analyses, respectively. The mean SF-Index for each interval of the first year on the waiting list and post-transplant are presented in Table 4. The first year on the waiting list represents 0.686 ± 0.11 QALYs compared with 0.710 ± 0.14 QALYs for the first year post-transplant (P = 0.047).
Table 4.
SF-Index and corresponding QALYs in different time intervals on waiting list and after transplantation
| Interval | n | SF-Index | QALYs |
|---|---|---|---|
| Baseline–Baseline + 6 months | 285 | 0.691 ± 0.11 | 0.345 ± 0.05 |
| Baseline + 6–Baseline + 12 months | 285 | 0.681 ± 0.12 | 0.340 ± 0.06 |
| QALYs 1 year on the waiting list | 0.686 ± 0.11 | ||
| Tx0–Tx + 2 months | 176 | 0.700 ± 0.10 | 0.117 ± 0.02 |
| Tx + 2 months–Tx + 6 months | 176 | 0.713 ± 0.14 | 0.238 ± 0.05 |
| Tx + 6 months–Tx + 12 months | 176 | 0.715 ± 0.16 | 0.357 ± 0.08 |
| QALYs 1 year post-engraftment | 0.710 ± 0.14 |
Values are presented as mean ± SD. Missing values were imputed as described in the ‘Materials and Methods’ section.
To explore the effect of imputation of missing SF-Index values described in the ‘Materials and Methods’ section, we performed a sensitivity analysis in which all patients observed <1 year were excluded. This analysis resulted in a QALYs of 0.713 ± 0.14 for the first year after transplantation and 0.686 ± 0.11 for 1 year on the waiting list. This indicates that our original method with imputation of missing values did not overestimate the positive effect of transplantation.
Cost per QALY
Based on data from a Swedish study [12], the expected cost for the first year after transplantation is €62 551. For patients on the waiting list, the expected cost is estimated to be €52 476. The cost per QALY for the first year after transplantation is consequently €62 551/0.710 = €88 100 compared with €52 476/0.686 = €76 495 for the first year on the waiting list.
Incremental cost-effectiveness ratio (ICER)
According to these figures, the ICER for 1 year after transplantation is (62 551–52 476)/(0.710–0.686) = €419 792/QALY (i.e. the cost for each QALY gained by transplantation).
DISCUSSION
It is important to understand the costs and benefits of transplantation to determine whether elderly patients with ESRD should be offered this treatment option. In the present study we found that self-perceived health in older kidney transplant recipients improves shortly after transplantation. In addition, our results indicate that kidney transplantation in older age offers the patient a QALYs gain after 1 year. However, according to the ICER, transplantation is not cost effective the first year after transplantation.
Jarl et al. [12] recently described that kidney transplantation saves from €41 000/year (Year 2 and 3) to €34 000/year (Year 10) compared with no transplantation, independent of patient age. In their analysis, the reduced costs after transplantation are evident from Year 2. A preliminary analysis of HRQoL data from the 64 patients who completed the 3-year survey indicates that the SF-Index remains at the same level at least within the first 3 years (data not shown). For this age group, estimated survival post-engraftment is, according to data from the Norwegian Renal Registry, 8 years compared with ∼4 years for those not receiving a transplant. According to Jarl et al. [12], 4-years survival on the waiting list constitutes a cost of €209 904, while 8 years survival post-engraftment constitutes a cost of €148 131. Consequently, it should be anticipated that kidney transplantation will be long-term cost effective since QALYs should be higher and costs lower. However, this should be confirmed in studies with longer observation times.
In a publication from 2008, Kontodimopoulos and Niakas [13] analysed lifelong QALYs in patients on renal replacement therapy by treatment modality. They included 642 patients on haemodialysis, 65 patients on peritoneal dialysis and 167 patients after kidney transplantation. The mean age was 58 years for dialysis patients and 44 years for transplant recipients. Cost per QALY for haemodialysis was €60 353, for peritoneal dialysis it was €54 504 and for kidney transplantation it was €45 523 the first year, declining to €21 322 at 3 years post-transplant, resulting in a lifelong cost of €11 981/QALY, which is lower than our estimates. This may be explained by inflation and different costs in Scandinavia and Greece. Perhaps the most important difference between the two studies is that while we used data from the same patients before and after transplantation, they used cross-sectional data obtained only once and from different cohorts. In our opinion, their design introduces an important selection bias exemplified by a significant age difference between transplant recipients and patients on dialysis. Calculation of the exact costs for each patient was not performed in any of the studies. Kontodimopoulos and Niakas [13] obtained national cost data from Greek dialysis facilities and from a report by the Hellenic National Transplant Organization, while we based our study on cost data obtained from our closest neighbouring country.
Offering kidney transplantation to an elderly population may result in increased waiting time for younger patients, thereby introducing increased costs to this group of patients who in many cases are employed and produce income to the society. However, increased use of organs from older deceased donors for older recipients [7, 8, 20] may counteract this negative effect since these organs often are found unsuitable for younger patients.
Except from a borderline significant difference at baseline, we did not find any difference in the SF-Index between pre-emptive patients and dialysis patients on the waiting list. A possible explanation may be that pre-emptive patients experience several symptoms of uraemia that influence their perception of health. Patients on dialysis should have fewer uraemic symptoms as the uraemic toxins are removed in dialysis, but on the other hand, their perception of health may be impaired by the dialysis treatment itself. It is also possible that the SF-Index is not sensitive enough to capture these subtle changes.
Our results support the practice of no formal upper age limit for acceptance to the transplant waiting list. Even though, according to our study, kidney transplantation is associated with higher costs the first year, it should be expected to be more cost effective than dialysis in the long run. It should be noted that the survival benefits described in previous studies are based on data published between 1999 and 2010 including patients who received their transplant in the early 1990s [1–3]. Since then, several medical improvements have been introduced, especially in the treatment of cardiovascular diseases [21], the most important cause of death in patients with advanced chronic kidney disease [22]. In addition, the prevalence of smoking has been markedly reduced in the same period, at least in the western world [23]. It is therefore possible that patients starting renal replacement therapy at that time had different risks than those starting renal replacement therapy today. Kaballo et al. [24] recently described that even though adult transplant recipients had better long-term survival than patients on dialysis, the time to ‘death risk equilibration’ was longer than that described in previous studies. In a recent publication from France, Legeai et al. [25] found no clear survival benefit of transplantation in recipients ≥70 years of age compared with patients remaining on the waiting list. In this setting, a cost–utility analysis demonstrating that kidney transplantation provides improved health for lower costs than dialysis is an important argument when discussing criteria for acceptance to transplantation.
Our study has some limitations. Most importantly, we did not have available costs for Norwegian patients but chose to use costs from a recent Swedish publication. This may reduce the accuracy of our results, but since Norway and Sweden are very similar both with respect to socio-economic status and organization of the health care system, it is reasonable to assume that the costs are comparable and that any bias should be evenly distributed between dialysis and transplantation. Furthermore, we have no post-transplant data from patients who lost their graft (n = 13). It would be reasonable to believe that these patients should perform worse than those with functioning grafts and consequently our results may overestimate the post-transplant values. Finally, there were some missing values and we therefore had to impute some values as well as exclude a number of patients from the QALYs analyses. This imputation obviously introduces an inaccuracy, but despite this, we strongly believe that our measurements are far more accurate than those based on a single measurement.
Finally, our main observation period is only 1 year and consequently we cannot make any firm conclusions about the cost–utility in a long-term scenario. However, others have reported that the costs post-transplant stay low for several years [12, 13], and despite a possible increase in costs at an earlier time in older recipients, the large difference between the costs makes it unlikely that cost per QALY over time would become higher in transplant recipients compared with dialysis patients.
An important strength is the before–after design that makes it possible to compare health utility pre- and post-transplant, patient by patient. Our data describe an almost linear decrease of the SF-Index during the last year before transplantation (Table 3, Figure 2) followed by a marked increase after transplantation. This change in the trend of patients’ self-perceived health is most likely a result of the transplantation, suggesting that the last year before transplantation is in fact a good indication of how the patients would have performed in the absence of transplantation.
CONCLUSION
The present study demonstrates that kidney transplantation improves health but also has higher costs per QALY at 1 year post-transplant compared with no transplantation in ESRD patients >65 years of age. Preliminary analyses suggest a favourable long-term effect in terms of health and costs per QALY. Further evaluations should be performed to address the long-term health economic effects, preferably combined with costs obtained from the same cohort.
ACKNOWLEDGEMENTS
Professor Anders Åsberg, leader of the Norwegian Renal Registry, provided survival data from the registry used for simulation of possible long-term effects.
FUNDING
The QUESTION65 received financial support from Extra Stiftelsen. Apart from this, no funding was received.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Wolfe RA, Ashby VB, Milford EL. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 2. Rao PS, Merion RM, Ashby VB. et al. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation 2007; 83: 1069–1074 [DOI] [PubMed] [Google Scholar]
- 3. Heldal K, Hartmann A, Grootendorst DC. et al. Benefit of kidney transplantation beyond 70 years of age. Nephrol Dial Transplant 2010; 25: 1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lønning K, Heldal K, Bernklev T. et al. Improved health-related quality of life in older kidney recipients 1 year after transplantation. Transplant Direct 2018; 4: e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branger P, Samuel U (eds). Annual Report 2017. Leiden: Eurotransplant International Foundation, 2017. https://www.eurotransplant.org/cms/mediaobject.php?file=Annual+Report+2017+HR10.pdf (5 Feb 2019, last accessed date)
- 6. Hart A, Smith JM, Skeans MA. et al. OPTN/SRTR 2016 annual data report: kidney. Am J Transplant 2018; 18(Suppl 1): 18–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lehner LJ, Kleinsteuber A, Halleck F. et al. Assessment of the kidney donor profile index in a European cohort. Nephrol Dial Transplant 2018; 33: 1465–1472 [DOI] [PubMed] [Google Scholar]
- 8. Frei U, Noeldeke J, Machold-Fabrizii V. et al. Prospective age-matching in elderly kidney transplant recipients—a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant 2008; 8: 50–57 [DOI] [PubMed] [Google Scholar]
- 9. Abramowicz D, Oberbauer R, Heemann U. et al. Recent advances in kidney transplantation: a viewpoint from the Descartes advisory board. Nephrol Dial Transplant 2018; 33: 1699–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minambres E, Suberviola B, Dominguez-Gil B. et al. Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant 2017; 17: 2165–2172 [DOI] [PubMed] [Google Scholar]
- 11. Foss S, Nordheim E, Sorensen DW. et al. First Scandinavian protocol for controlled donation after circulatory death using normothermic regional perfusion. Transplant Direct 2018; 4: e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jarl J, Desatnik P, Peetz Hansson U. et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clin Kidney J 2018; 11: 283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kontodimopoulos N, Niakas D.. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients' life expectancy. Health Policy 2008; 86: 85–96 [DOI] [PubMed] [Google Scholar]
- 14. Hays RD, Kallich JD, Mapes DL. et al. Development of the Kidney Disease Quality of Life (KDQOL) instrument. Qual Life Res 1994; 3: 329–338 [DOI] [PubMed] [Google Scholar]
- 15. Lonning K, Midtvedt K, Bernklev T. et al. Changes in health related quality of life in older candidates waiting for kidney transplantation. Nephrology (Carlton) 2018; 23: 948–956 [DOI] [PubMed] [Google Scholar]
- 16. Brazier J, Roberts J, Deverill M.. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002; 21: 271–292 [DOI] [PubMed] [Google Scholar]
- 17. Brazier JE, Roberts J.. The estimation of a preference-based measure of health from the SF-12. Med Care 2004; 42: 851–859 [DOI] [PubMed] [Google Scholar]
- 18. McCabe C, Brazier J, Gilks P. et al. Using rank data to estimate health state utility models. J Health Econ 2006; 25: 418–431 [DOI] [PubMed] [Google Scholar]
- 19. Kharroubi SA, Brazier JE, Roberts J. et al. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ 2007; 26: 597–612 [DOI] [PubMed] [Google Scholar]
- 20. Foss A, Heldal K, Scott H. et al. Kidneys from deceased donors more than 75 years perform acceptably after transplantation. Transplantation 2009; 87: 1437–1441 [DOI] [PubMed] [Google Scholar]
- 21. Ibanez B, James S, Agewall S. et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2017; 70: 1082. [DOI] [PubMed] [Google Scholar]
- 22. Thompson S, James M, Wiebe N. et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015; 26: 2504–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benjamin EJ, Virani SS, Callaway CW. et al. Heart disease and stroke statistics–2018 update: a report from the American Heart Association. Circulation 2018; 137: e67–e492 [DOI] [PubMed] [Google Scholar]
- 24. Kaballo MA, Canney M, O'Kelly P. et al. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin Kidney J 2018; 11: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Legeai C, Andrianasolo RM, Moranne O. et al. Benefits of kidney transplantation for a national cohort of patients aged 70 years and older starting renal replacement therapy. Am J Transplant 2018; 18: 2695–2707 [DOI] [PubMed] [Google Scholar]