Abstract
Three major guidelines deal with blood pressure thresholds and targets for antihypertensive drug therapy in chronic kidney disease (CKD) patients: the 2012 Kidney Disease: Improving Global Outcomes Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease; the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults; and the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. However, a careful reading of the three guidelines leaves the practicing physician confused about the definition of CKD, how hypertension and secondary hypertension should be diagnosed in CKD patients and what the blood pressure thresholds, targets and compelling indications of antihypertensive drug therapy should be for this population. Current guidelines refer to different CKD populations and propose different definitions of hypertension, different thresholds to initiate antihypertensive therapy in CKD patients and different BP targets compelling antihypertensive drug use. The different bodies producing guidelines should work together towards a unified definition of CKD, a unified concept of hypertension and unified BP thresholds and targets for hypertensive drug therapy for CKD patients. Otherwise they risk promoting confusion and therapeutic nihilism among physicians and patients.
Keywords: blood pressure targets, blood pressure thresholds, chronic kidney disease, hypertension, renin–angiotensin system, treatment
INTRODUCTION
Three major guidelines deal with blood pressure (BP) thresholds and targets for hypertensive drug therapy in chronic kidney disease (CKD) patients: the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease; the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults; and the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) Guidelines for the Management of Arterial Hypertension [1–3]. However, a careful reading of the three guidelines leaves the practicing physician confused about the definition of CKD, how hypertension and secondary hypertension should be diagnosed in CKD patients and what the BP thresholds and targets for antihypertensive drug therapy should be for this population. We now discuss the similarities and differences between these guidelines and call for unified basic concepts that prevent confusion among patients and physicians.
WHAT IS CKD?
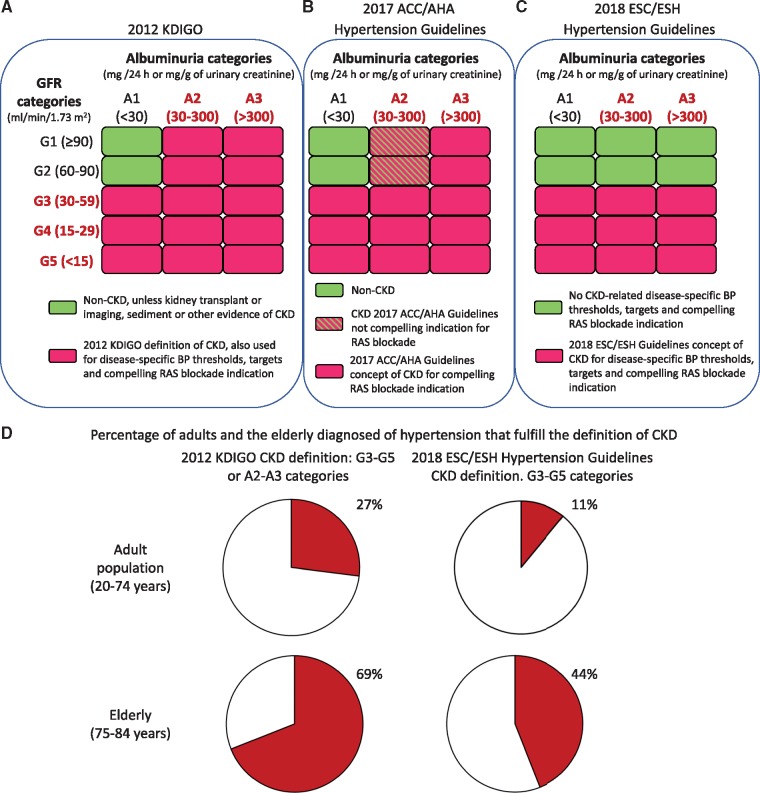
The current international consensus concept of CKD by the KDIGO 2012 states that CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health [4]. Individual criteria, on their own, allow the diagnosis of CKD. These are a decreased glomerular filtration rate (GFR) (<60 mL/min/1.73 m2) or evidence of kidney damage such as albuminuria [albumin excretion rate ≥30 mg/24 h, urinary albumin:creatinine ratio (UACR) ≥30 mg/g], urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging or a history of kidney transplantation. To rephrase, CKD may be diagnosed in patients with normal renal function, but this requires that they have additional evidence of kidney disease, such as pathological albuminuria. Although the definition is very precise and was supposed to provide a unified concept and language, it has often been misinterpreted [5]. Thus high-quality journals such as the New England Journal of Medicine use the concept ‘CKD Stage 1 or 2’ to represent individuals with an estimated GFR (eGFR) >60 mL/min/1.73 m2 in the absence of any demonstrated evidence of CKD: they apply the term CKD to individuals without CKD, that is, individuals who are healthy from the kidney point of view and have normal renal function [5, 6]. The confusion is further compounded by the different concepts of CKD embedded in guidelines from specialties other than nephrology [7]. However, it is most surprising that BP guidelines use concepts of CKD different than the consensus of KDIGO [2, 3] (Figure 1A–C). It would be desirable that they explicitly refer to the 2012 KDIGO definition and if they do want to single out specific CKD categories, they should do so throughout the manuscript and make clear at the start of the CKD section and throughout to what categories they are referring (CKD G3–G5, or CKD G3–G5 and/or A3) and not equate ‘CKD’ with just specific categories within the wider CKD concept [4].
FIGURE 1.
Different concepts of CKD used in recent hypertension guidelines. (A) CKD categories according to KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease [4]. A diagnosis of CKD can be made in the presence of a GFR <60 mL/min/1.73 m2 or in the presence of albuminuria >30 mg/24h (UACR >30 mg/g) or in the presence of other evidence of kidney injury persisting for >3 months. (B) The 2017 ACC/AHA guideline does not clarify the concept of CKD that they are using, and they do not reference the 2012 KDIGO definition of CKD. However, CKD patients have specific BP thresholds and targets, while patients with patients with CKD G3–G5 or A3 category additionally have a compelling indication for RAS blockade [2]. In drawing the figure, we have assumed that the 2017 ACC/AHA guideline refers to the 2012 KDIGO definition of CKD. (C) The 2018 ESC/ESH guidelines concept of CKD [3]. (D) Percentage of the adult (20–74 years old) and elderly (75–84 years old) hypertensive German population that fulfils each definition of CKD [8]. The 75- to 84-year-olds are representative of the >80-year-old population cited by the 2018 ESC/ESH guidelines to propose BP thresholds and targets for CKD patients [3]. In this study, hypertension was defined as a systolic BP ≥140 mmHg, a diastolic BP ≥90 mmHg or the use of antihypertensive drugs [8].
In the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults, the CKD section does not provide a definition of CKD when it states that adults with hypertension and CKD should be treated to a BP goal of <130/80 mmHg and the 2012 KDIGO definition of CKD is not cited among the references [2]. In agreement with the KDIGO definition, kidney transplant is considered CKD without any further consideration of albuminuria or GFR and it is recommended they be treated like other CKD patients. However, only a subset of CKD patients, defined as category G3 or higher or G1 or G2 with A3 albuminuria (≥300 mg/day or UACR ≥300 mg/g) have a compelling indication for renin–angiotensin system (RAS) blockade. Thus CKD categories G1 and G2 with albuminuria category A2 (30–299 mg/g) are not the subjects of any specific antihypertensive drug indications. Further confusion is created earlier in the guideline, in section 8.1.2, BP Treatment Threshold and the Use of CVD Risk Estimation to Guide Drug Treatment of Hypertension: ‘Use of BP-lowering medication is recommended for primary prevention of cardiovascular disease (CVD) in adults with no history of CVD and with an estimated 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥10% and a systolic BP of 130 mmHg or higher or a diastolic BP of 80 mmHg or higher’. Thus the reader is referred to an online calculator of the ACC/AHA Pooled Cohort Equations (http://tools.acc.org/ASCVD-Risk-Estimator/) to estimate the 10-year risk of atherosclerotic CVD. This calculator allows clinicians to introduce information on the presence of diabetes, but as of 5 August 2019, not the presence of CKD. Thus any risk calculation will not take into account the presence of CKD. The fact that CKD is missing for the recommended online calculator is not discussed in the text in section 8.1.2 dealing with risk estimation. However, a footnote to the treatment algorithm indicates, ‘Note that patients with diabetes mellitus or CKD are automatically placed in the high-risk category’. In any case, what is understood by ‘CKD’ is not clarified in this footnote.
In the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension, CKD is defined as an eGFR <60 mL/min/1.72 m2 with or without proteinuria in a footnote to Figure 6 of the text that shows the drug treatment strategy for hypertension in CKD patients [3]. This is consistent with other references to CKD in the same text and corresponds to the 2012 KDIGO categories G3–G5 and thus excludes CKD patients with normal renal function (categories G1/A2, G1/A3, G2/A2 and G2/A3) [3]. Figure 1D provides an approximation of the impact of the different CKD definitions on the population that is covered by recommendations provided by guidelines that use different concepts of CKD. Curiously, at some point, this guideline uses the concept CKD ‘grade’ (e.g. Figure 1 on page 3034 of the document) to refer to the current 2012 KDIGO concept of category. In other sections, the term used is stage (e.g. page 3033 of the document), which corresponds to the older Kidney Disease Outcomes Quality Initiative nomenclature. However, only the KDIGO manuscript is cited in the references. The indiscriminate and exchangeable use of the terms grade and stage is confusing when the current KDIGO term is category.
WHAT IS HYPERTENSION?
The two major hypertension guideline bodies provide different definitions of hypertension, and this is also confusing for patients, medical students and physicians.
The 2017 ACC/AHA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults defines hypertension as an office systolic BP ≥130 or an office diastolic BP ≥80 mmHg [2], while for the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension, hypertension is an office systolic BP ≥140 and/or office diastolic BP ≥90 mmHg [3]. Essentially this means that a patient diagnosed with hypertension in the USA because of a BP of 135/85 mmHg would no longer be considered to have hypertension after moving to Europe. Furthermore, the 2017 ACC/AHA guideline [2] recognize Stages 1 and 2 hypertension and the 2018 ESC/ESH guidelines [3] recognize Grades 1, 2 and 3 hypertension, but the thresholds in mmHg for these diagnoses do not match.
To complicate matters further, the 2018 ESC/ESH guidelines [3] also provide definitions of hypertension based on daytime ambulatory, nighttime ambulatory, 24 h ambulatory and home mean BP (Table 1). For example, for systolic BP, threshold values for a diagnosis range from ≥120 to ≥135 mmHg and for diastolic BP, from ≥70 mmHg to ≥85 mmHg, depending on the method of BP assessment chosen.
Table 1.
BP thresholds to define hypertension, depending on the country where you live or how the BP was measured
| Systolic BP (mmHg) | Diastolic BP (mmHg) | Criterion |
|---|---|---|
| ≥140 | ≥90 | Office BP, 2018 ESC/ESH |
| ≥135 | ≥85 | Home BP, daytime ambulatory BP, 2018 ESC/ESH |
| ≥130 | ≥80 | 24h mean ambulatory BP, 2018 ESC/ESH |
| Office BP, 2017 ACC/AHA. Equivalent to Home BP, daytime ambulatory BP | ||
| ≥125 | ≥75 | 24h mean ambulatory BP equivalent to 2017 ACC/AHA office BP definition of hypertension |
| ≥120 | ≥70 | Nighttime ambulatory BP, 2018 ESC/ESH |
| ≥110 | ≥65 | Nighttime ambulatory BP equivalent to 2017 ACC/AHA office BP definition of hypertension |
Either the systolic or the diastolic BP value is diagnostic of hypertension. The 2018 ESC/ESH guidelines [3] provide a table with non-office definitions, while the 2017 ACC/AHA guideline [2] provides a table with corresponding values of BP for home BP, office BP, and daytime, nighttime and 24 h mean ambulatory BP. The 2018 ESC/ESH guidelines are shown in blue and the 2017 ACC/AHA guideline is in red.
At least both guidelines agree on the equivalence between office BP and other measurements of BP, although there is no logical lineal correspondence between the values in the different techniques that allow extrapolation of BP values sitting between those provided (Figure 2).
FIGURE 2.
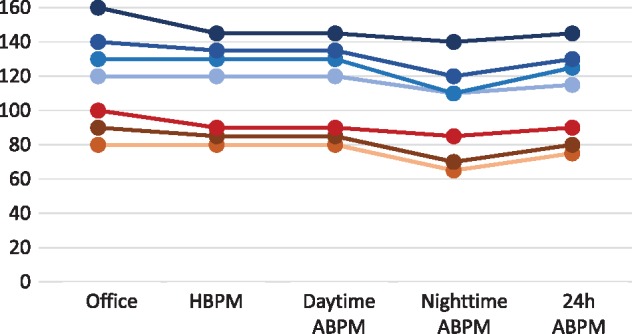
Corresponding values of systolic BP and diastolic BP assessed as office BP, home BP monitoring (HBPM) or ambulatory BP monitoring (ABPM: daytime, nighttime and 24 h) [2]. Please note that there is no logical lineal correspondence and thus it is difficult to extrapolate BP values between those provided. The figure is not meant to represent thresholds to diagnose hypertension. Systolic BP values are shown as shades of blue and diastolic BP values as shades of red/brown.
WHAT ARE THE BP THRESHOLDS TO INITIATE HYPERTENSIVE DRUG THERAPY IN CKD PATIENTS?
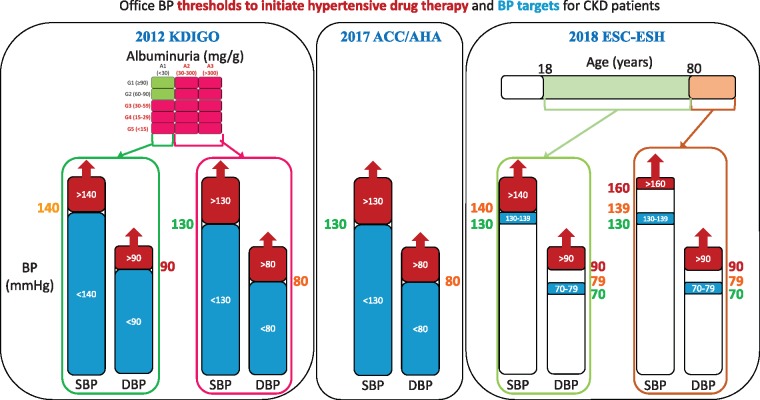
There are also major differences between the guidelines regarding BP thresholds to initiate antihypertensive drug therapy and specifically regarding thresholds for CKD patients (Figure 3). A major difference relates to the elderly. For the 2017 ACC/AHA guideline [2], individuals ≥80 years of age should be treated when systolic BP is ≥130 mmHg and/or diastolic BP ≥80 mmHg, independent of the presence of CKD, while in the 2018 ESC/ESH guidelines [3], for individuals ≥80 years of age the threshold for initiation of antihypertensive therapy is systolic BP ≥160 mmHg or diastolic BP ≥90 mmHg, again independent of the presence of CKD.
FIGURE 3.
Different thresholds to initiate antihypertensive drug therapy and therapeutic targets in CKD patients. (A) The 2012 KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease [1]. (B) The 2017 ACC/AHA guideline [2]. (C) The 2018 ESC/ESH guidelines [3]. Please note that thresholds and therapeutic targets differ between guidelines and the curious situation for the discrepant thresholds and therapeutic targets recommended by the 2018 ESC/ESH guidelines, especially for the elderly. Please note that the 2012 KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease and the 2017 ACC/AHA guideline provide no range of target values, so there is no lower limit for the recommended systolic or diastolic BP. Red colouring of the columns represents above-threshold BP. Blue colour represents within-target BP. Individual numerical values in red represent the highest values of the three guidelines for the same concept (systolic or diastolic BP), in orange represent intermediate values and in green represent the lowest values of the three guidelines for the same concept. For the KDIGO and ACC/AHA guidelines, the same values are thresholds and targets. For the ESC-ESH guidelines, thresholds and targets do not match.
In the 2017 ACC/AHA guideline, not all patients with a diagnosis of hypertension merit pharmacological therapy. Thus therapy is restricted to those meeting the European concept of hypertension (systolic BP ≥140 mmHg and/or ≥90 mmHg) or to those having an American diagnosis of hypertension (systolic BP ≥130 mmHg and/or diastolic BP ≥ 80 mmHg) that additionally have either CVD or a 10-year ASCVD risk ≥10% as per the ACC/AHA Pooled Cohort Equations [2]. This level of ASCVD risk is found in patients with diabetes or CKD and in patients ≥80 years of age. In this regard, the ACC/AHA Pooled Cohort Equations are validated for US adults ages 45–79 years in the absence of concurrent statin therapy [2]. The guideline specifically states that for those ≥80 years of age, the 10-year ASCVD risk is generally >10%, and thus the systolic BP threshold for antihypertensive drug treatment for patients >79 years of age is 130 mmHg.
For the 2018 ESC/ESH guidelines, the office BP thresholds for treatment are grossly equivalent to the thresholds used to diagnose hypertension. That is, a diagnosis of hypertension (systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg) means that therapy for hypertension should be prescribed when there are no associated diseases or when the patient has diabetes or CKD [3]. However, there are two exceptions:
For patients with coronary artery disease or stroke, treatment may be considered when systolic BP is 130–140 mmHg, except if they are ≥80 years of age.
For individuals ≥80 years of age, the threshold to prescribe treatment is systolic BP ≥160 and/or diastolic BP ≥90 mmHg in all cases, independent of the presence of associated conditions. Basically this means that the therapeutic implications of a diagnosis of hypertension are different for the elderly than for younger individuals.
In summary, in the 2018 ESC/ESH guidelines, the diastolic BP threshold for therapy is always 90 mmHg, but the systolic BP threshold ranges from ≥130 to ≥160 mmHg depending on age and associated conditions. Focusing on CKD patients, therapy for hypertension is indicated when systolic BP is ≥140 mmHg and/or diastolic BP is ≥90 mmHg, except for individuals ≥80 years of age, in whom the systolic BP threshold is ≥160 mmHg. The thresholds for younger individuals and, above all, the threshold for those ≥80 years of age are different from the 2017 ACC/AHA guideline for CKD patients—systolic BP ≥130 mmHg and/or diastolic BP ≥80 mmHg, which are independent of age (Figure 3).
The thresholds of both general guidelines also differ from those of the 2012 KDIGO for CKD patients: systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg for A1 albuminuria and systolic BP ≥130 mmHg and/or diastolic BP ≥80 mmHg for albuminuria A2 or A3, independent of GFR (Figure 3) [1].
WHAT ARE THE BP TARGETS FOR CKD PATIENTS ON HYPERTENSIVE DRUG THERAPY?
The BP target for both the 2012 KDIGO and 2017 ACC/AHA guidelines is to bring BP below the thresholds that trigger prescription therapy [1, 2] (Figure 3). However, the 2018 ESH/ESC guidelines differ from this apparently logical approach [3]. It is difficult to grasp why nephrologists would let the BP of elderly CKD patients rise to >160/90 mmHg before prescribing antihypertensive medication, but once this is prescribed, they have a target BP of 130–139/70–79 mmHg, as suggested by the 2018 ESH/ESC guidelines [3]. And it is even more difficult to understand why the 2018 ESH/ESC guidelines have a higher threshold to initiate antihypertensive medication in those >80 years of age, while this population is, again, because of age, automatically considered as very high risk by the 2017 ACC/AHA guidelines and thus the threshold to initiate therapy is lowered to 130/80 mmHg [2].
Additionally, different guidelines use different concepts of CKD when providing recommendations on compelling indications for specific antihypertensive drugs. The 2012 KDIGO guideline suggests RAS blockade for patients with a UACR 30–300 mg/g and recommends RAS blockade for a UACR >300 mg/g, independent of eGFR values [1]. In contrast, the 2017 ACC/AHA guideline excludes CKD patients with an eGFR > 60 mL/min/1.73 m2 that have a UACR of 30–300 mg/g [2] and the 2018 ESC/ESH guidelines exclude those with an eGFR >60 mL/min/1.73 m2 from the compelling indication of RAS blockade for CKD patients [3].
WHAT IS THE METHOD USED TO ASSESS BP THRESHOLDS AND TARGETS?
While guidelines provide a series of considerations regarding the value of ambulatory and home BP monitoring in correctly classifying patients into hypertension, white-coat and masked hypertension, and even the 2018 ESH/ESC guidelines [3] provide hypertension diagnostic criteria for these other approaches, the key thresholds for initiating therapy and BP targets are mainly provided in terms of office BP.
HOW IS HYPERTENSION SECONDARY TO CKD DIAGNOSED?
An interesting and, from our point of view, insufficiently addressed issue is how to differentiate hypertension-induced kidney injury from hypertension secondary to CKD. By defect, guidelines appear to attribute any CKD in a hypertensive patient to hypertension, unless demonstrated otherwise. The 2018 ESH/ESC guidelines state, ‘The diagnosis of hypertension-induced renal damage is based on the finding of reduced renal function and/or the detection of albuminuria’. [3] Thus, apparently for them, the coexistence of hypertension and decreased eGFR and/or presence of pathological albuminuria is diagnostic of hypertension-induced kidney injury rather than setting up a diagnostic work-up for kidney disease–induced hypertension.
According to the 2017 ACC/AHA guidelines, a specific, remediable cause of hypertension can be identified in ∼10% of adult patients with hypertension [2]. Renal parenchymal disease is reported to have a prevalence of 1–2%, however, the denominator (all hypertensive individuals?) for such prevalence is not clarified. In any case, the prevalence is much lower than for renovascular disease, which is reported as 5–34% depending on the clinical situation (hypertension alone, 5%; hypertension starting dialysis, 22%; hypertension and peripheral vascular disease, 28%; hypertension in the elderly with congestive heart failure, 34%). The low contribution of renal parenchymal disease to secondary hypertension is surprising, given that 10% of the adult population has CKD and the prevalence of CKD is >60% of those >80 years of age [8, 9]. This means that for the immense majority of patients with both hypertension and CKD, CKD is not thought to be causing hypertension. The guideline states that renal parenchymal disease may be suspected by the presence of urinary tract infections; obstruction, haematuria; urinary frequency and nocturia; analgesic abuse; family history of polycystic kidney disease; elevated serum creatinine; abnormal urinalysis; an abdominal mass (polycystic kidney disease) or skin pallor [2]. Renal ultrasound is considered a screening test.
The 2018 ESH/ESC guidelines suggest that 5–15% of hypertensive patients have secondary hypertension [3]. Surprisingly, it goes on to list four causes of secondary hypertension that may be present in ≥10% of hypertensive patients, potentially driving the prevalence of secondary hypertension to 45%. It lists parenchymal kidney disease as a cause in 2–10% of hypertensive patients. This is up to 10-fold higher than the figure in the 2017 ACC/AHA guideline. Renal parenchymal disease is listed as a key contributor to secondary hypertension at all ages. However, among those ≥65 years of age, it is estimated that 5–10% of hypertensive patients have one of three causes of hypertension (atherosclerotic renovascular disease, renal parenchymal disease and thyroid disease). Thus the prevalence of renal parenchymal disease as a cause of hypertension in this age group is estimated at <5–10%, well below the estimated prevalence of CKD in the general population in this age range. The guideline indicates that suggestive symptoms and signs of a CKD caudation include diabetes, haematuria, proteinuria, nocturia, anaemia or renal mass in adult polycystic CKD and evaluations include plasma creatinine and electrolytes, eGFR, urine dipstick for blood and protein, UACR and renal ultrasound.
Overall, in our opinion, the contribution of CKD as a driver of hypertension is not adequately clarified by the guidelines: they do not agree on the percentage of hypertensive patients who have hypertension secondary to CKD, and although tests are listed to uncover parenchymal kidney disease, the criteria to assign CKD as the cause of hypertension are unclear as opposed to being coexistent conditions or CKD being secondary to hypertension. In this regard, from 27 to 69% of adult hypertensives have CKD (Figure 1D) [8]. Thus current guidelines suggest that in the majority of hypertensive patients with CKD, CKD is not contributing to hypertension: according to the 2017 ACC/AHA guideline, the lack of contribution of CKD to hypertension is at least 15- to 35-fold more common than CKD contributing to hypertension, with the fold-change range depending on age [2], and according to the 2018 ESH/ESC guidelines, CKD not contributing to hypertension is at least 3- to 7-fold more common than CKD contributing to hypertension [3].
WHY OLDER DEFINITIONS OF HYPERTENSIVE NEPHROPATHY MAY BE OUTDATED
Hypertension and CKD are closely linked. Thus the diagnostic conundrum in a hypertensive CKD patient is whether hypertension caused CKD or CKD caused hypertension. This latter possibility would mean that hypertension is considered to be secondary to CKD. A third possibility would be to consider both entities coexistent and unrelated, and that, although theoretically possible, is unlikely. Reading the diagnostic criteria for hypertensive nephrosclerosis, it becomes clear that the whole field of the causal relationship between hypertension and CKD must be rethought and that CKD is likely a more frequent cause of hypertension than what is presented in guidelines or textbooks. Thus, for example, according to UpToDate,
the diagnosis of hypertensive nephrosclerosis is generally inferred from the characteristic clinical features and the exclusion of other kidney diseases…. Affected patients usually have a long history of hypertension that is typically accompanied by left ventricular hypertrophy, a relatively normal urine sediment, small kidneys, and, if previous information is available, slowly progressive renal insufficiency with gradually increasing proteinuria that is usually nonnephrotic…. Most important from a clinical viewpoint, the hypertension precedes the development of either proteinuria or renal insufficiency, and there is no other obvious cause of renal disease [10].
Reading this definition, one is confronted with the thought that it is describing CKD of unknown cause. Thus, for two patients with the same characteristics, if a cause of CKD is obvious, it would be called CKD-induced hypertension, but if the cause is not obvious (i.e. CKD of unknown cause) then it is called hypertensive nephrosclerosis, which would be most CKD patients of whatever cause that have reached this stage in which the kidneys are small. The fact that hypertension ‘precedes the development of either proteinuria or renal insufficiency’ does not mean that hypertension is causing either of them, since pathological albuminuria is already diagnostic of CKD and may precede overt proteinuria by decades and a diagnosis of renal insufficiency (eGFR <60 mL/min) means that CKD has been progressing, also potentially over decades, leading to a progressive decrease in GFR from ∼120 mL/min to <60 mL/min [5, 11]. Thus CKD is currently diagnosed much earlier in the course of kidney disease (e.g. when normal GFR is still normal but albuminuria is already pathological, such as values persistently ∼40 mg/g) than chronic kidney insufficiency was diagnosed in the 20th century (e.g. serum creatinine well above the upper limit of the normal range, independent of muscle mass). Thus it is not enough that hypertension precedes kidney insufficiency, it should also precede the earliest evidence of CKD to start thinking about attributing CKD to hypertension and not the other way around.
WHAT DID THE KDIGO CONTROVERSIES CONFERENCE 2019 SAY?
The conclusions of a KDIGO Controversies Conference on Blood Pressure in CKD were published in 2019 [12]. They were disappointing. Basically the 2012 KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease [1] should be revised, but there are many sources of uncertainty and this will not be easy. Difficulties include establishing conversion factors to translate a casual BP value into a standardized BP value, the lack of adequately powered randomized controlled trials of BP control on clinical outcomes that have targeted ambulatory or home BP in the CKD or general adult population and how to translate the findings from the Systolic Blood Pressure Intervention Trial into BP thresholds and targets, which should be reconsidered. In this regard, the choice of thresholds and targets for lower-risk populations will inform the need to define higher-risk subpopulations that require different, more stringent targets. The key conclusion was thus that the 2012 KDIGO BP guideline is outdated.
WHAT CAN BE DONE ABOUT IT?
The existence of multiple different definitions of CKD to label a hypertensive patient as having CKD for therapeutic decision-making purposes as well as the existence of multiple different BP thresholds to initiate antihypertensive drug therapy and of multiple targets for such therapy creates confusion as physicians are exposed to different guidelines in manuscripts or meetings. It is difficult to keep track of the subtleties regarding the different definitions of CKD and thresholds and targets for such populations. It is likely that the final result will be a mix-up of guidelines: certain populations defined in one guideline may be applied thresholds and targets meant for a different ‘CKD’ population by another guideline. This chaos may eventually promote therapeutic nihilism: if there are so many different thresholds or targets supported by a careful review of the evidence by experts, then any threshold or target may be correct or maybe none of them are correct. A larger danger lies in patient perceptions of the issue. Until recently, patients were content with following physician advice. But now they access online information, including different guidelines. An interconnected world means that patients have access, as do physicians, to the online guidelines. They also may realize the discrepancies and may become painfully aware of the chaotic situation. This may decrease their trust in the medical community or individual doctors.
FUNDING
Research by the authors was supported by FIS CP14/00133, PI16/02057, PI18/01366, PI19/00588, PI19/00815, DTS18/00032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071), ISCIII-RETIC REDinREN RD016/0009 Fondos FEDER, FRIAT, Sociedad Española de Nefrología, Comunidad de Madrid B2017/BMD-3686 CIFRA2-CM.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Kidney Disease: Improving Global Outcomes Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2: 337–414 [Google Scholar]
- 2. Whelton PK, Carey RM, Aronow WS. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71: 1269–1324 [DOI] [PubMed] [Google Scholar]
- 3. Williams B, Mancia G, Spiering W. et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104 [DOI] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2012; 3: 1–150 [Google Scholar]
- 5. Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez E. et al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019; 12: 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White WB, Saag KG, Becker MA. et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378: 1200–1210 [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Prado R, Castillo-Rodriguez E, Velez-Arribas FJ. et al. Creatinine clearance is not equal to glomerular filtration rate and Cockcroft-Gault equation is not equal to CKD-EPI collaboration equation. Am J Med 2016; 129: 1259–1263 [DOI] [PubMed] [Google Scholar]
- 8. Bruck K, Stel VS, Gambaro G. et al. CKD prevalence varies across the European general population. J Am Soc Nephrol 2016; 27: 2135–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevens LA, Li S, Wang C. et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2010; 55: S23–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mann JH. Clinical Features, Diagnosis, and Treatment of Hypertensive Nephrosclerosis 15 July 2019. www.uptodate.com (5 August 2019, date last accessed)
- 11. Sanchez-Nino MD, Sanz AB, Ramos AM. et al. Clinical proteomics in kidney disease as an exponential technology: heading towards the disruptive phase. Clin Kidney J 2017; 10: 188–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheung AK, Chang TI, Cushman WC. et al. Blood pressure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 95: 1027–1036 [DOI] [PubMed] [Google Scholar]