Abstract
Globally, chronic kidney disease (CKD) is an emerging public health challenge but accurate data on its true prevalence are scarce, particularly in poorly resourced regions such as sub-Saharan Africa (SSA). Limited funding for population-based studies, poor laboratory infrastructure and the absence of a validated estimating equation for kidney function in Africans are contributing factors. Consequently, most available studies used to estimate population prevalence are hospital-based, with small samples of participants who are at high risk for kidney disease. While serum creatinine is most commonly used to estimate glomerular filtration, there is considerable potential bias in the measurement of creatinine that might lead to inaccurate estimates of kidney disease at individual and population level. To address this, the Laboratory Working Group of the National Kidney Disease Education Program published recommendations in 2006 to standardize the laboratory measurement of creatinine. The primary objective of this review was to appraise implementation of these recommendations in studies conducted in SSA after 2006. Secondary objectives were to assess bias relating to choice of estimating equations for assessing glomerular function in Africans and to evaluate use of recommended diagnostic criteria for CKD. This study was registered with Prospero (CRD42017068151), and using PubMed, African Journals Online and Web of Science, 5845 abstracts were reviewed and 252 full-text articles included for narrative analysis. Overall, two-thirds of studies did not report laboratory methods for creatinine measurement and just over 80% did not report whether their creatinine measurement was isotope dilution mass spectroscopy (IDMS) traceable. For those reporting a method, Jaffe was the most common (93%). The four-variable Modification of Diet in Renal Disease (4-v MDRD) equation was most frequently used (42%), followed by the CKD Epidemiology Collaboration (CKD-EPI) equation for creatinine (26%). For the 4-v MDRD equation and CKD-EPI equations, respectively, one-third to one half of studies clarified use of the coefficient for African-American (AA) ethnicity. When reporting CKD prevalence, <15% of studies fulfilled Kidney Disease: Improving Global Outcomes criteria and even fewer used a population-based sample. Six studies compared performance of estimating equations to measured glomerular filtration rate (GFR) demonstrating that coefficients for AA ethnicity used in the 4-v MDRD and the CKD-EPI equations overestimated GFR in Africans. To improve on reporting in future studies, we propose an ‘easy to use’ checklist that will standardize reporting of kidney function and improve the quality of studies in the region. This research contributes some understanding of the factors requiring attention to ensure accurate assessment of the burden of kidney disease in SSA. Many of these factors are difficult to address and extend beyond individual researchers to health systems and governmental policy, but understanding the burden of kidney disease is a critical first step to informing an integrated public health response that would provide appropriate screening, prevention and management of kidney disease in countries from SSA. This is particularly relevant as CKD is a common pathway in both infectious and non-communicable diseases, and multimorbidity is now commonplace, and even more so when those living with severe kidney disease have limited or no access to renal replacement therapy.
Keywords: albuminuria, chronic kidney disease, creatinine, estimated and measured glomerular filtration rate, prevalence, systematic review
INTRODUCTION
In sub-Saharan Africa (SSA), a double burden of infectious and non-communicable diseases contributes to a potentially high risk for chronic kidney disease (CKD). However, the true prevalence of CKD remains difficult to quantify [1, 2]. A systematic review of the epidemiology of CKD in SSA concluded that most studies were of medium to low quality due to poor sampling methods and unreliable laboratory measurements of kidney function [1]. Many of these studies were conducted in urban hospitals with participants who had multiple risk factors for CKD, and proteinuria was the most common measure of kidney function [1]. Given that convenience sampling may lead to overestimating the burden of CKD at population level, and that the diagnostic criteria used to define CKD were sub-optimal in many of the studies from the review, the reasons for this low research quality merit consideration.
First, the funding and infrastructure required for population-based CKD prevalence studies is substantial and, even in the most well-resourced environments, conducting these studies is challenging [2]. Second, in the absence of validated, affordable, point of care diagnostics for kidney disease, for studies to conform to the internationally accepted Kidney Disease: Improving Global Outcomes (KDIGO) guidelines requires (i) laboratory measurement of serum creatinine using an isotope dilution mass spectroscopy (IDMS)-traceable standard reference material for creatinine; (ii) calculation of the glomerular filtration rate (GFR) using the serum creatinine; (iii) measurement of urine protein or albumin from a spot urine sample, preferably laboratory quantified as an albumin:creatinine ratio; and (iv) in the absence of clinical evidence that confirms chronicity, repeating these serum and urine measurements after a minimum period of 3 months [3, 4]. These requirements impose substantial logistic, infrastructural and financial hurdles for clinical researchers, particularly in resource-limited settings where the burden of CKD is projected to be highest.
For any study using KDIGO criteria, access to accurate diagnostic laboratory services is essential as even small variations in creatinine measurement can impact on population prevalence estimates [2]. This is underappreciated by clinician scientists and may reflect poor interdisciplinary collaboration with chemical pathologists. For example, the older colorimetric picric acid (Jaffe) method is less accurate but cheaper than the recently developed enzymatic method. While the enzymatic method is recommended, in SSA most laboratories use the Jaffe method, for which a correction factor should be applied [1]. To reduce inter-laboratory measurement bias, the Laboratory Working Group of the National Kidney Disease Education Program (NKDEP) in the USA published guidelines in 2006 that recommended use of an IDMS-traceable standard reference material for creatinine measurement [5]. Consequently, the four-variable Modification of Diet in Renal Disease (4-v MDRD) equation was re-expressed for IDMS-traceability and the CKD Epidemiology Collaboration (CKD-EPI) equations are based on the use of an IDMS-traceable method [6, 7]. Adherence to standard internal and external quality assurance procedures are additional obligatory steps that mitigate laboratory-induced bias. In SSA and other low and middle income settings, reliable diagnostic laboratory infrastructure cannot be assumed. The lack of study-specific standardization in sampling and measurement of creatinine is considered the greatest obstacle to determining the global prevalence of CKD [2, 8].
Further bias may be introduced through the choice of estimating equation for GFR. Initially, the National Kidney Foundation guidelines recommended the 4-v MDRD equation for estimating GFR [3]. This equation was derived from a relatively small study sample in the USA with a coefficient that corrected for a higher creatinine observed in African-Americans (AAs). A similar correction coefficient for AA ethnicity was derived for use with the CKD-EPI equation, which is now the preferred equation in the revised KDIGO guidelines for 2014 [4]. In studies from SSA, when the coefficients for AA ethnicity are used for either of these two equations, they consistently overestimate GFR [9–15]. In the absence of appropriate, validated estimating equations for African populations, some studies have omitted the coefficients for AA ethnicity when using the 4-v MDRD and CKD-EPI equations [16, 17]. Researchers need to be cognizant of the limitations imposed by these equations and the bias that could be introduced into their results.
The primary objective of this review was to appraise reporting of laboratory methods for creatinine measurement in studies utilizing creatinine to assess kidney function in SSA. The secondary objectives were to assess bias relating to: choice of equations used to estimate GFR; use of coefficients for AA ethnicity for the 4-v MDRD and CKD-EPI equations; criteria used to diagnose CKD; and study design and sampling strategies.
MATERIALS AND METHODS
Search strategy and selection criteria
This narrative systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42017068151) and completed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) together with the revised quality assessment of diagnostic accuracy studies (QUADAS-2) guidelines [18–20]. The period selected for review included all original research studies published from 31 January 2008 to 31 December 2018. This was based on the assumption that after the NKDEP guidelines were published in 2006, this would set the standard for the widespread implementation of IDMS-traceable creatinine assays in diagnostic laboratories by 2008—and we would see a similar trend in studies from SSA [5]. For studies that determined the prevalence of CKD, the recommended criteria for diagnosis of CKD were first published in 2002 and subsequently updated in 2012 [3, 4]. Likewise, we anticipated that these guidelines would inform the choice of criteria for clinical studies.
The online databases for PubMed, African Journals Online and Web of Science were searched using the relevant medical subject headings (Supplementary data, Appendix S1). Based on the title and abstract, all studies from the SSA region that assessed kidney function in adults were evaluated according to inclusion and exclusion criteria agreed upon by the team conducting the systematic review (Supplementary data, Appendix S1). Only those abstracts with studies with full-text articles, available in English, were selected for the final analysis.
Quality assessment and data extraction
The online database search generated abstracts for screening. J.A.G. and M.v.D. independently screened abstracts from African Journals Online and Web of Science. There were no additional references identified through bibliography searches. Results were compared and differences of opinion referred to J.F. or H.R.E. Similarly, J.F. and H.R.E. independently screened abstracts from PubMed and followed the same process with differences referred to J.A.G. and M.v.D. Duplicates were removed and the team agreed on a list of eligible abstracts for which full-text articles were sourced by J.A.G. and H.R.E. For each full-text article, data were extracted by two authors who worked independently (J.F., J.A.G., H.R.E., M.v.D. and R.K.). Each author then compared their data extraction to the second author and discrepancies were resolved or referred to the remaining authors for review. If needed, J.F. contacted authors via email for clarification of study-specific information and incorporated the feedback for those who responded.
The QUADAS-2 assessment sheet (Supplementary data, Appendix S2) was used as a template by the team to pilot the data extraction process. After conducting the pilot, a revised form, adapted for the specific needs of this study, was generated and accepted by the group for use. This was formulated into a study-specific data extraction sheet (Supplementary data, Appendix S3). In summary, where appropriate for each full-text study, the data were abstracted as follows: (i) was GFR measured or estimated, and by which method; (ii) was creatinine measured, and by which method, and was the method IDMS-traceable to a standard reference material for creatinine; (iii) was cystatin C measured; (iv) did the study determine CKD prevalence—if so, by which criteria, was chronicity confirmed with repeat measurements; and (v) study design, sample selection and sample size. The estimating equations for creatinine, cystatin and creatinine-cystatin, and CKD criteria are defined in Supplementary data, Appendix S4. Data were analyzed using simple descriptive statistics including frequency and percentage tabulation for continuous variables. A narrative analysis was considered more appropriate for the aim of this systematic review and a meta-analysis was therefore not conducted.
Performance of eGFR equations
For those studies with measured GFR (mGFR) and comparisons of performance of the different estimated GFR (eGFR) equations, the relative performance of these equations was assessed using: (i) bias: median of difference between estimated and mGFR; 95% confidence interval (CI), when available; and (ii) P30: percentage of eGFR values within 30% of the (gold standard) mGFR; 95% CI, when available.
RESULTS
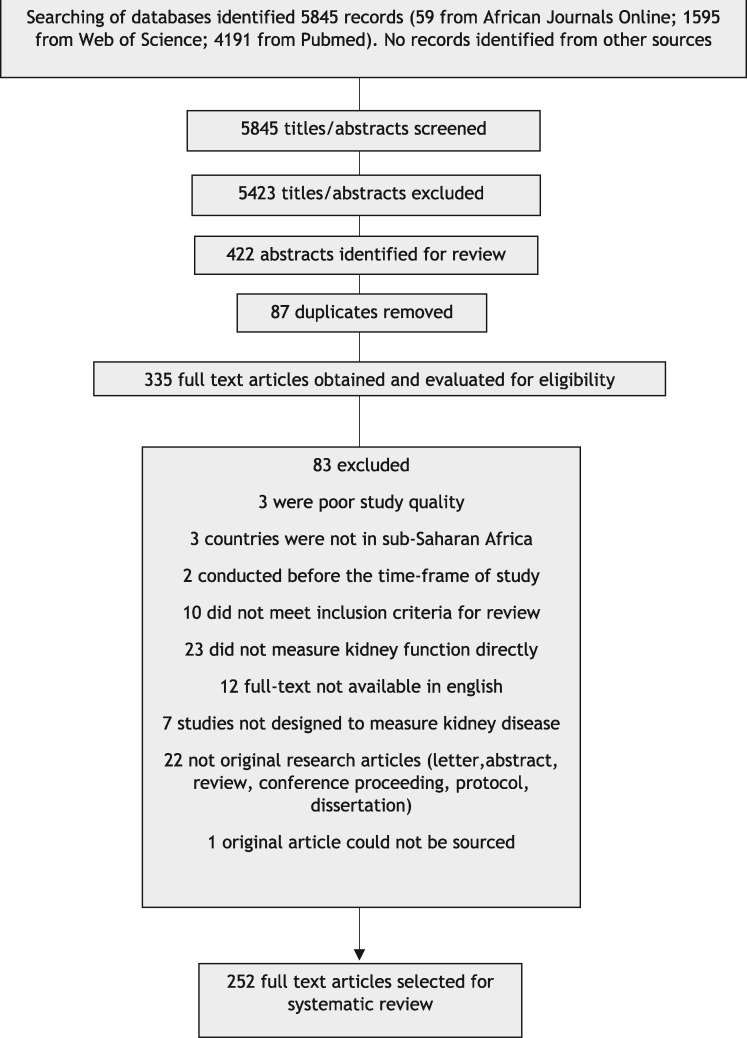
From the online database searches, there were 5845 records published during the review period. The procedure followed for study identification and selection is summarized in Figure 1. The final number of full-text articles assessed as eligible for inclusion into this systematic review totaled 252 (Supplementary data, Appendix S5). The results are presented as follows: (i) laboratory method for creatinine measurement; (ii) estimating equations for eGFR; (iii) mGFR using a gold standard method, comparison of the performance of eGFR equations in relation to mGFR and the impact of coefficients for AA ethnicity; (iv) diagnostic criteria for CKD; and (v) quality of the studies.
FIGURE 1.
Flowchart for study identification and selection.
Laboratory method for creatinine measurement
The laboratory method for creatinine measurement was not reported in two-thirds of studies (159/252; 63.1%). For those that included a method, the Jaffe method was by far the most common (80/86; 93.0%). Only six studies described an enzymatic method. Most studies (206/252; 81.7%) did not state whether their laboratory was using an IDMS-traceable standard reference material for creatinine measurement. When stipulated, IDMS-traceable assays were more common (34/39; 87.2%) than non-IDMS traceable assays, of which there were only five (Table 1).
Table 1.
Reporting of laboratory creatinine measurement and eGFR equations
| Laboratory creatinine measurement (n = 252 studiesa) | |
| Creatinine method | Jaffe/enzymatic 80 (31.7%)/6 (2.4%) |
| Not stated 159 (63.1%) | |
| Not measured 7 (2.8%) | |
| Creatinine method | IDMS-traceable 34 (13.5%) |
| Non-IDMS-traceable 5 (2.0%) | |
| Not stated 206 (81.7%) | |
| Not measured 7 (2.8%) | |
| eGFR equationsa (n = 363 eGFR equationsb) | |
| Creatinine clearance (n = 9; 2.5%) | BSAd normalized (4/9) |
| BSA not normalized (3/9) | |
| BSA not stated (2/9) | |
| Cockcroft–Gault (n = 85; 23.4%) | BSA normalized (29/85) |
| BSA not normalized (28/85) | |
| BSA not stated (28/85) | |
| Non-IDMS-traceable 4-v MDRD (n = 26; 7.2%) | + Coefficient for AA ethnicity (14/26) |
| − Coefficient for AA ethnicity (5/26) | |
| Coefficient not stated (7/26) | |
| IDMS-traceable 4-v MDRD (n = 46; 12.7%) | + Coefficient for AA ethnicity (23/46) |
| − Coefficient for AA ethnicity (15/46) | |
| Coefficient not stated (8/46) | |
| 4-v MDRD (not stated) (n = 74; 20.4%) | + Coefficient for AA ethnicity (8/74) |
| − Coefficient for AA ethnicity (9/74) | |
| Coefficient not stated (57/74) | |
| CKD-EPIc (creatinine) (n = 94; 25.9%) | + Coefficient for AA ethnicity (39/94) |
| − Coefficient for AA ethnicity (32/94) | |
| Coefficient not stated (23/94) | |
| CKD-EPIc (creatinine + cystatin C) (n = 6; 1.7%) | + Coefficient for AA ethnicity (3/6) |
| − Coefficient for AA ethnicity (3/6) | |
| Other (n = 23; 6.3%) | eGFR equation not specified (17/23) |
| Different eGFR equation used (6/23) | |
Percentages rounded to one decimal point and may not sum to 100%.
Of 252 studies, 21 did not use an eGFR method. Of the remaining studies (231), some evaluated more than one eGFR method, thus totaling 363 eGFR equations.
CKD-EPI equation for creatinine alone, or creatinine and cystatin C, or cystatin C alone.
BSA (body surface area), normalized to 1.73 m2 [21].
Estimating equations for GFR
Most studies used an estimating equation to assess kidney function (231/252; 91.6%) and some used more than one, so that eGFR equations were used in 363 instances. Of the available equations, the 4-v MDRD was most frequently used (146/363; 40.2%), followed by the CKD-EPI equation for creatinine (94/363; 25.9%) and Cockcroft–Gault (85/363; 23.4%). When the 4-v MDRD equation was used, one-third used the 4-v MDRD re-expressed for IDMS traceability (46/146; 31.5%), half did not stipulate which version was used (74/146; 50.7%) and the remainder used the original 4-v MDRD (prior to the introduction of an IDMS-traceable standard reference material for creatinine). The AA coefficient was used for a third of the 4-v MDRD equations (45/146; 30.8%) and almost half (72/146; 49.3%) did not stipulate if this was used. For the CKD-EPI creatinine equation, more studies used the AA coefficient (39/94; 41.5%) and a quarter did not clarify if this was used (23/94; 24.5%) (Table 1 andSupplementary data, Appendix S4).
mGFR using a gold standard method, comparison of the performance of eGFR equations in relation to mGFR and the impact of coefficients for AA ethnicity
There were 10 studies that measured GFR using a gold standard reference method, 4 from South Africa and 1 each from the Democratic Republic of the Congo, Ghana, Ivory Coast, Kenya, Seychelles and Sudan [9–13, 15, 22–25]. The Kenyan study and one study from South Africa focused on adult participants with HIV infection [12, 13]. This was particularly relevant for the SSA region because of widespread use of tenofovir-containing antiretroviral therapy regimens as first-line treatment. The methods used to measure GFR included iohexol on dried blood spots, iohexol plasma excretion, technetium-99m diethylenetriamine penta-acetic acid (DTPA), chromium-51 ethylenediamine tetra-acetic acid (EDTA), inulin and 24-h urinary creatinine clearance. Six studies compared the performance of currently recommended eGFR equations to a reference mGFR method. The impact of using the coefficient for AA ethnicity for the 4-v MDRD and CKD-EPI equations was also evaluated. Overall, most studies demonstrated that when compared with the mGFR method that was used, inclusion of the coefficient for AA ethnicity resulted in overestimation of eGFR in Africans (Table 2). Some studies compared the relative performance of one or more eGFR equations without reference to a gold standard mGFR. Since the scientific validity of this practice is unsubstantiated, no further analysis was conducted.
Table 2.
Studies from SSA with mGFR comparing performance of eGFR equations with/without coefficients for AA ethnicity
| Study | Sample size | Self-reported ethnicity; HIV status | mGFR method | mGFR (mL/min/ 1.73 m2) | eGFR equation | Bias with AA ethnicity coefficient % (95% CI) | Bias without AA ethnicity coefficient % (95% CI) | P30 with AA ethnicity coefficient % (95% CI) | P30 without AA ethnicity coefficient % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| South Africa 2008 [9] | 100 | Black African; 20 HIV positive | 51Cr-EDTA | 61.5 | 4-v MDRDa | 27 | 5 | 52 | 74 |
| South Africa 2012 [11] | 148 | Black African; HIV negative | 99mTc-DTPA | 38 | 4-v MDRDb | Not reported | Not reported | 50 | 55 |
| Kenya 2013 [12] | 99 | Black African; HIV positive | Iohexol blood spot | 115 | 4-v MDRDb | 18 | −3 | 73 | 83 |
| CKD-EPI(SCr)c | 10 | −4 | 82 | 85 | |||||
| South Africa 2016 [13] | 100 | Black African; HIV positive | 51Cr-EDTA | 92.5 | 4-v MDRDa | 38.4 (27.5; 49.3) | 14.2 (5.2; 23.2) | 43.3 | 59.8 |
| CKD-EPI(SCr)c | 33.7 (25.0; 42.4) | 15.3 (7.8; 22.8) | 41.2 | 62.9 | |||||
| CKD-EPI(SCrCys)d | 11.5 (5.4; 17.6) | 2.9 (−2.9; 8.8) | 73.0 | 78.0 | |||||
| Ivory Coast 2018 [24] | 237 | Black African; HIV negative | Iohexol plasma excretion | 103 | CKD-EPI(SCr)c | NA | NA | NA | NA |
| e FAScreat | NA | NA | NA | NA | |||||
| Democratic Republic of the Congo 2018 [15] | 93 | Black African; HIV status not declared | Iohexol plasma excretion | 92.0 | 4-v MDRDa | 13.6 (8.0; 19.2) | −4.9 (−9.6; −0.2) | 79.6 (71.2; 87.9) | 86.0 (78.8; 94.0) |
| CKD-EPI(SCr)c | 17.2 (13.3; 21.2) | 2.3 (−1.3; 5.8) | 73.1 (63.9; 82.3) | 81.7 (73.7; 89.7) | |||||
| CKD-EPI(SCrCys)d | 9.0 (5.9; 12.0) | 1.5 (−1.4; 4.4) | 87.1 (80.2; 94.0) | 92.5 (87.0; 97.9) | |||||
| CKD-EPI(Cys)f | 1.5 (−1.8; 4.7) | 91.4 (85.6; 97.2) | |||||||
Diagnostic criteria for CKD
Most studies (162/252; 64.5%) defined CKD using a broad range of criteria that have been summarized in Table 3. Fewer than 15% of studies confirmed chronicity by repeating the out of range test for eGFR and/or albuminuria/proteinuria after the minimum recommended period of 3 months, a requirement for diagnosing CKD as per KDIGO guidelines. Notably, most studies that fulfilled the KDIGO requirements were published in the latter half of the period under review [26–38].
Table 3.
Studies evaluating CKD in SSA (n = 162)
| Number of studiesa,bn = 162, n (%) | Parameter used to define CKD | Concordance with KDIGO definition of CKD |
||
|---|---|---|---|---|
| Creatinine-based eGFR equation | Urine protein or albumin | Confirmation of chronicity | ||
| 4 (2.5) | Serum creatinine |

|

|

|
| 1 (0.6) | Serum creatinine + urine protein |

|

|

|
| 67 (41.3) | Serum creatinine-based eGFR |

|

|

|
| 8 (4.9) | Serum creatinine-based eGFR + follow up measurement at ≥3 months |

|

|

|
| 22 (13.6) | Serum creatinine-based eGFR + urine albumin |

|

|

|
| 38 (23.5) | Serum creatinine-based eGFR + urine protein |

|

|

|
| 1 (0.6) | Serum creatinine-based eGFR + urine albumin + urine protein |

|

|

|
| 12 (7.4) | Serum creatinine-based eGFR + urine protein + follow up measurement/s at ≥3 months |

|

|

|
| 3 (1.9) | Urine albumin |

|

|

|
| 5 (3.0) | Urine protein |

|

|

|
| 1 (0.6) | Urine protein + follow up measurement at ≥3 months |

|

|

|
One study was excluded as the definition used for CKD was unclear.
Percentages have been rounded to one decimal point and might not sum to 100%.
Quality of studies
Most study designs were cross-sectional (172/252; 68.3%), fewer were case-controlled (41/252; 16.3%) and the methodology was unclear in the remainder (39/252; 15.4%). Many studies reported ‘prevalent’ CKD, but this was restricted to hospital- or clinic- based convenience samples and often focused on participants at high risk for kidney disease, for example, those with sickle cell disease, diabetes, hypertension, obesity, HIV and cardiac failure. For true population prevalence of CKD, there were eight randomly sampled population-based studies that determined prevalent CKD [10, 17, 39–44]. None of the population-based studies reported incident CKD.
DISCUSSION
There is scope for improving the quality of studies on kidney function in SSA. This includes aspects of study design and sampling, reporting of laboratory methods for creatinine measurement and IDMS traceability, detailing choices for GFR equations that include coefficients for AA ethnicity, and diagnosing CKD using appropriate criteria. While this is the first review of its kind for SSA, similar findings have been reported in Europe, Mexico, Uruguay and India [8, 45].
There are published NKDEP guidelines for the laboratory reporting of creatinine; however, two-thirds of our studies did not report the method of creatinine measurement and even fewer reported whether the method was IDMS-traceable to a standard reference material for creatinine. This limited insights into the extent of implementation of laboratory standards for creatinine-based testing of kidney function, but our findings reflect a need for better study-specific interdisciplinary collaboration between chemical pathologists, epidemiologists, public health and clinical scientists. Collaboration would deepen the scientific rationale and strengthen the methodological components of studies that characterize kidney disease, particularly at population level.
Many studies applied an estimating equation for GFR, but it is noteworthy that Cockcroft–Gault was still relatively frequently used, despite its omission from clinical practice guidelines since 2002. Perhaps this is due to the long history of this equation and its ease of use in clinical settings. Because of the time period for this review, it would be expected, as we have confirmed, that the 4-v MDRD equation was the most frequently used equation, but most studies did not identify which version of the equation was used in relation to IDMS traceability. Depending on which equation was used [CKD-EPI(serum creatinine, SCr) and/or 4-v MDRD], up to half of studies did not state whether the coefficients for AA ethnicity were used. In this regard, two recent studies from the Democratic Republic of the Congo and Ivory Coast (both using iohexol plasma excretion to measure GFR) deserve mention. The former, supporting prior findings from South and Eastern Africa, confirmed that omitting coefficients for AA ethnicity with CKD-EPI(SCr) and 4-v MDRD equations improved accuracy. Together, these studies highlight the critical importance of validating eGFR equations in populations in which they are recommended for use [9, 12, 13, 15]. The Ivorian study goes a few steps further, where for the first time in West Africans, normal reference ranges for GFR are now available and the performance of the Full Age Spectrum for creatinine (FAScreat) equation was validated. Originally derived from northern Caucasian populations, the FAScreat equation incorporates adjustment for age and gender as a Q value. In the Ivorian study, using Q values derived for West Africans, the performance of FAScreat was better than CKE-EPI(SCr) and requires no adjustment for ethnicity [25, 46]. If the FAScreat equation is validated in other regions of SSA and proven to be superior to the currently recommended CKD-EPI(SCr) equation, this provides strong support for changing current eGFR equations recommended for use in KDIGO clinical practice guidelines [4]. More broadly, remembering that performance of CKD-EPI(SCr) has been questioned in Asia, it is incumbent on policy makers—including KDIGO, to prioritize use of validated population-appropriate eGFR equations particularly when relating to diagnosis of CKD—given the global health implications [46, 47].
The gold standard reference methods used for mGFR also require some reflection due to the potential biases that might arise from their use [49–51]. In Europe, iohexol is the preferred method while in the USA, iothalamate is most commonly used. Recently, it has been suggested that iohexol may be the most practical and accurate measure of GFR for a few reasons: it is stable at a wide range of temperatures and has a very good clinical safety profile; iohexol is more accurate than iothalamate (which can systematically overestimate GFR by 10–15% due to tubular secretion); there is very little difference between laboratory techniques for measuring iohexol, for example when comparing high-performance liquid chromatography with ultraviolet detection and liquid chromatography-tandem mass spectrometry, whereas differences have been demonstrated with iothalamate; and there is an external quality control program for iohexol to aid standardizing interlaboratory variation in measurements which does not exist for iothalamate [48]. It is critical to contextualize this for SSA, as the 4-v MDRD and CKD-EPI eGFR equations were all developed using iothalamate and the coefficient for AA ethnicity would further overestimate GFR. For the few studies in this review that compared mGFR with eGFR, the reference methods differed, but none used iothalamate and three used iohexol (two intravenously for measurement of plasma excretion and one with dried blood spots) [12, 15]. While iohexol blood spots may be relatively inferior to intravenous administration of iohexol, the use of dried blood spots was possibly the most pragmatic, on the assumption that access to laboratory support was unlikely in rural Kenya.
This systematic review highlights potential sources of bias inherent in the assessment of kidney function in clinical studies in SSA. This has created the opportunity to increase awareness of the requirements for laboratory-based creatinine assays, the appropriate choice of estimating equations for calculating GFR and the appropriate use of diagnostic criteria for CKD. In response, we propose an ‘easy to use’ checklist for researchers as a guide for the reporting of kidney function in SSA (Table 4). We hope this will minimize bias and strengthen future studies conducted in the region. In addition, inferring population prevalence of CKD in SSA from small convenience samples of individuals at high risk for developing CKD must be cautioned, as the risk of overestimating CKD is substantial.
Table 4.
Recommendations for reporting kidney function in SSA populations: the African Research of Kidney Disease (ARK) checklist for researchers
| mGFR (gold standard reference method)—method and biomarker (51Cr-EDTA; 99mTc-DTPA; inulin, iohexol, iothalamate) |
|
| Laboratory creatinine method—include all the following: |
|
| Estimating equations for GFR—state which equation was used: |
|
|
|
| Diagnosis of CKD using KDIGO criteria [4]—include the following: |
| True prevalence requires a randomized population-based sample: describe the sampling strategy |
| KDIGO Clinical Practice Guidelines (2012) are recommended for diagnosis of CKD and require testing for: |
|
| Recommendation: In SSA, for CKD-EPI equation—omit coefficient for AA ethnicity [9–13, 24] |
aRe-expressed using an IDMS-traceable assay to a standard reference material.
bUse of 4-v MDRD and Cockcroft–Gault equations not recommended.
cSupporting evidence can be findings on renal ultrasound; and/or proof of pre-existing kidney disease from medical records or prior urine and serum test results.
While the focus of this systematic review has been clinical research, the implications of our findings have much broader application for the management of kidney disease in SSA. This encompasses individual patient care in the setting of acute and chronic kidney disease, home-based monitoring of CKD as a component of an integrated care model for chronic disease management, and public health policy for screening and prevention of CKD in SSA. None of this can be realized without affordable and accurate diagnostics for kidney disease. To achieve this, validated population-appropriate estimating equations for glomerular function need to be prioritized, as well as innovative approaches to accurate and affordable point of care diagnostics for assessing kidney function and diagnosing CKD.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
There was no specific funding to conduct this systematic review. However, this research was undertaken as part of a broader multicenter collaborative study between South Africa, Uganda, Malawi and the London School of Hygiene and Tropical Medicine, which is collectively identified as the African Research in Kidney Disease (ARK) Network. This is jointly funded by the South African Medical Research Council, with funding from the South African National Department of Health, MRC UK (via the Newton Fund) and GlaxoSmithKline Africa Non-Communicable Disease Open Lab Grant [Project Number: 8111 (Uganda and Malawi) and 074 (South Africa)]. The funder had no role in study design, data collection and analysis or decision to publish. Authors retained control of the final content of the publication.
A.N.W. is supported by the Fogarty International Centre of the National Institutes of Health under Award Number K43TW010698. This article describes the views of the authors and does not necessarily represent the official views of the National Institutes of Health (USA).
L.A.T. is funded by a Wellcome Intermediate Clinical Fellowship (reference: WT101143MA).
AUTHORS’ CONTRIBUTIONS
J.A.G., M.v.D., H.R.E., R.K. and J.F. reviewed full-text articles and extracted the data. J.F. conducted the narrative analysis, wrote the first draft of the paper and incorporated revisions into the final draft. H.R.E. compiled recommendations for reporting of studies that assess kidney function. H.R.E., J.A.G., R.K., S.N., S.T., L.A.T., M.v.D. and A.N.W. contributed to revising the first draft, editing and review of the final draft of the article.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
Supplementary Material
REFERENCES
- 1. Stanifer JW, Jing B, Tolan S. et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2014; 2: e174–e181. [DOI] [PubMed] [Google Scholar]
- 2. Coresh J, Hu JR, Bello AK. et al. Action plan for determining and monitoring the prevalence of chronic kidney disease. Kidney Int Suppl 2017; 7: 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levey AS, Coresh J, Bolton K. et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 4.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 5. Myers GL, Miller WG, Coresh J. et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5–18 [DOI] [PubMed] [Google Scholar]
- 6. Levey A, Coresh J, Greene T. et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Int Med 2006; 145: 247–254 [DOI] [PubMed] [Google Scholar]
- 7. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Int Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brück K, Jager KJ, Dounousi E. et al. Methodology used in studies reporting chronic kidney disease prevalence: a systematic literature review. Nephrol Dial Transplant 2015; 30: iv6–iv16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Deventer HE, George JA, Paiker JE. et al. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clin Chem 2008; 54: 1197–1202 [DOI] [PubMed] [Google Scholar]
- 10. Eastwood JB, Kerry SM, Plange-Rhule J. et al. Assessment of GFR by four methods in adults in Ashanti, Ghana: the need for an eGFR equation for lean African populations. Nephrol Dial Transplant 2010; 25: 2178–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madala ND, Nkwanyana N, Dubula T. et al. Predictive performance of eGFR equations in South Africans of African and Indian ancestry compared with 99mTc-DTPA imaging. Int Urol Nephrol 2012; 44: 847–855 [DOI] [PubMed] [Google Scholar]
- 12. Wyatt CM, Schwartz GJ, Owino Ong’or W. et al. Estimating kidney function in HIV-infected adults in Kenya: comparison to a direct measure of glomerular filtration rate by iohexol clearance. PLoS One 2013; 8: e69601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seape T, Gounden V, van Deventer HE. et al. Cystatin C-and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem 2016; 53: 58–66 [DOI] [PubMed] [Google Scholar]
- 14. Moodley N, Hariparshad S, Peer F. et al. Evaluation of the CKD-EPI creatinine based glomerular filtration rate estimating equation in Black African and Indian adults in KwaZulu-Natal, South Africa. Clin Biochem 2018; 59:43–49. [DOI] [PubMed] [Google Scholar]
- 15. Bukabau JB, Sumaili EK, Cavalier E. et al. Performance of glomerular filtration rate estimation equations in Congolese healthy adults: the inopportunity of the ethnic correction. PLoS One 2018; 13: e0193384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glaser N, Deckert A, Phiri S. et al. Comparison of various equations for estimating GFR in Malawi: how to determine renal function in resource limited settings? PLoS One 2015; 10: e0130453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stanifer JW, Maro V, Egger J. et al. The epidemiology of chronic kidney disease in Northern Tanzania: a population-based survey. PLoS One 2015; 10: e0124506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liberati A, Altman DG, Tetzlaff J. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whiting PF, Rutjes AWS, Westwood ME. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Int Med 2011; 155: 529–536 [DOI] [PubMed] [Google Scholar]
- 20.Systematic Review of the Assessment of Kidney Function Using Measured and Estimated Glomerular Filtration Rate in Sub-Saharan Africans [Internet], 2017. https://www.crd.york.ac.uk/PROSPERO/display_record.php? RecordID=68151 (11 March 2019, date last accessed)
- 21. Du Bois D, Du Bois E.. A formula to estimate the approximate surface area if height and weight are known. Arch Int Med 1917; 17: 863–871 [PubMed] [Google Scholar]
- 22. van Deventer HE, Paiker JE, Katz IJ. et al. A comparison of cystatin C-and creatinine-based prediction equations for the estimation of glomerular filtration rate in black South Africans. Nephrol Dial Transplant 2011; 26: 1553–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wuerzner G, Pruijm M, Maillard M. et al. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. Am J Kidney Dis 2010; 56: 303–312 [DOI] [PubMed] [Google Scholar]
- 24. Osman AO, Elmadani AE.. Comparison of slope-intercept with single plasma sample methods in estimating glomerular filtration rate using radionuclides. Saudi J Kidney Dis Transplant 2014; 25: 321. [DOI] [PubMed] [Google Scholar]
- 25. Yayo E, Ayé M, Yao C. et al. Measured (and estimated) glomerular filtration rate: reference values in West Africa. Nephrol Dial Transplant 2017; 33: 1176–1180 [DOI] [PubMed] [Google Scholar]
- 26. Madala ND, Dubula T, Assounga AGH. et al. Association of kidney function and waist circumference with uric acid levels in South Africans. Metab Syndr Relat Disord 2017; 15: 500–506 [DOI] [PubMed] [Google Scholar]
- 27. Adugna T, Merga H, Gudina EK.. Impaired glomerular filtration rate, high grade albuminuria and associated factors among adult patients admitted to tertiary Hospital in Ethiopia. BMC Nephrol 2018; 19: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amoako YA, Laryea DO, Bedu-Addo G. et al. Clinical and demographic characteristics of chronic kidney disease patients in a tertiary facility in Ghana. Pan Afr Med J 2014; 18:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arogundade F, Sanusi A, Hassan M. et al. An appraisal of kidney dysfunction and its risk factors in patients with sickle cell disease. Nephron Clin Pract 2011; 118: c225–c231 [DOI] [PubMed] [Google Scholar]
- 30. Babua C, Kalyesubula R, Okello E. et al. Cardiovascular risk factors among patients with chronic kidney disease attending a tertiary hospital in Uganda: cardiovascular topics. Cardiovasc J Afr 2015; 26: 177–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cailhol J, Nkurunziza B, Izzedine H. et al. Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: a cross-sectional study. BMC Nephrol 2011; 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ekiti ME, Zambo JB, Assah FK. et al. Chronic kidney disease in sugarcane workers in Cameroon: a cross-sectional study. BMC Nephrol 2018; 19: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamadou B, Boombhi J, Kamdem F. et al. Prevalence and correlates of chronic kidney disease in a group of patients with hypertension in the Savanah zone of Cameroon: a cross-sectional study in Sub-Saharan Africa. Cardiovasc Diagn Ther 2017; 7: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaze FF, Kengne AP, Magatsing CT. et al. Prevalence and determinants of chronic kidney disease among hypertensive Cameroonians according to three common estimators of the glomerular filtration rate. J Clin Hypertens 2016; 18: 408–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madala ND, Thusi GP, Assounga AG. et al. Characteristics of South African patients presenting with kidney disease in rural KwaZulu-Natal: a cross sectional study. BMC Nephrol 2014; 15: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Namuyimbwa L, Atuheire C, Okullo J. et al. Prevalence and associated factors of protein-energy wasting among patients with chronic kidney disease at Mulago hospital, Kampala-Uganda: a cross-sectional study. BMC Nephrol 2018; 19: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Onodugo O, Ulasi I, Ijoma C. et al. Predictors of autonomic dysfunction among predialysis chronic kidney disease patients in Nigeria. Niger J Clin Pract 2018; 21: 932–938 [DOI] [PubMed] [Google Scholar]
- 38. Marie PH, Moussa O, Francois K. et al. Prevalence and associated factors of chronic kidney disease among patients infected with human immunodeficiency virus in Cameroon. Iran J Kidney Dis 2018; 12: 268–274 [PubMed] [Google Scholar]
- 39. Kalyesubula R, Nankabirwa JI, Ssinabulya I. et al. Kidney disease in Uganda: a community based study. BMC Nephrol 2017; 18: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsha TE, Yako YY, Rensburg MA. et al. Chronic kidney diseases in mixed ancestry south African populations: prevalence, determinants and concordance between kidney function estimators. BMC Nephrol 2013; 14: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaze FF, Meto DT, Halle MP. et al. Prevalence and determinants of chronic kidney disease in rural and urban Cameroonians: a cross-sectional study. BMC Nephrol 2015; 16: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seck SM, Doupa D, Guéye L. et al. Chronic kidney disease epidemiology in northern Senegal: a cross-sectional study. Iran J Kidney Dis 2014; 8: 286. [PubMed] [Google Scholar]
- 43. Oluyombo R, Ayodele O, Akinwusi P. et al. A community study of the prevalence, risk factors and pattern of chronic kidney disease in Osun State, South West Nigeria. West Afr J Med 2013; 32: 85–92 [PubMed] [Google Scholar]
- 44. Sumaili EK, Krzesinski JM, Zinga CV. et al. Prevalence of chronic kidney disease in Kinshasa: results of a pilot study from the Democratic Republic of Congo. Nephrol Dial Transplant 2008; 24: 117–122 [DOI] [PubMed] [Google Scholar]
- 45. Ruiz-Arenas R, Sierra-Amor R, Seccombe D. et al. A summary of worldwide national activities in chronic kidney disease (CKD) testing. EJIFCC 2017; 28: 302–314 [PMC free article] [PubMed] [Google Scholar]
- 46. Pottel H, Hoste L, Dubourg L. et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 2016; 31: 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim BS, Lee YK, Choi HY. et al. Is the new GFR equation using inulin clearance a more accurate method for Asian patients? Clin Nephrol 2015; 84: 331–338 [DOI] [PubMed] [Google Scholar]
- 48. Wang J, Xie P, Huang J-M. et al. The new Asian modified CKD-EPI equation leads to more accurate GFR estimation in Chinese patients with CKD. Int Urol Nephrol 2016; 48: 2077–2081 [DOI] [PubMed] [Google Scholar]
- 49. Seegmiller JC, Burns BE, Schinstock CA. et al. Discordance between iothalamate and iohexol urinary clearances. Am J Kidney Dis 2016; 67: 49–55 [DOI] [PubMed] [Google Scholar]
- 50. Delanaye P, Ebert N, Melsom T. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: how to measure glomerular filtration rate with iohexol? Clin Kidney J 2016; 9: 682–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Inker LA, Levey AS, Coresh J.. Estimated glomerular filtration rate from a panel of filtration markers—hope for increased accuracy beyond measured glomerular filtration rate? Adv Chronic Kidney Dis 2018; 25: 67–75 [DOI] [PubMed] [Google Scholar]
- 52. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Levey AS, Bosch JP, Lewis JB. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Int Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 54. Cockcroft DW, Gault MH.. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.