Abstract
Background
Acute interstitial nephritis (AIN) is a renal injury causing renal function deterioration and requiring renal replacement therapy (RRT) in a substantial number of cases. Therapy is based on withdrawal of suspicious causative drugs or the underlying diseases and/or steroid application if renal function is not restored after cessation of the underlying condition. Hard clinical evidence for augmenting steroid therapy is not available.
Methods
We reviewed the course and diagnosis for >20 years among all 1126 biopsied samples of our tertiary renal centre.
Results
49 (4.4%) were diagnosed with primary AIN, corresponding to an annual incidence of 1/100 000 population; 17 out of 49 biopsy-proven AIN patients required short-term or long-term (n = 5) RRT. According to a combined outcome criterion of coming off dialysis and/or reaching serum creatinine <200 µmol/L, 19 patients reached recovery whereas 20 did not. Among 39 patients with a comprehensive clinical and histopathological data set, presence of cortical scars, AIN histological activity (acute leucocyte infiltrates) and proteinuria were baseline parameters discriminating significantly between groups with or without recovery. No associations with the presence of specific drugs were found. Therapeutic use of steroids was associated with a lower probability of recovery (P = 0.008), presumably due to inclusion bias.
Conclusions
Following our basic finding of the importance of histopathological parameters of acuity associated with recovery, we argue for the inauguration of grading measures to characterize this issue quantitatively and make it usable for future controlled investigations. Finally, we provide a suggestion for a therapeutic algorithm in the management of AIN.
Keywords: C-reactive protein, cortex, dialysis, interstitial, nephritis, nephrology, scars, steroid
INTRODUCTION
Acute interstitial nephritis (AIN) is a renal injury causing a decrease in renal function and is hallmarked by a leucocyte infiltrate in renal interstitium [1, 2]. Toxic and allergy-causing compounds such as all kinds of medication [3–25] or environmental substances, infectious agents, i.e. bacteria (Legionella, Leptospirosa, Streptococci, Corynebacteriae, Mycoplasma, etc.) and viruses (hantavirus, measles, Epstein–Barr virus, cytomegalovirus, HIV) [26], and a variety of autoimmune disorders [27] (e.g. systemic lupus erythematosus [28, 29], Sjögren's syndrome, sarcoidosis [30], immunoglobulin G4-related disease) play a key role in the pathogenesis of this disease. Among the drugs triggering AIN, there has been increasing awareness about the role of proton pump inhibitors (PPIs) [3, 14, 21, 22, 31, 32] in addition to non-steroidal anti-inflammatory drugs (NSAIDs) [3] and antibiotics [13] as causative agents and even inducers of chronic kidney disease (CKD) [33].
Diagnosing AIN is generally challenging. The triad of rash, fever and eosinophilia is rarely seen together (<10%) [34]. There is no specific symptom with a useful predictive value for AIN. The occurrence of drug rash is often interpreted as a hint of AIN in association with acute kidney failure, but actually, it is only seen in 15–50% of cases [35] and mostly in antibiotic exposure, and rarely in patients on PPIs or NSAIDs.
The predictive value of fractional excretion of sodium is lower than generally thought to be. Urine microscopy can be misleading and urine eosinophils can be found in a variety of kidney diseases besides AIN. In the largest recent study, 63 out of 91 patients with biopsy-proven AIN had no evidence of urine eosinophils at all, leaving urine eosinophils with low sensitivity in diagnosing AIN [36]. The diagnostic performance of other modalities like imaging procedures (ultrasound, computed tomography scan, gallium scintigraphy) is not satisfactory. The only definite diagnostic tool is kidney biopsy.
All variations of outcomes starting from mild and intermittent acute kidney injury (AKI), including partial recovery, up to progression to end-stage renal disease (ESRD) necessitating renal replacement therapy (RRT; 5–10% of cases) can be seen. Therapy is usually based on steroids with primary cessation of the suspected causing agent. For augmenting steroid therapy, no randomized controlled trial (RCT) is available and observations surveying outcomes are inconclusive. There are case series supporting steroid therapy in which the time to recovery or the proportion of patients remaining on dialysis was lower if steroid therapy was applied [37]. Recently a large retrospective study from London with 187 cases of AIN (158 treated with steroids) suggested a benefit of steroid use with greater improvement in estimated glomerular filtration rate and fewer patients progressing to ESRD [38]. Other retrospective series demonstrated no benefit from steroid therapy [1, 39].
To survey our experience spanning two decades with AIN, we identified all biopsy-proven AIN between 1992 and 2012 in the metropolitan area of Leipzig (Saxony, Germany) and retrospectively analysed symptoms, causes, therapy regimes and renal outcomes. We aimed to identify the risks and therapeutical factors influencing the course of AIN.
MATERIALS AND METHODS
Patient population, data acquisition and handling
Over the observation period from 1992 to 2012, two generations of senior nephrologists were in charge of indicating renal biopsy in all presenting patients. A biopsy was performed in patients primarily presented with acute kidney failure or in patients with suspected AIN who did not respond to drug cessation and/or steroid therapy. Overall biopsy incidence rose over the years from 18 biopsies in 1992 to 84 in 2012, yielding a total of 1126 biopsy cases over the 20-year period of investigation. The hospital is one of two renal centres in Leipzig and covers 50% of the overall metropolitan population of 700 000 people.
Registry structure
After the biopsy procedure, all tissue samples were shipped to a specialized kidney histopathology laboratory (University Hospital Hamburg-Eppendorf, Div. of Renal Histopathology). For clinical diagnosis, a written report was generated. All reports were subjected to a structured register (n = 1126). The reports were collected in 2014 and classified by predefined diagnosis and presence of the following histopathological features: nephrosclerosis, cortical scars, focal inflammation, destructive injury, tubulopathy, haematuria and leucocyturia. AIN ‘activity’ was classified into:
High activity: biopsies showing only acute inflammatory infiltrates.
Intermediate activity: biopsies showing both acute infiltrates but also signs of chronic changes.
No activity: biopsies showing non-active AIN with mainly chronic damage of expired AIN.
Clinical records were retrieved and classified for C-reactive protein, proteinuria, serum creatinine (Crea), urine cytological findings, presence of the presumably causing medication and application of steroid therapy.
Outcome parameter
To investigate the outcomes, a combined recovery criterion composed of changing renal function and a remaining necessity for RRT was created. Patients who reached (known or anticipated) pre-AIN baseline renal function were considered to have ‘complete recovery’. Those coming-off dialysis and/or reaching Crea <200 µmol/L were considered to have ‘partial recovery’ and those with no improvement in renal function and/or remaining on RRT were considered to have ‘no recovery’.
Statistics
Descriptive statistics of patient data with pseudonym ID numbers were produced using standard procedures with the SPSS version 13.0 program package. In logistic regression analyses, covariates were analysed by a non-conditional overall model using outcome as ‘dependent’. Group comparison of categorical values was computed by contingency tables and chi-square, while numerical values were compared by one-factorial analysis of variance and independent t-test. Time-on-RRT analysis was conducted by univariate procedures (Kaplan–Meier) and dichotomized for trimodal population strata depending on baseline status of activity and use of steroids. Start time was the first dialysis date (survival plots) and end time was the last dialysis date. All events leading to the loss of follow-up other than endpoints (change to life-sustaining renal function, and end of observation in December 2014) were censored and indicated by crosses within the survival plot lines. Survival plots were truncated at 10 years.
RESULTS
Among 49 patients with biopsy-proven AIN during the observation period (out of a total number of 1126 renal biopsies), a complete data set of outcome surrogates could be identified in 39 patients. The finding of 49 AIN patients over the 20-year period corresponded to an annual incidence of 1 patient per 100 000 inhabitants and 4.4% of patients with indication to perform a biopsy.
Baseline anthropometric results by the presence of recovery are given in Table 1. Patients achieving complete or partial recovery had an average baseline Crea of 119 ± 48 µmol/L compared with 174 ± 61 µmol/L (P = 0.85) in patients not reaching recovery. The average maximal Crea was not different amongst patients with any kind of recovery versus patients with no recovery (415 ± 295 µmol/L versus 543 ± 223 µmol/L, P = 0.34). There was no difference in blood pressure or body mass index (BMI) between those two groups. Proteinuria was significantly higher in patients with no recovery than in patients with any kind of recovery (3.22 ± 5.03 g/g Crea versus 1.43 ± 2.15 g/g Crea, P = 0.003).
Table 1.
Baseline characteristics of the population by groups of AKI recovery
| Recovery |
|||
|---|---|---|---|
| Anthropometric feature | Any (n = 30) | No (n = 9) | P |
| Age (years) | 51.5 ± 20.4 | 52.4 ± 18.6 | 0.49 |
| Male | 15 (50) | 7 (77.8) | 0.14 |
| Crea baseline (µmol/L) | 119 ± 48.3 | 174 ± 61.1 | 0.85 |
| BP (mmHg) | 150/84 ± 29.8/12.4 | 159/82 ± 25.1/12.3 | 0.46/0.7 |
| BMI (kg/m2) | 28 ± 6.7 | 25.4 ± 5.9 | 0.75 |
| Proteinuria (g/g Crea) | 1.43 ± 2.15 | 3.22 ± 5.03 | 0.003 |
| Steroid therapy | 13 (43.4) | 8 (88.8) | 0.008 |
| Pulse | 2 (6.7) | 4 (44.4) | |
| Weight-adapted | 11 (36.7) | 4 (44.4) | |
Values are represented as n (%) or mean ± SD.
In total, 17 patients needed RRT. Eight biopsy-proven AIN patients had intermittent dialysis no longer than 2 weeks, four patients between 2 weeks and 30 months and five patients remained on maintenance dialysis until the end of observation (Table 2). A 78-year-old man died 2 years after coming off dialysis due to renal carcinoma (considered partial recovery) and a 79-year-old woman died after AIN on dialysis due to fungal septicaemia (considered no recovery). Including patients coming off dialysis, 20 patients reached complete recovery, 10 partial recovery and 9 no recovery; 37% of patients reaching any kind of recovery needed temporary RRT compared with 67% of patients in need of RRT with no recovery (P < 0.001).
Table 3.
Baseline features of renal histology and urine cytology by groups of AKI recovery
| Recovery |
|||
|---|---|---|---|
| Patients exhibiting …, n (%) | Any (n = 30) | No (n = 9) | P |
| Nephrosclerosis | 10 (33.3) | 6 (66.7) | 0.08 |
| Cortical scars | 16 (53.3) | 9 (100) | 0.01 |
| Destructive injury | 23 (76.7) | 7 (77.8) | 0.94 |
| Tubulopathy | 21 (70) | 6 (66.7) | 0.85 |
| Haematuria | 16 (53.3) | 6 (66.7) | 0.35 |
| Leucocyturia | 9 (30) | 3 (33.3) | 0.63 |
| Focal inflammation | 17 (56.7) | 5 (55.6) | 0.92 |
| Active AIN | 27 (70) | 4 (44.4) | 0.01 |
| High, n (%) | 8 (26.7) | 2 (22.2) | |
| Intermediate, n (%) | 19 (63.3) | 2 (22.2) | |
Values are represented as n (%).
The presence of cortical scars (53% versus 100%, P = 0.01) and active interstitial inflammation (70% versus 44%, P = 0.01) were histological baseline parameters discriminating significantly between groups with or without AIN recovery (Table 3). There were no differences in the presence of nephrosclerosis or tubulopathy and no difference in the presence of urinary markers, e.g. haematuria or leucocyturia.
Table 4.
Baseline medication (suspected drugs) by groups of AKI recovery
| Recovery |
|||
|---|---|---|---|
| Patients receiving …, n (%) | Any (n = 30) | No (n = 9) | P |
| Antibiotics | 6 (20) | 1 (11.1) | 0.54 |
| NSAIDs | 5 (16.7) | 0 (0.0) | 0.19 |
| PPI | 8 (26.7) | 5 (55.6) | 0.11 |
| Angiotensin-receptor blocker | 5 (16.7) | 3 (33.3) | 0.28 |
| ACE-inhibitor | 9 (30) | 5 (55.6) | 0.16 |
| Allopurinol | 5 (16.7) | 3 (33.3) | 0.28 |
| Loop diuretics | 6 (20) | 2 (22.2) | 0.88 |
Values are represented as n (%).
Among the drugs used by patients with diagnosed biopsy-proven AIN in our cohort (Table 4), RAAS inhibitors were the most common medication (22 out of 39 patients in the entire cohort with no difference between recovery groups). Antibiotics (20% versus 11%, P = 054) and NSAIDs (17% versus 0%, P = 0.19) were more often used in patients that achieved some kind of recovery compared with patients with no recovery. PPIs, on the other hand, were more often found in patients with worse renal outcome compared with patients achieving some kind of recovery (56% versus 27%, P = 0.11).
Table 2.
Outcome characteristics by groups of AKI recovery
| Recovery |
|||
|---|---|---|---|
| Outcome, n (%) | Any (n = 30) | No (n = 9) | P |
| Necessitating RRT, n (%) | 11 (36.7) | 6 (66.7) | <0.0001 |
| Time on RRT (years) | 0.47 (0.85) | 4.6 ± 3.14 | 0.006 |
| Crea max (µmol/L) | 415 ± 295 | 543 ± 223 | 0.34 |
| Crea last (µmol/L) | 178 ± 125 | 406 ± 192 | 0.023 |
Values are represented as n (%) or mean ± SD.
By logistic regression of baseline variables, using recovery as ‘dependent’, baseline Crea remained in the model (P = 0.048) and the presence of scars showed a tendency to influence a forward conditional outcome model (P = 0.08). No associations of other anthropometric parameters, nor presence of specific drugs (Table 4), except a trend for NSAIDs and PPIs, were found. Steroids were more often applied to patients with the outcome ‘no recovery’ than to patients with some kind of recovery (89% versus 43%, P = 0.008). Therapeutic use of steroids was associated with a lower probability of recovery (P = 0.008).
DISCUSSION
The present observation provides a long-term and population-based insight into the epidemiology and prognosis of AIN while being retrospective in nature, incorporating all the pitfalls of such a design. It is the only investigation providing a prognostic evaluation by detailed analysis of patient’s time on dialysis. The annual AIN incidence of 1/100 000 is comparable to recent population-based data found in Scotland [40] and France [16], and earlier data from Germany [41]. With 17 patients temporarily needing and 5 patients remaining on RRT, AIN requiring biopsy diagnosis must be considered as a severe and life-threatening disease, comparable to crescentic glomerulonephritis. Suspected causes of AIN were similar to earlier data showing suspicious drugs present in >70% of patients. It is, however, intriguing to suppose the effects of drugs like PPI because they are used in a multitude of patients and the question of causality is hard to decide. This is best elucidated by our result, that inhibitors of the renin–angiotensin system were the most common drugs used in patients diagnosed with AIN. Only one nested-case epidemiological study provides stronger evidence of a 70% higher incidence of AKI in patients treated with PPIs [42]. Our study found NSAIDs to be used more frequently in patients recovering from AIN–AKI, although this trend was not statistically significant. The trend for worse renal outcomes among patients using PPIs and better outcomes for users of NSAIDs and antibiotics may be explained by a higher awareness of treating physicians of the causative association between NSAIDs/antibiotics and AIN and lower awareness between the link of PPIs with AIN. The latter might lead to delay in diagnosis and failure to stop the causative drug, and therefore worsen renal outcome.
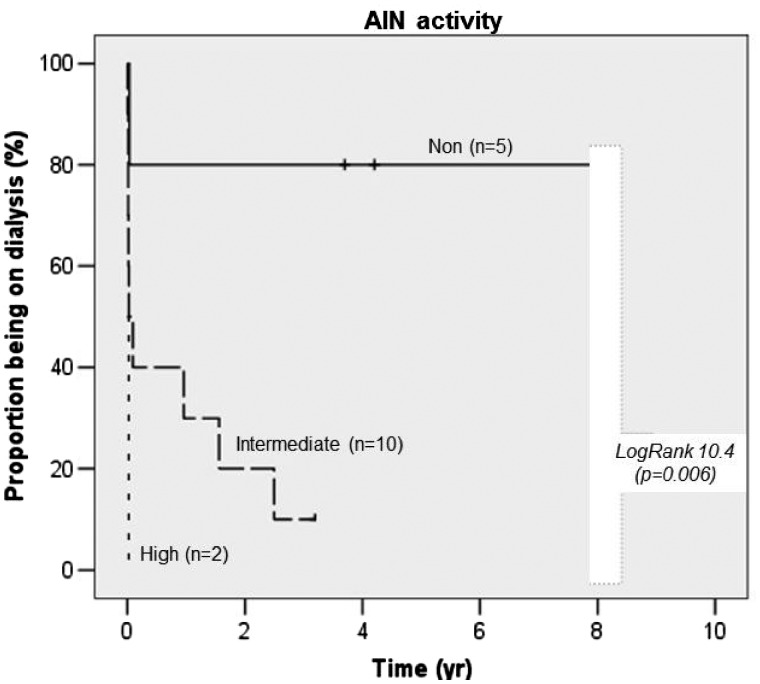
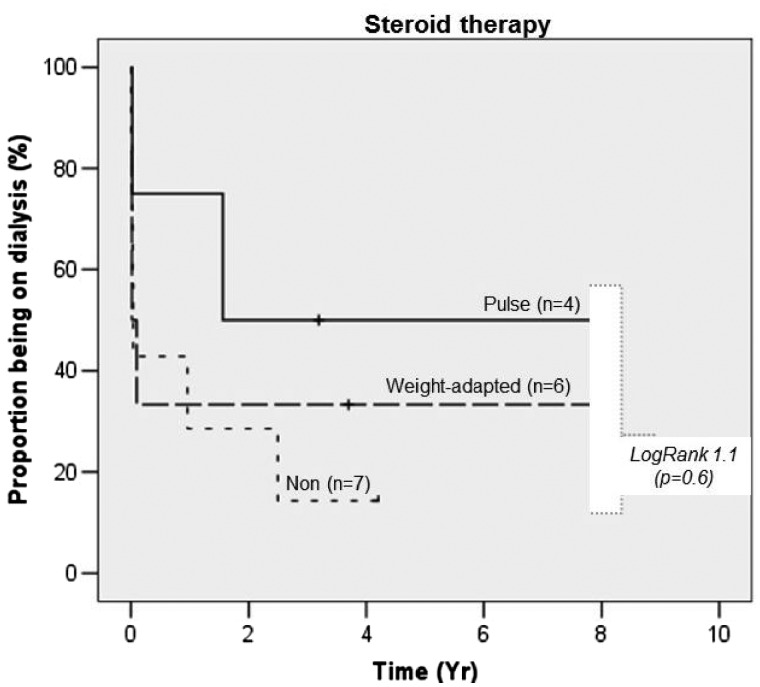
Renal cortex scars and proteinuria were significantly associated with a worse renal outcome (not reaching recovery) even given the small numbers of observation, which was similarly described in another German study [41]. AIN histological activity (without scars) was associated with recovery showing more acute leucocyte infiltrations in patients recovering from AIN and those coming off RRT if needed (Figure 1). Steroids were more frequently used in those patients not reaching AIN recovery and/or coming off RRT (Figure 2). This finding has to be interpreted cautiously, with awareness of significant indication bias. More severe disease is more likely to trigger steroids use independent of inflammatory acuity, at least given current clinical considerations. We confirm results from other studies by failing to show a positive effect of steroid use on renal outcomes.
FIGURE 1.
Proportion of patients remaining on dialysis by activity grade.
FIGURE 2.
Proportion of patients remaining on dialysis by steroid use.
In clinical settings, steroids are widely used particularly in patients without renal improvement after cessation of the presumably causative drug, supposedly without much regard to acuity. However, from a pathophysiological point of view, looking at the renal leucocyte infiltrate before deciding on immunosuppressive therapy makes a lot of sense. The histopathological hallmark of AIN consists of an interstitial inflammatory infiltrate composed mainly of lymphocytes together with plasma cells and macrophages. Glucocorticoids (GCs) are potent inhibitors of those inflammatory processes. Their anti-inflammatory and immunomodulatory effects are mediated predominantly by genomic mechanisms, either by direct binding of the GC/GC-receptor complex to GC responsive elements in the promoter region of genes or by non-genomic mechanisms, responsible for some regulating effects, which arise a few minutes after administration [43]. In this way, they inhibit several proinflammatory molecules, such as cytokines, chemokines, arachidonic acid metabolites and adhesion molecules. Therefore, GCs can reduce and reverse the mechanism of tubulointerstitial inflammation and additionally exhibit direct effects on the infiltrating lymphocytes, e.g. by induction of apoptosis [44, 45].
Questions remain on how to manage therapy with special focus on how to adjust therapy decisions to histopathological features (acuity versus scaring), when to treat (waiting for renal improvement after cessation of the presumably causative drug or proceeding with treatment after clinical suspicion of AIN), and what dosage of steroids should be used and for how long. Concerning steroid timing, a recent RCT in biopsy-proven AIN revealed no outcome difference in patients randomized to oral or pulse prednisolone with treatment duration for >3 weeks followed by rapid tapering over another 3 weeks [46]. A significant benefit of steroid usage in patients with an early start of treatment within 7 days after the withdrawal of the offending drug was suggested [47]. Another study emphasizes worse prognosis with delayed steroid treatment and most interestingly points out no benefit from longer duration of steroid therapy (>3 weeks) on outcome [37]. More acute onset of disease with signs of acute inflammation in biopsy without extensive chronic damage had a higher probability of renal recovery [37]. This is in line with our result of the association of AIN histological activity with recovery and the higher likelihood of coming off RRT and recovery from AIN in those patients with more acute leucocyte infiltrations.
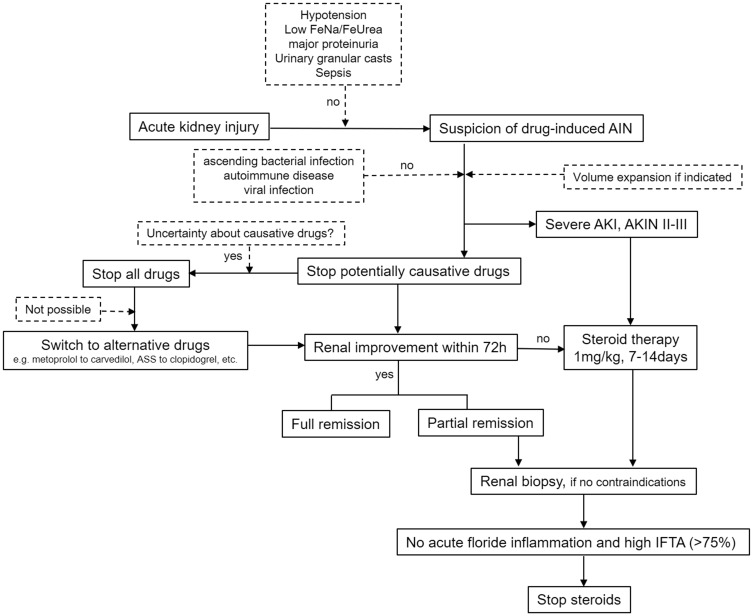
Due to the lack of any hard evidence and the low likelihood of having an RCT on AIN therapy soon, we would like to propose an algorithm for clinical management in cases with suspected drug-induced AIN (Figure 3). This algorithm is our personal opinion but it might help develop currently lacking widely accepted therapy guidelines on AIN.
FIGURE 3.
Suggested opinion-based algorithm on practical clinical management in suspected AIN. FeUrea, fractional excretion of Urea; FeNa, fractional excretion of sodium; AKIN, Acute Kidney Injury Network; IFTA, interstitial fibrosis and tubular atrophy.
We do support an important role for renal biopsy for gaining a definite diagnosis and prognostic information. Renal biopsy is a safe procedure, especially in experienced centres, and complication rates can be as low as 2% [48]. The risk of complications is higher in the setting of AKI [49], mainly due to the presence of increased risk factors [50]. We do postpone renal biopsy for 5–7 days in the presence of aspirin medication (while already treating the patient in case of suspected AIN with severe AKI) even though this practice is not supported by hard evidence and some data suggest no elevated bleeding risk in performing renal biopsy without stopping aspirin [51]. Currently, there is no consensus on the interpretation of the available data and guidelines that standardize biopsy practices are lacking [52].
Of course, our investigation suffers from several weaknesses. First, similar to basically all studies on AIN, it employs retrospective risk factor retrieval and therapy. With such an approach, no high-grade clinical evidence, particularly regarding the usefulness of therapy, can be reached. Because this study adds a further negative observation on steroid therapy success in AIN, we see an urgent need to conduct a collaborating multicentre, randomized trial on this question. Secondly, although we found some disproportionate distribution of risk factors and compounds (NSAIDs), these findings are hard to rate because of the small incidence over the observation period of 20 years. It is therefore necessary to combine the design of such a trial with a structured registry evaluating the systematic occurrence of symptoms and histological features in a satisfactory number of patients (n > 300). Thirdly, following our finding of the importance of cortex scars and AIN activity (acuity of infiltrates), we want to stress the perspective utility of a histological scoring system (comparable to Banff or Oxford scales in other diseases) for a quantitative description of interstitial findings.
In summary, we present an observational study evaluating clinical presentation, histological features, therapy and outcome of AIN in a renal centre for >20 years. We found proteinuria, presence of renal cortex scars and acuity of histological inflammation to be the significant prognostic factors. Steroid therapy had an inverse association with outcome, presumably due to inclusion bias. Therapy for AIN should not be delayed, as the highest chance of recovery can be attributed to the early stages of the disease with acute inflammatory signs and low chronic damage in the biopsy.
ACKNOWLEDGEMENTS
We greatly appreciate the contribution of Prof. Udo Helmchen and Prof. Thorsten Wiech, former and current head of Division of Renal Histopathology, University Hospital Hamburg-Eppendorf, Hamburg, Germany, in providing histopathological analysis, and of Florian Fahr for collecting registry data on renal histopathological results. We also thank Ursula Perrier for her language support.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Bhaumik SK, Kher V, Arora P.. Evaluation of clinical and histological prognostic markers in drug-induced acute interstitial nephritis. Ren Fail 1996; 18: 97–104 [DOI] [PubMed] [Google Scholar]
- 2. Buysen JG, Houthoff HJ, Krediet RT. et al. Acute interstitial nephritis: a clinical and morphological study in 27 patients. Nephrol Dial Transplant 1990; 5: 94–99 [DOI] [PubMed] [Google Scholar]
- 3. Leonard CE, Freeman CP, Newcomb CW. et al. Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf 2012; 21: 1155–1172 [DOI] [PubMed] [Google Scholar]
- 4. Alexopoulos E. Drug-induced acute interstitial nephritis. Ren Fail 1998; 20: 809–819 [DOI] [PubMed] [Google Scholar]
- 5. Linton AL, Clark WF, Driedger AA. et al. Acute interstitial nephritis due to drugs: review. Ann Intern Med 1980; 93: 735–741 [DOI] [PubMed] [Google Scholar]
- 6. Pommer W, Offermann G, Schultze G. et al. [ Acute interstitial nephritis caused by drugs]. Dtsch Med Wochenschr 2008; 108: 783–788 [DOI] [PubMed] [Google Scholar]
- 7. Appel GB. A decade of penicillin related acute interstitial nephritis–more questions than answers. Clin Nephrol 1980; 13: 151–154 [PubMed] [Google Scholar]
- 8. Augusto J-F, Sayegh J, Simon A. et al. A case of sulphasalazine-induced DRESS syndrome with delayed acute interstitial nephritis. Nephrol Dial Transplant 2009; 24: 2940–2942 [DOI] [PubMed] [Google Scholar]
- 9. Baldwin D, Prince M, Marshall S. et al. Regulation of insulin receptors: evidence for involvement of an endocytotic internalization pathway. Proc Natl Acad Sci USA 1980; 77: 5975–5978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burkhart R, Shah N, Lewin M.. Sildenafil induced acute interstitial nephritis. Case Rep Nephrol 2015; 2015: 731284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calvino J, Romero R, Pintos E. et al. Mesalazine-associated tubulo-interstitial nephritis in inflammatory bowel disease. Clin Nephrol 1998; 49: 265–267 [PubMed] [Google Scholar]
- 12. Chatzikyrkou C, Hamwi I, Clajus C. et al. Biopsy proven acute interstitial nephritis after treatment with moxifloxacin. BMC Nephrol 2010; 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Agati VD, Theise ND, Pirani CL. et al. Interstitial nephritis related to nonsteroidal anti-inflammatory agents and beta-lactam antibiotics: a comparative study of the interstitial infiltrates using monoclonal antibodies. Mod Pathol 1989; 2: 390–396 [PubMed] [Google Scholar]
- 14. Geevasinga N, Coleman PL, Webster AC. et al. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol 2006; 4: 597–604 [DOI] [PubMed] [Google Scholar]
- 15. Kaye WA, Passero MA, Solomon RJ. et al. Cimetidine-induced interstitial nephritis with response to prednisone therapy. Arch Intern Med 1983; 143: 811–812 [PubMed] [Google Scholar]
- 16. Leven C, Hudier L, Picard S. et al. Prospective study of drug-induced interstitial nephritis in eleven French nephrology units. Presse Med 2014; 43: e369–e376 [DOI] [PubMed] [Google Scholar]
- 17. Markowitz GS, Tartini A, D’Agati VD.. Acute interstitial nephritis following treatment with anorectic agents phentermine and phendimetrazine. Clin Nephrol 1998; 50: 252–254 [PubMed] [Google Scholar]
- 18. Murray KM, Keane WR.. Review of drug-induced acute interstitial nephritis. Pharmacotherapy 1992; 12: 462–467 [PubMed] [Google Scholar]
- 19. Perazella MA, Markowitz GS.. Drug-induced acute interstitial nephritis. Nat Rev Nephrol 2010; 6: 461–470 [DOI] [PubMed] [Google Scholar]
- 20. Pitone JM, Santoro JJ, Biondi RJ. et al. Cimetidine-induced acute interstitial nephritis. Am J Gastroenterol 1982; 77: 169–171 [PubMed] [Google Scholar]
- 21. Sampathkumar K, Ramalingam R, Prabakar A. et al. Acute interstitial nephritis due to proton pump inhibitors. Indian J Nephrol 2013; 23: 304–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sierra F, Suarez M, Rey M. et al. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26: 545–553 [DOI] [PubMed] [Google Scholar]
- 23. Smith WR, Neill J, Cushman WC. et al. Captopril-associated acute interstitial nephritis. Am J Nephrol 1989; 9: 230–235 [DOI] [PubMed] [Google Scholar]
- 24. Soni N, Harrington JW, Weiss R. et al. Recurrent acute interstitial nephritis induced by azithromycin. Pediatr Infect Dis J 2004; 23: 965–966 [DOI] [PubMed] [Google Scholar]
- 25. Woodroffe AJ, Weldon M, Meadows R. et al. Acute interstitial nephritis following ampicillin hypersensitivity. Med J Aust 1975; 1: 65–68 [DOI] [PubMed] [Google Scholar]
- 26. Mirabella M, Taramasso L, Nicolini LA. et al. Successful recovery of associated interstitial nephritis and focal segmental glomerulosclerosis in patients with HCV and HIV treated with sofosbuvir and daclatasvir and revision of literature. Clin Nephrol Case Stud 2018; 6: 31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez-Perez JC, Cruz-Alamo M, Perez-Aciego P. et al. Clinical and immune aspects of idiopathic acute tubulointerstitial nephritis and uveitis syndrome. Am J Nephrol 1995; 15: 386–391 [DOI] [PubMed] [Google Scholar]
- 28. Park MH, D’Agati V, Appel GB. et al. Tubulointerstitial disease in lupus nephritis: relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis. Nephron 1986; 44: 309–319 [DOI] [PubMed] [Google Scholar]
- 29. Yung S, Chan TM.. Molecular and immunological basis of tubulo-interstitial injury in lupus nephritis: a comprehensive review. Clin Rev Allergy Immunol 2017; 52: 149–163 [DOI] [PubMed] [Google Scholar]
- 30. Mignon F, Mery JP, Mougenot B. et al. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984; 13: 219–245 [PubMed] [Google Scholar]
- 31. Harmark L, van der Wiel HE, de Groot MCH. et al. Proton pump inhibitor-induced acute interstitial nephritis. Br J Clin Pharmacol 2007; 64: 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simpson IJ, Marshall MR, Pilmore H. et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology (Carlton) 2006; 11: 381–385 [DOI] [PubMed] [Google Scholar]
- 33. Xie Y, Bowe B, Li T. et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2016; 27: 3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker RJ, Pusey CD.. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2004; 19: 8–11 [DOI] [PubMed] [Google Scholar]
- 35. Perazella MA. Diagnosing drug-induced AIN in the hospitalized patient: a challenge for the clinician. Clin Nephrol 2014; 81: 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muriithi AK, Leung N, Valeri AM. et al. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int 2015; 87: 458–464 [DOI] [PubMed] [Google Scholar]
- 37. Fernandez-Juarez G, Perez JV, Caravaca-Fontán F. et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol 2018; 13: 1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prendecki M, Tanna A, Salama AD. et al. Long-term outcome in biopsy-proven acute interstitial nephritis treated with steroids. Clin Kidney J 2017; 10: 233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koselj M, Kveder R, Bren AF. et al. Acute renal failure in patients with drug-induced acute interstitial nephritis. Ren Fail 1993; 15: 69–72 [DOI] [PubMed] [Google Scholar]
- 40. Valluri A, Hetherington L, Mcquarrie E. et al. Acute tubulointerstitial nephritis in Scotland. QJM 2015; 108: 527–532 [DOI] [PubMed] [Google Scholar]
- 41. Schwarz A, Krause PH, Kunzendorf U. et al. The outcome of acute interstitial nephritis: risk factors for the transition from acute to chronic interstitial nephritis. Clin Nephrol 2000; 54: 179–190 [PubMed] [Google Scholar]
- 42. Klepser DG, Collier DS, Cochran GL.. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol 2013; 14: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buttgereit F, Straub RH, Wehling M. et al. Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum 2004; 50: 3408–3417 [DOI] [PubMed] [Google Scholar]
- 44. Lanza L, Scudeletti M, Puppo F. et al. Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol 2007; 103: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Webster JC, Huber RM, Hanson RL. et al. Dexamethasone and tumor necrosis factor-alpha act together to induce the cellular inhibitor of apoptosis-2 gene and prevent apoptosis in a variety of cell types. Endocrinology 2002; 143: 3866. [DOI] [PubMed] [Google Scholar]
- 46. Kim MJ, Heim M, Mayr M.. Effect of corticosteroids during ongoing drug exposure in pantoprazole-induced interstitial nephritis. Nephrol Dial Transplant 2010; 25: 1716–1719 [DOI] [PubMed] [Google Scholar]
- 47. González E, Gutiérrez E, Galeano C. et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008; 73: 940–946 [DOI] [PubMed] [Google Scholar]
- 48. Tøndel C, Vikse BE, Bostad L. et al. of Percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin J Am Soc Nephrol 2012; 7: 1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corapi KM, Chen JLT, Balk EM. et al. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis 2012; 60: 62–73 [DOI] [PubMed] [Google Scholar]
- 50. Korbet SM, Gashti CN, Evans JK. et al. Risk of percutaneous renal biopsy of native kidneys in the evaluation of acute kidney injury. Clin Kidney J 2018; 11: 610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lees JS, McQuarrie EP, Mordi N. et al. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin Kidney J 2017; 10: 573–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lees JS, McQuarrie EP, Mackinnon B.. Renal biopsy: it is time for pragmatism and consensus. Clin Kidney J 2018; 11: 605–609 [DOI] [PMC free article] [PubMed] [Google Scholar]