Abstract
Background
Secondary hyperparathyroidism (SHPT) is frequent in haemodialysis (HD) patients. Oral cinacalcet-hydrochloride (HCl) decreases parathyroid hormone (PTH); however, real-life PTH data, according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, are still lacking. Our goal is to assess the percentage of cinacalcet-HCl-treated HD patients with controlled SHPT (PTH <9× upper limit of the normal range) after 12 months (M12) of treatment.
Methods
This is a retrospective observational study in HD patients with SHPT treated by cinacalcet-HCl between 2005 and 2015 and dialysed in seven French HD centres using the same database (Hemodial™).
Results
The study included 1268 patients with a mean (standard deviation) follow-up of 21 ± 12 months. Their mean dialysis vintage was 4.3 ± 5.6 years. PTH values were available and exploitable at M12 in 50% of them (645 patients). Among these patients, 58.9% had controlled (mean PTH of 304 ± 158 pg/mL) and 41.1% uncontrolled SHPT (mean PTH of 1084 ± 543) at M12. At the baseline, patients with controlled SHPT were older (66 ± 15 versus 61 ± 17 years), and had lower PTH (831 ± 346 versus 1057 ± 480 pg/mL) and calcaemia (2.18 ± 0.2 versus 2.22 ± 0.19 mmol/L) than uncontrolled patients. In multivariate analysis, these three factors still remained significantly associated with controlled SHPT.
Conclusion
In this real-life study, 41.1% of HD patients with SHPT treated with cinacalcet-HCl remained with a PTH above the KDIGO recommended target after 12 months of treatment. Apart from the possibility of non-compliance, the severity of SHPT appears to be a major factor determining the response to cinacalcet-HCl treatment, reinforcing the importance of treating SHPT at earlier stages.
Keywords: cinacalcet, compliance, KDIGO, Mimosa study, secondary hyperparathyroidism
INTRODUCTION
Secondary hyperparathyroidism (SHPT) is a common, serious and progressive complication of chronic kidney disease (CKD) [1]. It is mainly characterized by high serum parathyroid hormone (PTH), parathyroid gland hyperplasia and disturbance in mineral metabolism, mainly hypocalcaemia and hyperphosphataemia [2]. It begins before end-stage renal disease (ESRD) and worsens over time in haemodialysis (HD) patients. SHPT is one of the most important components of CKD mineral and bone disorder (CKD–MBD) syndrome [3]. This syndrome associates three major domains: biochemical abnormalities (calcium, phosphate, PTH, vitamin D, klotho, fibroblast growth factor 23 and sclerostin), cardiovascular calcifications and bone abnormalities. The ancient histomorphometric concept of renal osteodystrophy (ROD) has been redefined by Kidney Disease: Improving Global Outcomes (KDIGO) and includes now skeletal fragility parameters such as bone volume and mineralization [4]. Besides the mineral abnormalities, SHPT leads to several consequences including ROD and progressive vascular calcifications, which are often associated with increased risk of cardiovascular diseases, skeletal fractures and death [5].
Initial therapy for SHPT in HD patients usually includes calcium salts, intestinal phosphate binders and vitamin D derivatives. The oral calcimimetic cinacalcet-hydrochloride (HCl) is often used later in the course of the disease in patients who fail to respond adequately to the previous treatments. Cinacalcet-HCl has been shown to be effective in reducing circulating PTH levels in HD patients with SHPT in several clinical trials [6]. One of these trials, the European Evaluation of the Clinical use of mimpara in Haemodialysis and peritoneal dialysis patients: an Observational (ECHO) study investigating the use and effectiveness of cinacalcet-HCl in real-world clinical practice, confirmed the efficacy of cinacalcet-HCl in reducing PTH [7]. In the French cohort of the ECHO study (485 patients from 44 centres), ∼30% of cinacalcet-HCl-treated patients reached the National Kidney Foundation-Kidney/Dialysis Outcomes Dialysis Initiative (NKF-K/DOQI) [4] recommended PTH target (between 150 and 300 pg/mL) after 12 months of treatment [8].
Some patients appear to be insensitive or to exhibit a hyporesponsiveness to cinacalcet-HCl treatment. Likewise, it has been demonstrated that the number, size and nodular hyperplasia aspect of the parathyroid glands are known to importantly predict the response to cinacalcet-HCl in patients with advanced SHPT [9, 10]. Other reasons for an insufficient response to cinacalcet-HCl could simply be the lack of compliance to treatment and/or deficient therapeutic education provided to these patients. Indeed, reduced persistence, compliance and/or adherence with oral medications are a common problem, especially in CKD patients [11], with non-adherence rates of 3–80% reported [12–14]. In a European retrospective observational study published recently [15], it was observed that 23% of the incident HD patients discontinued their cinacalcet-HCl treatment after 1 year. This is an extremely important issue since the inconclusive results of the EValuation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE) study could hypothetically be due, to some extent, to lack of compliance [16]. Accordingly, the effect of cinacalcet-HCl was more pronounced among older patients (>65 years), in whom there was a higher frequency of cardiovascular events but who were perhaps also more compliant.
Taking into account that (i) KDIGO [5], which recommends maintaining circulating PTH levels in the range of two to nine times the upper limit of the normal range (ULN) of the assay, is less conservative that the previous NKF-K/DOQI [7], (ii) that the ECHO study was performed based on the NKF-K/DOQI recommended target values for PTH and (iii) that there is a lack of French data on this subject, it seemed appropriate to generate real-life data on PTH control in HD patients with SHPT treated with cinacalcet-HCl.
MATERIALS AND METHODS
Study design, data source, population and objectives
The Mimosa study is an observational multicentre study based on retrospective medical data analysis of a group of HD patients treated with cinacalcet-HCl between January 2005 and December 2015. Medical, biological and therapeutical data were properly registered on the same shared database (Hemodial™ System) for all these patients.
Seven HD centres using this database throughout France accepted to participate and provide data to be extracted and analysed (AURA Paris; Diaverum Paris; Clinique du Landy St Ouen; Polyclinique du Languedoc Narbonne; Hôpital privé d’Athis-Mons; Centre hospitalier Roubaix; Hôpital privé de Thiais). Patients’ inclusion criteria were (i) age >18 years, (ii) ESRD chronically treated by HD and (iii) SHPT treated with cinacalcet-HCl, which had been initiated between January 2005 and December 2015.
The planned follow-up for the whole population was 3 years. The main goal of the Mimosa study was to assess the percentage of cinacalcet-HCl-treated HD patients with controlled SHPT according to the KDIGO guidelines, that is, serum PTH <9× the ULN after a 12 month follow-up in real-life conditions; secondary objectives were (i) to determine the PTH course when treated with cinacalcet-HCl during 36-month (M36) follow-up, (ii) to identify the factors associated with uncontrolled SHPT after 12 months of treatment with cinacalcet-HCl and (iii) to evaluate the percentage of patients treated with cinacalcet-HCl with a decrease of >30% and >50% in serum PTH levels versus baseline after 12 months of therapy.
Data collected
No specific assessment was requested for the study as all data were collected from the Hemodial™ database according to particular specifications, including quality control.
No administrative authorizations or ethical committees (CNIL (Commission Nationale de l'Informatique et des Libertés), CCTIRS (Comité Consultatif sur le traitement de l'information en matière de recherche dans le domaine de la santé)) were necessary because of the anonymous data. It was impossible to return to the patient file in case of missing data because of the anonymous data. All volunteer centres agreed with this study.
Baseline data (Month 0, M0) were defined as the date of first prescription of cinacalcet-HCl. For all biological data, the value considered for M0 was the closest before cinacalcet-HCl treatment initiation. Biological values corresponding to Month 3 (M3)–Month 36 (M36) were considered with a window of ±1 month except for 12 months (M12) (±2 months). The ‘End of study’ (EOS) time was defined as last data registered M36 after first prescription of cinacalcet-HCl (M36, ±2 months) or the last data collected until the date of death, renal transplantation, lost to follow-up or change in HD centre if this date was <36 months after baseline date.
All centres had the same biological method of serum PTH measurement; therefore, the threshold defining controlled SHPT base on KDIGO guidelines was serum PTH between 130 (2×ULN) and 600 (9×ULN) pg/mL.
Statistical analysis
Data management and all statistical analyses were performed using SAS® V9.3 software (NC, USA) and Stata. Statistical analysis was performed in the groups of patients for which data were available. The analyses were first descriptive and univariate, then comparative according to some specific groups of patients. Multivariate analyses were also conducted to describe the factors associated with PTH control or decrease after 12 months of cinacalcet-HCl treatment. Continuous variables were described by mean, standard deviation (SD), minimum and maximum, median and interquartile range (IQR); qualitative variables by frequencies. Continuous variables were compared between groups by Student t-tests in case of normal distribution and Wilcoxon–Mann–Whitney test otherwise. Qualitative variables were compared between groups using the Pearson Chi-squared test if all theoretical sample sizes were ≥5 or using the Fisher’s test if <5. Percentage calculation did not include missing data. A logistic regression model was also performed to identify factors that independently correlated with uncontrolled SHPT after a 1-year treatment with cinacalcet. Significant factors in univariate analyses (P < 0.10) were included in the model by a stepwise procedure.
RESULTS
Flow chart
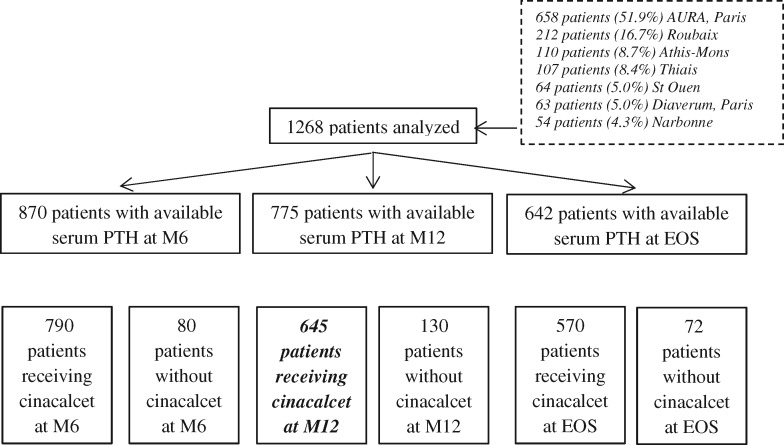
A total of 2151 patients were identified in the Hemodial™ database, but 883 were excluded from the analysis because they did not meet the analysis criteria, that is, one serum PTH value at baseline and at least another PTH value during the follow-up available in the database (n = 793) or baseline serum PTH <300 pg/mL (n = 90). Thus, data from 1268 patients were retained for analysis with a mean 21.2 ± 12.6 month follow-up for this whole population. The pattern of patients is detailed in Figure 1: overall, we identified 775 patients with available serum PTH at the beginning and at M12, of whom 645 patients who were still treated with cinacalcet-HCl after 12 months and for whom PTH values were available at that time in the database; 130 patients had suspended the drug (75 patients for side effects, 28 patients for interaction with a new prescribed drug and 27 patients for hypocalcaemia) (Figure 1). This constitutes the analysed population for the primary objective of the study.
FIGURE 1.
Flow chart of patient enrollment and exclusion. Among the 1268 enrolled patients, 378 patients were excluded for the analysis at M6 because of lack of PTH value; moreover, 80 patients in this group were no longer taking cinacalcet-HCl at M6. At M12, another additional group of 95 was excluded for the same reason, and among them 130 patients were not taking cinacalcet-HCl. Overall, we identified 652 patients who could be analysed at the end of the 36 month period of the study. EOS, end of study.
Population characteristics
The main characteristics of the 1268 patients analysed are presented and compared with the characteristics of the 645 patients included in the primary analysis in Table 1. No clinically significant differences are observed between these two populations except for the proportion of males, which is slightly higher in the latter. Their mean age was 64 years. The nephropathy was recorded as being related to diabetes mellitus for 24% of the patients, glomerulonephritis for 26%, nephroangiosclerosis for 19% and unknown for 11% of them. At the time of cinacalcet-HCl treatment initiation, most of the patients were dialysed for >4 years and had severe SHPT with a mean serum PTH of 915 pg/mL, with a majority of the patients having a serum PTH > 600 pg/mL.
Table 1.
Population characteristics
| Parameters | Overall population, N=1268 | Population considered for analysis at M12, N=645 |
|---|---|---|
| Demographic data | ||
| Male, n (%) | 721 (56.9) | 389 (60.3) |
| Age, mean ± SD (years) | 63.8 ± 15.9 | 63.5 ± 15.6 |
| <40 years (%) | 8.6 | 9.1 |
| 40–55 (%) | 20 | 19.5 |
| 55–70 (%) | 30.9 | 33.2 |
| 70–85 (%) | 32.6 | 31.5 |
| ≥85 years (%) | 7.9 | 6.7 |
| BMI, mean ± SD (kg/m²) | 25.3 ± 5.5 | 25.5 ± 5.4 |
| Obese, n (%) | 193 (16.5) | 103 (16.9) |
| Disease history, n (%) | ||
| Diabetic nephropathy | 306 (24.2) | 164 (25.4) |
| Nephroangiosclerosis | 246 (19.4) | 138 (21.4) |
| Glomerulonephritis | 325 (25.7) | 150 (23.3) |
| Interstitial nephritis | 161 (12.7) | 82 (12.7) |
| Polycystic kidney disease | 90 (7.1) | 36 (5.6) |
| Other or unknown | 139 (11.0) | 75 (11.6) |
| Duration of dialysis,a mean ± SD (years) | 4.3 ± 5.6 | 4.4 ± 5.7 |
| HD/peritoneal dialysis (%) | 97.5/2.5 | 97.5/2.5 |
| Biological data at M0 | ||
| Serum PTH, mean ± SD (pg/mL) | 915 ± 469 | 924 ± 421 |
| Serum PTH, median (IQR) (pg/mL) | 805 (621–1065) | 824 (649–1090) |
| Serum PTH, pg/mL (%) | ||
| <600 | 287 (22.6) | 119 (18.4) |
| 600–1000 | 597 (47.1) | 321 (49.8) |
| ≥1000 | 384 (30.3) | 205 (31.8) |
| Serum calcium, mean ± SD (mmol/L) | 2.19 ± 0.21 | 2.20 ± 0.19 |
| Serum phosphate, mean ± SD (mmol/L) | 1.64 ± 0.56 | 1.68 ± 0.57 |
Percentages are based on the total number of patients in each column for whom the answer is available. aAt the time of cinacalcet treatment start.
PTH control after 12 months of treatment
Among the 645 patients still treated with cinacalcet-HCl after 12 months and with serum PTH dosages available, 380 (58.9%) had serum PTH levels controlled according to KDIGO guidelines (i.e. <9× ULN) at M12 and 265 (41.1%) patients uncontrolled SHPT (serum PTH ≥9× ULN). Based on PTH decrease between baseline and M12, these numbers were, respectively, 369 (57.2%) and 276 (42.8%) patients for the decrease or not of >30%, and 265 (41.1%) and 380 (58.9%) patients for the decrease or not of >50%.
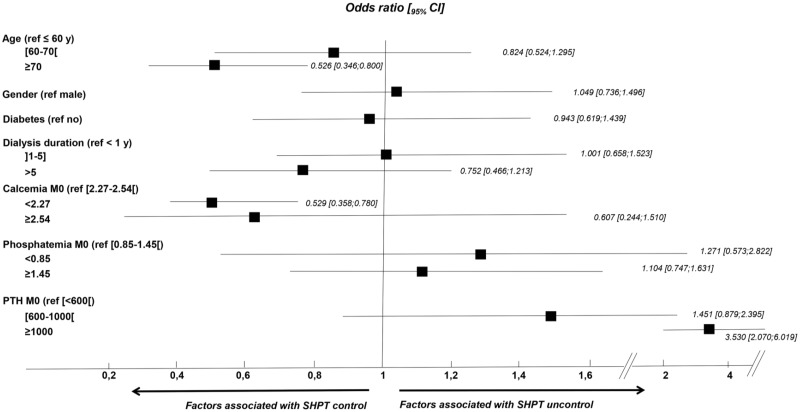
The main characteristics of these two groups of patients are presented in Table 2. In univariate analysis, those with controlled SHPT based on KDIGO guidelines were older, had a higher body mass index (BMI), a less severe SHPT at the time of cinacalcet-HCl treatment initiation and a lower serum calcium and phosphate levels at baseline than those who remained uncontrolled after 12 months of treatment. In multivariate analysis, the factors that remained associated with uncontrolled SHPT (based on KDIGO guidelines) after 12 months of treatment were younger age and baseline higher serum calcium and PTH levels (Figure 2). The same analysis was conducted to assess associated factors with SHPT control based on PTH decrease of >30% or 50% at M12 versus baseline with the same results (data not shown).
Table 2.
Population characteristics according to SHPT control (based on KDIGO guidelines) at M12
| Parameters | Uncontrolled treated patients at M12 (PTH ≥9×ULN) N=265 | Controlled treated patients at M12 (PTH <9×ULN) N=380 | P-value |
|---|---|---|---|
| Male, n (%) | 158 (59.6) | 231 (60.8) | 0.7657 |
| Age, mean ± SD (years) (%) | 60.6 ± 16.5 | 65.5 ± 14.7 | <0.0001 |
| <40 | 12.5 | 6.8 | |
| 40–55 | 25.3 | 15.5 | |
| 55–70 | 31.3 | 34.5 | |
| 70–85 | 25.3 | 35.8 | |
| ≥85 | 5.7 | 7.4 | |
| BMI, mean ± SD (kg/m²) | 24.9 ± 5.5 | 25.9 ± 5.3 | 0.0191 |
| Obese, n (%) | 34 (13.3) | 69 (19.5) | |
| Diabetes mellitus, n (%) | 64 (24.2) | 100 (26.3) | 0.5345 |
| Dialysis duration, mean ± SD (years) (%) | 4.1 ± 5.2 | 4.5 ± 6.1 | 0.9517 |
| 0–1 | 31.3 | 29.7 | |
| 1–5 | 44.2 | 42.6 | |
| >5 | 24.5 | 27.6 | |
| Biological data at baseline | |||
| Serum PTH, mean ± SD (pg/mL) | 1057 ± 480 | 831 ± 346 | <0.0001 |
| Serum PTH, median (IQR) (pg/mL) | 965 (718–1261) | 759 (611–975) | |
| Serum PTH, pg/mL (%) | |||
| <600 | 12.1 | 22.9 | |
| 600–1000 | 41.9 | 55.3 | |
| ≥1000 | 46.0 | 21.8 | |
| Serum calcium, mean ± SD (mmol/L) | 2.22 ± 0.19 | 2.18 ± 0.20 | 0.0213 |
| Serum phosphate, mean ± SD (mmol/L) | 1.73 ± 0.57 | 1.63 ± 0.56 | 0.0351 |
| Biological data at M12 | |||
| Serum PTH, mean ± SD (pg/mL) | 1084 ± 543 | 304 ± 158 | <0.0001 |
| Serum PTH, median (IQR) (pg/mL) | 911 (725–1203) | 288 (178–436) | |
| Serum calcium, mean ± SD (pg/mL) | 2.19 ± 0.20 | 2.19 ± 0.23 | 0.9430 |
| Serum phosphate, mean ± SD (mmol/L) | 1.55 ± 0.63 | 1.40 ± 0.56 | 0.0013 |
Percentages are based on the total number of patients in each column for whom the answer is available.
FIGURE 2.
Odds ratios of factors associated with the response to cinacalcet-HCl. Multivariate analysis showing the odds ratios [95% confidence interval (CI)] of the factors that remained associated with uncontrolled SHPT after 12 months of treatment by cinacalcet-HCl were younger age and higher baseline serum calcium and PTH levels.
Most of the patients were initiated with cinacalcet-HCl 30 mg/day and the mean dosage of cinacalcet-HCl increased afterwards to reach a mean dosage of 53.9 ± 29.3 mg/day in the 645 patients still treated at M12. The mean dosage of cinacalcet-HCl at M12 was higher in patients with uncontrolled SHPT (i.e. PTH ≥9×ULN) than in patients with controlled SHPT [respectively, 59.5 ± 32.4 versus 49.9 ± 26.3 mg per day (P < 0.0001)]. Concomitant therapies included for 35% of the patients calcium supplementation at a mean dosage of 2.4 ± 1.9 g/day, 52% received phosphate binders at a mean dosage of 4.6 ± 3.7 g/day (the most common being sevelamer), 45% received active vitamin D analogs at a mean dosage of 4.6 ± 3.7 µg/week and at least 42% received natural vitamin D at a mean dosage of 27 160 ± 808 UI/week.
PTH course during the overall follow-up
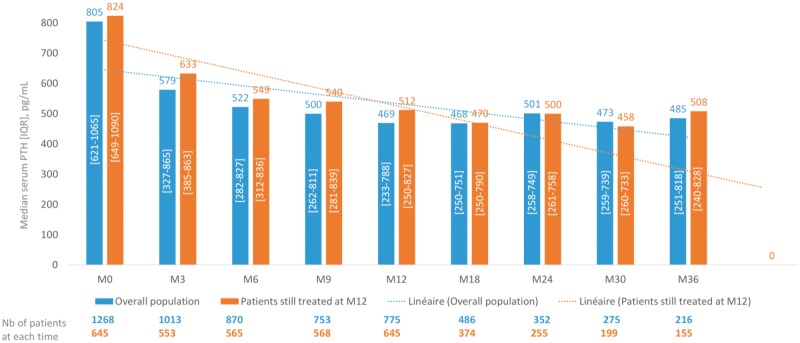
Figure 3 shows the PTH course during the 3-year follow-up of the 1268 patients included in the analysis: overall, severe SHPT was observed at the time of treatment initiation, followed by rapid decrease of serum PTH levels and a relative stabilization observed during the whole follow-up. The same figure is observed for the 645 patients still treated with cinacalcet-HCl at M12, for whom serum PTH course has been analysed.
FIGURE 3.
PTH course during the study. This figure shows the course of mean serum PTH levels, and the number of patients at each time point, during the 3-year follow-up for the 1268 patients included in the analysis.
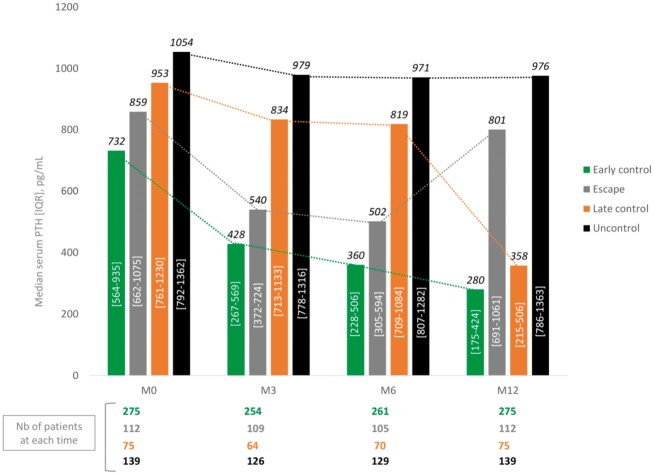
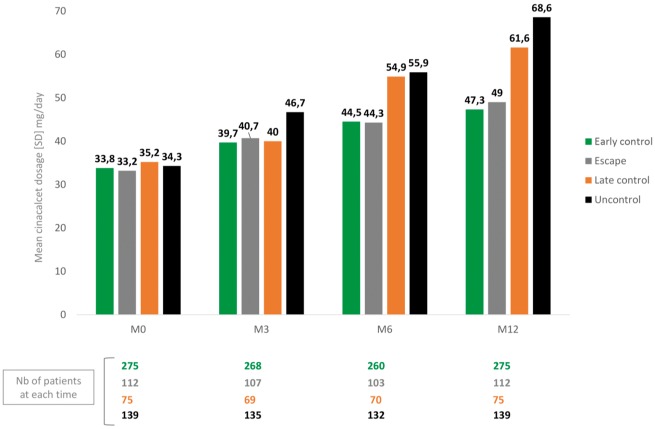
Considering the group of patients still treated with cinacalcet-HCl at M12 and for whom serum PTH dosages were available in the database at M0, M3/M6 and M12 (44 pts were excluded because of missing PTH at M3 or M6), we were able to identify four different groups of patients: those who were rapidly controlled (n = 275, 45.8%), those who were rapidly controlled but escaped after 6 months of treatment (n = 112, 18.6%), those with late control (n = 75, 12.5%) and those who remain uncontrolled during 12 months of treatment (n = 139, 23.1%) (Figure 4). The main characteristics of these patients are presented in Table 3. Patients in the ‘early control’ group seem to be older with a less severe SHPT at baseline. No clinically significant difference exists between the three other groups except for the patients in the ‘escape’ group who also seem to be younger than the others. The mean cinacalcet-HCl dosages at each time and in each group of patients are presented in Figure 5: as expected, the dosages increased more intensively in the ‘uncontrolled’ group of patients than in the others.
FIGURE 4.
Four different PTH trajectories. This figure shows the course of mean serum PTH levels for four different groups of patients: those who were rapidly controlled, those who were rapidly controlled but escaped after 6 months of treatment, those with late control and those who remain uncontrolled during 12 months of treatment.
Table 3.
Population characteristics according to serum PTH course between baseline and M12
| Parameters | Early control, N=275 | Escape, N=112 | Late control, N=75 | Uncontrolled, N=139 | |
|---|---|---|---|---|---|
| Male (%) | 167 (60.7) | 62 (55.4) | 42.0 (56) | 84 (60.4) | |
| Age, mean ± SE (years) (%) | 65.7 ± 14.3 | 61.3 ± 15.6 | 62.5 ± 15.7 | 60.4 ± 17.2 | |
| <40 | 6.9 | 10.7 | 9.3 | 13.7 | |
| 40–55 | 13.8 | 25.0 | 21.3 | 25.9 | |
| 55–70 | 34.5 | 33.9 | 38.7 | 28.1 | |
| 70–85 | 37.8 | 25.0 | 24.0 | 25.9 | |
| ≥85 | 6.9 | 5.4 | 6.7 | 6.5 | |
| BMI, mean ± SD (kg/m²) | 25.8 ± 5.3 | 24.7 ± 6.2 | 26.0 ± 5.1 | 25.0 ± 5.0 | |
| Obese, n (%) | 47 (18.5) | 14 (13.1) | 13 (18.6) | 19 (14.0) | |
| Diabetes mellitus, n (%) | 68 (24.7) | 32 (28.6) | 22 (29.3) | 30 (21.6) | |
| Dialysis duration, mean ± SD (years) (%) | 4.3 ± 5.7 | 3.8 ± 4.9 | 5.0 ± 7.2 | 4.5 ± 5.7 | |
| 0–1 | 29.5 | 28.6 | 34.7 | 33.8 | |
| 1–5 | 44.0 | 50.0 | 36.0 | 38.8 | |
| >5 | 26.5 | 21.4 | 29.3 | 27.3 | |
| Biological data at baseline | |||||
| Serum PTH, mean ± SD (pg/mL) | 787 ± 315 | 946 ± 431 | 1040 ± 391 | 1140 ± 492 | |
| Serum PTH, pg/mL (%) | |||||
| <600 | 26.5 | 17.9 | 4.0 | 7.2 | |
| 600–1000 | 55.3 | 48.2 | 56.0 | 37.4 | |
| ≥1000 | 18.2 | 33.9 | 40.0 | 55.4 | |
| Serum calcium, mean ± SD (mmol/L) | 2.18 ± 0.20 | 2.21 ± 0.19 | 2.21 ± 0.19 | 2.22 ± 0.19 | |
| Serum phosphate, mean ± SD (mmol/L) | 1.61 ± 0.55 | 1.65 ± 0.62 | 1.78 ± 0.61 | 1.80 ± 0.50 | |
Percentages are based on the total number of patients in each column for whom the answer is available.
FIGURE 5.
Mean daily cinacalcet-HCl dose in the four groups of different PTH trajectories. This figure shows the daily mean dose of cinacalcet-HCl in the four groups of patients with different PTH trajectories.
DISCUSSION
The main results of this study demonstrated that, in real-life clinical practice, 59% of dialysis patients with SHPT respond satisfactorily after 12 months of treatment by cinacalcet-HCl and maintained PTH values within the KDIGO recommended range. However, the other 41% of patients remained with PTH values above the upper limit recommended by KDIGO. Moreover, these patients with uncontrolled PTH were significantly younger and had higher PTH and calcaemia at baseline when compared with controlled patients.
The present study provides important information regarding the response and the way cinacalcet-HCl is used in real-life clinical practice in France. Its observational design and the use of the same Hemodial™ database allowed the assumption that all patients were harmoniously treated and monitored according to local practice at each one of the seven dialysis centres. There was no particularly or significantly different trend in demographic, clinical and biochemical data observed between centres. The studied population was also representative of the 45 000 French dialysis patients as evidenced by their comparable mean age, gender distribution, BMI, type of nephropathy and dialysis vintage as in the French registry Sytème d’Information Multi-Source pour l’Insuffisance Rénale Terminale [17].
At first glance, one of the most unexpected finding of this study was the irregularity in the implementation of KDIGO recommendations, in particular the frequency of PTH monitoring [4]. While KDIGO recommended assessing PTH every 3 months in CKD-5D patients, half of the patients could not be analysed at the M12 time point because of unmeasured or missing PTH data.
IQR serum PTH levels at the baseline in the present study ranged from 649 to 1090 pg/mL, reflecting a SHPT that could be considered as moderate in two-third of patients and as severe in the other third (PTH >1000 pg/L in 31% of them), and comparable to the distribution observed in many cinacalcet-HCl clinical trials, including ECHO, EVOLVE, BONAFIDE and PARADIGMA [7, 18, 19], but slightly more severe than in other studies including ACHIEVE, IMPACT, OPTIMA, ADVANCE and INCIDENT, where median baseline PTH was on average around 500–550 pg/mL [19–24]. At baseline, 45% of patients were receiving active vitamin D analogues and 52% intestinal phosphate binders, with sevelamer being the most common type of phosphate binders used. The use of these medications was slightly lower than that of other studies, certainly reflecting the national real-life clinical practice [25].
The primary criterion for the evaluation of cinacalcet-HCl efficacy in the control of SHPT has always been the decrease of serum PTH levels by ≥30% after 6 and 12 months of treatment. The results of the Mimosa study are consistent, confirmatory and extend these data. The proportion of patients whose serum PTH levels decreased by at least 30 and 50% at M12 were 57.2% and 41.1%, respectively. These results are ∼10% lower than those observed in prospective and more controlled studies such as OPTIMA [22], ECHO [7] and INCIDENT [24], where the proportion of patients with PTH decrease of 30% at M6 was of 71, 66 and 63%, respectively. This lower response seen in Mimosa study could partially be explained by the more severe SHPT and a longer duration of treatment with a part of non-compliance in our patients and cannot be associated with the prescription of a lower daily dose of cinacalcet-HCl. Indeed, the mean daily cinacalcet-HCL dose was of 54 mg, which was comparable to that of most controlled studies (OPTIMA: 57 mg/day, INCIDENT: 52 mg/day) [22, 24].
As previously mentioned and as demonstrated by the Mimosa results, ∼30–40% of HD patients treated by cinacalcet-HCL either do not decrease their serum PTH by 30% or remain with PTH values above 9×ULN. Importantly, not all resistant and/or hyporesponder patients behaved the same way. Four types of PTH trajectory could be identified: (i) PTH rapidly decreased from the cinacalcet-HCL initiation and remained controlled; (ii) PTH rapidly decreased but escaped after 6 months of treatment; (iii) PTH remained high during the first 6 months and then significantly decreased; and (iv) PTH remained high and uncontrolled during the 12 months of treatment (Figure 4). Patients with the first PTH trajectory were older and had a less severe SHPT at baseline, which might explain the rapid response to cinacalcet-HCl. No clinical or biochemical significant differences existed between the three other groups except for the patients with the fourth trajectory who were younger than the others. As expected, mean daily dose of cinacalcet-HCl increased more intensively in this group of patients than in the others.
What could be the reasons for the resistance or hyporesponsiveness to cinacalcet-HCL in these HD patients? Despite the absence of evidence in the present study, it could be hypothesized that (i) ionic and metabolic disorders [negative calcium balance, persistent hyperphosphataemia, hypomagnesaemia, metabolic acidosis, low vitamin D and decreased parathyroid calcium-sensing receptor (CaR), vitamin D receptor (VDR) and klotho/FGF-R], (ii) genetic abnormalities (polymorphisms/mutations in the CaR, VDR and other genes such as Arrestins, Dorphins, Filamin A, Ramps, G proteins, etc.) and (iii) compliance (incomplete or non-adherence to oral medication, intolerance and/or undesirable side effects) could be involved in the poor response to cinacalcet-HCl [11, 26].
The last point is certainly one of the most common and realistic causes of the poor response to cinacalcet-HCl in these patients. It has been demonstrated that 23% of incident HD patients discontinued cinacalcet-HCl treatment after 1 year of follow-up [15]. Indeed, the medical management of SHPT in HD patients is complex. Its chronic condition and the high number of associated comorbidities and lifelong medication regimens decrease compliance and increase medication-related undesirable adverse reactions and wrongly administered medications. These problems are ranked among the most common causes for hospitalization and mortality [27] and are deemed to be preventable in >70% of the cases [28]. Dialysis patients often do not have enough therapeutic education, which would allow them to improve their persistence, compliance and adherence with oral medication. Any delivered but not-taken cinacalcet-HCl treatment incurs both direct and indirect cost, resulting in a clear inefficacy. Thus, preventive studies evaluating the clinical, biochemical and economic impact of pharmacotherapeutic educational programme and follow-up are highly needed in this patient population. One of the possibilities to bypass the compliance in HD patients could be the prescription of calcimimetics administered intravenously. They could be considered as an opportunity to improve outcomes by optimizing treatment for uncontrolled SHPT, but this must be further evaluated [29].
The main limitation of this study is its retrospective and observational design, which resulted in several missing data, especially regarding other medications used for the treatment of SHPT and a loss of approximately half of the patient population at M12, mainly because of the unavailable PTH data. However, to our knowledge, this is the largest French ‘real-life’ study to assess the impact of cinacalcet-HCl pharmaceutical intervention in HD patients with SHPT patients.
In conclusion, in this clinical real-life study, 41.1% of HD patients with SHPT treated with cinacalcet-HCl remained with PTH values above the upper limit recommended by KDIGO guidelines after 12 months of treatment. Apart from the possibility of non-compliance, the severity of SHPT appears to be a major factor determining the response to cinacalcet-HCl treatment, reinforcing the importance to treat SHPT at earlier stages.
ACKNOWLEDGEMENTS
We would like to thank the Association des Néphrologues du Nord Parisien as well as all Mimosa study investigators from the seven dialysis centres for their collaboration and Philippe Petit for the extraction of all data from the Hemodial database. This study was partly presented as a poster at the ERA-EDTA meeting in May 2018.
FUNDING
The Mimosa study and present analysis were sponsored by Amgen Europe.
AUTHORS’ CONTRIBUTIONS
J.R. and P.U.-T. contributed to general editing and wrote the introduction, methods, results and conclusion, compiled the tables and figures, and carried out reference formatting. C.E. wrote the statistical methods and contributed to the results analysis and interpretation. D.T., V.G., A.H., O.C., H.H. and T.G. contributed to the results analysis and interpretation, discussion and general editing.
CONFLICT OF INTEREST STATEMENT
P.U.-T. received lecture fees from Amgen and Fresenius and travel support from Amgen and Hemotech. He is also principal investigator in clinical trials from Abbvie, Amgen, Astellas and GSK laboratories. J.R. received lecture fees from Amgen and Vifor, and travel support from Amgen and Vifor.
REFERENCES
- 1. Drueke TB. Hyperparathyroidism in chronic kidney disease In: De Groot LJ, Chrousos G, Dungan K. et al. (eds). Endotext. South Dartmouth, MA: MDText.com, Inc., 2000 [Google Scholar]
- 2. Arnold A, Brown MF, Ureña P. et al. Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest 1995; 95: 2047–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cozzolino M, Urena-Torres P, Vervloet MG. et al. Is chronic kidney disease-mineral bone disorder (CKD–MBD) really a syndrome? Nephrol Dial Transplant 2014; 29: 1815–1820 [DOI] [PubMed] [Google Scholar]
- 4.KDIGO. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009: S1–S130 [DOI] [PubMed] [Google Scholar]
- 5. Ketteler M, Block GA, Evenepoel P. et al. Executive summary of the 2017 KDIGO chronic kidney disease–mineral and bone disorder (CKD–MBD) guideline update: what’s changed and why it matters. Kidney Int 2017; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 6. Urena P, Frazao JM.. Calcimimetic agents: review and perspectives. Kidney Int Suppl 2003: S91–S96 [DOI] [PubMed] [Google Scholar]
- 7. Urena P, Jacobson SH, Zitt E. et al. Cinacalcet and achievement of the NKF/K-DOQI recommended target values for bone and mineral metabolism in real-world clinical practice—the ECHO observational study. Nephrol Dial Transplant 2009; 24: 2852–2859 [DOI] [PubMed] [Google Scholar]
- 8. Urena P, Fouque D, Brunet P. et al. [Cinacalcet treatment for secondary hyperparathyroidism in dialysis patients in real-world clinical practice—the ECHO observational study: French experience]. Nephrol Ther 2012; 8: 527–533 [DOI] [PubMed] [Google Scholar]
- 9. Hirai T, Nakashima A, Takasugi N. et al. Association of nodular hyperplasia with resistance to cinacalcet therapy for secondary hyperparathyroidism in hemodialysis patients. Ther Apher Dial 2010; 14: 577–582 [DOI] [PubMed] [Google Scholar]
- 10. Komaba H, Nakanishi S, Fujimori A. et al. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol 2010; 5: 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burnier M, Pruijm M, Wuerzner G. et al. Drug adherence in chronic kidney diseases and dialysis. Nephrol Dial Transplant 2015; 30: 39–44 [DOI] [PubMed] [Google Scholar]
- 12. Browne T, Merighi JR.. Barriers to adult hemodialysis patients’ self-management of oral medications. Am J Kidney Dis 2010; 56: 547–557 [DOI] [PubMed] [Google Scholar]
- 13. Covic A, Rastogi A.. Hyperphosphatemia in patients with ESRD: assessing the current evidence linking outcomes with treatment adherence. BMC Nephrol 2013; 14: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmid H, Hartmann B, Schiffl H.. Adherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: a critical review of the literature. Eur J Med Res 2009; 14: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Francisco ALM, Gillespie IA, Gioni I. et al. Anti-parathyroid treatment effectiveness and persistence in incident haemodialysis patients with secondary hyperparathyroidism. Nefrologia 2016; 36: 164–175 [DOI] [PubMed] [Google Scholar]
- 16. Chertow GM, Block GA, Correa-Rotter R. et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012; 367: 2482–2494 [DOI] [PubMed] [Google Scholar]
- 17. Lassalle M, Ayav C, Frimat L. et al. The essential of 2012 results from the French Renal Epidemiology and Information Network (REIN) ESRD registry. Nephrol Ther 2015; 11: 78–87 [DOI] [PubMed] [Google Scholar]
- 18. Chertow GM, Parfrey PS.. Cinacalcet for cardiovascular disease in patients undergoing dialysis. N Engl J Med 2013; 368: 1844–1845 [DOI] [PubMed] [Google Scholar]
- 19. Wetmore JB, Gurevich K, Sprague S. et al. A randomized trial of cinacalcet versus vitamin D analogs as monotherapy in secondary hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol 2015; 10: 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fishbane S, Shapiro WB, Corry DB. et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008; 3: 1718–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ketteler M, Martin KJ, Cozzolino M. et al. Paricalcitol versus cinacalcet plus low-dose vitamin D for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: study design and baseline characteristics of the IMPACT SHPT study. Nephrol Dial Transplant 2012; 27: 1942–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Messa P, Macario F, Yaqoob M. et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 2008; 3: 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raggi P, Chertow GM, Torres PU. et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011; 26: 1327–1339 [DOI] [PubMed] [Google Scholar]
- 24. Urena-Torres P, Bridges I, Christiano C. et al. Efficacy of cinacalcet with low-dose vitamin D in incident haemodialysis subjects with secondary hyperparathyroidism. Nephrol Dial Transplant 2013; 28: 1241–1254 [DOI] [PubMed] [Google Scholar]
- 25. Pelletier S, Roth H, Bouchet J-L. et al. [Changes in mineral and bone disorder management in a French cohort of hemodialysis patients between 2008 and 2012: The National Bone and Mineral Metabolism observatory (Photo-Graphe 2 and 3)]. Nephrol Ther 2016; 12: 171–177 [DOI] [PubMed] [Google Scholar]
- 26. Torres PU, Prié D, Beck L. et al. New therapies for uremic secondary hyperparathyroidism. J Ren Nutr 2006; 16: 87–99 [DOI] [PubMed] [Google Scholar]
- 27. Saran R, Bragg-Gresham JL, Rayner HC. et al. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int 2003; 64: 254–262 [DOI] [PubMed] [Google Scholar]
- 28. Baena MI, Faus MJ, Fajardo PC. et al. Medicine-related problems resulting in emergency department visits. Eur J Clin Pharmacol 2006; 62: 387–393 [DOI] [PubMed] [Google Scholar]
- 29. Block GA, Bushinsky DA, Cunningham J. et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017; 317: 146–155 [DOI] [PubMed] [Google Scholar]