Abstract
Background
The number of patients on waiting lists for repeated kidney transplantation has increased. However, retransplanted patients have a greater surgical and immunological risk than first-time kidney recipients.
Methods
We retrospectively analysed all kidney recipients that underwent third, fourth or fifth kidney transplantation (Group 3+) at the University Hospital Essen, Essen, Germany from October 1973 to January 2017. A historical cohort of recipients retransplanted with a second kidney (Group 2) served as the control. Donor and recipient demographic data, cold ischaemia time (CIT), warm ischaemia time, overall operation time and methods, transplantectomy of previous kidney grafts, incidence of surgical and immunological complications as well as patient- and death-censored survival were analysed.
Results
We identified 108 recipients transplanted with the third, fourth or fifth renal allograft. Patients with more than one transplantation had significantly higher surgical risk due to atherosclerosis (P = 0.002) and higher immunological risk due to higher panel reactive antibody levels preoperatively (current panel reactive antibody P = 0.004; highest panel reactive antibody value P = 0.0001). Group 3+ patients had more often undergone previous transplant nephrectomy (P = 0.0001). There was a significant difference in CIT (P = 0.009), overall operative time (P = 0.0001) and post-transplantation thrombotic events (P = 0.02). We could not demonstrate any differences in graft and patient survival.
Conclusion
Third, fourth and fifth transplant recipients are a high-risk patient cohort. Our results suggest that patient survival after more than three renal transplantations is similar to that of second graft recipients. This supports the concept of repeated kidney retransplantations.
Keywords: graft survival, immunological complications, kidney retransplantation, patient survival, surgical complications
INTRODUCTION
In recent decades, many advances have been made in kidney transplantation, including the introduction of more effective immunosuppressant drugs and further development of surgical procedures, resulting in significant improvement of renal allograft survival. However, after kidney transplantation, graft loss because of chronic rejection still constitutes a major problem [1]. The number of patients on waiting lists for repeated kidney transplantation has increased to almost 20% of all waitlisted patients in our centre. Several transplant centres list recipients for third, fourth and fifth kidney transplantations; it is known, however, that retransplanted patients have a greater surgical and immunological risk than first-time kidney recipients [2, 3].
Surgical procedures on previously operated iliac fossae can present additional technical challenges. Relevant scarring and fibrosis may make identification of correct tissue planes more difficult, necessitating vascular surgery and risking complications. Cold ischaemia time (CIT) and warm ischaemic time (WIT) may be extended because of the complex and prolonged anastomotic procedures and have been associated with longer overall operative time, impacting morbidity [4]. Several retransplantation approaches are known, including the retroperitoneal or intraperitoneal technique. Variations of the arterial anastomoses with the extern, intern or common iliac arteries or direct connection with the aorta are well described. Venous drainage through the iliac veins or the vena cava inferior is performed routinely [5, 6].
Besides the surgical difficulties, immunological sensitization associated with a previous graft still presents a major challenge to retransplantation. Higher panel reactive antibody (PRA) levels are associated with hyperacute rejection, delayed graft function (DGF) and poor graft survival rates [7]. It is still not clear whether transplantectomy of previous renal grafts affects long-term kidney retransplantation graft survival [8–11].
Data for patient and graft outcomes in second, third, fourth and fifth renal transplantations are limited. Significantly decreased graft survival was reported when compared with first kidney transplants [5]. Nevertheless, repeated kidney transplantation still offers a significant survival benefit for the recipient over remaining on dialysis [12]. Therefore this study aimed to analyse patients and graft survival after kidney retransplantation.
MATERIALS AND METHODS
Study population
We included all adult patients who underwent third, fourth or fifth kidney transplantation at the University Hospital Essen, Essen, Germany from October 1973 to January 2017. Data were prospectively collected through the Eurotransplant database and the local patient database and retrospectively evaluated for this study. A randomly chosen historical cohort of recipients re-transplanted with a second kidney served as the control, therefore excluding the bias of surgical as well as immunological benefits of the first transplantation. Patients who underwent multiorgan transplantation and paediatric recipients were excluded. The study protocol was approved by the local ethics committee. Due to the retrospective study design, informed consent was waived.
The following characteristics were considered for the analysis: donor age; donor body mass index (BMI); kidney donor risk index (KDRI); kidney donor profile index (KDPI) specified for the year 2016; CIT; WIT; overall operation time (OPT); human leucocyte antigen (HLA) A, B or DR mismatches; recipient age; gender; underlying kidney disease; comorbidities; living or deceased donor kidney transplantation; previous kidney transplantation; highest (hPRA) and current PRA (cPRA) levels; transplantectomy of previous kidney grafts; incidence of reoperation for bleeding and vascular thrombotic events; infectious complications; rejection of the allograft and patient- and death-censored graft survival.
Surgical technique
Recipients were preoperatively screened by nephrologists, cardiologists, urologists, transplant surgeons and anaesthesiologists. The extraperitoneal approach of the iliac fossa was performed in most of the patients. The renal vein was anastomosed to the external or common iliac vein or the inferior vena cava. The vascular anastomoses were performed in an end-to-side fashion. The renal artery was then anastomosed to the external iliac artery. If this artery was not suitable for a recurrent vascular anastomosis, then the common or internal iliac artery was used in an end-to-side or end-to-end fashion. Donor ureter and recipient bladder were anastomosed by an extravesical approach as described by Gregoir [13]. A 7-French stent was inserted in the ureter as standard care. In case of a severely fibrotic or scarred iliac fossae, repeated transplantations were performed intraperitoneally without any major difference in the vascular or ureteral anastomotic techniques.
Immunosuppressive treatment
Immunosuppression was based on the ongoing studies at the time of transplantation and included induction and maintenance immunosuppression; however, this changed over the years. Typically, initial immunosuppression consisted of an induction agent, calcineurin inhibitors, mycophenolic acid and steroids. Acute rejection was defined as biopsy-proven histological changes treated with intravenously applied methylprednisolone shot therapy or plasmapheresis and/or intravenous immunoglobulin treatment depending on the cellular or humoral rejection. Progressive irreversible graft failure under continued immunosuppressive therapy was described as chronic graft loss. All patients were followed pre- and postoperatively at our outpatient kidney transplant clinic.
Definition of DGF
DGF was defined as the need for at least one haemodialysis session during the first week post-transplant [14].
Statistical analysis
Data were expressed as mean and standard error of the mean or median and range as appropriate. All data were tested for normality using the method of Kolmogorov–Smirnov. Applied statistical tests were dependent on the underlying data and were performed with t-tests or Mann–Whitney tests and Fisher’s exact test or chi-square test as appropriate.
Univariable and multivariable analyses were performed with logistic regression and Cox proportional hazard models. Variables with P < 0.1 in univariable analysis were included in a stepwise mixed multivariable regression analysis. Odds ratios were obtained from hazard models. Missingness of data was handled by case exclusion. Differences of P < 0.05 were considered statistically significant. Statistical analyses were performed using JMP (John’s Macintosh Project) (version 10.0.0; SAS Institute, Cary, NC, USA) and SPSS (Statistical package for the social sciences) (version 24.0.0.0; IBM, Armonk, NY, USA).
RESULTS
We identified 3998 patients who underwent kidney transplantation at our centre between October 1973 and January 2017. Among these, 91, 16 and 1 patient received a third, fourth and fifth transplantation, respectively (Group 3+, n = 108). We identified 347 patients that underwent their second kidney transplantation. As the control group, we randomly selected 108 patients who had received their second kidney transplantation (Group 2) and were not identical to any patient from Group 3+.
Donor characteristics
Mean donor age, BMI and gender were similar in both groups. We performed 14 (13%) living related kidney transplantations as the first retransplantation and 11 (10.2%) as the third, fourth or fifth transplantation. Both groups were comparable with regards to median KDRI [Group 2: 1.07 (range 0.6–2.8) versus Group 3+: 1.05 (0.6–3.1)] and median KDPI [Group 2: 57% (range 3–100) versus Group 3+: 54% (1–100)]. Details are given in Table 1.
Table 1.
Univariable analysis of donor characteristics
| Group 2 | Group 3+ | P-value | |
|---|---|---|---|
| (n = 108) | (n = 108) | ||
| Gender, n (%) | |||
| Male | 58 (53.7) | 57 (52.8) | 0.89 |
| Female | 50 (46.3) | 51 (47.2) | |
| Age (years), mean (SD) | 43.9 (±1.7) | 43.2 (±1.5) | 0.72 |
| BMI, mean (SD) | 25.7 (±0.46) | 24.2 (±0.3) | 0.01 |
| Living-related kidney transplantation, n (%) | 14 (11) | 11 (10.2) | 0.52 |
| KDRI median (range) | 1.07 (0.6–2.8) | 1.05 (0.6–3.1) | 0.2 |
| KDPI (%) median (range) | 57 (3–100) | 54 (1–100) | 0.19 |
Data are expressed as mean and standard deviation and median and range. P < 0.05.
Recipient characteristics
Recipient demographic data are shown in Table 2. There was no statistical difference between the groups with regards to age, gender and BMI. Group 3+ patients demonstrated a high prevalence of vascular comorbidities. In particular, peripheral atherosclerosis was observed significantly more often than in patients undergoing the first retransplantation [Group 3+: n = 23 (21.5%) versus Group 2: n = 5 (5.7%), P = 0.002]. Group 3+ recipients had significantly higher PRA levels at the time of transplantation [cPRA Group 2: 0% (range 0–95) versus Group 3+: 5% (0–92), P = 0.004] as well as hPRA levels compared with patients with one transplantation in their history [hPRA Group 2: 5% (range 0–100) versus Group 3+: 32% (0–100), P = 0.0001].
Table 2.
Univariable analysis of recipient characteristics
| Group 2 | Group 3+ | P-value | |
|---|---|---|---|
| (n = 108) | (n = 108) | ||
| Gender, n (%) | |||
| Male | 58 (53.7) | 57 (52.8) | 0.89 |
| Female | 50 (46.3) | 51 (47.2) | |
| Age (years), mean (SD) | 43.9 (±1.7) | 43.2 (±1.5) | 0.72 |
| BMI (kg/m2), mean (SD) | 25.7 (±0.46) | 24.2 (±0.3) | 0.01 |
| cPRA (%), median (range) | 0 (0–95) | 5 (0–92) | 0.004 |
| hPRA (%), median (range) | 5 (0–100) | 32 (0–100) | 0.0001 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 19 (20.7) | 28 (26.2) | 0.36 |
| Cardiac disease | 35 (38.9) | 43 (40.2) | 0.853 |
| Atherosclerosis | 5 (5.7) | 23 (21.5) | 0.002 |
| COPD | 6 (6.6) | 8 (7.6) | 0.78 |
| HCV | 12 (13.5) | 31 (28.7) | 0.01 |
Data are expressed as mean and standard deviation and median and range. P < 0.05.
COPD, chronic obstructive pulmonal disease; HCV, hepatitis C virus.
We removed one or more transplanted kidneys more often in Group 3+ patients than in Group 2 patients [Group 2: n = 45 (47.9%) versus Group 3+: n = 87 (82.1%), P = 0.0001]. Data are shown in Table 3.
Table 3.
Univariable analysis of operative and post-operative data
| Group 2 | Group 3+ | P-value | |
|---|---|---|---|
| (n = 108) | (n = 108) | ||
| CIT (h), mean (SD) | 15.4 (± 0.7) | 18.1 (± 0.7) | 0.009 |
| WIT (min) | 31.0 (± 1.1) | 31.2 (± 1.4) | 0.89 |
| OPT (min) | 149 (± 4.8) | 187 (± 6.9) | 0.0001 |
| Simultaneous transplantectomy, n (%) | 2 (2.1) | 20 (18.7) | 0.0001 |
| Kidney graft position, n (%) | |||
| Extraperitoneal | 107 (98.9) | 93 (86.1) | |
| Intraperitoneal | 1 (1.1) | 15 (13.9) | 0.0001 |
| Venous anastomoses, n (%) | 0.0070 | ||
| External iliac vein | 104 (96) | 90 (83) | |
| Common iliac vein | 3 (3) | 13 (12) | |
| Inferior vena cava | 1 (1) | 5 (5) | |
| Arterial anastomoses, n (%) | 0.0006 | ||
| External iliac artery | 95 (88) | 69 (64) | |
| Common iliac artery | 11 (10) | 35 (32) | |
| Internal iliac artery | 1 (1) | 2 (2) | |
| Abdominal aorta | 1 (1) | 2 (2) | |
| Ureter anastomoses, n (%) | 0.0484 | ||
| Ureteroneocystostomy | 95 (88) | 103 (95) | |
| Uretero-ureteral | 1 (1) | 2 (2) | |
| Other | 12 (11) | 3 (3) |
Data are expressed as mean and standard deviation. P < 0.05.
Kidney transplantation
There was no significant difference between the two groups in terms of WIT [Group 2: mean 31 min (SD ± 1.1) versus Group 3+: 31.2 (±1.4), P = 0.89]. The mean length of CIT, just like OPT, was significantly longer in recipients with more than one retransplantation [CIT: Group 2: mean 15.4 h (SD ± 0.7) versus Group 3+: 18.1 (±0.76), P = 0.009; OPT: Group 2: mean 149 min (SD ± 4.8) versus Group 3+: 187 (±6.9), P = 0.0001].
Routinely in our centre, the renal allograft is placed in the extraperitoneal position. We decided on the intra-abdominal position in one case in Group 2 and in 15 cases in Group 3+ [n = 1 (1.1%) versus n = 15 (13.9%), P = 0.0001]. There were significantly more uncommon vascular anastomosis sites performed in Group 3+. Detailed procedural data are presented in Table 3.
Post-operative surgical complications
Surgical treatment for complications was required in 14 (19.2%) recipients after first retransplantation and 31 patients (31.3%) (P = 0.07) with a third, fourth or fifth transplantation. Besides bleeding complications [Group 2: n = 11 (15.1%) versus Group 3+: n = 20 (20.2%), P = 0.39], thrombotic complications [Group 2: n = 2 (2.7%) versus Group 3+: n = 13 (13.1%), P = 0.02] and urine leakage [Group 2: n = 2 (2.7%) versus Group 3+: n = 3 (3%), P = 0.89] were identified. None of the patients underwent any procedure because of peripheral sclerosis or vascular injury of the lower extremities. More recipients with a third, fourth or fifth allograft were treated in the intensive care unit (ICU) post-operatively than recipients from Group 2 [Group 2: n = 14 (13%) versus Group 3+: n = 23 (21.3%), P = 0.07]. There was no difference in the length of the ICU stay [Group 2: 1 day (1–3) versus Group 3+: 1 day (1–11), P = 0.15].
Post-operative immunological complications
Analysis of HLA mismatches between donor and recipient detected no significant differences in the three major localizations (HLA-A, -B, -DR) in Groups 2 and 3+ (0 mismatches: Group 2: n = 15 versus Group 3+: n = 14, P = 1.000; 6 mismatches: Group 2: n = 2 versus Group 3+: n = 1, P = 1.000).
Biopsy-proven rejections were documented in 31 (39.2%) recipients in Group 2 and 40 (37%) in Group 3+ (P = 0.2465). Treatment consisted of a cortisone shot (n = 21 versus n = 26, P = 1.000), plasmapheresis (n = 7 versus n = 10, P = 1.000) or immunoglobulin therapy (n = 13 versus n = 6, P = 0.0153), if needed, in escalating order.
Patient and graft outcomes
Occurrence of DGF was not significantly different between the groups [Group 2: n = 17 (21.8%) versus Group 3+: n = 24 (24.7%), P = 0.65].
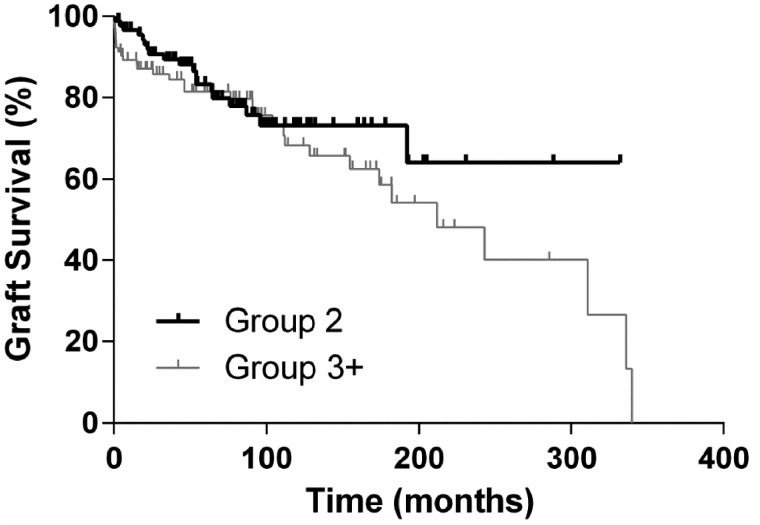
Graft survival 30 days, 12 months and 5 years after first retransplantation was 89.6, 86.7 and 74.6%, respectively. For patients undergoing a second or more retransplantation, the graft survival rates after 30 days, 12 months and 5 years were 94.4, 85.5 and 69.6%, respectively. As the reason for graft failure, chronic glomerular injury or chronic rejection could be detected in 15 patients in Group 2 and 44 patients in Group 3+ (P < 0.0001). Comparison of graft survival between the groups demonstrated similar outcomes (P = 0.16). In multivariate analysis, recipient age and biopsy-proven rejections were identified as independent risk factors for graft survival in Group 2 and previous transplantectomy, KDPI and biopsy-proven rejections in Group 3+. Detailed results are depicted in Tables 4 and 5.
Table 4.
Multivariable Cox proportional hazard analysis for graft survival after second kidney transplantation
| Risk ratioa (95% CI) | P-value | ||
|---|---|---|---|
| Age | 0.97 (0.94–1.00) | 0.05 | |
| Biopsy-proven rejection | 0.29 (0.12–0.7) | 0.01 |
Risks referring to change of 1 U in the regressor. P < 0.05.
Table 5.
Multivariable Cox proportional hazard analysis for graft survival after third or more kidney transplantations
| Risk ratioa (95% CI) | P-value | ||
|---|---|---|---|
| hPRA | 1.01 (1.002–1.023) | 0.16 | |
| Previous transplantectomy | 0.44 (0.21–0.92) | 0.03 | |
| KDPI | 1.02 (1.004–1.03) | 0.01 | |
| Biopsy-proven rejection | 2.27 (1.05–4.9) | 0.04 |
Risks referring to change of 1 U in the regressor. P < 0.05.
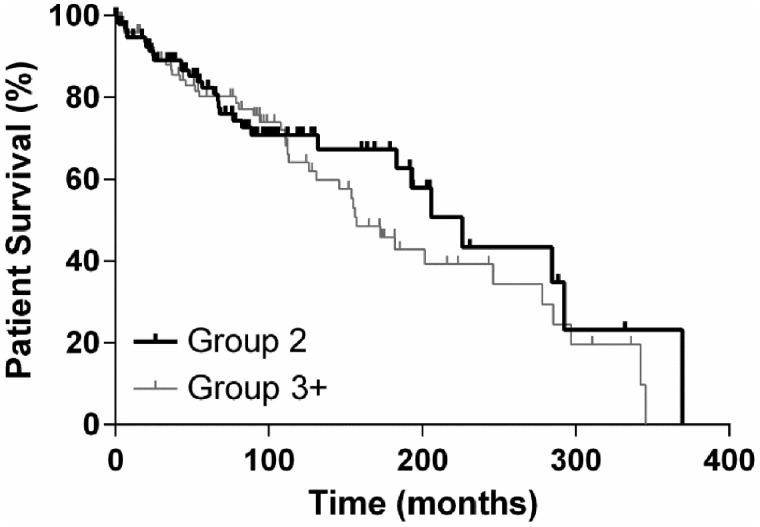
Patient survival after 30 days, 12 months and 5 years was 100, 95 and 86%, respectively, in Group 2 and 100, 96 and 86%, respectively, in Group 3+ (P = 0.3276). Graft and patient survival are demonstrated in Kaplan–Meier curves in Figures 1 and 2.
FIGURE 1.
Graft survival.
FIGURE 2.
Patient survival.
DISCUSSION
In our study, outcomes were not different between patients undergoing their second kidney transplantation compared with those undergoing their third, fourth or fifth transplantation. Graft survival after 30 days, 12 months and 5 years as well as the rate of DGF were similar in both groups. However, it has to be acknowledged that outcomes are inferior compared with first kidney transplantations [3].
With the present state of severe organ shortages, the concept of repeated transplantations has been debated [5]. While results are reported to be inferior compared with first kidney transplantations, patients’ quality of life and overall health care costs are still improved [15]. Therefore it is accepted practice to perform repeated renal transplantations. Nowadays, >20% of patients on the waiting list have had a prior kidney transplantation and 2.7% of patients are registered for a third or subsequent transplantation [14, 15]. In our study, most recipients of kidney retransplantations were notably younger than kidney recipients in general, probably representing a positive selection of patients suitable to undergo the procedure once more in spite of assumed higher rates of perioperative risks.
Surgical difficulties encountered by retransplantations after the second graft have been investigated before [3, 12, 16]. In part, such technical difficulties are based on severe calcifications of the recipients’ aortoiliac vessels. In the present cohort, peripheral vascular disease was observed in every fifth patient. Moreover, adhesions and scarring in the retroperitoneal space make vascular dissection more challenging. Therefore an intraperitoneal approach is carried out routinely in some centres for patients undergoing their second transplantation in order to decrease vascular complications. We prefer to place the graft in the iliac fossa, even in cases of several preceding transplantations. Our results demonstrate that this is possible in most cases. Nevertheless, we placed the kidney graft significantly more frequently intra-abdominally in Group 3+ compared with Group 2 (15 versus 1, P = 0.0001).
Repeated surgery of the iliac fossa has been shown to be associated with increased operation times and blood loss as well as vascular and ureteric complications [6]. Kienzl-Wagner et al. [2] reported higher rates of surgical complications after third, fourth and fifth kidney transplantations, whereas blood loss and anastomotic times were not analysed. In our study, surgical complications were not significantly different between the groups. However, operative time and CIT were significantly longer in patients who had received more than two kidney transplantations. Moreover, we observed an increased risk of thrombotic events of renal vessels, possibly due to the utilization of unusual localizations for the anastomoses. Our data clearly demonstrate that surgical failure is an unusual cause of graft loss in recipients of three or more kidneys, supporting the concept that kidney retransplantations are justified.
We detected a high rate of high PRAs in recipients of a third or more kidneys, which was subsequently followed by a high frequency of biopsy-proven acute rejections. The high incidence of rejection (39% in Group 2 versus 50% in Group 3+) is explained by 30% of our patients being hyperimmunized. Our results are comparable to others reported in the literature, with the rate of biopsy-proven rejection ranging from 28% to 45% after repeated kidney transplantation [5].
Recently, much attention has been focused on renal transplantation in highly sensitized patients. PRAs have been considered to be an independent risk factor for graft loss and for patient death in several studies [5, 12, 17]. Even in the absence of specific anti-donor antibodies, high PRAs are followed by inferior outcomes [7]. There is a trend in our data as well, indicating that higher PRAs are associated with poor long-term graft function, although this did not reach statistical significance. Other data described no differences in terms of acute rejection episodes and graft survival according to PRAs [16, 18].
Elevated PRA levels can be reduced by prolonged immunosuppressive regimes after renal allograft failure without transplant nephrectomy [10]. This approach should be taken into account for all recipients with failing allografts who might be suitable for a future transplantation to minimize immunological risk. Otherwise, transplant nephrectomies might be performed. Without generally accepted indications for transplant nephrectomy, the rate of removal of grafts from recipients after renal transplantation varies widely from 0.5% to 43% [19, 20]. Schleicher et al. [21] described a negative influence of primary allograft nephrectomy on the second transplant outcome. Our treatment strategy is to perform transplant nephrectomy in case of early technical allograft failures and in symptomatic patients. Simultaneous transplant nephrectomy is sometimes considered during kidney retransplantation due to space issues. In the present series, transplant nephrectomy was performed in >80% of recipients with three or more kidney transplantations and almost 20% simultaneously during the next transplantation. This approach was not associated with an increased rate of DGF.
Any benefit of transplant nephrectomy should be weighed against the serious risks of this procedure. Lucarelli et al. [9] demonstrated higher surgical complication rates among patients who underwent nephrectomy prior to retransplantation compared with those who did not. Other studies suggest avoiding simultaneous transplantectomy and retransplantation to reduce the operative risk [10, 21, 22].
In patients with renal allograft failure and a first retransplantation, transplant nephrectomy should mainly be performed if symptoms (haemorrhage, infection, rejection) occur. In recipients needing a third or fourth transplantation, transplant nephrectomy could be recommended to minimize surgical complications during future transplantations.
We demonstrated a similar 1-month, 1-year and 5-year graft and patient survival after the first retransplantation and second or more retransplantations. A Swiss study showed survival rates at 1 and 5 years of 75 and 60%, respectively, for second grafts, inferior to our results for second grafts (89.6 and 74.6%) and comparable to our third or more kidney transplantation results [23]. Patient survival is generally high in studies on kidney retransplantations [24].
The limitations of this study are the small sample size and its retrospective single-centre design. To account for the common clinical selection bias, we compared our third/fourth/fifth transplant group with a control group of recipients undergoing their second transplantation, most likely to a naive iliac fossa of the other side.
CONCLUSION
In conclusion, this study provides insights into the surgical and immunological challenges of kidney retransplantation. Our data confirmed previous results, suggesting that patient survival rates after three or more renal transplantations were statistically not different from those of second graft recipients. In spite of the shortage of donor organs, outcomes, manageable complications and economic considerations support multiple kidney retransplantations and encourage us to continue with this procedure.
ACKNOWLEDGEMENTS
We are indebted to all of our co-workers at the University Hospital Essen who supported the skillful and assiduous care of our patients during therapy.
AUTHORS’ CONTRIBUTIONS
All the authors read and approved the final manuscript and are responsible for the integrity of the data and the accuracy of the data analysis. T.B. designed the study and wrote the article. P.H. collected data. S.R. analysed data and provided intellectual content. A.G. revised the article. J.W.T. supervised the project. G.M.K. provided intellectual content. D.P.H. analysed the data and gave final approval.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Port FK, Wolfe RA, Mauger EA. et al. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270: 1339–1343 [PubMed] [Google Scholar]
- 2. Kienzl-Wagner K, Mark W, Maglione M. et al. Single-center experience with third and fourth kidney transplants. Transpl Int 2011; 24: 780–786 [DOI] [PubMed] [Google Scholar]
- 3. Koch M, Kantas A, Ramcke K. et al. Surgical complications after kidney transplantation: different impacts of immunosuppression, graft function, patient variables, and surgical performance. Clin Transplant 2015; 29: 252–260 [DOI] [PubMed] [Google Scholar]
- 4. Barocci S, Valente U, Fontana I. et al. Long-term outcome on kidney retransplantation: a review of 100 cases from a single center. Transplant Proc 2009; 41: 1156–1158 [DOI] [PubMed] [Google Scholar]
- 5. Blanco M, Medina J, Gonzalez E. et al. Third kidney transplantation: a permanent medical-surgical challenge. Transplant Proc 2009; 41: 2366–2369 [DOI] [PubMed] [Google Scholar]
- 6. Ooms LSS, Roodnat JI, Dor FJMF. et al. Kidney retransplantation in the ipsilateral iliac fossa: a surgical challenge. Am J Transplant 2015; 15: 2947–2954 [DOI] [PubMed] [Google Scholar]
- 7. Kawase T, Tojimbara T, Niki R. et al. Successful third kidney transplantation with intensive immunosuppression in a highly sensitized recipient. Transplant Proc 2008; 40: 2428–2430 [DOI] [PubMed] [Google Scholar]
- 8. Schachtner T, Otto NM, Stein M. et al. Transplantectomy is associated with presensitization with donor-reactive T cells and graft failure after kidney retransplantation: a cohort study. Nephrol Dial Transplant 2018; 33: 889–896 [DOI] [PubMed] [Google Scholar]
- 9. Lucarelli G, Vavallo A, Bettocchi C. et al. Impact of transplant nephrectomy on retransplantation: a single-center retrospective study. World J Urol 2013; 31: 959–963 [DOI] [PubMed] [Google Scholar]
- 10. Lin J, Wang R, Xu Y. et al. Impact of renal allograft nephrectomy on graft and patient survival following retransplantation: a systematic review and meta-analysis. Nephrol Dial Transplant 2018; 33: 700–708 [DOI] [PubMed] [Google Scholar]
- 11. Dinis P, Nunes P, Marconi L. et al. Kidney retransplantation: removal or persistence of the previous failed allograft? Transplant Proc 2014; 46: 1730–1734 [DOI] [PubMed] [Google Scholar]
- 12. Halawa A. The third and fourth renal transplant; technically challenging, but still a valid option. Ann Transplant 2012; 17: 125–132 [DOI] [PubMed] [Google Scholar]
- 13. Gregoir W. [Congenital vesico-ureteral reflux]. Acta Urol Belg 1962; 30: 286–300 [PubMed] [Google Scholar]
- 14. Irish WD, Ilsley JN, Schnitzler MA. et al. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant 2010; 10: 2279–2286 [DOI] [PubMed] [Google Scholar]
- 15. Rao PS, Schaubel DE, Wei G. et al. Evaluating the survival benefit of kidney retransplantation. Transplantation 2006; 82: 669–674 [DOI] [PubMed] [Google Scholar]
- 16. Hagan C, Hickey DP, Little DM.. A single-center study of the technical aspects and outcome of third and subsequent renal transplants. Transplantation 2003; 75: 1687–1691 [DOI] [PubMed] [Google Scholar]
- 17. Magee JC, Barr ML, Basadonna GP. et al. Repeat organ transplantation in the United States, 1996–2005. Am J Transplant 2007; 7(5 Pt 2): 1424–1433 [DOI] [PubMed] [Google Scholar]
- 18. Mouquet C, Benalia H, Chartier-Kastler E. et al. [Renal retransplantation in adults. Comparative prognostic study]. Prog Urol 1999; 9: 239–243 [PubMed] [Google Scholar]
- 19. Anton-Perez G, Gallego-Samper R, Marrero-Robayna S. et al. Transplantectomy following renal graft failure. Nefrologia 2012; 32: 573–578 [DOI] [PubMed] [Google Scholar]
- 20. Abouljoud MS, Deierhoi MH, Hudson SL. et al. Risk factors affecting second renal transplant outcome, with special reference to primary allograft nephrectomy. Transplantation 1995; 60: 138–144 [PubMed] [Google Scholar]
- 21. Schleicher C, Wolters H, Kebschull L. et al. Impact of failed allograft nephrectomy on initial function and graft survival after kidney retransplantation. Transpl Int 2011; 24: 284–291 [DOI] [PubMed] [Google Scholar]
- 22. Ott U, Busch M, Steiner T. et al. Renal retransplantation: a retrospective monocentric study. Transplant Proc 2008; 40: 1345–1348 [DOI] [PubMed] [Google Scholar]
- 23. Etienne T, Goumaz C, Ruedin P. et al. Renal retransplantation in Switzerland: poor HLA matching of first and subsequent allografts does not appear to affect overall graft survival. Transpl Int 1992; 5(Suppl 1): S65–S66 [DOI] [PubMed] [Google Scholar]
- 24. Pour-Reza-Gholi F, Nafar M, Saeedinia A. et al. Kidney retransplantation in comparison with first kidney transplantation. Transplant Proc 2005; 37: 2962–2624 [DOI] [PubMed] [Google Scholar]