Abstract
Background
Women with chronic kidney disease (CKD) are at increased risk of superimposed pre-eclampsia (SPE). Accurate identification of SPE is challenging. We hypothesized that specific components of the renin–angiotensin–aldosterone system (RAAS) would discriminate between CKD and SPE. The aim of the study was to establish differences in circulating and intrarenal RAAS in women with CKD with and without SPE and compare these to normotensive controls (NCs) and women with pre-eclampsia (PE).
Methods
White European NC women (n = 20), women with PE (n = 9), normotensive CKD without SPE (n = 8) and with SPE (n = 11) were recruited in the third trimester. Plasma renin, plasma and urine total angiotensinogen (AGT) concentrations were quantified by enzyme-linked immunosorbent assay, urinary tetrahydroaldosterone (TH-aldo) concentration by gas chromatography-mass spectrometry and placental growth factor (PlGF) by immunoassay.
Results
Urinary TH-aldo:creatinine ratios were lower in women with PE or SPE compared with NC or women with CKD (P < 0.05 for all). The same group differences were observed for plasma active renin and PlGF concentrations (P < 0.05 for all). Urine total AGT was higher in women with PE compared with NC (P < 0.05) and urine TH-aldo:urine AGT was lower (P < 0.05). However, women with SPE had lower urinary AGT concentrations compared with women with PE (P < 0.05). No differences in plasma total AGT were observed between groups.
Conclusions
Women with SPE have a lower urinary TH-aldo:creatinine ratio, lower plasma active renin and lower PlGF concentrations than women with CKD, comparable to women with PE without pre-existing disease, suggestive of similar pathophysiology. These data suggest disruption of the RAAS pathway in SPE similar to PE. Exploration of the predictive value of RAAS components for adverse pregnancy events in women with CKD is required.
Keywords: chronic kidney disease, hypertension in pregnancy, pre-eclampsia, renin-angiotensin-aldosterone system, urinary angiotensinogen
INTRODUCTION
Pre-eclampsia (PE) is characterized by new-onset hypertension and proteinuria at ≥20 weeks of gestation [1]. This human-specific syndrome affects 2–8% of all pregnancies, and is a leading cause of maternal and perinatal morbidity and mortality [2]. Superimposed PE (SPE) is the development of PE in a patient with chronic hypertensive vascular or renal disease [3]. Women with chronic kidney disease (CKD) are at increased risk of SPE, reported to occur in 22–75% of pregnancies [4]. Diagnosis of SPE using arterial hypertension and proteinuria is difficult and of limited clinical use because both may pre-exist in women with CKD even without SPE [5]. Inaccurate diagnosis may lead to unnecessary iatrogenic preterm delivery.
The renin–angiotensin–aldosterone system (RAAS) is known to be an important regulator of blood pressure, sodium and fluid homeostasis. Angiotensinogen (AGT) is the only substrate for renin, which cleaves a 10 amino acid peptide from its N-terminus, angiotensin I (Ang I), which is subsequently cleaved to the biologically active Ang II [6]. Ang II binds to specific receptors, triggering a broad range of biological actions impacting virtually every organ in the body. Ang II also stimulates the secretion of the hormone aldosterone from the adrenal cortex [7]. Aldosterone causes the tubular epithelial cells of the kidneys to increase the reabsorption of sodium ions from the tubular fluid back into the blood, while at the same time causing them to excrete potassium or hydrogen ions into the tubular fluid, which will become urine. Tetrahydroaldosterone (TH-aldo) is the major metabolite of aldosterone, and its urinary excretion is used to estimate aldosterone secretion. Plasma and urinary aldosterone concentrations are elevated, while plasma renin activity and Ang II are reduced, in patients with CKD [8, 9].
A healthy pregnancy involves both renal and systemic haemodynamic adaptations, which allow continual renal retention of sodium and water and substantial, cumulative, plasma volume expansion from very early in gestation; these changes are all ‘physiological’ in pregnancy and are a result of the activation of the RAAS [10, 11]. In PE, plasma volume expansion has been reported to be 13% lower than in normal pregnancy and this precedes increases in blood pressure [12, 13]. In women with established PE, plasma aldosterone, renin and Ang II concentrations are all suppressed compared with normotensive pregnant controls [14–18]. However, an intriguing prospective study in 1969 [19] suggested second-trimester activation of the RAAS, both basally and in response to sodium depletion, in women who went on to develop ‘toxaemia’, but this response has never been validated.
Placental growth factor (PlGF) is an angiogenic protein synthesized primarily by syncytiotrophoblasts, which increases in the blood of normotensive pregnant women until 26–30 weeks of gestation and then falls towards term [20]. Low PlGF concentrations have been reported in women with PE, possibly due to the rise in soluble fms-like tyrosine kinase-1 (sFlt-1), an anti-angiogenic soluble protein that binds PlGF [21], thus preventing interaction with endothelial receptors. Recently, we have shown that PlGF could be used as a potential marker to assist clinical decision-making in women with SPE [22]. We and others have also previously reported PlGF associations with the components of the RAAS [23, 24]; in particular, aldosterone can stimulate PlGF in placental (BeWo) and adrenocortical (H295R) cell lines [24].
We hypothesized that altered concentrations of RAAS components and lower PlGF concentrations would discriminate pregnant women with CKD alone from those with CKD and SPE. The aim of the study was to compare plasma active renin, total plasma and urinary AGT, urinary TH-aldo and plasma PlGF concentrations in pregnant White European women with CKD with and without SPE, and in normotensive pregnant controls and women with PE without pre-existing disease.
MATERIALS AND METHODS
Cohort and sample collection
Samples were obtained from participants who were recruited to a multicentre (Imperial College and King’s Health Partners) study of a PE, CKD and SPE cohort between June 2009 and September 2013, following written informed consent. Full details of definitions of each group can be found in a previous report [22]. A pragmatic approach was adopted; all samples from White European women that were available for analysis within the cohort were selected and analysed, and all data presented. Due to the known differences in the RAAS in Afro-Caribbean women [25–27], only White European women were selected. The groups that were examined consisted of pregnant normotensive control (NC) participants (n = 20) and pregnant women with PE (n = 9), normotensive CKD without SPE (CKD; n = 8) and SPE (on background of CKD; n = 11) as defined previously [22] and summarized in Table 1. Exclusion criteria were age <18 or >50 years, an inability or unwillingness to give informed consent, known HIV, Hepatitis B or C positive or a multi-foetal pregnancy. Ethical approval was provided by the National Research Ethics Service (11/LO/1776), and the study was performed in accordance with the guidelines of the Declaration of Helsinki.
Table 1.
Definitions used for classification of women—adapted from [22]
| Definition | Criteria |
|---|---|
| NCs | • No risk factors for PE; • no history of PE, hypertension, diabetes mellitus, renal disease, connective tissue disease or anti-phospholipid antibody syndrome; • systolic blood pressure <140 mmHg; • diastolic blood pressure <90 mmHg; • no or trace protein or less on dipstick analysis of midstream urine; • not in labour |
| PE | • Gestational hypertension (≥140/90 mmHg after 20 weeks’ gestation) AND proteinuria of >300 mg protein over 24 h (or protein:creatinine ratio of >30 mg/mmol) |
| Normotensive CKD |
|
| SPE |
|
Venous blood samples were collected in the third trimester (>28 weeks of gestation) and plasma aliquots stored at −80°C. Midstream urine samples were collected into sterile containers, centrifuged at 1400 g (10 min), and stored in multiple aliquots at −80°C within 3 h of collection.
Assay analyses
All samples were analysed masked to clinical outcome.
Plasma active renin concentrations
Plasma active renin concentrations were assayed, blinded to outcome, in duplicate by enzyme-linked immunosorbent assay (ELISA) (IBL, Hamburg, Germany). Intra- and inter-assay coefficients of variation were 4 and 11.6%, respectively.
Plasma and urine AGT concentrations
Plasma and urine AGT concentrations were measured, blinding to outcome group, in duplicate by ELISA (IBL, Japan). Plasma samples were diluted 1:10 000 and urine samples between 1:4 and 1:30, using buffer provided within the kit. Intra- and inter-assay coefficient of variation for plasma were 4.2 and 14.1% and for urine were 4.8 and 11.6%, respectively.
Plasma PlGF concentrations
PlGF was measured in plasma samples for a previous larger study [22]. Briefly, plasma samples were analysed using the Triage PlGF kit (Alere, San Diego, CA, USA). The assay used a fluorescently labelled recombinant murine monoclonal antibody, with the range of detection of between 12 and 1300 pg/mL. The coefficient of variations for standard controls (85 and 1300 pg/mL) was 12.8 and 13.2%, respectively.
Urinary aldosterone
Urinary TH-aldo was analysed by gas chromatography-mass spectrometry (GC-MS) according to a modified method, originally described by Shackleton [29] and applied by us as reported earlier [30]. Measurement of TH-aldo rather the aldosterone was conducted as we used the mass spectrometry-based assays that are able to accurately measure individual steroid hormone concentrations. Many of the conventional assays for aldosterone, such as the radioimmunoassays and ELISAs, are compromised by significant cross-reactivity and lack of specificity [30].
Sample preparation consisted of pre-extraction, enzymatic hydrolysis, extraction from the hydrolysis mixture, derivatization and gel filtration. Medroxyprogesterone (2.5 μg) was added as recovery standard to 1.5 mL urine. The sample was extracted on a Sep Pak C18 column, dried, reconstituted in 0.1 M acetate buffer (pH 4.6) and hydrolysed with powdered Helix pomatia enzyme (12.5 mg) and 12.5 μL of β-glucuronidase/arylsulfatase liquid enzyme at 55°C for 3 h. The resulting free steroids were extracted on a Sep Pak C18 cartridge and 0.15 μg of 3β5β-TH-aldo was added as a standard for derivatization and chromatography. The samples were derivatized to form methyloxime-trimethylsilyl ethers. The derivatives were purified by gel filtration on Lipidex 5000 columns. Samples were analysed on a gas chromatograph 6890 N equipped with a mass selective detector 5973 N (Agilent, La Jolla, CA, USA) during a temperature-controlled run over 35 min by selected ion monitoring. One specific ion was monitored for each compound analysed. A known amount of 3α5β-TH-aldo was measured on a regular basis to act as a calibration standard. The recovery of the analysis was checked with medroxyprogesterone and results were corrected for the loss during sample preparation; all samples had recoveries >80%. The urinary concentration of 3α5β-TH-aldo was normalized against urinary creatinine concentration.
Urinary creatinine
Urinary creatinine concentrations were measured with the enzymatic creatinine method (Roche Diagnostics Inc.).
Statistical analysis
All tests were performed using SPSS version 22. Summary data are presented as means±standard deviation (SD) or median and interquartile range (IQR) as appropriate. The Kruskal–Wallis test followed by Mann–Whitney U-test was used for multiple group analysis. The Student’s t-test or Mann–Whitney U-tests were used depending on the distribution of the data, after testing using the Kolmogorov–Smirnov test. The null hypothesis was rejected where P < 0.05.
RESULTS
Baseline demographic and pregnancy outcome data are presented in Table 2. There were no demographic differences between women with CKD, who did or did not experience SPE other than significantly lower blood pressures compared with women with SPE (P < 0.05). Further detailed data regarding the whole cohort have been previously published [22].
Table 2.
Participant demographics and pregnancy outcome data
| Parameter | NC (n=20) | PE (n=9) | CKD without SPE (n=8) | SPE (n=11) |
|---|---|---|---|---|
| Age at booking, mean ± SD, years | 31.0 ± 6.9 | 30.0 ± 6.7 | 36.0 ± 7.1 | 33.0 ± 4.7 |
| BMI at booking, mean ± SD, kg/m2 | 24.0 ± 5.9a | 32.4 ± 8.1 | 30.3 ± 7.6 | 27.2 ± 4.1 |
| Nulliparous, n (%) | 13 (68) | 5 (63) | 3 (60) | 8 (73) |
| CKD Stage, n (%) | – | – |
|
|
| ≥2+ proteinuria at booking | – | – | 3 (38) | 2 (18) |
| Serum creatinine (µmol/L) | – | – | 72 (64, 81) | 59 (44, 81) |
| Smoking, n (%) | 7 (35) | 1 (11) | 4 (50) | 3 (27) |
| Gestational age at sampling, median (IQR), weeks | 35.0 (33.0–37.3)a,b | 36.6 (35.1–38.3) | 37.5 (36.4–38.7)d | 33.0 (27.6–34.4) |
| Caesarean section, n (%) | 2 (10) | 6 (67) | 3 (38) | 8 (73) |
| Highest SBP, mean ± SD, mmHg | 110 ± 11.7a,b,c | 149 ± 10.1 | 123 ± 13.1d | 150 ± 14.1 |
| Highest DBP, mean ± SD, mmHg | 68 ± 7.5a,b,c | 96 ± 7.1 | 87 ± 13.5 | 97 ± 5.3 |
| Highest 24-h urine collection protein, median (IQR), g/24 h | – | 0.7 (0.4–1.2) | 0.2 (0.2–0.6) | 0.5 (0.3–0.7) |
| Highest protein urine PCR, median (IQR) | – | 47 (25–92) | 91 (13–213) | 115 (83–280) |
| Gestational age at delivery, median (IQR), weeks | 40.0 (39.6–40.6)a,b | 38.0 (37.4–38.7) | 39.4 (37.4–38.7)d | 36.6 (34.0–38.0) |
| Birth weight, median (IQR), g | 3505 (3150–3910)a,b | 2960 (2200–3120) | 3325 (3190–3915) | 2390 (1584–3050) |
| Birth weight centile, median (IQR) | 49.5 (30.5–64) | 23.0 (14.5–42) | 30.5 (22.5–44) | 14.0 (6.5–56.5) |
P < 0.05 between NCs and PE.
P < 0.05 between NCs and SPE.
P < 0.05 between NCs and CKD.
P < 0.05 between CKD and SPE. BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; PCR, protein:creatinine ratio.
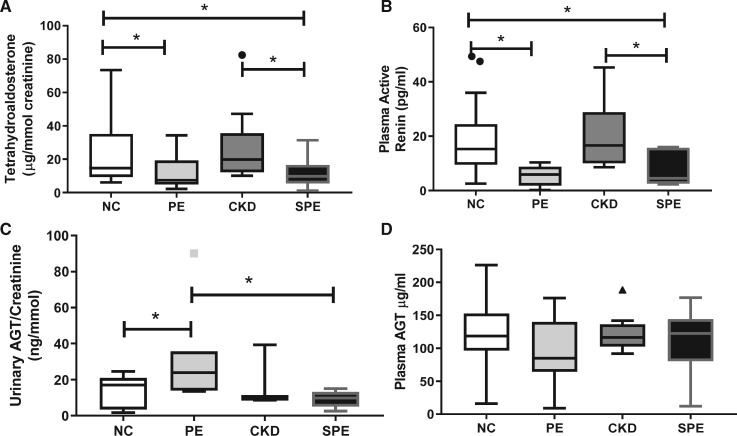
Urine TH-aldo:creatinine ratios and plasma active renin (Kruskal–Wallis, both P < 0.05) were significantly different between groups. TH-aldo:creatinine ratios were significantly lower in both PE and SPE compared with the NC and women with CKD without SPE (P < 0.05 for all; Figure 1A).
FIGURE 1.
Maternal (A) urinary aldosterone; (B) plasma active renin; (C) urinary AGT concentrations; and (D) plasma AGT concentrations in women with an NC pregnancy, PE pregnancy, CKD without SPE or CKD with SPE. Data represented as median (IQR) with outliers shown as symbols (filled circle); *P < 0.05.
Plasma active renin concentrations were also lower in PE and SPE women compared with NC women (P < 0.05 for all; Figure 1B). In addition, the SPE women had significantly lower concentrations compared with the CKD women who did not develop SPE (Figure 1B), but there were no differences between women with CKD alone and PE.
Urinary total AGT:creatinine ratios were significantly different between groups (Kruskal–Wallis, P < 0.05); levels were higher in PE compared with NC (P = 0.03). Interestingly, urinary total AGT:creatinine ratios in women with SPE were significantly lower than women with PE (P = 0.01); no differences were found between the CKD group and all other groups (P > 0.05; Figure 1C).
Although women with PE appeared to have lower plasma AGT concentrations than NC, no significant differences were seen between groups (Kruskal–Wallis, P > 0.1; Figure 1D).
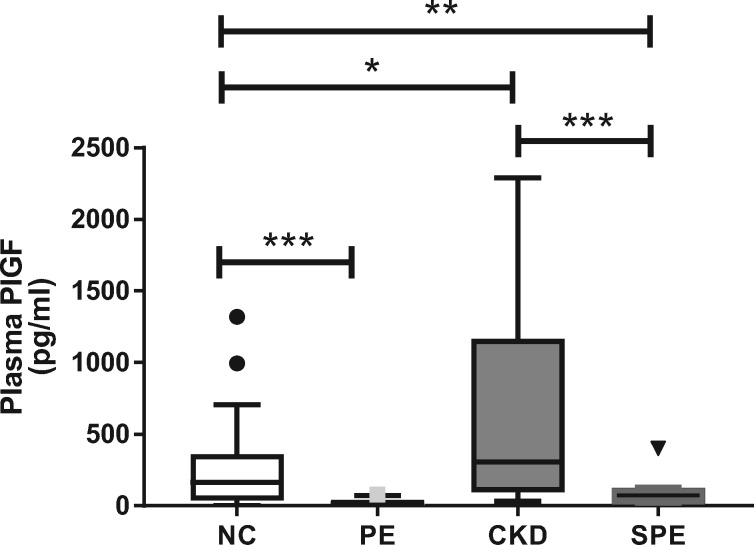
The Kruskal–Wallis test indicated a highly significant difference in plasma PlGF concentrations (P < 0.0001; Figure 2) between groups. Subsequent Mann–Whitney U-tests confirmed that PlGF concentrations were significantly lower in both women with PE and SPE compared with the NC and CKD women, respectively (P < 0.05 for all; Figure 2).
FIGURE 2.
Maternal plasma active PlGF concentration in women with a NC pregnancy, PE pregnancy, CKD without SPE or CKD with SPE. PlGF was measured in plasma samples from a previous larger study [22]. Data represented as median (IQR) with outliers shown by symbols; *P < 0.05; **P < 0.001; ***P < 0.0001.
DISCUSSION
The novel finding in this study is that women with CKD with SPE have reduced urinary TH-aldo excretion and plasma active renin concentration compared with those with CKD without SPE. This parallels previous observations that women with PE without pre-existing disease have suppressed RAAS activation compared with NCs. During pregnancy, there is a 30–50% expansion in extracellular fluid and a 30–40% increase in plasma volume, which is an essential component of pregnancy-associated augmentation in organ perfusion [31]. The RAAS is activated very early in pregnancy, proposed to be a consequence of the ‘underfilling’ of the circulation [10] and leading to water and sodium retention. Plasma volume expansion is less pronounced in women who subsequently develop PE without pre-existing disease and a decreased expansion can be detected as early as Weeks 14–17 of gestation [32], although it is not known whether this is primary or secondary.
Plasma active renin concentrations are elevated in healthy pregnancy, probably as a compensatory mechanism in response to the fall in vascular resistance and blood pressure at the beginning of pregnancy. Renin synthesis has been demonstrated to be suppressed in women with established PE [15]. Interestingly, a small (n = 58), but prospective, study reported non-significant activation of the RAAS between Weeks 13 and 27, both basally and in response to sodium depletion, in women who went on to develop ‘toxaemia’ although a majority of these women probably had (non-proteinuric) gestational hypertension [33]. In addition, a recent study has also reported similar changes in the RAAS in patients with chronic hypertension and SPE [34]. Furthermore, a study of plasma volume throughout pregnancy in initially normotensive women reported that in 29 of 40 women who subsequently developed hypertension, early volume expansion was followed by significant volume contraction in the third trimester, before blood pressure rose [35]. It remains unclear why renin levels are low despite reduced plasma volume in women with established PE, but proposed mechanisms include elevated atrial natriuretic peptide concentrations in PE [36], increased sensitivity to Ang II and AT1R-autoantibodies [37] or reduced vascular endothelial growth factor (VEGF) availability, which has been shown to suppress renin [38]. Low VEGF availability could also lead to impaired adrenal aldosterone release, secondary to changes in the fenestrated endothelium of the juxtaglomerular apparatus, which we have previously described [39].
Reduced plasma active renin concentrations in women with both PE and SPE despite suppressed plasma active renin concentrations in women with CKD without SPE compared with NC suggest this reduction to be a consequence of the underlying pathophysiology of PE, and an appropriate response to the hypertension. This finding is in keeping with previous reports of maintained plasma renin activity in women who developed de novo gestational hypertension without significant proteinuria [40–42]. However, a variety of animal studies of the (initially) subpressor infusion of Ang II either systemically or directly into the renal artery showed persistent changes in both systemic and renal resistance vasculature (see [43]). This accords with Folkow’s original suggestion in the 1950s that an apparently very small stimulus may be enough to trigger vascular hypertrophy and hypertension in susceptible individuals, which could be maintained after the stimulus ceased (see [44]). By analogy with this, the early stimulation of the RAAS in all pregnancies might, in susceptible women, lead to vascular changes, which result in hypertension.
Urinary TH-aldo concentrations are also relatively lower in pregnancies complicated by PE compared with NCs and are likely to contribute to decreased volume expansion and subsequent poor placental perfusion. We have previously reported urinary TH-aldo levels to be correlated with placental weight and proliferation of cultured trophoblasts is increased upon aldosterone stimulation [24]. Our finding that urinary TH-aldo: creatinine ratios are also lower in SPE compared with women with CKD who did not develop SPE further suggests that this finding is a PE-specific alteration. It is important to highlight that previous measurements of plasma aldosterone using radioimmunoassays or ELISA are hampered by high cross-reactivity with other metabolites, especially progesterone [30], whereas the validated, highly sensitive GC-MS of the major metabolite, TH-aldo, used in this study resolves such issues. In addition, urinary excretion must be considered a better representation of the overall temporal exposure to aldosterone and AGT.
Lower PlGF concentrations in women with both PE and SPE compared with those with CKD without SPE and NCs is consistent with our previous findings [22] and further highlights this as a useful diagnostic tool for both PE and SPE. The similar pattern of changes seen for PlGF with renin and aldosterone strengthens previously identified associations of PlGF and RAAS activation [24, 45]. Further work to fully elucidate mechanisms of regulation between angiogenic imbalance and the RAAS in early pregnancy is required.
The higher urinary AGT concentrations seen in women with PE could be related to renally synthesized AGT, which spills over into the urine, where it can be measured. Importantly, this suggests the potential local action of intrarenally generated Ang II, capable of fine-tuning intrarenal sodium reabsorption, and highlights the potential importance of examining the intra-renal RAAS, as well as the circulating RAAS when considering the origin of hypertension. This is supported by our recent reports of the presence of AGT expression in kidney biopsies collected from pregnant women [46]. This finding requires further work, especially as studies into intra-renal AGT in human pregnancy are only just beginning to appear [47]. Unlike women with PE without pre-existing disease, urinary AGT concentrations were the same in women with CKD with or without SPE, and considerably lower in both than in either group with normal renal function (Figure 1). This was unexpected since non-pregnant patients with CKD have been reported to have increased urinary AGT excretion (e.g. [48]). It may be that pre-existing hypertension blunts the necessary response of renal AGT synthesis to pregnancy, which could contribute to the inadequate plasma volume expansion seen in pregnant women with chronic hypertension [35]. The reduced urinary TH-aldo: urine AGT ratio in PE women suggests a disruption of the RAAS system as the increased AGT is not linked to higher aldosterone concentrations.
The absence of differences in plasma AGT concentrations between groups may be due to quantification of total AGT rather than oxidized and reduced forms separately, which have been shown to be different between NCs and PE [49]. In addition, others have reported that high-molecular mass AGT is a complex eosinophil major basic protein (proMBP) with normal AGT [50]. It has been reported that the kinetics of cleavage by renin of proMBP-AGT are impaired and may contribute to the reduced renin concentrations observed in PE [51].
This study was restricted to samples from White European women due to the known differences in the RAAS in Afro-Caribbean women [25–27]. A detailed analysis was restricted to samples from White European women due to the known differences in the RAS in Afro-Caribbean women, of which there were only a small number of samples, and thus were excluded.
A limitation of the study includes the challenge of confirming the diagnosis of SPE in women with CKD; however, confirmation of diagnosis by two independent adjudicators and pre-specified diagnosis criteria reduced this uncertainty. This further emphasizes the need to find other more suitable tools.
In conclusion, the findings of this study allow us to propose that reduced PlGF concentrations (and thus reduced translocation of the VEGF to the active receptor, secondary to increased sFlt-1) in women who develop PE, including SPE, is associated with reduced renin synthesis and also less VEGF-derived adrenal aldosterone synthesis, which in turn results in lower urinary aldosterone concentrations. Renin activity and aldosterone synthesis are dissociated in pregnancy [52] and AGT concentrations become rate-limiting for Ang II production [53]. Ang II thus cannot be adequately upregulated with secondary increase in aldosterone in response to plasma volume reduction in PE. Furthermore, these data suggest urinary TH-aldo:creatinine ratios and plasma active renin in addition to PlGF may be useful discriminators between pre-existing CKD and SPE.
ACKNOWLEDGEMENTS
We thank the women who participated in the study and the midwives/doctors whose support made this study possible. We thank Dr Bernhard Dick for assistance with the GC-MS.
FUNDING
We acknowledge support from the British Heart Foundation and European Renal Association-European Dialysis and Transplant Association (ERA-EDTA). This work was produced by H.D.M. under the terms of a BHF Basic Science Intermediate Basic Science Fellowship (FS/15/32/31604) and an ERA-EDTA Long-Term Fellowship (LTF 137-2013).
AUTHORS’ CONTRIBUTIONS
L.O.K., H.D.M., F.B.P. and M.G.M. conceived and designed the experiments. H.D.M. and L.O.K. performed the experiments. L.O.K., H.D.M., F.B.P. and M.G.M. analysed the data. L.O.K. and H.D.M. contributed reagents/materials/analysis tools. H.D.M., L.O.K., F.B.P. and M.G.M. wrote the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Brown MA, Lindheimer MD, de Swiet M. et al. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001; 20: IX–XIV [DOI] [PubMed] [Google Scholar]
- 2. Steegers EAP, von Dadelszen P, Duvekot JJ. et al. Pre-eclampsia. Lancet 2010; 376: 631–644 [DOI] [PubMed] [Google Scholar]
- 3. Brown MA, Magee LA, Kenny LC. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2018; 13: 291–310 [DOI] [PubMed] [Google Scholar]
- 4. Williams D, Davison J.. Chronic kidney disease in pregnancy. Br Med J 2008; 336: 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crews DC, Plantinga LC, Miller ER. et al. Prevalence of chronic kidney disease in persons with undiagnosed or prehypertension in the United States. Hypertension 2010; 55: 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu C, Lu H, Cassis LA. et al. Molecular and pathophysiological features of angiotensinogen: a mini review. N Am J Med Sci 2011; 4: 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yee AH, Burns JD, Wijdicks EF.. Cerebral salt wasting: pathophysiology, diagnosis, and treatment. Neurosurg Clin N Am 2010; 21: 339–352 [DOI] [PubMed] [Google Scholar]
- 8. Schulz A, Jankowski J, Zidek W. et al. Absolute quantification of endogenous angiotensin II levels in human plasma using ESI-LC-MS/MS. Clin Proteomics 2014; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terata S, Kikuya M, Satoh M. et al. Plasma renin activity and the aldosterone-to-renin ratio are associated with the development of chronic kidney disease: the Ohasama Study. J Hypertens 2012; 30: 1632–1638 [DOI] [PubMed] [Google Scholar]
- 10. Chapman AB, Abraham WT, Zamudio S. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 1998; 54: 2056–2063 [DOI] [PubMed] [Google Scholar]
- 11. Ganzevoort W, Rep A, Bonsel GJ. et al. Plasma volume and blood pressure regulation in hypertensive pregnancy. J Hypertens 2004; 22: 1235–1242 [DOI] [PubMed] [Google Scholar]
- 12. Gallery ED, Brown MA.. Volume homeostasis in normal and hypertensive human pregnancy. Baillieres Clin Obstet Gynaecol 1987; 1: 835–851 [DOI] [PubMed] [Google Scholar]
- 13. de Haas S, Ghossein-Doha C, van Kuijk SMJ. Physiological adaptation of maternal plasma volume during pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017; 49: 177–187 [DOI] [PubMed] [Google Scholar]
- 14. Uddin MN, Horvat D, Jones RO. et al. Suppression of aldosterone and progesterone in preeclampsia. J Matern Fetal Neonatal Med 2015; 28: 1296–1301 [DOI] [PubMed] [Google Scholar]
- 15. Verdonk K, Visser W, Van Den Meiracker AH. et al. The renin-angiotensin-aldosterone system in pre-eclampsia: the delicate balance between good and bad. Clin Sci 2014; 126: 537–544 [DOI] [PubMed] [Google Scholar]
- 16. Symonds EM, Pipkin F, Craven DJ.. Changes in the renin-angiotensin system in primigravidae with hypertensive disease of pregnancy. Br J Obstet Gynaecol 1975; 82: 643–650 [DOI] [PubMed] [Google Scholar]
- 17. Shojaati K, Causevic M, Kadereit B. et al. Evidence for compromised aldosterone synthase enzyme activity in preeclampsia. Kidney Int 2004; 66: 2322–2328 [DOI] [PubMed] [Google Scholar]
- 18. Hanssens M, Keirse MJ, Spitz B. et al. Angiotensin II levels in hypertensive and normotensive pregnancies. Br J Obstet Gynaecol 1991; 98: 155–161 [DOI] [PubMed] [Google Scholar]
- 19. Gordon RD, Parsons S, Symonds EM.. A prospective study of plasma-renin activity in normal and toxaemic pregnancy. Lancet 1969; 1: 347–349 [DOI] [PubMed] [Google Scholar]
- 20. Levine RJ, Maynard SE, Qian C. et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350: 672–683 [DOI] [PubMed] [Google Scholar]
- 21. Robinson CJ, Johnson DD, Chang EY. et al. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 2006; 195: 255–259 [DOI] [PubMed] [Google Scholar]
- 22. Bramham K, Seed PT, Lightstone L. et al. Diagnostic and predictive biomarkers for pre-eclampsia in patients with established hypertension and chronic kidney disease. Kidney Int 2016; 89: 874–885 [DOI] [PubMed] [Google Scholar]
- 23. Anton L, Merrill DC, Neves LAA. et al. Angiotensin II and angiotensin-(1-7) decrease sFlt1 release in normal but not preeclamptic chorionic villi: an in vitro study. Reprod Biol Endocrinol 2010; 8: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eisele N, Albrecht C, Mistry HD. et al. Placental expression of the angiogenic placental growth factor is stimulated by both aldosterone and simulated starvation. Placenta 2016; 40: 18–24 [DOI] [PubMed] [Google Scholar]
- 25. He FJ, Markandu ND, Sagnella GA. et al. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension 1998; 32: 820–824 [DOI] [PubMed] [Google Scholar]
- 26. Williams SF, Nicholas SB, Vaziri ND. African Americans, hypertension and the renin angiotensin system. World J Cardiol 2014; 6: 878–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rooyen JM, Poglitsch M, Huisman HW. et al. Quantification of systemic renin-angiotensin system peptides of hypertensive black and white African men established from the RAS-Fingerprint(R). J Renin Angiotensin Aldosterone Syst 2016; 17: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KDOQ1 . Chapter 1: definition and classification of CKD. Kidney Int Suppl 2013; 3: 19–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shackleton CH. Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. J Steroid Biochem Mol Biol 1993; 45: 127–140 [DOI] [PubMed] [Google Scholar]
- 30. Mistry HD, Eisele N, Escher G. et al. Gestation-specific reference intervals for comprehensive spot urinary steroid hormone metabolite analysis in normal singleton pregnancy and 6 weeks postpartum. Reprod Biol Endocrinol 2015; 13: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chesley LC. Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol 1972; 112: 440–450 [DOI] [PubMed] [Google Scholar]
- 32. Salas SP, Marshall G, Gutiérrez BL. et al. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 2006; 47: 203–208 [DOI] [PubMed] [Google Scholar]
- 33. August P, Lenz T, Ales KL. et al. Longitudinal study of the renin-angiotensin-aldosterone system in hypertensive pregnant women: deviations related to the development of superimposed preeclampsia. Am J Obstet Gynecol 1990; 163 (5 Pt 1): 1612–1621 [DOI] [PubMed] [Google Scholar]
- 34. Malha L, Sison CP, Helseth G. et al. Renin-angiotensin-aldosterone profiles in pregnant women with chronic hypertension. Hypertension 2018; 72: 417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gallery ED, Hunyor SN, Gyory AZ.. Plasma volume contraction: a significant factor in both pregnancy-associated hypertension (pre-eclampsia) and chronic hypertension in pregnancy. Q J Med 1979; 48: 593–602 [PubMed] [Google Scholar]
- 36. Miyamoto S, Shimokawa H, Sumioki H. et al. Circadian rhythm of plasma atrial natriuretic peptide, aldosterone, and blood pressure during the third trimester in normal and preeclamptic pregnancies. Am J Obstet Gynecol 1988; 158: 393–399 [DOI] [PubMed] [Google Scholar]
- 37. Morgan L, Crawshaw S, Baker PN. et al. Functional and genetic studies of the angiotensin II type 1 receptor in pre-eclamptic and normotensive pregnant women. J Hypertens 1997; 15 (12 Pt 1): 1389–1396 [DOI] [PubMed] [Google Scholar]
- 38. Kappers MHW, van Esch JHM, Sluiter W. et al. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010; 56: 675–681 [DOI] [PubMed] [Google Scholar]
- 39. Gennari-Moser C, Khankin EV, Escher G. et al. Vascular endothelial growth factor-A and aldosterone: relevance to normal pregnancy and preeclampsia. Hypertension 2013; 61: 1111–1117 [DOI] [PubMed] [Google Scholar]
- 40. Symonds EM, Andersen GJ.. The effect of bed rest on plasma renin in hypertensive disease of pregnancy. J Obstet Gynaecol Br Commonw 1974; 81: 676–681 [DOI] [PubMed] [Google Scholar]
- 41. Kaaja RJ, Moore MP, Yandle TG. et al. Blood pressure and vasoactive hormones in mild preeclampsia and normal pregnancy. Hypertens Pregnancy 1999; 18: 173–187 [DOI] [PubMed] [Google Scholar]
- 42. Gallery EDM, Stokes GS, Györy AZ. et al. Plasma renin activity in normal human pregnancy and in pregnancy-associated hypertension, with reference to cryoactivation. Clin Sci (Lond) 1980; 59: 49–53 [DOI] [PubMed] [Google Scholar]
- 43. Edgley A, Kett M, Anderson W.. ‘Slow pressor’ hypertension from low-dose chronic angiotensin II infusion. Clin Exp Pharmacol Physiol 2001; 28: 1035–1039 [DOI] [PubMed] [Google Scholar]
- 44. Lever AF. Slow developing pressor effect of angiotensin II and vascular structure. J Hypertens Suppl 1993; 11: S27–S28 [PubMed] [Google Scholar]
- 45. Eisele N, Gennari-Moser C, Albrecht C. et al. Does aldosterone participate in placental angiogenesis via PlGF? Pregnancy Hypertens 2012; 2: 245. [DOI] [PubMed] [Google Scholar]
- 46. Kurlak LO, Broughton Pipkin F, Gardner DS. et al. Intrarenal angiotensinogen (AGT), potassium, glomerular endotheliosis and hypertension in pregnancy In: SA Karumanchi, A Hennessy (eds). International Society for the Study of Hypertension in Pregnancy. Berlin: Elsevier, 2017, OP40 [Google Scholar]
- 47. Pringle K, Lumbers E, Sykes S. et al. The intrarenal renin-angiotensin system and pregnancy outcome. Pregnancy Hypertens 2013; 3: 79–80 [DOI] [PubMed] [Google Scholar]
- 48. Juretzko A, Steinbach A, Hannemann A. et al. Urinary angiotensinogen and renin excretion are associated with chronic kidney disease. Kidney Blood Press Res 2017; 42: 145–155 [DOI] [PubMed] [Google Scholar]
- 49. Zhou A, Carrell RW, Murphy MP. et al. A redox switch in angiotensinogen modulates angiotensin release. Nature 2010; 468: 108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kløverpris S, Skov LL, Glerup S. Formation of high-molecular-weight angiotensinogen during pregnancy is a result of competing redox reactions with the proform of eosinophil major basic protein. Biochem J 2013; 449: 209–217 [DOI] [PubMed] [Google Scholar]
- 51. Brown MA, Nicholson E, Gallery ED.. Sodium-renin-aldosterone relations in normal and hypertensive pregnancy. Br J Obstet Gynaecol 1988; 95: 1237–1246 [DOI] [PubMed] [Google Scholar]
- 52. Brown MA, Broughton Pipkin F. et al. The effects of intravenous angiotensin II upon blood pressure and sodium and urate excretion in human pregnancy. J Hypertens 1988; 6: 457–464 [DOI] [PubMed] [Google Scholar]
- 53. Tryon ES, Tewksbury AD.. Kinetic anlaysis of the reaction of human renin with low and high molecular weight human angiotensinogen In: American Society of Biological Chemists 75th Annual Meeting and the American Association of Immunologists 68th Annual Meeting. St Louis, MO, 1984: Abstract 43, p. 1854. Bethesda, MD: Federation Proceedings. [Google Scholar]