Abstract
Chronic non-communicable diseases have become a pandemic public problem in the 21st century, causing enormous burden on the economy, health and quality of life of societies. The role of a chronic inflammatory state in the pathogenesis of chronic disease has been more comprehensively recognized by recent findings. The new paradigm ‘metaflammation’ focuses on metabolism-induced (high fat or fructose-based diet or excessive calorie intake) chronic inflammation. There is a close correlation between the increased incidence of chronic kidney disease (CKD) and chronic heart failure with both increased inflammatory marker levels and western-type diet. In this review we describe the concept of metaflammation, its role in the development of CKD and chronic heart disease, the molecular and signalling pathways involved and the therapeutic consequences.
Keywords: cardiovascular disease, diet, epigenetics, kidney disease, Klotho, metaflammation, pentoxifylline
INTRODUCTION
Excessive nutrient intake and chronic non-communicable metabolic diseases are prominent hallmarks of the 21st century. Noticeably, chronic heart failure (CHF) is one of the largest pandemic health problems, involving >26 million people worldwide [1]. Its incidence and prevalence are increasing in association with modern lifestyles. It is estimated that 8 million patients have CHF in the USA alone, and its prevalence is expected to increase by 46% by 2030. In 2012, its economic burden on the USA reached $31 billion and is expected to increase by 127% in 2012–30 [1]. Likewise, chronic kidney disease (CKD) represents another major public health problem imposing remarkable economic and social burdens globally. CKD also has indirect impacts on society by increasing the severity of morbidities, including cardiovascular disease (CVD), diabetes, hypertension, human immunodeficiency virus and malaria [2, 3]. Indeed, CKD is an independent risk factor for CVD morbidity and mortality [4–6]. Remarkably, the Global Burden of Disease 2015 study directly attributed 1.2 million deaths, 19 million disability-adjusted life years and 18 million years of life lost from CVD to CKD [3, 7]. The Global Burden of Disease study further detected 5–10 million annual deaths from CKD. Indeed, the worldwide prevalence of CKD is 3–18%, translating to 500 million patients [8]. In some countries, CKD was estimated to become the second leading cause of death after Alzheimer’s disease by 2100 [9]. Knowledge of the aetiology and pathophysiology of CKD and CHF is required to tailor more efficient, targeted and individualized treatments [10].
In recent years, multiple studies have supported a pathogenic role of systemic chronic inflammatory conditions on many chronic diseases: inflammaging [11–15]. Inflammation is essential to protect the body against pathogens and also to promote healing, remodelling and renewal of tissues. However, the outcome of inflammatory activation depends on its duration and severity and whether it is appropriate for the nature and magnitude of the insult. During evolution, acute and adequate inflammatory responses provided a survival advantage and defense against pathogens. However, chronic, sterile and low-grade inflammation does not have a role in evolutionary development. The naturally protective inflammatory state can become an unfavourable condition that promotes further tissue injury in the long term [16, 17].
Recently a new paradigm called metaflammation (also termed metainflammation) has emerged and is suggested to play a role in various chronic diseases including CHF and CKD. In this review we first define metaflammation and then explore the role of metaflammation in the development and progression of CHF and CKD and the therapeutic implications.
Metaflammation
PubMed first recorded use of the term metaflammation in 2008 [18]. More recently, it was defined as a chronic low-grade inflammatory state induced by alterations in metabolism [17]. The complex link between body nutrition status, metabolic pathways and immune signalling has been preserved, with roots back to Drosophila, as an evolutionary adaptation to starvation. For example, activation of innate immune system components such as tumour necrosis factor (TNF) and Toll-like receptors (TLRs) blocks insulin pathway signalling through c-Jun N-terminal kinase (JNK) and myeloid differentiation primary response gene (MyD88) pathways. This may be one of the multiple links between the inflammatory state and nutritional status [17]. As early as 1983, TNF-α secreted from macrophages was shown to induce insulin resistance in adipocytes, supporting a close link between inflammation and metabolic syndrome [19]. The presence of macrophage infiltration in obese mice adipose tissue further supported this hypothesis and became an important milestone to understand the pathophysiology of metabolic syndrome [17]. Free fatty acid exposure triggers polarization of adipose tissue macrophages to M1-type macrophages, which inhibit insulin function [17]. The transformation of resident adipose tissue quiescent macrophages to active macrophages initiates further recruitment of macrophages and secretion of inflammatory molecules, including monocyte chemoattractant protein-1, interleukin-6 (IL-6) and TNF [20]. Insulin resistance has been linked to immune system dysfunctions, including thymic atrophy, impaired number and function of regulatory T cells (Tregs), natural killer cells, and dendritic cells [21]. Additionally, in obese mice, adipose tissue regulatory B cells are decreased. These cells secrete IL-10 and transforming growth factor β1 (TGF-β1), which have anti-inflammatory properties [21]. These observations may have therapeutic consequences. As an example, induction or transfer of CD4+ latency-associated peptide Tregs as immunotherapy decreased inflammation in adipose tissue, hepatosteatosis and pancreatic islet cell hyperplasia [22].
The role of endoplasmic reticulum (ER) dysfunction in metaflammation
The ER is a key organelle in the regulation of cellular and metabolic homeostasis. Maintenance of the proteome consistency is required for cell homoeostasis [23]. In the presence of misfolded proteins, the ER unfolded protein response is activated to decrease cellular stress via increasing proper protein folding, chaperone availability and misfolded protein clearance. ER stress induces nuclear factor kappa light chain enhancer of activated B cells (NF-κB) signalling that regulates the immune and inflammatory response system ranging from haematopoietic cell production to cytokine secretion. ER stress results in an excessive inflammatory response, and the ER has an essential role in lipid homoeostasis and regulation of insulin-induced signalling pathways [23, 24]. In this regard, obesity-induced ER stress impaired insulin signalling through serine phosphorylation of insulin receptor substrate 1 and overactivation of JNK via inositol requiring enzyme 1 [25]. An oral chemical chaperone (4-phenyl butyric acid or dimethylsulphoxide) reduced ER stress, decreased hepatosteatosis and improved insulin sensitivity [26].
Inflammasome and metaflammation
The inflammasome is composed of multimeric proteins that activate caspase-1 cleavage of pro-inflammatory cytokines IL-1β and IL-18 in the presence of danger-associated molecular patterns (DAMPs), infection, cellular/tissue damage or metabolic dysregulation, resulting in pro-inflammatory cell death (i.e. pyroptosis) [27]. Inflammasome and associated caspase and IL systems are key modulators of metabolism and adipocyte function. Caspase-1 is overexpressed during adipocyte differentiation. Mice deficient in caspase-1 or nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) (inflammasome component) had better insulin sensitivity and adipocyte oxidation/metabolism and mitochondrial function than wild-type mice [28]. Indeed, caspase-1 or inflammasome inhibitors have been proposed as novel treatments for metabolic syndrome [28].
High mobility group box 1 (HMGB1) is one component of chromatin that stabilizes nucleosomes and changes DNA configuration to facilitate transcription [29]. HMGB1 is released by necrotic cells and activates the inflammasome in macrophages by binding to receptors for advanced glycation end products (RAGEs) [29, 30]. In this regard, anti-HMGB1 antibodies reduced weight gain and liver inflammation, further supporting a role for inflammation in metabolic syndrome [30]. Indeed, HMGB1 is significantly elevated in CKD and correlates with glomerular filtration rate (GFR) as well as with markers of inflammation and malnutrition [31].
Epigenetics in metaflammation
Epigenetic changes [DNA methylation, post-translational histone modifications and microRNAs (miRNAs)] modulate gene expression and thus inflammatory and metabolic signalling [32]. Epigenetic changes may be responsible for ‘metabolic memory’, particularly in adipose tissue macrophages, which is one of the key elements driving low-grade chronic inflammation [20]. Epigenetic changes facilitate the expression or repression of certain genes both acutely and chronically. Since epigenetic modifications may be transferred to daughter cells, there is the potential to perpetuate changes in gene transcription. This may explain metabolic memory and long-term effects of acute tissue injury. As an example, both inflammatory mediators and albuminuria decrease kidney expression of the cardioprotective, anti-ageing and anti-inflammatory hormone through epigenetic changes that may be concerned with NF-κB [33, 34]. For example, kidney Klotho downregulation persists long after renal function has been recovered in acute kidney injury [33]. Indeed, Klotho suppression has been proposed as a key contributor to inflammaging in CKD [35] and epigenetic modulation of Klotho expression improves kidney injury [36]. Epigenetic regulators in response to inflammatory signals also regulate insulin resistance. Overexpression of the epigenomic co-repressor G protein pathway suppressor 2 in obese mice improves insulin resistance and reduces inflammation and macrophage recruitment in adipose tissue [20].
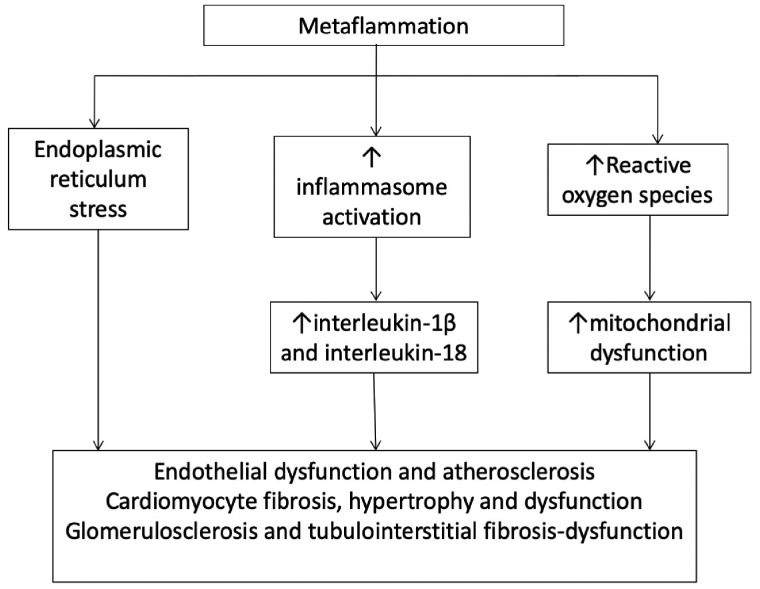
Inflammation and metabolic disorders interplay with genetic and epigenetic factors and have a key role in the development of chronic non-transmissible diseases (Figure 1). Below, we separately discuss these issues in cardiorenal disorders, including CHF and CKD.
FIGURE 1.
Postulated mechanisms between inflammation, insulin resistance, ER stress and systemic diseases.
The adenosine monophosphate–activated protein kinase (AMPK) pathway also plays an important role in the metabolism, inflammation and CHF–CKD cascade. Recent studies suggest that AMPK might act as an inflammatory repressor, and inhibition of AMPK increases inflammation, especially in obese humans [37]. In the same line, AMPK activation might suppress inflammation by inhibition of the NF-κB pathway and overexpression of AMPK inhibits the NF-κB pathway in endothelial cells [38]. Furthermore, adiponectin decreased angiotensin II–induced cardiac hypertrophy by decreasing NF-κB activity in rats, but this beneficial effect disappeared after AMPK inhibition [39]. Experimental studies showed that the cardioprotective effect of metformin requires AMPK activation [40, 41]. Last but not the least, the cardioprotective effect of statins might also be mediated at the molecular level by increasing AMPK and nitric oxide production in human umbilical vein endothelial cells and in mouse aortas and myocardium [42]. Thus the AMPK pathway may be another mediator of metabolism inflammation, CKD and CVD, and this issue needs further investigation.
Chronic heart disease
Recent evidence has shown a close relation between increased inflammatory markers and CHF [43–45]. There is an ongoing discussion of whether systemic inflammation plays a role in the pathogenesis of CHF, is a consequence of CHF, or both, contributing to a ‘vicious cycle’ of disease severity [46]. For example, the serum TNF level increases in the decompensation phase of CHF and is an independent prognostic factor for both cardiac and non-cardiac-related mortality in CHF [16, 47]. Additionally, TNF and IL-1β downregulate Ca+2-related gene expression, including sarcoplasmic reticulum Ca+2 ATPase and Ca+2 release channels; impair the efficiency of gap junction mechanisms, leading to dysregulated electrical conduction and unsynchronized contraction and have a negative inotropic effect on myocytes, concluding with eccentric cardiomyocyte hypertrophy, myocardial fibrosis, ventricular dilation and ultimate CHF [47, 48]. Inflammation- or CKD-driven Klotho deficiency also contributes to cardiomyopathy, and recombinant α-Klotho prevents and retards uraemic cardiomyopathy [49].
TNF-like weak inducer of apoptosis (TWEAK) is a member of the TNF cytokine superfamily that activates the Fn14 receptor and activates both the canonical (as TNF) and the non-canonical (unlike TNF) pathways of NF-κB [50, 51]. In addition to decreasing Klotho through epigenetic mechanisms, TWEAK directly promotes cardiac dysfunction and failure through downregulation of the mitochondrial biogenesis regulator PGC1α, an action of TWEAK that also contributes to CKD [52, 53], and increases vascular calcification [54]. In keeping with this, higher serum TWEAK levels are associated with the severity of coronary artery disease, particularly in CKD Stages 2 and 3 patients [55], and predict mortality in haemodialysis patients [56]. Liver TWEAK signalling is differentially activated by palm oil in association with insulin resistance [57] and in response to a high-fat diet, and it contributes to liver steatosis, fibrosis and inflammation and insulin resistance [58].
In addition to IL-1β and TNF superfamily cytokines [59], IL-6 also contributes to CHF. Thus it decreases myocardial contractility and promotes cardiac hypertrophy and fibrosis leading to diastolic dysfunction by signalling through Gp130 to activate Janus kinase/signal transducer and activator of transcription 3 [47, 60]. IL-6 is the cytokine that best correlates with adverse cardiovascular outcomes in CKD [61]. However, C-reactive protein (CRP) is the marker of systemic inflammation most commonly used in clinical practice, and it associates with diabetes mellitus, atherosclerosis and CHF. A direct role of CRP on CV injury has been suggested [62]. CHF and inflammation may be further linked through endothelial dysfunction and haemodynamic dysregulation via improper endothelial activation and increased vascular stiffness [14, 16, 63–65]. Inflammation-induced vascular permeability may cause fluid extravasation and further amplify cardiac dysfunction [16]. Additionally, venous congestion due to CHF may trigger more pro-inflammatory cytokine release, increase endotoxin absorption from the gut and promote kidney dysfunction that, in turn, further promotes inflammation and increased gut permeability [66, 67]. CHF is also associated with an altered gut microbiota that correlates with systemic inflammation in CHF [46, 68, 69].
Alteration of gut microbiota is another factor linking metabolic disorders, inflammation and chronic diseases such as CKD and CHF. Vaziri et al. [70] showed that the microbial composition of the gut is highly different in end-stage renal disease (ESRD) patients compared with healthy subjects. Due to changes like diet and colonic transit time in CKD patients, microbial metabolism moves towards a predominantly proteolytic fermentation. The percentage of bacterial families possessing urease, uricase and indole- and p-cresol-forming enzymes increase, whereas the percentage of bacterial families possessing butyrate-forming enzymes decreases in CKD patients. This increase of protein fermentation occurs at the expense of carbohydrate fermentation and disrupted epithelial barrier. This suggests that altered microbial composition and metabolism may have a role in the dysfunctional epithelial barrier, which is associated with bacterial translocation and endotoxinaemia, increases in systemic inflammation and the risk for CVD [71]. This altered dysfunction of the intestinal barrier in CKD. This pathophysiologic process is now considered one of the mechanisms explaining high cardiovascular burden in CKD patients.
With time, the vicious cycle between inflammation and CHF becomes more and more prominent and eventually it may be unbreakable [16]. Both TLRs and RAGEs are elemental pattern recognition receptors for detection of DAMPs, such as HMGB1 and others. The metabolic syndrome–associated chronic inflammatory state induces activation of TLR- and NF-κB-associated inflammatory responses on cardiac myocytes, contributing to insulin resistance, inflammation and further cardiomyocyte injury [46].
Metaflammation also contributes to endothelial dysfunction, vascular calcification and atherosclerosis [72]. Dyslipidaemia originating from metabolic syndrome [including decreased levels of high-density lipoprotein and increased levels of triglycerides, total cholesterol and low-density lipoprotein (LDL)] is an independent risk factor for adverse cardiovascular outcomes [73, 74]. Atherogenic adipokines are also released by adipose tissue [72].
Metaflammation is also associated with mitochondrial dysfunction, decreasing energy availability and increasing mitochondrial reactive oxygen species (ROS) production [75]. Indeed, energy metabolism is thought to be a key driver of diabetic cardiomyopathy, and it has been proposed that an improved fuel profile is a key contributor to the improved outcomes of patients on sodium-glucose co-transporter 2 inhibitors [76].
Epigenetic modifications and CHF
Differentiating healthy cardiomyocytes during development and failing or stressed cardiomyocytes share epigenetic modification patterns [77]. During CHF, epigenetic modifications regulate the expression of transcription factors, angiogenic factors and natriuretic factors, among others [77–79]. Since there is an association of increased DNA methylation levels with pathological hypertrophy and impaired contractility in failing cardiomyocytes, inhibition of DNA methylation has been proposed as a treatment option for CHF since it has the potential to reverse pathologic norepinephrine and Ca+2-induced cardiac fibrosis [79]. DNA methylation inhibitors are already in clinical use for malignancy [32].
Histone post-translational modifications have also been associated with metabolic syndrome, inflammation and heart disease. Of >50 known histone post-translational modifications, acetylation and methylation are the best characterized. Histone acetylation modulates gene expression associated with cardiomyocyte function, inflammation and fibrosis [79]. Sirtuins are histone/protein deacetylases that regulate mitochondrial function and cell survival. Specifically, SIRT1 and SIRT3 protect cardiomyocytes from stress [80]. Histone deacetylase inhibitors decrease cardiac fibrosis and hypertrophy and improved myofilament Ca+2 sensitivity [81]. Trimethylation of histone H3 on lysine 4 or lysine 9 were markedly different between normal hearts and heart failure both in experimental animals and in humans [82]. However, the potential therapeutic consequences of this observation remain unexplored.
Altered messenger RNA expression also contributes to heart failure. As an example, miR-208a, which is released into the circulation during heart injury, is associated with the expression of the α- myosin heavy chain and is a regulator of cardiac hypertrophy and conduction [83]. miR-208a is required for normal cardiac function [84], but when overexpressed, it induced hypertrophy and arrhythmia [84]. In this regard, targeting miR-208a by anti-miR protected Dahl hypertensive rats from hypertension-induced heart failure [85]. Interestingly, miR-208a targets mediator complex subunit 13 (MED13), one of components of the mediator complex that regulates fatty acid, cholesterol, lipid and cellular energy homoeostasis, increasing energy expenditure [79, 86]. Cardiac-specific overexpression of MED13 or pharmacologic inhibition of miR-208a (leading to MED13 upregulation) in mice conferred resistance to high-fat diet-induced obesity and showed an improvement in systemic insulin sensitivity and glucose tolerance [86]. In summary, these studies demonstrated a close relationship between the epigenetic regulation of cardiac function and metabolic diseases and a potential of miR208-α inhibition in the treatment of cardiac and chronic metabolic diseases. Upregulation of miR-23, miR24 and miR-195 was also associated with cardiac hypertrophy and ischaemic cardiomyopathy through the MAPK signalling pathway [79, 87, 88]. The number and types of heart failure-related miRNA or other epigenetic changes are rapidly increasing and may provide novel therapeutic targets for CHF.
CKD
The role of chronic inflammation on the pathogenesis of CKD and its complications is widely recognized [12, 89–91]. CKD is a cause of low-grade chronic inflammation and chronic inflammation decreases kidney Klotho expression and increases oxidative stress, adhesion molecules, aberrant matrix composition and fibrosis of renal tissue, leading to progressive renal dysfunction [92].
Oxidative stress may have a role in the progression of kidney disease independent from the aetiology. The formation of ROS occurs both in the renal cortex and medulla. Increased ROS has various effects, such as microinflammation, which is frequently seen in CKD [93, 94]. Oxidative stress has been considered the link between inflammation and CVD in CKD [95]. Oxidative stress is closely related to endothelial dysfunction and endothelial dysfunction, in turn, is closely related to arterial hypertension, arteriosclerosis and heart failure [96]. Undoubtedly, the most elaborated role of oxidative stress is acknowledged in diabetic nephropathy (DN). This issue is very important since DN is the most common form of CKD. Especially in DN, mitochondrial dysfunction has been well demonstrated [97] and antioxidant supplementation was beneficial in DN [98, 99].
Patients with metabolic syndrome have an increased frequency of renal vascular sclerosis, tubulointerstitial fibrosis and tubular atrophy [100, 101]. Despite the strong association of metaflammation and metabolic syndrome with CKD, further studies are needed to elucidate the exact pathogenesis and potential novel therapeutic implications. Several questions remain to be clarified: Does CKD per se originate from metaflammation? Does chronic inflammation accelerate a pre-existent CKD? Does CKD worsen already existing metaflammation? Is this a vicious cycle—and at what stage of CKD can it be arrested?
The observations regarding oxidative stress, CKD and CVD are not confined in the context of diabetes and metabolic disorders. The role of oxidative stress–related CVD is also evident in other types of CKD. For example, a recent review by Andries et al. [102] clearly demonstrated that excessive oxidative stress is closely associated with endothelial dysfunction and hypertension, even in the early stages of autosomal dominant polycystic kidney disease. Endothelial nitric oxide synthase uncoupling due to increased asymmetric dimethylarginine and mitochondrial dysfunction are postulated mechanisms linking oxidative stress and CVD in autosomal dominant polycystic kidney disease.
Among the drivers of tissue injury in the CKD metainflammatory environment, recent data have focused on the NLRP3 inflammasome, which contributes to western diet and fructose-induced renal inflammation, renal cholesterol accumulation and hyperuricaemia, as evidenced by protection in Nlrp3 knockout mice [103, 104]. Tubular cell lipotoxicity induced by continuous exposure to LDL cholesterol and long-term overnutrition was characterized by intralysosomal lipid accumulation, lysosomal dysfunction, oxidative stress and tubular dysfunction leading to NLRP3 inflammasome activation that inhibited the sirtuin-1/LKB1/AMPK pathway to dampened lipid breakdown, leading to a vicious cycle of further phospholipid accumulation, oxidative stress and mitochondrial damage [105]. In this regard, in a transcriptomic study of a large cohort (n = 95) of normal and fibrotic human kidney tubules, inflammation and metabolism were the top dysregulated pathways in the diseased kidneys. Specifically, tubulointerstitial fibrosis samples had lower expression of key enzymes and regulators of fatty acid oxidation and higher intracellular lipid deposition. Indeed, fatty acid oxidation is the key energy source in tubular cells and inhibition of fatty acid oxidation in tubule epithelial cells caused ATP depletion, cell death, dedifferentiation and intracellular lipid deposition [106].
The Chronic Renal Insufficiency Cohort (CRIC) study established the link between impaired renal function [lower estimated glomerular filtration rate (eGFR)] or kidney injury (higher albuminuria) and levels of inflammatory markers, including IL-1β, IL-6, TNF, CRP and fibrinogen [92]. There is evidence for all of them being more than markers of risk by contributing to the pathogenesis of CKD progression and cardiovascular complications [11]. CRP may have deleterious effects on renal blood flow through the NF-κB pathway in endothelial cells leading to endothelial dysfunction and triggering vasoconstriction through recruitment of angiotensin-II and endothelin 1, and decreasing nitric oxide synthesis [107]. Higher CRP and TNF levels were independently associated with accelerated renal function loss [108] as well as with decreased survival in haemodialysis patients [109, 110]. Fibrinogen may increase blood viscosity and decrease microvascular flow as well as being a mitogen for renal fibroblasts [52].
Further evidence for altered lipid metabolism in CKD progression and vascular injury is derived from the pleiotropic actions of statins, which, beyond LDL reduction, can interfer with intracellular prenylation processes, displaying anti-inflammatory and anti-fibrotic properties [111–113]. Thus, in CKD patients, atorvastatin decreased levels of inflammatory marker (CRP, IL-1β and TNF) and improved cardiovascular function independent of its impact on lipid profile in CKD [114].
Epigenetics and CKD
In recent years, evidence has emerged on the role and importance of epigenetic modifiers on both embryonic kidney development and acute kidney injury and CKD [115]. Abnormalities in DNA methylation, histone modifications and miRNA have been described and shown to contribute to CKD progression. Genome-wide studies (GWASs) observed prominent differences in DNA methylation of CpG sites between DN and healthy subjects [116]. Demethylation of genes for methylenetetrahydrofolate reductase and connective tissue growth factor was a predisposing factor for the development of DN [116]. Indeed, the differentially methylated regions were associated with profibrotic genes [116]. Also, hyperglycaemia and oxidant stress increase the expression of the histone methyl transferase ‘SET7’ that facilitates the transcription of profibrotic genes in DN [117]. Furthermore, changes in methylation patterns of mitochondria-associated genes (peptidase, mitochondrial β subunit; translation elongation factor, mitochondrial; AU RNA binding methylglutaconyl-CoA hydratase) are seen in DN, suggesting the connection between mitochondrial dysfunction and DN [117].
Ledo et al. [118] investigated novel genes in the vicinity of CKD-associated single nucleotide polymorphisms (SNPs). They showed a strong correlation between SNPs (FAM47E, PLXDC1, ACSM2A/B, ACSM5 and MAGI2) and candidates for CKD development [118]. In another study, Xu et al. [119] identified three genes (NAT8B, CASP9 and MUC1) that have a causal effect on eGFR [119]. Parsa et al. [120] performed a GWAS in the CRIC study participants. CKD progression defined as change in eGFR over time among 1331 blacks and 1476 whites with CKD status. They identified 12 SNPs among black patients and 6 SNPs among white patients. Among blacks without diabetes, rs653747 in LINC00923 was associated with ESRD. In contrast, rs931891 in LINC00923 associated with an eGFR decrease in white patients without diabetes.
Histone deacetylase inhibitors reduced inflammation, fibrosis and expression of α-smooth muscle actin, collagen I, fibronectin, TGF-β1 and NF-κB [117]. In addition to the well-known acetylation and methylation of histones, a role for histone crotonylation in nephroprotection was recently described [121]. Crotonate is a short-chain fatty acid produced by the gut microbiota [122]. Finally, various miRNAs have been implicated in the pathogenesis of DN, including miRNA-25, miRNA-23b, miRNA-21, miRNA-29a, miRNA-146a and others [123].
Role of mitochondrial dysfunction in CKD
Mitochondria have major regulatory functions in energy, Ca+2 and iron homoeostasis, inflammation, cell death pathways and oxidative stress and mitochondrial dysfunction and have received attention as a potential therapeutic target in kidney disease [124]. In particular, proximal tubular epithelial cells have high energy requirements and oxygen consumption to support molecule secretion and reabsorption. Metabolic syndrome and diet may disrupt this balance. As an example, even transient postprandial hyperglycaemia may increase the glucose load in proximal tubules and increase oxygen and energy requirements to reabsorb filtered glucose, which through coupling to sodium reabsorption also contributes to CHF [125]. Moreover, hyperglycaemia can directly induce mitochondrial oxidative stress, contributing to endothelial and tubular dysfunction, podocyte injury and activation of apoptosis [126, 127]. DM-induced mitochondrial DNA oxidative stress causes endothelial dysfunction and podocyte loss via endothelin 1/endothelin 1 receptor type A signalling [126]. Treatment with the mitochondrial-targeted potent antioxidant mitoTEMPO improved endothelial injury, albuminuria and glomerular sclerosis [126]. Additional mitochondrial protective strategies are undergoing clinical trials in kidney and CVD [124].
Future perspectives
The increasing evidence of an impact of diet-induced chronic inflammatory state on the initiation of chronic diseases has led to the design of clinical trials targeting inflammation in CKD and CVD (Figure 2). These range from open-label investigator-initiated trials of non-expensive drugs to large pharma-sponsored trials. As examples, pentoxifylline has anti-inflammatory actions and in DN it decreased albuminuria, preserved GFR, decreased circulating and urinary TNF and preserved Klotho levels in the circulation and in urine, in line with a Klotho-preserving action in cells exposed to inflammatory mediators of albumin [34, 128]. In another example, low-dose aspirin increased the anti-inflammatory lipid 15-epi-lipoxin A4 in CKD patients and decreased coronary and renal events [129]. In this regard, several anti-inflammatory drugs are undergoing clinical evaluation in DN [130]. On the other side of the spectrum, biological agents targeting specific cytokines have been proposed for nephro and cardiovascular protection [59]. The most striking demonstration of the feasibility and efficacy of this approach was the Canakinumab Anti-inflammatory Thrombosis Outcomes Study in patients with previous myocardial infarction and evidence of systemic inflammation [131]. Targeting IL-1β with canakinumab reduced non-fatal myocardial infarction, non-fatal stroke and cardiovascular death [131]. Additional approaches include the use of low-dose immunosuppressive agents. In experimental animals, mycophenolate mofetil reduced renal inflammation, oxidative stress, endothelial dysfunction and hypertension [132, 133]. Although mycophenolate mofetil is used in clinical practice to treat autoimmune diseases, including primary glomerulonephritis, vasculitis and lupus nephritis, as well as kidney transplant recipients, its potential use as a non-specific anti-inflammatory agent in non-immune CKD has not yet been tested. Another non-specific immunosuppressant, low-dose methotrexate, has been tested for cardiovascular protection. However, among patients with stable atherosclerosis, low-dose methotrexate did not reduce IL-1β, IL-6 or CRP or cardiovascular events [134]. Antioxidant treatments to reduce mitochondrial oxidative stress are another potential treatment option [124]. Lastly, epigenetic modulation is coming of age. Modulators of DNA methylation and post-translational histone modifications are already in clinical use, mainly in the oncology context [32]. Additionally, inhibitors of bromodomain and extraterminal proteins, which are readers of epigenetic clues, have been successfully used in experimental kidney injury and early clinical trials for CVD and CKD have been promising [135]. RNA interference approaches, either small-interfering RNAs or miRNA inhibitors (anti-miRs) or mimics have been tested clinically [136]. Patisiran is an RNA inhibitor that improved multiple clinical manifestations of hereditary transthyretin amyloidosis, a disease characterized by neurological cardiac and kidney manifestations, and was approved by regulatory authorities in the summer of 2018 [137]. While this is a very specific condition, these studies validate the feasibility of the approach. In any case, renal safety should be monitored in routine clinical practice, given the kidney safety signals from miravirsen, an anti-miR-122 for hepatitis C virus infection [138]. Finally, dietary modification or the use of prebiotic or probiotic approaches is another possibility [139].
FIGURE 2.
The interaction of the macroenvironment (in this case, western diet and lifestyle) and genetic predisposition determines the magnitude and type of metabolic alterations, which in turn drives a change in the microenvironment through epigenetic modifications, oxidative stress, mitochondrial and lysosomal dysfunction and an inflammatory response, leading to progressive tissue injury. Several positive feedback loops form vicious circles that increase the severity of tissue injury and dysfunction, leading to cardiac and kidney disease progression. All contributing factors and pathways may be subject to therapeutic manipulation.
CONCLUSION
Chronic inflammation induced by excessive nutrient intake and metabolic syndrome has reached epidemic proportions in the 21st century and is contributing to the increased prevalence and mortality from CVD and CKD.
Metainflammation is defined as a chronic low-grade inflammatory state induced by alterations in metabolism and is linked to CKD and CHF. Recent advances have identified key mediators and pathways that link altered metabolism and chronic inflammation with target organ injury. These include mitochondrial dysfunction, oxidative stress, genetic and epigenetic changes and altered gut microbiota. These recent findings have led to the design of novel therapeutic strategies, some of which have proved successful in Phase 3 clinical trials. A more detailed understanding of the cellular and molecular pathways involved will increase the array of therapeutic tools that may stem the current negative trend in mortality from CKD and CHF.
ACKNOWLEDGEMENTS
We would like to thank Prof. Giuseppe Remuzzi and Prof. Daniela Macconi for editing the manuscript. M.K. gratefully acknowledges use of the services and facilities of the Koç University Research Center for Translational Medicine, funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget.
FUNDING
Research by A.O. was funded by FIS ISCIII FEDER funds PI16/02057, ISCIII-RETIC REDinREN RD16/0009, EUTOX, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM, Fundación Renal Iñigo Alvarez de Toledo M.K. gratefully acknowledges use of the services and facilities of the Koç University Research Center for Translational Medicine, funded by the Presidency of Turkey, Presidency of Strategy and Budget.
AUTHORS’ CONTRIBUTIONS
M.K., A.Y., B.A. and A.S. are involved in writing the manuscript. P.S., A.W., A.O. and M.K contributed to preparation and editing of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Savarese G, Lund LH.. Global public health burden of heart failure. Cardiac Fail Rev 2017; 3: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang V, Vilme H, Maciejewski ML. et al. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol. 2016; 36: 319–330 [DOI] [PubMed] [Google Scholar]
- 3. Luyckx VA, Tonelli M, Stanifer JW.. The global burden of kidney disease and the sustainable development goals. Bull World Health Org 2018; 96: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoccali C, Vanholder R, Massy ZA. et al. The systemic nature of CKD. Nat Rev Nephrol 2017; 13: 344–358 [DOI] [PubMed] [Google Scholar]
- 5. Vanholder R, Fouque D, Glorieux G. et al. Clinical management of the uraemic syndrome in chronic kidney disease. Lancet Diab Endocrinol 2016; 4: 360–373 [DOI] [PubMed] [Google Scholar]
- 6. Sag AA, Covic A, London G. et al. Clinical imaging of vascular disease in chronic kidney disease. Int Urol Nephrol 2016; 48: 827–837 [DOI] [PubMed] [Google Scholar]
- 7. Menon V, Gul A, Sarnak MJ.. Cardiovascular risk factors in chronic kidney disease. Kidney Int 2005; 68: 1413–1418 [DOI] [PubMed] [Google Scholar]
- 8. Glassock RJ, Warnock DG, Delanaye P.. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol 2017; 13: 104. [DOI] [PubMed] [Google Scholar]
- 9. Ortiz A, Sanchez-Nino MD, Crespo-Barrio M. et al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2018; 39: 29–34. [DOI] [PubMed] [Google Scholar]
- 10. Afsar B, Rossignol P, van Heerebeek L. et al. Heart failure with preserved ejection fraction: a nephrologist-directed primer. Heart Fail Rev 2017; 22: 765–773 [DOI] [PubMed] [Google Scholar]
- 11. Afsar B, Turkmen K, Covic A. et al. An update on coronary artery disease and chronic kidney disease. Int J Nephrol 2014; 2014: 767424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yilmaz MI, Solak Y, Covic A. et al. Renal anemia of inflammation: the name is self-explanatory. Blood Purif 2011; 32: 220–225 [DOI] [PubMed] [Google Scholar]
- 13. Kanbay M, Ikizek M, Solak Y. et al. Uric acid and pentraxin-3 levels are independently associated with coronary artery disease risk in patients with stage 2 and 3 kidney disease. Am J Nephrol 2011; 33: 325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanbay M, Yilmaz MI, Sonmez A. et al. Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol 2011; 33: 298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yilmaz MI, Solak Y, Saglam M. et al. The relationship between IL-10 levels and cardiovascular events in patients with CKD. Clin J Am Soc Nephrol 2014; 9: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colombo PC, Ganda A, Lin J. et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev 2012; 17: 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017; 542: 177–185 [DOI] [PubMed] [Google Scholar]
- 18. Forsythe LK, Wallace JM, Livingstone MB.. Obesity and inflammation: the effects of weight loss. Nutr Res Rev 2008; 21: 117–133 [DOI] [PubMed] [Google Scholar]
- 19. Pekala P, Kawakami M, Vine W. et al. Studies of insulin resistance in adipocytes induced by macrophage mediator. J Exp Med 1983; 157: 1360–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan R, Toubal A, Goñi S. et al. Loss of the co-repressor GPS2 sensitizes macrophage activation upon metabolic stress induced by obesity and type 2 diabetes. Nat Med 2016; 22: 780–791 [DOI] [PubMed] [Google Scholar]
- 21. Nishimura S, Manabe I, Takaki S. et al. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metabol 2013; 18: 759–766 [DOI] [PubMed] [Google Scholar]
- 22. Ilan Y, Maron R, Tukpah AM. et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA 2010; 107: 9765–9770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bettigole SE, Glimcher LH.. Endoplasmic reticulum stress in immunity. Annu Rev Immunol 2015; 33: 107–138 [DOI] [PubMed] [Google Scholar]
- 24. Fu S, Watkins Steven M, Hotamisligil Gökhan S.. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metabol 2012; 15: 623–634 [DOI] [PubMed] [Google Scholar]
- 25. Ozcan U, Cao Q, Yilmaz E. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461 [DOI] [PubMed] [Google Scholar]
- 26. Ozcan U, Yilmaz E, Ozcan L. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006; 313: 1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Latz E, Xiao TS, Stutz A.. Activation and regulation of the inflammasomes. Nat Rev Immunol 2013; 13: 397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stienstra R, Joosten LA, Koenen T. et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 2010; 12: 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andersson U, Erlandsson-Harris H, Yang H. et al. HMGB1 as a DNA-binding cytokine. J Leukoc Biol 2002; 72: 1084–1091 [PubMed] [Google Scholar]
- 30. Montes VN, Subramanian S, Goodspeed L. et al. Anti-HMGB1 antibody reduces weight gain in mice fed a high-fat diet. Nutr Diabetes 2015; 5: e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruchfeld A, Qureshi AR, Lindholm B. et al. High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD). Mol Med 2008; 14: 109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fontecha-Barriuso M, Martin-Sanchez D, Ruiz-Andres O. et al. Targeting epigenetic DNA and histone modifications to treat kidney disease. Nephrol Dial Transplant 2018; 33: 1875–1886 [DOI] [PubMed] [Google Scholar]
- 33. Moreno JA, Izquierdo MC, Sanchez-Nino MD. et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 2011; 22: 1315–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandez-Fernandez B, Izquierdo MC, Valino-Rivas L. et al. Albumin downregulates Klotho in tubular cells. Nephrol Dial Transplant 2018; 33: 1712–1722 [DOI] [PubMed] [Google Scholar]
- 35. Izquierdo MC, Perez-Gomez MV, Sanchez-Nino MD. et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant 2012; 27(Suppl 4): iv6–10 [DOI] [PubMed] [Google Scholar]
- 36. Liao HK, Hatanaka F, Araoka T. et al. In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell 2017; 171: 1495–1507. e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang S, Song P, Zou MH.. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci 2012; 122: 555–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katerelos M, Mudge SJ, Stapleton D. et al. 5-aminoimidazole-4-carboxamide ribonucleoside and AMP-activated protein kinase inhibit signalling through NF-κB. Immunol Cell Biol 2010; 88: 754–760 [DOI] [PubMed] [Google Scholar]
- 39. Wang C, Li L, Zhang ZG. et al. Globular adiponectin inhibits angiotensin II-induced nuclear factor κB activation through AMP-activated protein kinase in cardiac hypertrophy. J Cell Physiol 2010; 222: 149–155 [DOI] [PubMed] [Google Scholar]
- 40. Zhou G, Myers R, Li Y. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zou MH, Kirkpatrick SS, Davis BJ. et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 2004; 279: 43940–43951 [DOI] [PubMed] [Google Scholar]
- 42. Sun W, Lee TS, Zhu M. et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation 2006; 114: 2655–2662 [DOI] [PubMed] [Google Scholar]
- 43. Voroneanu L, Siriopol D, Apetrii M. et al. Prospective validation of a screening biomarker approach combining amino-terminal pro-brain natriuretic peptide with galectin-3 predicts death and cardiovascular events in asymptomatic hemodialysis patients. Angiology 2018; 69: 449–455 [DOI] [PubMed] [Google Scholar]
- 44. Hogas S, Bilha SC, Branisteanu D. et al. Potential novel biomarkers of cardiovascular dysfunction and disease: cardiotrophin-1, adipokines and galectin-3. Arch Med Sci 2017; 13: 897–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hogas S, Schiller A, Voroneanu L. et al. Predictive value for galectin 3 and cardiotrophin 1 in hemodialysis patients. Angiology 2016; 67: 854–859 [DOI] [PubMed] [Google Scholar]
- 46. Van Linthout S, Tschöpe C.. Inflammation – cause or consequence of heart failure or both? Curr Heart Fail Rep 2017; 14: 251–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shirazi LF, Bissett J, Romeo F. et al. Role of inflammation in heart failure. Curr Atheroscler Rep 2017; 19: 27. [DOI] [PubMed] [Google Scholar]
- 48. Finkel M, Oddis C, Jacob T. et al. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 1992; 257: 387–389 [DOI] [PubMed] [Google Scholar]
- 49. Hu MC, Shi M, Gillings N. et al. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 2017; 91: 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sanz AB, Izquierdo MC, Sanchez-Nino MD. et al. TWEAK and the progression of renal disease: clinical translation. Nephrol Dial Transplant 2014; 29(Suppl 1): i54–i62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poveda J, Tabara LC, Fernandez-Fernandez B. et al. TWEAK/Fn14 and non-canonical NF-κB signaling in kidney disease. Front Immunol 2013; 4: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jain M, Jakubowski A, Cui L. et al. A novel role for tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in the development of cardiac dysfunction and failure. Circulation 2009; 119: 2058–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ruiz-Andres O, Suarez-Alvarez B, Sanchez-Ramos C. et al. The inflammatory cytokine TWEAK decreases PGC-1α expression and mitochondrial function in acute kidney injury. Kidney Int 2016; 89: 399–410 [DOI] [PubMed] [Google Scholar]
- 54. Henaut L, Sanz AB, Martin-Sanchez D. et al. TWEAK favors phosphate-induced calcification of vascular smooth muscle cells through canonical and non-canonical activation of NFκB. Cell Death Dis 2016; 7: e2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Azak A, Akdoğan MF, Denizli N. et al. Soluble TWEAK levels are independently associated with coronary artery disease severity in patients with stage 2–3 kidney disease. Int Urol Nephrol 2014; 46: 411–415 [DOI] [PubMed] [Google Scholar]
- 56. Carrero JJ, Ortiz A, Qureshi AR. et al. Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin J Am Soc Nephrol 2009; 4: 110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hernandez EA, Kahl S, Seelig A. et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J Clin Invest 2017; 127: 695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bennett G, Strissel KJ, DeFuria J. et al. Deletion of TNF-like weak inducer of apoptosis (TWEAK) protects mice from adipose and systemic impacts of severe obesity. Obesity (Silver Spring) 2014; 22: 1485–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Afsar B, Covic A, Ortiz A. et al. The future of IL-1 targeting in kidney disease. Drugs 2018; 78: 1073–1083 [DOI] [PubMed] [Google Scholar]
- 60. Hilfiker-Kleiner D, Shukla P, Klein G. et al. Continuous glycoprotein-130-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation 2010; 122: 145–155 [DOI] [PubMed] [Google Scholar]
- 61. Elewa U, Sanchez-Nino MD, Martin-Cleary C. et al. Cardiovascular risk biomarkers in CKD: the inflammation link and the road less traveled. Int Urol Nephrol 2012; 44: 1731–1744 [DOI] [PubMed] [Google Scholar]
- 62. Araújo JP, Lourenço P, Azevedo A. et al. Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Cardiac Fail 2009; 15: 256–266 [DOI] [PubMed] [Google Scholar]
- 63. Kanbay M, Afsar B, Gusbeth-Tatomir P. et al. Arterial stiffness in dialysis patients: where are we now? Int Urol Nephrol 2010; 42: 741–752 [DOI] [PubMed] [Google Scholar]
- 64. Kanbay M, Afsar B, Siriopol D. et al. Endostatin in chronic kidney disease: associations with inflammation, vascular abnormalities, cardiovascular events and survival. Eur J Intern Med 2016; 33: 81–87 [DOI] [PubMed] [Google Scholar]
- 65. Yilmaz MI, Siriopol D, Saglam M. et al. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int 2014; 86: 1213–1220 [DOI] [PubMed] [Google Scholar]
- 66. Afsar B, Ortiz A, Covic A. et al. Focus on renal congestion in heart failure. Clin Kidney J 2016; 9: 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Castillo-Rodriguez E, Pizarro-Sanchez S, Sanz AB. et al. Inflammatory cytokines as uremic toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins (Basel) 2017; 9: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kanbay M, Onal EM, Afsar B. et al. The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol 2018; 50: 1453–1466 [DOI] [PubMed] [Google Scholar]
- 69. Castillo-Rodriguez E, Fernandez-Prado R, Esteras R. et al. Impact of altered intestinal microbiota on chronic kidney disease progression. Toxins (Basel) 2018; 10: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vaziri ND, Wong J, Pahl M. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013; 83: 308–315 [DOI] [PubMed] [Google Scholar]
- 71. Meijers B, Jouret F, Evenepoel P.. Linking gut microbiota to cardiovascular disease and hypertension: lessons from chronic kidney disease. Pharmacol Res 2018; 133: 101–107 [DOI] [PubMed] [Google Scholar]
- 72. Van Gaal LF, Mertens IL, De Block CE.. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444: 875–880 [DOI] [PubMed] [Google Scholar]
- 73. Dincer N, Dagel T, Afsar B. et al. The effect of chronic kidney disease on lipid metabolism. Int Urol Nephrol 2019; 51: 265–277 [DOI] [PubMed] [Google Scholar]
- 74. Bulbul MC, Dagel T, Afsar B. et al. Disorders of lipid metabolism in chronic kidney disease. Blood Purif 2018; 46: 144–152 [DOI] [PubMed] [Google Scholar]
- 75. Ayoub KF, Pothineni NVK, Rutland J. et al. Immunity, inflammation, and oxidative stress in heart failure: emerging molecular targets. Cardiovasc Drugs Ther 2017; 31: 593–608 [DOI] [PubMed] [Google Scholar]
- 76. Ferrannini E, Mark M, Mayoux E.. CV protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care 2016; 39: 1108–1114 [DOI] [PubMed] [Google Scholar]
- 77. Meder B, Haas J, Sedaghat-Hamedani F. et al. Epigenome-wide association study identifies cardiac gene patterning and a novel class of biomarkers for heart failure. Circulation 2017; 136: 1528–1544 [DOI] [PubMed] [Google Scholar]
- 78. Movassagh M, Choy MK, Knowles David A. et al. Distinct epigenomic features in end-stage failing human hearts. Circulation 2011; 124: 2411–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang J, Xu WW, Hu SJ.. Heart failure: advanced development in genetics and epigenetics. BioMed Res Int 2015; 2015: 352734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pillai VB, Sundaresan NR, Kim G. et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 2010; 285: 3133–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gupta MP, Samant SA, Smith SH. et al. HDAC4 and PCAF bind to cardiac sarcomeres and play a role in regulating myofilament contractile activity. J Biol Chem 2008; 283: 10135–10146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kaneda R, Takada S, Yamashita Y. et al. Genome-wide histone methylation profile for heart failure. Genes Cells 2009; 14: 69–77 [DOI] [PubMed] [Google Scholar]
- 83. Wang GK, Zhu JQ, Zhang JT. et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 2010; 31: 659–666 [DOI] [PubMed] [Google Scholar]
- 84. Callis TE, Pandya K, Seok HY. et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 2009; 119: 2772–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Montgomery RL, Hullinger TG, Semus HM. et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 2011; 124: 1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Grueter CE, van Rooij E, Johnson BA. et al. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell 2012; 149: 671–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ikeda S, Kong SW, Lu J. et al. Altered microRNA expression in human heart disease. Physiol Genom 2007; 31: 367–373 [DOI] [PubMed] [Google Scholar]
- 88. Van Rooij E, Sutherland LB, Liu N. et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 2006; 103: 18255–18260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Carrero JJ, Stenvinkel P.. Inflammation in end-stage renal disease–what have we learned in 10 years? Semin Dial 2010; 23: 498–509 [DOI] [PubMed] [Google Scholar]
- 90. Yerlikaya A, Bulbul MC, Afsar B. et al. Iron in kidney and heart failure: from theory to practice. Int Urol Nephrol 2018; 50: 481–493 [DOI] [PubMed] [Google Scholar]
- 91. Kanbay M, Perazella MA, Kasapoglu B. et al. Erythropoiesis stimulatory agent-resistant anemia in dialysis patients: review of causes and management. Blood Purif 2010; 29: 1–12 [DOI] [PubMed] [Google Scholar]
- 92. Gupta J, Mitra N, Kanetsky PA. et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 2012; 7: 1938–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Daenen K, Andries A, Mekahli D. et al. Oxidative stress in chronic kidney disease. Pediatr Nephrol 2019; 34: 975–991 [DOI] [PubMed] [Google Scholar]
- 94. Nistala R, Whaley-Connell A, Sowers JR.. Redox control of renal function and hypertension. Antioxid Redox Signal 2008; 10: 2047–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Himmelfarb J, Stenvinkel P, Ikizler TA. et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002; 62: 1524–1538 [DOI] [PubMed] [Google Scholar]
- 96. Popolo A, Autore G, Pinto A. et al. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic Res 2013; 47: 346–356 [DOI] [PubMed] [Google Scholar]
- 97. Galvan DL, Green NH, Danesh FR.. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int 2017; 92: 1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tang Y, Yang Q, Lu J. et al. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem 2010; 21: 237–246 [DOI] [PubMed] [Google Scholar]
- 99. Parham M, Amini M, Aminorroaya A. et al. Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial. Rev Diabet Stud 2008; 5: 102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Singh AK, Kari JA.. Metabolic syndrome and chronic kidney disease. Curr Opin Nephrol Hypertens 2013; 22: 198–203 [DOI] [PubMed] [Google Scholar]
- 101. de Vries AP, Ruggenenti P, Ruan XZ. et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol 2014; 2: 417–426 [DOI] [PubMed] [Google Scholar]
- 102. Andries A, Daenen K, Jouret F. et al. Oxidative stress in autosomal dominant polycystic kidney disease: player and/or early predictor for disease progression? Pediatr Nephrol 2019; 34: 993–1008 [DOI] [PubMed] [Google Scholar]
- 103. Yerlikaya A, Dagel T, King C. et al. Dietary and commercialized fructose: sweet or sour? Int Urol Nephrol 2017; 49: 1611–1620 [DOI] [PubMed] [Google Scholar]
- 104. Bakker PJ, Butter LM, Kors L. et al. Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int 2014; 85: 1112–1122 [DOI] [PubMed] [Google Scholar]
- 105. Rampanelli E, Orsó E, Ochodnicky P. et al. Metabolic injury-induced NLRP3 inflammasome activation dampens phospholipid degradation. Sci Rep 2017; 7: 2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kang HM, Ahn SH, Choi P. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 2015; 21: 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol 2009; 24: 1445–1452 [DOI] [PubMed] [Google Scholar]
- 108. Tonelli M, Sacks F, Pfeffer M. et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 2005; 68: 237–245 [DOI] [PubMed] [Google Scholar]
- 109. Cohen SD, Phillips TM, Khetpal P. et al. Cytokine patterns and survival in haemodialysis patients. Nephrol Dial Transplant 2010; 25: 1239–1243 [DOI] [PubMed] [Google Scholar]
- 110. Akchurin OM, Kaskel F.. Update on inflammation in chronic kidney disease. Blood Purif 2015; 39: 84–92 [DOI] [PubMed] [Google Scholar]
- 111. Kanbay M, Yildirir A, Bozbas H. et al. Statin therapy helps to control blood pressure levels in hypertensive dyslipidemic patients. Ren Fail 2005; 27: 297–303 [PubMed] [Google Scholar]
- 112. Kanbay M, Turgut F, Covic A. et al. Statin treatment for dyslipidemia in chronic kidney disease and renal transplantation: a review of the evidence. J Nephrol 2009; 22: 598–609 [PubMed] [Google Scholar]
- 113. Ferro CJ, Mark PB, Kanbay M. et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 2018; 14: 727–749 [DOI] [PubMed] [Google Scholar]
- 114. Goicoechea M, de Vinuesa SG, Lahera V. et al. Effects of atorvastatin on inflammatory and fibrinolytic parameters in patients with chronic kidney disease. J Am Soc Nephrol 2006; 17(12 Suppl 3): S231–S235 [DOI] [PubMed] [Google Scholar]
- 115. Shiels PG, McGuinness D, Eriksson M. et al. The role of epigenetics in renal ageing. Nat Rev Nephrol 2017; 13: 471–482 [DOI] [PubMed] [Google Scholar]
- 116. Beckerman P, Ko YA, Susztak K.. Epigenetics: a new way to look at kidney diseases. Nephrol Dial Transplant 2014; 29: 1821–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wanner N, Bechtel-Walz W.. Epigenetics of kidney disease. Cell Tissue Res 2017; 369: 75–92 [DOI] [PubMed] [Google Scholar]
- 118. Ledo N, Ko YA, Park AS. et al. Functional genomic annotation of genetic risk loci highlights inflammation and epithelial biology networks in CKD. J Am Soc Nephrol 2015; 26: 692–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Xu X, Eales JM, Akbarov A. et al. Molecular insights into genome-wide association studies of chronic kidney disease-defining traits. Nat Commun 2018; 9: 4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Parsa A, Kanetsky PA, Xiao R. et al. Genome-wide association of CKD progression: the chronic renal insufficiency cohort study. J Am Soc Nephrol 2017; 28: 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ruiz-Andres O, Sanchez-Nino MD, Cannata-Ortiz P. et al. Histone lysine crotonylation during acute kidney injury in mice. Dis Model Mech 2016; 9: 633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ruiz-Andres O, Sanchez-Nino MD, Moreno JA. et al. Downregulation of kidney protective factors by inflammation: role of transcription factors and epigenetic mechanisms. Am J Physiol Renal Physiol 2016; 311: F1329–F1340 [DOI] [PubMed] [Google Scholar]
- 123. Liu Y, Li H, Liu J. et al. Variations in microRNA-25 expression influence the severity of diabetic kidney disease. J Am Soc Nephrol 2017; 28: 3627–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tabara LC, Poveda J, Martin-Cleary C. et al. Mitochondria-targeted therapies for acute kidney injury. Expert Rev Mol Med 2014; 16: e13. [DOI] [PubMed] [Google Scholar]
- 125. Hahn K, Ejaz AA, Kanbay M. et al. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol 2016; 12: 711–712 [DOI] [PubMed] [Google Scholar]
- 126. Qi H, Casalena G, Shi S. et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes 2017; 66: 763–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Galvan DL, Green NH, Danesh FR.. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int 2017; 92: 1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Navarro-Gonzalez JF, Sanchez-Nino MD, Donate-Correa J. et al. Effects of pentoxifylline on soluble klotho concentrations and renal tubular cell expression in diabetic kidney disease. Diabetes Care 2018; 41: 1817–1820 [DOI] [PubMed] [Google Scholar]
- 129. Goicoechea M, de Vinuesa SG, Quiroga B. et al. Aspirin for primary prevention of cardiovascular disease and renal disease progression in chronic kidney disease patients: a multicenter randomized clinical trial (AASER study). Cardiovasc Drugs Ther 2018; 32: 255–263 [DOI] [PubMed] [Google Scholar]
- 130. Perez-Gomez MV, Sanchez-Nino MD, Sanz AB. et al. Targeting inflammation in diabetic kidney disease: early clinical trials. Expert Opin Investig Drugs 2016; 25: 1045–1058 [DOI] [PubMed] [Google Scholar]
- 131. Ridker PM, Everett BM, Thuren T. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131 [DOI] [PubMed] [Google Scholar]
- 132. Bravo Y, Quiroz Y, Ferrebuz A. et al. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol 2007; 293: F616–F23 [DOI] [PubMed] [Google Scholar]
- 133. Herrera J, Ferrebuz A, MacGregor EG. et al. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 2006; 17(12 Suppl 3): S218–S225 [DOI] [PubMed] [Google Scholar]
- 134. Ridker PM, Everett BM, Pradhan A. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2018; 380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Suarez-Alvarez B, Morgado-Pascual JL, Rayego-Mateos S. et al. Inhibition of bromodomain and extraterminal domain family proteins ameliorates experimental renal damage. J Am Soc Nephrol 2017; 28: 504–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Titze-de-Almeida R, David C, Titze-de-Almeida SS.. The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm Res 2017; 34: 1339–1363 [DOI] [PubMed] [Google Scholar]
- 137. Adams D, Gonzalez-Duarte A, O'Riordan WD. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018; 379: 11–21 [DOI] [PubMed] [Google Scholar]
- 138. Sanchez-Nino MD, Ortiz A.. HCV infection and miravirsen. N Engl J Med 2013; 369: 877–878 [DOI] [PubMed] [Google Scholar]
- 139. Castillo-Rodriguez E, Fernandez-Prado R, Esteras R. et al. Impact of altered intestinal microbiota on chronic kidney disease progression. Toxins (Basel) 2018; 10: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]