Abstract
Cystinosis is a rare autosomal recessive disease causing cystine deposition in all tissues, primarily affecting the kidneys. There are few published reports of pregnancy in women with cystinosis, and little evidence is available regarding optimal management. Kidney transplantation and cystine-depleting therapy have transformed the prognosis of cystinosis, and pregnancy is increasingly considered. The evidence base for cystinosis management in pregnancy, therefore, requires expansion. We report three successful pregnancy outcomes and five early pregnancy losses in two women with cystinosis. The challenges of pregnancy in patients with cystinosis are discussed. Pre-pregnancy planning and antenatal management in a specialist renal obstetric clinic are paramount.
Keywords: chronic kidney disease; cysteamine; cystinosis; end-stage renal disease, pregnancy
BACKGROUND
Cystinosis is a rare autosomal recessive disorder characterized by widespread cystine deposition, leading to end-stage renal disease (ESRD) by early adulthood [1]. The advent of kidney transplantation and cystine-depleting therapy (cysteamine) have led to improved life expectancy and restored fertility, and pregnancy is increasingly considered for this patient group. Extra-renal manifestations such as hypothyroidism, muscle wasting and diabetes are commonplace and further complicate pregnancy. Expansion of the evidence base is required, and optimum management requires consideration.
PATIENT 1
A 26-year-old female with ESRD and mild photophobia secondary to cystinosis received a live donor renal transplant in 2002. Pre-pregnancy estimated glomerular filtration rate (eGFR) was 74 mL/min/1.73 m2, albumin/creatinine ratio (ACR) 9.5 mg/mmol and blood pressure (BP) 120/85 mmHg. Medication included amlodipine, tacrolimus, prednisolone, azathioprine, folic acid and cysteamine eye drops. Oral cysteamine was suspended after a positive pregnancy test. At 6 weeks’ gestation, she commenced low-dose aspirin for preeclampsia prophylaxis.
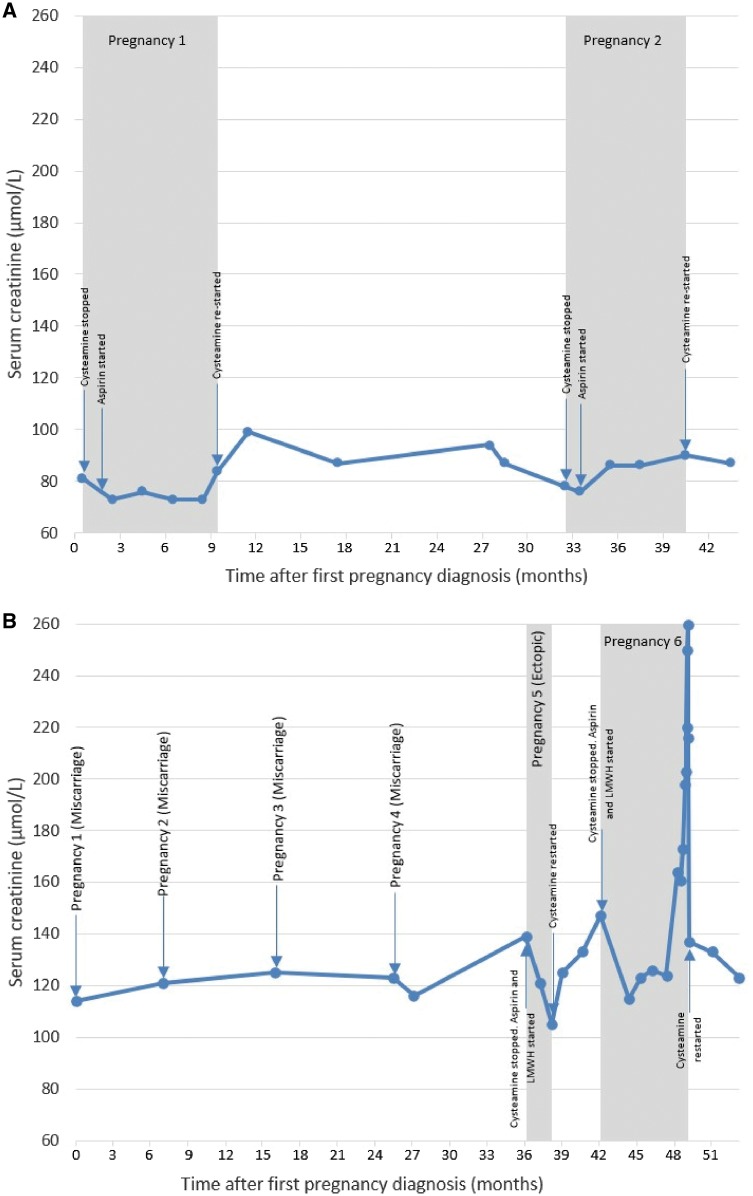
Pregnancy progressed with stable graft function and BP. Glucose tolerance testing and thyroid function remained normal at 26 weeks. She underwent planned induction of labour at 37 weeks, and delivered a healthy female weighing 3203 g by Caesarean section after labour failed to progress. She remained well postnatally with no evidence of deterioration in renal function or cystinosis progression (Figure 1A).
FIGURE 1.
(A) Change in serum creatinine over time—Patient 1. (B) Change in serum creatinine over time—Patient 2. LMWH, low molecular weight heparin.
A second pregnancy followed 2 years later and was managed with the same medication changes and regular renal obstetric review. She underwent planned Caesarean at 37+4 weeks and delivered a female weighing 3374 g who required a 10-day stay on neonatal intensive care (NICU) for respiratory infection. Patient 1 opted to bottle-feed both babies to allow immediate postnatal reintroduction of oral cysteamine.
PATIENT 2
A 33-year-old female with cystinosis complicated by ESRD, hypothyroidism, corneal crystal deposition and restrictive lung defect due to respiratory muscle weakness. She received a cadaveric kidney transplant in 1998. Immunosuppression comprised tacrolimus and prednisolone. Pre-pregnancy eGFR was 40 mL/min/1.73 m2 and she was normotensive with minimal proteinuria (ACR 3.6 mg/mmol).
Obstetric history included four previous first trimester miscarriages. No structural causes for infertility were identified on pelvic imaging; however, she was twice tested as borderline positive for lupus anticoagulant. On confirmation of her fifth pregnancy, cysteamine was stopped and aspirin and enoxaparin prophylaxis commenced. Vaginal bleeding and abdominal pain at 7 weeks’ gestation prompted a transvaginal ultrasound that confirmed ectopic pregnancy requiring laparoscopic salpingectomy.
A further pregnancy 5 months later was managed with the same medication changes. A gradual rise in creatinine occurred from 25 weeks’ gestation (baseline 133–185 µmol/L), without proteinuria or hypertension. Tacrolimus levels remained therapeutic, and ultrasound showed mild transplant hydronephrosis (likely pregnancy-related; ureteric obstruction is uncommon in pregnant transplant patients).
Creatinine peaked to 260 at 29 weeks although BP and ACR remained stable. She was admitted for observation and underwent emergency Caesarean section at 29+5 weeks due to fetal distress and maternal respiratory compromise secondary to advancing gestation and pre-existing cystinosis-induced respiratory muscle weakness. She delivered a female weighing 1207 g requiring an 8-week NICU admission. Rapidly rising BP and worsening renal function prompted postpartum treatment for pre-eclampsia. Renal function was comparable to pre-pregnancy levels at 6 months postpartum (Figure 1B).
DISCUSSION
There are few published cases of pregnancy in cystinosis (Table 1), with adverse maternal and fetal events being commonplace. Potential challenges include cephalopelvic disproportion due to maternal short stature, gestational diabetes, hypothyroidism and respiratory muscle weakness. Furthermore, kidney transplantation, chronic hypertension and diabetes substantially increased the risk of adverse outcome [8].
Table 1.
Summary of published cases of pregnancy in cystinosis patients
| References | Maternal age at delivery (years) | Modality of RRT | Cysteamine prior to pregnancy (yes/no) | Cysteamine during pregnancy (yes/no) | Pregnancy/neonatal complications | Gestation (weeks + days) | Mode of delivery | Requirement for NICU (yes/no) |
|---|---|---|---|---|---|---|---|---|
| Reiss et al. [2] | 20 | Transplant | No | No | Mild preeclampsia, Group B strep amnionitis, skin abscess, UTI | 35 + 3 | Caesarean section | No |
| Andrews and Sacks [3] | 30 | Transplant | Yes | No | Preeclampsia, cephalopelvic, disproportion | 33 + 5 | Caesarean section | No |
| Haase et al. [4] | 21 | Haemodialysis | Yes | Yes | Premature rupture of membranes, polyhydramnios | 31 + 5 | Vaginal | Yes (3 days) |
| Ramappa and Pyatt [5] | Not known | Haemodialysis | Yes | No | Stillbirth, pregnancy- associated cardiomyopathy causing cardiac failure postnatally | 25 | Vaginal | NA |
| Chuang et al. [6] | 25 | Transplant | No | No | None | Not known | Vaginal | No |
| Lindsay et al. [7] | 31 | Haemodialysis, previous failed transplant | Unknown | No | Intra-uterine growth restriction, infantile death (severe broncho-pulmonary dysplasia) | 24 + 2 | Caesarean section | Yes (113 days) |
RRT, renal replacement therapy; UTI, urinary tract infection.
Cysteamine is usually stopped in pregnancy because of potential teratogenicity [9]. However, without treatment, cystine can accumulate and may adversely affect uteroplacental function [2], causing progressive maternal organ damage. The risk of accelerated progression of cystinosis complications is unknown although a minimal period of treatment cessation is considered optimal [10]. We recommend stopping cysteamine only after confirmation of pregnancy and advising against breastfeeding to minimize the cysteamine-free period (excretion in breast milk remains unknown).
Both patients were reviewed in a pre-pregnancy renal obstetric counselling clinic and risks of pregnancy were discussed. Both were also seen intensively throughout pregnancy in a renal obstetric clinic with expertise in managing complex pregnancies. Patient 1 demonstrates that uncomplicated pregnancy is possible in cystinosis without deterioration in graft function. More advanced pre-pregnancy renal impairment, maternal age and significant extra-renal manifestations of cystinosis increased pregnancy risks in Patient 2.
Successful pregnancy is possible for women with cystinosis although expert renal obstetric pre-pregnancy and antenatal review are vital. This report significantly adds to the current evidence base, although further expansion and long-term follow-up of patients are required to better guide treatment decision-making and evaluate the effect of interrupting treatment on disease progression.
ACKNOWLEDGEMENTS
Written informed consent was obtained from both the subjects of this case report. The authors wish to thank them both for agreeing to participate in this work.
AUTHORS’ CONTRIBUTIONS
H.B. reviewed and revised the initial draft and wrote the final version of the manuscript. J.P.-J. wrote the initial draft and checked the final version of the manuscript. E.K. and G.L. reviewed the initial draft and checked the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract form.
REFERENCES
- 1. Nesterova G, Gahl WA.. Cystinosis: the evolution of a treatable disease. Pediatr Nephrol 2013; 28: 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beckman DA, Mullin JJ, Assadi FK.. Developmental toxicity of cysteamine in the rat: effects on embryo-fetal development. Teratology 1998; 58: 96–102 [DOI] [PubMed] [Google Scholar]
- 3. Reiss RE, Kuwabara T, Smith ML. et al. Successful pregnancy despite placental cystine crystals in a woman with nephropathic cystinosis. New Engl J Med 1988; 319: 223–226 [DOI] [PubMed] [Google Scholar]
- 4. Berryhill A, Bhamre S, Chaudhuri A. et al. Cysteamine in renal transplantation: A report of two patients with nephropathic cystinosis and the successful re-initiation of cysteamine therapy during the immediate post-transplant period. Pediatr Transplant 2016; 20: 141–145 [DOI] [PubMed] [Google Scholar]
- 5. Andrews PA, Sacks SH.. Successful pregnancy in cystonisos. JAMA 1994; 272: 1327–1328 [DOI] [PubMed] [Google Scholar]
- 6. Haase M, Morgera S, Bamberg C. et al. Successful pregnancies in dialysis patients including those suffering from cystinosis and familial Mediterranean fever. J Nephrol 2006; 19: 677–681 [PubMed] [Google Scholar]
- 7. Ramappa AJ, Pyatt JR.. Pregnancy-associated cardiomyopathy occurring in a young patient with nephropathic cystinosis. Cardiol Young 2010; 20: 220–222 [DOI] [PubMed] [Google Scholar]
- 8. Deshpande NA, James NT, Kucirka LM. et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant 2011; 11: 2388–2404 [DOI] [PubMed] [Google Scholar]
- 9. Chuang Y-W, Wen M-C, Wu M-J. et al. Follow-up and treatment of renal transplantation with nephropathic cystinosis in Central Taiwan. Transplant Proc 2012; 44: 80–82 [DOI] [PubMed] [Google Scholar]
- 10. Lindsay M, Haqqie S, El-Hage L. et al. Pregnancy in a patient with cystinosis and end stage kidney disease on haemodialysis. Am J Kidney Dis 2015; 65: A55 [Google Scholar]