Abstract
Background
Several renal biopsy registries in Europe have shown geographical and temporal variations in the patterns of renal diseases. However, there is a lack of current data on trends of renal disease in Central Europe.
Methods
After exclusion of transplant and re-biopsies, the renal biopsy registry of the German RWTH Aachen University Hospital included data of 1208 biopsies over a period of 24 years (1990–2013). Trends in the biopsy rate and diagnosis of glomerular diseases were analysed.
Results
The average annual biopsy incidence was 6.1 biopsies per 100 000 population. The frequency of kidney biopsies increased significantly over the years (P < 0.001). Primary glomerulonephritis (GN) accounted for nearly two-thirds (58.4%) of all native kidney biopsies, and immunoglobulin A-nephropathy (IgAN) was the leading histological diagnosis (34.7%) followed by necrotizing GN (RPGN) at 18.7%. IgAN increased 2-fold over the study periods (+195%, P < 0.001). Focal segmental glomerulosclerosis accounted for 6.1% of all diagnoses, and its frequency rose to 3.9-fold (+388%, P < 0.001). Lupus nephritis showed a doubling in incidence (P = 0.0499), while acute tubular necrosis decreased to 3.5-fold (P = 0.0008). All other disease entities failed to exhibit linear trends over time. In children, the most common pathologies were IgAN (26.1%) and minimal change disease (21.7%), whereas RPGN (19.4%) dominated in the group of patients >60 years.
Conclusion
IgAN was the most common primary glomerular disease in our centre and its prevalence increased over 24 years.
Keywords: CKD, FSGS, glomerulonephritis, IgA nephropathy, kidney biopsy
INTRODUCTION
Several renal biopsy registries in Europe have shown geographical and temporal variations in the patterns of renal diseases, and different evolutions of incidence rates over the years have been reported. In most European countries, immunoglobulin A-nephropathy (IgAN) is the most common histologically diagnosed primary renal disease [1–8]. However, in some countries, other diseases like membranous nephropathy [9, 10] or membranoproliferative glomerulonephritis (GN) [11] are ranked first. The incidence of focal segmental glomerulosclerosis (FSGS) varies worldwide. FSGS is the most common idiopathic glomerular disease in adults particularly in centres of North America [12–16] and South America [17, 18], and some studies have reported a growing incidence of FSGS [13, 15], particularly in the black and Hispanic population [14, 17, 19]. Similar increases are less well documented in the Caucasian population [20].
Data on biopsy-proven renal diseases in Germany and German-speaking countries are scarce, in contrast to Italy [3, 5], Denmark [22, 23], Spain [2], Lithuania [8] and Czech Republic [1], where large national renal biopsy registries exist. In addition, temporal trends in incidences have been reported in only a few studies. The aim of this retrospective study was to analyse the epidemiology and changing patterns of renal diseases based on the histological diagnosis from a single centre in Central Europe over a period of 24 years.
MATERIALS AND METHODS
Patients and histological specimens
The results of all native renal biopsies performed at the University Hospital Aachen, Germany during the period of January 1990 to December 2013 were analysed retrospectively. To allow a comparison with previous reports, the patients were divided into three groups according to age: children were ≤15 years, adults 16–60 years and the elderly >60 years. In addition, to compare the diseases in detail, the adults were split into three further age groups consisting of 16–30, 31–45 and 46–60 years.
The following data were registered for each patient: name, date of birth, sex, date of biopsy and primary leading histological diagnosis. Other histological diagnoses such as secondary diagnoses were not considered. Biopsies of transplant kidneys were excluded. Patients with re-biopsies (more than one biopsy) were also excluded.
Given that all biopsies were performed by a single centre with a relatively fixed biopsy policy, indications for renal biopsies did not change substantially during that period. The following criteria constituted an indication for renal biopsy at the RWTH Aachen University Hospital: nephrotic syndrome, nephritic syndrome, impaired glomerular filtration rate of uncertain origin with or without significant proteinuria (defined as >1 g/day) and persistent acute renal failure of undetermined origin. The specimens of renal biopsies obtained at the RWTH Aachen University Hospital were sent to the Department of Pathology of the University Medical Center Hamburg-Eppendorf from 1990 to 1998 and to the Department of Cellular and Molecular Pathology, German Cancer Research Center (DKFZ) in Heidelberg, Germany from 1999 to 2013 for histological interpretation and diagnoses. All biopsy specimens were stained and analysed at both sites by identical methods using light microscopy, immunohistology and electron microscopy. Where appropriate, specific (immuno-)staining was performed. All biopsies were evaluated by two nephropathologists (U.H., H.-J.G.) only.
Histological diagnoses
For the analyses, the histological diagnoses were divided into six major categories: primary glomerular disease, secondary glomerular disease, acute and chronic tubulointerstitial nephritis or acute tubular necrosis (TIN), vascular nephropathy, hereditary nephritis and miscellaneous pathologies.
Primary GN included IgAN, membranous glomerulonephritis (MGN), FSGS, global sclerosis, necrotizing GN (rapidly progressive glomerulonephritis, RPGN), minimal change disease (MCD), membranoproliferative GN types 1 and 2, non-IgA mesangioproliferative nephropathy, endocapillary GN and thin basement membrane nephropathy.
Secondary GN contained lupus nephritis, amyloidosis, diabetic nephropathy, secondary FSGS, Goodpasture’s syndrome, cholesterol embolism, IgA vasculitis (Henoch–Schönlein purpura), C3-GN and light-chain deposition disease. TIN was classified into acute TIN, chronic TIN, interstitial nephritis and fibrosis, granulomatous TIN and myeloma cast nephropathy.
Hypertensive nephrosclerosis, thrombotic microangiopathy, haemolytic uraemic syndrome, ischaemic glomerulosclerosis, renal infarction, arteritis, eclampsia-induced nephropathy and renal cortical necrosis were pooled under the term ‘vascular nephropathy’.
Hereditary disorders included Alport syndrome, Fabry disease and other hereditary glomerulopathies.
Miscellaneous diagnoses were, for example, renal tumours, normal renal tissue or non-classifiable diseases.
Time periods
The data were analysed from 1 January 1990 to 31 December 2013. For better comparison, three periods of 8 years each, that is, 1990–97, 1998–2005 and 2006–13, were chosen. To determine the biopsy rates, the complete population of the catchment area of the University Hospital Aachen in the years 1995, 2001 and 2011 was used. Data for 1995 and 2001 were based on estimates of the Federal Statistical Office in Germany, and the population in 2011 was determined by population census. The population of the catchment area of the University Hospital Aachen varied from 815 000 to 832 000 to 809 000, respectively, over the three periods analysed. The catchment area was calculated as follows: the county Aachen (707 km2 with 547 861 inhabitants in the mean) and the rural community surrounding Aachen (altogether 829 km2 and 270 883 inhabitants) were considered, that is, altogether 818 744 inhibitants in 1536 km2. The population density of 533 inhabitants/km2 is above the German (231 inhabitants/km2) and the European average (approximately 117 inhabitants/km2). The RWTH Aachen University Hospital is the only hospital in this area where renal biopsies were performed.
Statistical analysis
Data were originally stored on Microsoft Office Access and on Microsoft Office Excel. Statistical analysis was performed using the SPSS statistical software package for Windows (Version 21.0, IBM® Corp.) and SAS Statistical Analysis Systems (Version 9.4). The annual incidence was defined as the number of new cases per year related to the catchment area, expressed as per hundred thousand population per year (php/year). Furthermore, Fisher’s exact test was used for categorical variables and a modified Pearson Chi-square test called ‘Cochrane-Armitage test for trend’ for continuous variables and their proportions. P < 0.05 were considered statistically significant.
RESULTS
Biopsies
All native renal biopsies performed at the RWTH Aachen University Hospital from January 1990 until December 2013 were analysed. In total, 2243 biopsies were performed. After excluding 944 transplant biopsies and 91 patients with re-biopsies, a total of 1208 patients with one native kidney biopsy were enrolled.
Frequency of renal biopsies
The biopsy rate showed a significant increase over 24 years (P < 0.001). Initially, the biopsy rate was stable in the first period (1990–97; 5.5 biopsies php/year) and in the second period (1998–2005; 5.5 biopsies php/year). It rose to 7.4 biopsies php/year in the last period (2006–13). As shown in Table 1, the renal biopsy rate is comparable to that of other European studies except for results from Romania and Serbia with lower rates.
Table 1.
Summary of the annual renal biopsy rate in European studies (modified from Naumovic et al. [31])
| Country (reference) | Type of registry | Follow-up (years) | Number of biopsies | Biopsy rate (php/year) | Population density (inhab./km2) (2002) |
|---|---|---|---|---|---|
| Spain [2] | National | 6 | 7016 | 4.8 | 81 |
| Finland [32] | Six centres | 24 | 3310 | 17.6 | 15 |
| Italy [3] | National | 7 | 15 461 | NA | 187 |
| Denmark [22] | National | 13 | 2380 | 4.0 | 125 |
| France [4] | Single centre | 27 | 1742 | 16.2 | 108 |
| Czech Republic [1] | National | 7 | 4004 | 4.4–6.9 | 129 |
| Romania [11] | Two centres | 10 | 606 | 1.1 | 94 |
| Serbia [31] | Single centre | 20 | 1626 | 1.1 | 84 |
| Northern Ireland [24] | Single centre | 30 | 2128 | 2.0–7.1 | 122 |
| Germany, present study | Single centre | 24 | 1208 | 6.1 | 231 |
Demographics
A total of 23 (2%) children, 809 (67%) adults and 376 (31%) elderly were biopsied; 63% of them were male. The average age of all patients increased from the first period to the last period (P < 0.002; Table 2). The percentage of patients >60 years rose from 28% in the first period to 35% in the last period (P = 0.027).
Table 2.
Demographics
| Category | All (%) | 1990–97 (%) | 1998–2005 (%) | 2006–13 (%) |
|---|---|---|---|---|
| Age (years) | 50 ± 17.5 | 48 ± 18.1 | 49 ± 16.5 | 52 ± 17.6 |
| Gender (m/f) | 759 (63) /449 (37) | 215(60)/143 (40) | 235 (64)/133 (36) | 309 (64)/173 (36) |
| Children (≤15 years) | 23 (2) | 14 (4) | 4 (1) | 5 (1) |
| Adults (16–60 years) | 809 (67) | 242 (68) | 260 (71) | 307 (64) |
| Elderly (>60 years) | 376 (31) | 102 (28) | 104 (28) | 170 (35) |
Given are mean ± SD or n (%). m, male; f, female.
Diagnostic categories
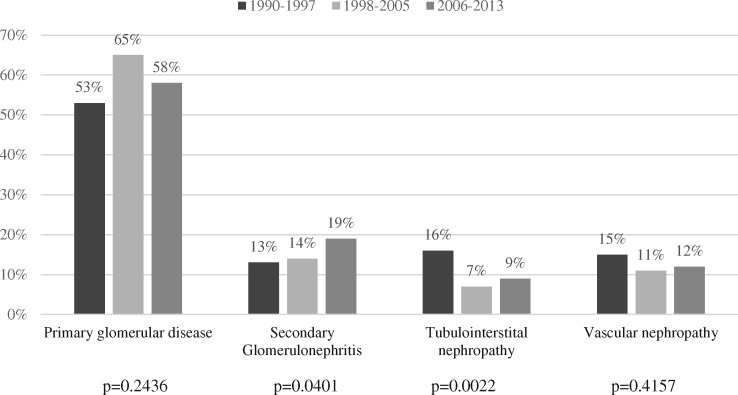
The majority of patients had a primary glomerular disease (58%), with male predominance (Table 3). The absolute numbers of primary GN showed a non-significant trend (P = 0.24; Figure 1), whereas the relative frequencies remained stable (53, 65 and 58% in the first, second and third period, respectively). The diagnosis of secondary GN increased over time (P = 0.04). The categories of vascular nephropathy, tubulointerstitial diseases, hereditary nephritis and miscellaneous diagnoses were stable across all 8-year time frames.
Table 3.
Distribution of histological diagnoses
| Category | Male (%) | n (%) |
|---|---|---|
| Primary GN | 65.5 | 706 (58.4) |
| Secondary GN | 51.2 | 190 (15.7) |
| Vascular nephropathy | 65.6 | 154 (12.8) |
| TIN | 60.6 | 127 (10.5) |
| Miscellanous pathologies | 70.0 | 20 (1.7) |
| Hereditary nephritis | 45.5 | 11 (0.9) |
| Total | 62.8 | 1208 (100) |
FIGURE 1.
Histological diagnoses divided into 8-year time frames (n = 1208).
Glomerular diseases
The most frequent glomerulopathies of all diagnoses (n = 1208), irrespective of their categories, were IgAN (20.3%), RPGN (10.9%), nephrosclerosis (10.3%), MGN (8.7%), MCD (6.1%) and FSGS (6.1%; Table 4).
Table 4.
Incidence of renal diseases in Aachen, Germany for a period of 24 years, divided into three 8-year time frames
| GN subtype | 1990–97 |
1998–2005 |
2006–13 |
All years |
Trend of incidence | ||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Incidence php/year | n (%) | Incidence php/year | n (%) | Incidence php/year | n (%) | Incidence php/year | P-value | |
| IgAN | 41 (11) | 0.6 | 83 (23) | 1.2 | 121 (25) | 1.9 | 245 (20) | 1.2 | <0.0001 |
| RPGN | 50 (14) | 0.8 | 41 (11) | 0.6 | 41 (9) | 0.6 | 132 (11) | 0.7 | 0.353 |
| NSc | 39 (11) | 0.6 | 34 (9) | 0.5 | 51 (11) | 0.8 | 124 (10) | 0.6 | 0.174 |
| MGN | 25 (7) | 0.4 | 46 (13) | 0.7 | 34 (7) | 0.5 | 105 (9) | 0.5 | 0.266 |
| FSGS | 8 (2) | 0.1 | 27 (7) | 0.4 | 39 (8) | 0.6 | 74 (6) | 0.4 | <0.0001 |
| MCD | 33 (9) | 0.5 | 20 (5) | 0.3 | 21 (4) | 0.3 | 74 (6) | 0.4 | 0.091 |
| LN | 13 (4) | 0.2 | 20 (5) | 0.3 | 25 (5) | 0.4 | 58 (5) | 0.3 | 0.0499 |
| aTIN | 25 (7) | 0.4 | 11 (3) | 0.2 | 7 (1) | 0.1 | 43 (4) | 0.2 | 0.0008 |
| Others | 124 (35) | 1.9 | 86 (23) | 1.3 | 143 (30) | 2.2 | 353 (29) | 1.8 | 0.1935 |
| Total | 358 (100) | 5.5 | 368 (100) | 5.5 | 482 (100) | 7.4 | 1208 (100) | 6.1 | <0.0001 |
NSc, nephrosclerosis; LN, lupus nephritis; aTIN, acute tubulointerstitial nephritis.
Primary and secondary GN
When analysing only patients with primary GN (n = 706), IgAN was the most common entity (34.7%) with a male predominance (72.2%). RPGN was diagnosed especially in the group of the elderly with an average ± SD age of 59.7 ± 15.4 years and was the second most frequent primary GN diagnosis (18.7%), followed by MGN (14.9%), MCD (10.5%), FSGS (7.8%), global glomerulosclerosis (5.0%) and endocapillary GN (3.3%).
In the analysis of secondary GN (n = 190) lupus nephritis was the most common glomerular disease, accounting for 30.5% of secondary GN, with a significant female predominance (72.4%). Amyloidosis and diabetic nephropathy were other frequent causes with 22.1 and 21.6%, followed by secondary FSGS (10%).
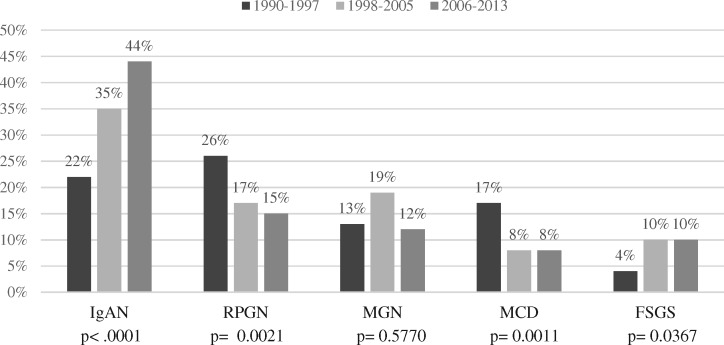
Trends with time
To evaluate changing patterns of kidney disease, diagnoses with the highest prevalence were analysed over time (Figure 2 and Table 4). While the incidence of IgAN rose 2-fold (+195%, P < 0.0001) and FSGS 3.9-fold (+388%, P < 0.0001), a 3.5-fold decrease of acute TIN (P = 0.0008) was detected. Lupus nephritis showed a doubling in incidence (P = 0.0499), whereas the incidences of nephrosclerosis, MGN and MCD remained relatively stable.
FIGURE 2.
Trend in primary GN over time (n = 706).
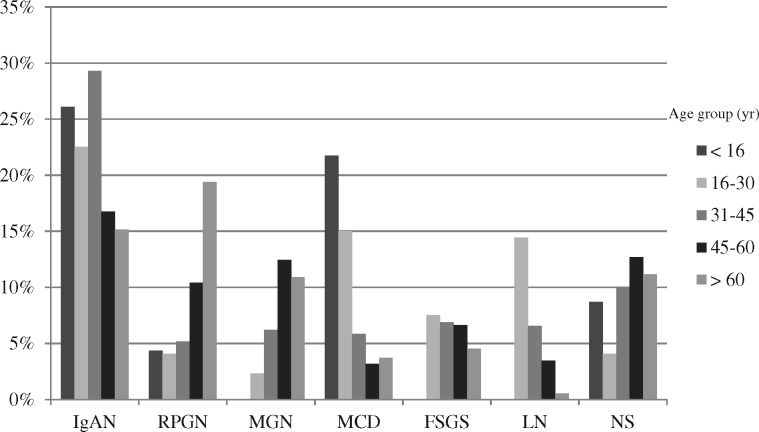
Trends with age
The peak incidence of IgAN was in the 31- to 45-year age category at 29.3% (see Figure 3). Overall, IgAN dominated in all age categories except in the >60 years age category, where RPGN ranked first with 19.4% and IgAN was second with 15.2%.
FIGURE 3.
Frequency of most common glomerular diseases by different age groups. LN, lupus nephritis; NS, nephrosclerosis.
Lupus nephritis affected patients of the younger generation and was most commonly diagnosed in the age group of 16–30 years (14.5%) with a mean age of 35.1 ± 12.5 years and a female predominance (72.4%; P < 0.001).
In children, the most common pathologies were IgAN (26.1%), MCD (21.7%), and nephrosclerosis and interstitial nephritis (each 8.7%).
DISCUSSION
In this study, we present current data on trends of renal diseases in a single-centre study in Germany over a period of 24 years. To the best of our knowledge, this is the first report of a current renal biopsy registry with analysis of trends in central Europe with such a long period of follow-up and number of biopsies. Studies from other countries reported geographical and temporal variations in the patterns of renal disease [1, 3, 10–13, 22–25]. We found a constant proportion of primary GN over the years of 58%, which is in line with other European studies [1–5, 11, 24]. Within the primary GN, IgAN made up 34.7% of the diagnoses and thus was the leading histological diagnosis of the renal biopsy database (20.3%).
One of our major findings is that the IgAN incidence increased significantly over the period of 24 years. IgAN is the most common renal disease in Europe [1–5] and in some Asian countries [27–28]. In the USA, IgAN frequency has been reported variably, with it being the leading diagnosis in some [20] but not all regions [12]. Concerning the risk factors for IgAN, both immunogenetic and environmental factors have been discussed to play a role. In France, the incidence of IgAN was stable over 27 years, suggesting that immunogenetic factors are of higher impact than environmental factors for the onset of IgAN [4] (unless environmental factors remain stable). However, in line with our study, Hanko and colleagues [24] also described an increase in IgAN incidence in Northern Ireland. What are the potential reasons for an increase in IgAN incidence? First, a higher biopsy rate could be the reason for an increased incidence as IgAN may occur subclinically. However, in contrast to the study of Hanko et al. [24], which noted a 4.7-fold increase of biopsy rates, we observed only a 1.4-fold increase of biopsy rate over the period. Consequently, if at all, increased biopsy frequency may contribute only a minor proportion to the increased IgAN incidence. Secondly, an altered biopsy indication could explain the changing patterns of renal diseases, but the stable biopsy policy and almost identical nephrologists in our centre over the study period as well as a consistent biopsy evaluation argue against this option. Thirdly, a recent study reported that socioeconomic deprivation was associated with biopsy-proven IgAN [29]. Though we did not determine socioeconomic factors in our study, deprivation as cause for an increased IgAN incidence seems unlikely as the region of Aachen did not undergo any major socioeconomic changes in recent decades. Fourthly, increased rates in metabolic syndrome could be a reason for increased IgAN incidence. However, this is highly speculative. Though we did not determine body mass index (BMI) in our patients, an increase in BMI from 25.1 kg/m2 in 1999 to 25.8 in 2013 has been reported in Germany (Federal Statistical Office, Germany). Fifthly, a combination of the abovementioned factors would also be a possible explanation for the increased IgAN incidence over time.
Our second major finding is an increase in the incidence of FSGS over time. Ethnic differences have been described in FSGS incidences where Afro-Americans show a clear predominance [14, 15]. In line with our finding, other studies also reported an increase in FSGS incidence [7, 12, 13, 20]. Interestingly, despite a predominance of Caucasian ethnicity (>80% white race), a study from Minnesota [20] also reported a higher FSGS incidence (17%) than ours (8%), which may be due to the fact that more obese patients were biopsied in Minnesota compared with our study. Contrary to our study, Hanko et al. [24] could not observe an increase in FSGS incidence. One potential reason for this difference could be that numbers of FSGS cases in Northern Ireland were likely too small to observe significant trends. Concerning underlying factors for an increasing FSGS incidence in a mainly Caucasian population, Swaminathan and collegues [20] discussed urbanization and increasing obesity as potential reasons. In fact, our area had a higher population density in comparison with other European regions. In addition, as outlined above, an increase in BMI was indeed reported in Germany between 1999 and 2013, but we have no specific information on our patients.
We found RPGN to be the most common glomerular disease in the elderly, with a significant increase over time. Hedger et al. [30] also reported an increased RPGN incidence with age. Brazdziute et al. [8] related the relative increase in crescentic GN to an improved clinical diagnosis of the disease. On the contrary, Swaminathan et al. [20] observed a non-significant decline of RPGN incidence over time. Reasons for our observation of an increased RPGN incidence with age remain highly speculative and could be related to an ageing population.
One limitation of our study is the lack of clinical data. Nevertheless, the strength of the present study is that the renal biopsy indication policy did not change over the years. Another limitation is the difficult comparison with other registries and centres because of the potential differences in biopsy rates with geographical and temporal variation. The reported incidences are usually based on the epidemiology of moderate and severe disease, whereas mild forms may be underdiagnosed. With a restricted number of cases, we may have missed smaller differences or trends in our study. Key strengths of our study include the long follow-up of 24 years, the evaluation of all biopsies using identical methods by two nephropathologists (senior and scholar) from the same school.
In conclusion, we noted a major absolute and relative increase in biopsy-confirmed IgAN at our centre. FSGS also exhibited a major relative increase although absolute numbers were much smaller compared with IgAN. While the continued obesity epidemic and an ageing population may in part explain the trends in FSGS incidence, the reasons underlying the marked increased in IgAN diagnosis remain unknown at present.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Rychlík I, Jancová E, Tesar V. et al. The Czech registry of renal biopsies. Occurence of renal diseases in the years 1994–2000. Nephrol Dial Transplant 2004; 19: 3040–3049 [DOI] [PubMed] [Google Scholar]
- 2. Rivera F, López-Gómez JM, Pérez-García R.. Frequency of renal pathology in Spain 1994–1999. Nephrol Dial Transplant 2002; 17: 1594–1602 [DOI] [PubMed] [Google Scholar]
- 3. Schena FP; The Italian Group of Renal Immunopathology. Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. Nephrol Dial Transplant 1997; 12: 418–426 [DOI] [PubMed] [Google Scholar]
- 4. Simon P, Ramee M-P, Boulahrouz R. et al. Epidemiologic data of primary glomerular diseases in western France. Kidney Int 2004; 66: 905–908 [DOI] [PubMed] [Google Scholar]
- 5. Gesualdo L, Di Palma AM, Morrone LF. et al. The Italian experience of the national registry of renal biopsies. Kidney Int 2004; 66: 890–894 [DOI] [PubMed] [Google Scholar]
- 6. Horvatic I, Tisljar M, Bulimbasic S. et al. Epidemiologic data of adult native biopsy-proven renal diseases in Croatia. Int Urol Nephrol 2013; 45: 1577–1587 [DOI] [PubMed] [Google Scholar]
- 7. Kurnatowska I, Jędrzejka D, Małyska A. et al. Trends in the incidence of biopsy-proven glomerular disease in the adult population in central Poland in the years 1990-2010. Kidney Blood Press Res 2012; 35: 254–258 [DOI] [PubMed] [Google Scholar]
- 8. Brazdziute E, Miglinas M, Gruodyte E. et al. Nationwide renal biopsy data in Lithuania 1994–2012. Int Urol Nephrol 2015; 47: 655–662 [DOI] [PubMed] [Google Scholar]
- 9. Polenakovic MH, Grcevska L, Dzikova S.. The incidence of biopsy-proven primary glomerulonephritis in the Republic of Macedonia - long term follow-up. Nephrol Dial Transplant 2003; 18: 26–27 [DOI] [PubMed] [Google Scholar]
- 10. Hsiao K-C. Ten-year registry of native kidney biopsy from a single center in Taichung. Acta Nephrologica 2012; 26: 68–73 [Google Scholar]
- 11. Covic A, Schiller A, Volovat C. et al. Epidemiology of renal disease in Romania: a 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant 2006; 21: 419–424 [DOI] [PubMed] [Google Scholar]
- 12. Braden GL, Mulhern JG, O'Shea MH. et al. Changing incidence of glomerular diseases in adults. Am J Kidney Dis 2000; 35: 878–883 [DOI] [PubMed] [Google Scholar]
- 13. Haas M, Spargo BH, Coventry S.. Increasing incidence of focalsegmental glomerulosclerosis among adult nephropathies: a 20-year renal biopsy study. Am J Kidney Dis 1995; 26: 740–750 [DOI] [PubMed] [Google Scholar]
- 14. Kitiyakara C, Eggers P, Kopp JB.. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 2004; 44: 815–825 [PubMed] [Google Scholar]
- 15. D’Agati V. The many masks of focal segmental glomerulosclerosis. Kidney Int 1994; 46: 1223–1241 [DOI] [PubMed] [Google Scholar]
- 16. Dragovic D, Rosenstock JL, Wahl SJ.. Changing incidence of glomerular diseases in adults. Am J Kidney Dis 2000; 35: 878–883 [DOI] [PubMed] [Google Scholar]
- 17. Arias LF, Henao J, Giraldo RD. et al. Glomerular diseases in a Hispanic population: review of a regional renal biopsy database. Sao Paulo Med J 2009; 127: 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bahiense-Oliveira M, Saldanha LB, Mota ELA. et al. Primary glomerular disease in Brazil (1979-1999): is the frequency of focal and segmental glomerulosclerosis increasing? Clinc Nephrol 2004; 61: 90–97 [DOI] [PubMed] [Google Scholar]
- 19. Nair R, Walker PD.. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int 2006; 69: 1455–1458 [DOI] [PubMed] [Google Scholar]
- 20. Swaminathan S, Leug N, Lager DJ.. Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol 2006; 1: 483–487 [DOI] [PubMed] [Google Scholar]
- 21. Braun N, Schweisfurth A, Lohöfener C. et al. Epidemiology of glomerulonephritis in Northern Germany. Int Urol Nephrol 2011: 43: 1117–1126 [DOI] [PubMed] [Google Scholar]
- 22. Heaf J. The Danish renal biopsies register. Kidney Int 2004; 66: 895–897 [DOI] [PubMed] [Google Scholar]
- 23. Heaf J, Løkkegaard H, Larsen S.. The epidemiology and prognosis of glomerulonephritis in Denmark 1985–1997. Nephrol Dial Transplant 1999; 14: 1889–1897 [DOI] [PubMed] [Google Scholar]
- 24. Hanko JB, Mullan RN, O’Rourke DM. et al. The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant 2009; 24: 3050–3054 [DOI] [PubMed] [Google Scholar]
- 25. Okpechi I, Swanepoel C, Duffield M. et al. Patterns of renal disease in Cape Town South Africa: a 10-year review of a single-centre renal biopsy database. Nephrol Dial Transplant 2011; 26: 1853–1861 [DOI] [PubMed] [Google Scholar]
- 26. Choi IJ, Jeong HJ, Han DS. et al. An analysis of 4, 514 cases of renal biopsy in Korea. Yonsei Med J 2001; 42: 247–254 [DOI] [PubMed] [Google Scholar]
- 27. Li LS, Liu ZH.. Epidemiological data of renal diseases from a single unit in China: analysis based on 13, 519 renal biopsies. Kidney Int 2004; 66: 920–923 [DOI] [PubMed] [Google Scholar]
- 28. Zhou F-D, Zhao M-H, Zou W-Z. et al. The changing spectrum of primary glomerular diseases within 15 years: a survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant 2009; 24: 870–876 [DOI] [PubMed] [Google Scholar]
- 29. McQuarrie EP, Mackinnon B, McNeice V. et al. The incidence of biopsy-proven IgA nephropathy is associated with multiple socioeconomic deprivation. Kidney Int 2014; 85: 198–203 [DOI] [PubMed] [Google Scholar]
- 30. Hedger N, Stevens J, Drey N. et al. Incidence and outcome of pauci-immune rapdily progressive glomerulonephritis in Wessex, UK: a 10-year retrospective study. Nephrol Dial Transplant 2000; 15: 1593–1599 [DOI] [PubMed] [Google Scholar]
- 31. Naumovic R, Pavlovic S, Stojkovic D. et al. Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant 2009; 24: 877–885 [DOI] [PubMed] [Google Scholar]
- 32. Wirta O, Mustonen J, Helin H. et al. Incidence of biopsy proven glomerulonephritis. Nephrol Dial Transplant 2002; 23: 193–200 [DOI] [PubMed] [Google Scholar]
- 33. van Paassen P, Van Breda Vriesman PJC, Van Rie H. et al. Signs and symptoms of thin basement membrane nephropathy: A prospective regional study on primary glomerular disease - The Limburg Renal Registry. Kidney Int 2004; 66: 909–913 [DOI] [PubMed] [Google Scholar]